Abstract
Exposure to ultraviolet B (UVB) irradiation from sunlight induces the upregulation of VEGF, a potent angiogenic factor that is critical for mediating angiogenesis-associated photodamage. However, the molecular mechanisms related to UVB-induced VEGF expression have not been fully defined. Here, we demonstrate that one of the catalytic subunits of the IκB kinase complex (IKK), IKKα, plays a critical role in mediating UVB-induced VEGF expression in mouse embryonic fibroblasts (MEFs), which requires IKKα kinase activity but is independent of IKKβ, IKKγ and the transactivation of NF-κB. We further show that the transcriptional factor AP-1 functions as the downstream target of IKKα that is responsible for VEGF induction under UVB exposure. Both the accumulation of AP-1 component, c-Fos and the transactivation of AP-1 by UVB require the activated IKKα located within the nucleus. Moreover, nuclear IKKα can associate with c-Fos and recruit to the vegf promoter regions containing AP-1-responsive element and then trigger phosphorylation of the promoter-bound histone H3. Thus, our results have revealed a novel independent role for IKKα in controlling VEGF expression during the cellular UVB response by regulating the induction of the AP-1 component and phosphorylating histone H3 to facilitate AP-1 transactivation. Targeting IKKα shows promise for the prevention of UVB-induced angiogenesis and the associated photodamage.
INTRODUCTION
Chronic exposure to solar UVB (280–320 nm wavelengths) irradiation is known to induce a variety of medical problems, including photoaging and skin cancers (1–3). Angiogenesis, the process of generating new capillary blood vessels, contributes largely to UVB-induced skin damage. It has been well demonstrated that UVB irradiation may induce vascular hyperpermeability, dermal blood vessel dilation and lead to an imbalance between angiogenic factors and inhibitors in the skin. The expression of vascular endothelial growth factor (VEGF), the main angiogenic factor, is increased by UVB exposure, which plays a critical role in mediating angiogenesis-associated photodamage and carcinogenesis (4,5). However, the molecular mechanisms related to UVB-induced VEGF expression are still largely unknown.
The IκB kinase (IKK) complex plays a critical role in the activation of the NF-κB pathway under physiological and pathological conditions (6). The two catalytic subunits of the IKK complex, IKKα and IKKβ, share structural similarity but trigger NF-κB activation by different mechanisms. IKKβ is involved in the activation of the canonical NF-κB pathway in response to inflammatory stimuli by inducing the phosphorylation and degradation of the NF-κB inhibitor, I-κB, which can subsequently release NF-κB and enable its nuclear translocation and activation; while IKKα plays a key role in activating the non-canonical NF-κB pathway upon B-cell activating factor (BAFF) or CD40 ligation by processing the NF-κB2/p100 precursor to the p52 subunit (6).
Besides the NF-κB and I-κB proteins, IKKα and IKKβ also target a variety of other substrates and therefore are involved in many physiological and pathological processes through NF-κB-dependent as well as independent mechanisms (6–8). Moreover, some specificity occurs between IKKα and IKKβ, because most of their substrates are exclusively regulated by one kinase but not the other (9–19).
Distinct IKKβ-independent roles of IKKα in the regulation of cell growth and immunity have been extensively described in recent studies (9–19). These novel functions of IKKα are mediated by the broad actions of its various downstream substrates. For example, IKKα is reported to phosphorylate histone H3 at certain NF-κB-dependent promoters in response to cytokine stimulation, which is critical for the optimal induction of the NF-κB-target genes expression (9,10). Moreover, IKKα also contributes to NF-κB transactivation by phosphorylating and activating the coactivator CBP and thereby switching the binding preference of CBP from p53 to NF-κB to favor the induction of NF-κB-mediated gene expression (11). In another report, IKKα is shown to be involved in derepressing NF-κB activity by phosphorylating and removing the corepressor SMRT from the chromatin, which facilitates NF-κB transcription (12,13). In contrast to these positive actions, IKKα is also reported to restricts NF-κB activation by triggering the turnover of NF-κB subunits p65 and c-Rel or suppressing the DNA-binding ability of p65 in response to inflammatory stimuli (14,15). These results have thus provided multiple lines of evidence for the specific roles of IKKα in the regulation of NF-κB transactivation. Moreover, some unique functions of IKKα that are unrelated to NF-κB activity have also been reported. For example, the studies on IKKα-null mice have revealed that IKKα is essential for the regulation of keratinocyte differentiation and the development of embryonic skin and skin cancers, which cannot be compensated for by IKKβ (16–18,20,21). In addition, IKKα can independently mediate tumor metastasis by inhibiting the transcription of the metastasis suppressor, Maspin (19). These results suggest that IKKα may play a considerably larger role that may go far beyond its well-known actions in the activation of the NF-κB pathway (7).
In the current study, we define a novel role for IKKα in mediating UVB-induced VEGF expression, which is independent of IKKβ, IKKγ and NF-κB activities, while eliciting c-Fos-dependent AP-1 transactivation. The established function of IKKα in mediating the inflammatory response to UVB exposure may provide a novel clue for the prevention of UVB-induced photodamage.
MATERIALS AND METHODS
Cells, plasmids and reagents
The wild-type (WT), IKKβ−/–, IKKα−/–, reconstituted IKKα−/–(IKKα) and IKKβ-KM mouse embryonic fibroblasts (MEFs), which are WT MEFs stably expressing the kinase mutant of IKKβ, have been described in previous studies (22,23). Both the VEGF- and HRE-luciferase reporter plasmids, in which the transcription of the luciferase reporter gene is driven by either ∼3.0 kb of the vegf promoter or the HIF1α-responsive elements, were provided by Dr Chuanshu Huang (New York University, USA). The AP-1-luciferase reporter plasmid was described in previous studies (23–25). The expression plasmids containing the IκBα mutant (HA-IκBα-AA) and the dominant-negative mutant of c-Jun (DN-c-Jun) have been described in previous studies (24,26). All expression plasmids for the IKKα mutants (including the deletion mutants, IKKα-NLS, IKKα-KM), WT c-Fos and the c-Fos mutant, c-Fos S32A were constructed by regular polymerase chain reaction (PCR) to obtain the DNA fragments. The details of the primers used for the construction are available upon request. Mouse p52 siRNA was ordered from Invitrogen-Life Technology (Beijing, China). Mouse IKKγ siRNA, mouse IKKα siRNA and their control siRNAs were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The antibodies against phospho-c-Jun (Ser73), c-Jun, phospho-c-Fos (Ser32), IKKα, IKKβ, p53, p52, GFP and β-actin were purchased from Cell Signaling Technology (Beverly, MA). The antibodies against HIF1α, c-Fos, IKKγ, HA and GAPDH were obtained from Santa Cruz Biotechnology. An anti-FLAG antibody was obtained from Sigma (St Louis, MO, USA). The anti-phospho-IKKα (Ser176/Ser180) antibody (07–837) was purchased from Millipore/Upstate (Billerica, MA, USA). MG132 and cyclohexamide (CHX) were purchased from Sigma (St. Louis, MO).
UVB apparatus and cell exposure
The UVB light source (CL-1000M Midrange Ultraviolet Crosslinker) was purchased from UVP Inc. (Upland, CA) and emitted UVB light at a wavelength of 302 nm. The cells were pretreated and irradiated at the desired doses of UVB irradiation as described previously (25–27).
Cell culture and transfection
Cl41 cells were maintained in modified Eagle's medium supplemented with 5% fetal bovine serum (FBS). All MEFs were maintained in Dulbecco's modified Eagle's medium supplemented with 10% FBS. The transfections were performed with the LipofectAMINE 2000 or LipofectAMINE™ RNAi MAX reagents (Life Technologies, Inc., Rockville, MD) according to the manufacturer's instructions. To establish the stable transfections, cultures were subjected to either G418 or hygromycin B selection. The stable transfectants were cultured in drug-free medium for at least two passages before being subjected to the various experiments.
Luciferase reporter assay
Cells were transiently or stably transfected with the luciferase reporter constructs. The luciferase activities were tested as described previously (26).
Reverse transcription polymerase chain reaction (RT–PCR)
Total RNA was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and cDNAs were synthesized with the ThermoScript™ RT–PCR system (Invitrogen, Carlsbad, CA, USA). To detect the induction of vegf and c-fos transcription, the following oligonucleotides were synthesized and used as specific primers to amplify the mouse vegf or c-fos cDNA: vegf: 5′-caacatcaccatgcagatcatg-3′ (forward) and 5′-tcaccgccttggcttgtcaca-3′ (reverse) and c-fos: 5′-ggtctcctccgtggccccat-3′ (forward) and 5′-cttgcaggcaggtcggtggg-3′ (reverse).
Enzyme-linked immunosorbent assay (ELISA)
VEGF production in the cell culture supernatants was quantified by a mouse VEGF immunoassay kit (R&D Systems, Inc., Minneapolis, MN, USA). In short, the cells were seeded in 24-well plates and cultured to 90–100% confluence. Cells were subjected to UVB exposure and the supernatants were collected at the indicated time points to detect the induction of VEGF production under UVB irradiation.
Western blot assay
Cellular protein extracts were prepared with cell lysis buffer (10 mM Tris–HCl, pH 7.4, 1% SDS, 1 mM Na3VO4) and resolved by SDS–PAGE. The signals were detected as described in our previous reports (22,23,25).
Construction of c-Fos shRNA expression plasmid and the establishment of the WT/c-Fos shRNA stable transfectants
The c-Fos shRNA expression plasmid was constructed by using the GeneSuppressor system (Imgenex Co., San Diego, CA). The target sequence of the mouse c-Fos shRNA was 5′-gcggagacagatcaacttgaa-3′. The construct was transfected into WT MEFs, and stable transfectants were established by G418 selection.
Immunoprecipitation
WT MEFs were incubated for 8 h following UVB exposure and lysed. Cell lysate was pre-cleared by incubation with protein A/G-agarose (Santa Cruz Biotechnology) and then incubated with 2 µg of anti-c-Fos antibody overnight at 4°C. Then, 40 µl of protein A/G-agarose were added to the complexes and incubated with agitation for another 1 h at 4°C. The samples were washed with the cell lysis buffer and subjected to the western blot assay. For the co-IP experiment, FLAG-wt IKKα or its mutant expression plasmids were transfected into 293T cells together with the GFP-c-Fos expression construct. Cell lysates were immunoprecipitated with an anti-GFP antibody and then the immunoprecipitants were subjected to the western blot assay with an anti-FLAG antibody.
ChIP assay
ChIP assay was performed using an EZ ChIP kit (Upstate Biotechnology) as described previously (25,27). To specifically amplify the regions containing the putative AP-1-responsive elements within the mouse VEGF promoter, PCR was performed with the following pairs of primers: Site A (−616 to −606): 5′-tcgtgccaacggtttgaggag-3′ (forward) and 5′-tctgtctgtccgtcagcgc-3′ (reverse); Site D (−2122 to −2115): 5′-agaagatgaaccgtaagcctag-3′ (forward) and 5′-aggcccttgttcctcctgatc-3′ (reverse); and Site E (−2900 to −2892): 5′-gagggttgagaatcacagctg-3′ (forward) and 5′-ggatggctctcttgtagcag-3′ (reverse). The following pair of ChIP primers was also designed to amplify the region covering the third exon of the vegf gene and was used as a negative control (NC) in this experiment. NC: 5′-gttcatggatgtctaccagcg-3′ (forward) and 5′-tgatgttgctctctgacgtgg-3′ (reverse).
RESULTS
UVB induces VEGF expression in the MEFs
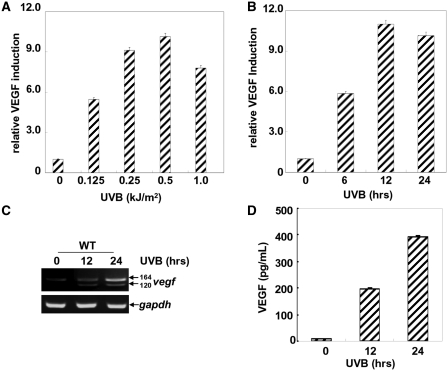
To determine the effects of UVB exposure on VEGF expression in the MEFs, a VEGF luciferase reporter plasmid containing ∼3.0 kb of the vegf promoter was transfected into WT MEFs, and then the stable transfectants were established. When these transfectants were exposed to different doses of UVB irradiation or a single dose of UVB irradiation for different time periods, we found both a dose- and time-dependent increase of vegf promoter-dependent luciferase activities in the WT MEFs (Figure 1A and B). The peak induction of VEGF expression can be observed upon 0.5 kJ/m2 of UVB irradiation (Figure 1A). Under the same exposure conditions, the inducible VEGF luciferase activity was detected at 6 h after irradiation and was sustained for 24 h (Figure 1B).
Figure 1.
UVB irradiation induces VEGF expression in MEFs. (A and B) A VEGF promoter-driven luciferase reporter plasmid was transfected into WT MEFs, and stable transfectants were established. The cells were exposed to different doses of UVB (A) or a single dose of UVB (0.5 kJ/m2) (B), and then VEGF luciferase activities were detected at 12 h (A) or the indicated time points (B) after UVB exposure. (C) WT MEFs were left untreated or treated with UVB irradiation (0.5 kJ/m2), and then the expression of vegf mRNAs were detected by an RT–PCR assay at the indicated time points after UVB exposure. (D) WT MEFs were exposed to UVB irradiation (0.5 kJ/m2), and then the production of VEGF in the culture supernatants was determined by ELISA at the indicated time points after UVB exposure.
Based on the alternative splicing of the eight coding exons, vegf mRNA exists as several different isoforms, of which, the vegf120, vegf164 and vegf188 isoforms are predominantly expressed (28). To further confirm the results obtained from the luciferase reporter assay, we analyzed the mRNA levels of the vegf isoforms in the absence or presence of UVB exposure by an RT–PCR assay. As shown in Figure 1C, the levels of vegf164 and vegf120 were increased significantly in response to UVB exposure. Furthermore, ELISA assay showed that a dramatic increase in VEGF production was observed in the culture supernatants of the MEFs within 24 h after UVB exposure (Figure 1D). Therefore, these results indicate that UVB effectively induces VEGF expression in the MEFs.
IKKα is involved in VEGF induction upon UVB exposure
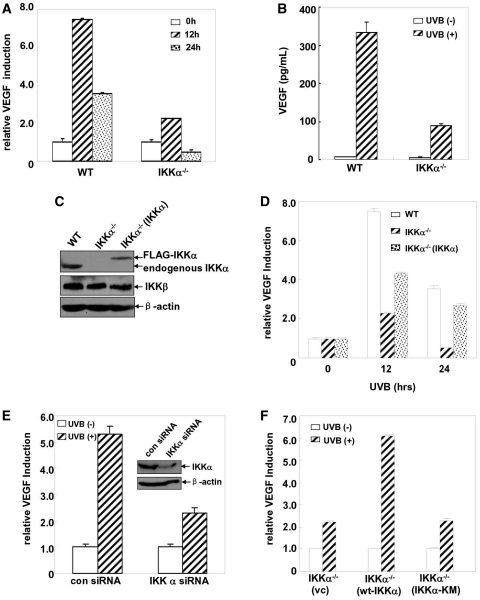
Data from our previous studies have demonstrated that IKKα can specifically mediate UVB-induced G0/G1 growth arrest and the apoptotic response independent of IKKβ in MEFs [(26) and Li, Y. et al. unpublished data], which indicates a specific role for IKKα in protecting against photodamage by UVB irradiation. Therefore, whether IKKα could mediate or protect against angiogenesis-related photodamage via regulating VEGF expression deserved further investigation. To this end, the VEGF luciferase reporter plasmid was stably transfected into the IKKα-null cells and then the induction of the VEGF luciferase activity was detected after UVB exposure. As shown in Figure 2A, we repeatedly observed a significant induction of the vegf promoter-dependent luciferase activity in the WT MEFs, while this response was suppressed in the IKKα−/– MEFs under the same conditions. ELISA assay further confirmed that the induction of VEGF production in the IKKα−/– MEFs was also significantly reduced compared with that in the WT cells (Figure 2B). These results indicate that IKKα is involved in UVB-induced VEGF expression in the MEFs.
Figure 2.
IKKα is required for UVB-induced VEGF expression. (A) WT and IKKα-null cells with the stable transfection of the VEGF promoter-driven luciferase reporter plasmid were treated with different doses of UVB and then the VEGF luciferase activities were detected at 12 h after UVB exposure. (B) WT and IKKα-null cells were exposed to UVB (0.5 kJ/m2) and then the production of VEGF in the culture supernatants 24 h after exposure was detected by ELISA. (C) The identification of the reconstituted IKKα−/–(IKKα) MEFs. (D) WT, IKKα−/– and IKKα−/–(IKKα) cells were transiently transfected with a VEGF promoter-driven luciferase reporter plasmid and then exposed to UVB (0.5 kJ/m2). Thirty-six hours after transfection, VEGF luciferase activities were detected at the indicated time points after UVB exposure. (E) Cl41 cells were transfected with a VEGF promoter-driven luciferase reporter plasmid in combination with mouse IKKα siRNA or the control siRNA. Thirty-six hours after transfection, the cells were subjected to UVB irradiation (0.5 kJ/m2) and the VEGF luciferase activities were detected at 12 h after UVB exposure. (F) IKKα-null cells were transfected with a VEGF promoter-driven luciferase reporter plasmid in combination with the control vector or WT IKKα or IKKα-KM expression plasmids, respectively. The cells were subjected to UVB irradiation (0.5 kJ/m2) 36 h after transfection, and the VEGF luciferase activities were detected at 24 h after UVB exposure.
To further confirm that the impairment of VEGF induction in the UVB-treated IKKα−/– MEFs was indeed due to the specific deficiency of ikkα expression rather than non-specific gene changes during the establishment of the IKKα−/– MEFs, we next detected whether VEGF induction could be restored in IKKα-reconstituted IKKα-null cells. As shown in Figure 2C and D, re-introduction of the ikkα gene into the IKKα−/– MEFs partially rescued the induction of VEGF expression. These findings indicate that IKKα plays a critical role in mediating UVB-induced VEGF expression in MEFs.
To clarify whether IKKα can exert a general effect on the regulation of VEGF expression in the cellular UVB response, we next detected the levels of VEGF induction in the UVB-treated mouse epidermal cells, Cl41, in the absence or presence of IKKα expression. We found that knockdown of IKKα expression in Cl41 cells significantly inhibited VEGF induction upon UVB exposure (Figure 2E). These data suggest that the role for IKKα in regulating VEGF induction might be a general effect of the UVB response.
To address whether IKKα kinase activity is required for mediating VEGF induction in the UVB response, we compared the induced VEGF expression in the wt IKKα to that of the kinase mutant of IKKα (IKKα-KM)-reconstituted IKKα−/– cells. As shown in Figure 2F, overexpression of IKKα-KM in the IKKα−/– cells failed to rescue the upregulation of VEGF expression to similar levels as the wt IKKα. Therefore, we conclude that IKKα kinase activity is required for the induction of VEGF expression in response to UVB irradiation.
The role for IKKα in mediating VEGF induction is independent of IKKβ, IKKγ and NF-κB activities
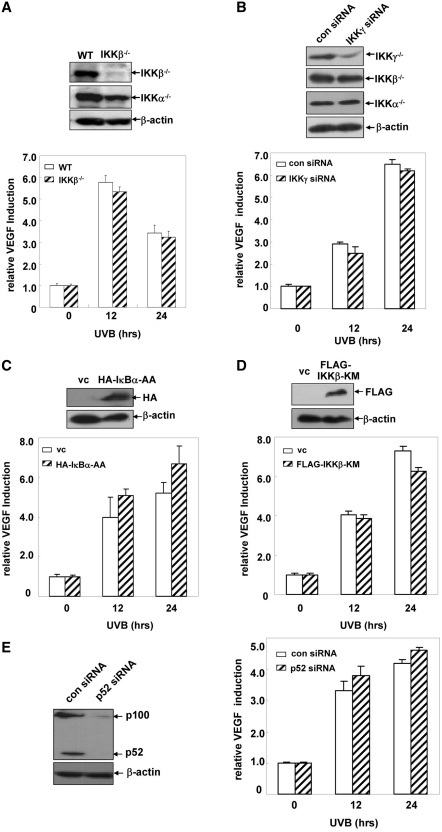
To clarify whether IKKα-dependent VEGF upregulation upon UVB exposure is related to IKKβ, IKKγ and NF-κB activities, we first compared the induction of VEGF expression in the WT and IKKβ−/– MEFs. As shown in Figure 3A, the levels of VEGF induction were similar in the UVB-treated WT and IKKβ−/– MEFs, which excluded the involvement of IKKβ in regulating VEGF expression in the UVB response. Then we investigated whether IKKγ contributed to UVB-induced VEGF expression by using IKKγ siRNA. As shown in Figure 3B, specific knockdown of IKKγ expression in the WT MEFs did not alter the induced expression of VEGF. Furthermore, C-terminal deletion mutant of IKKα with the defective IKKγ-binding domain rescued VEGF induction in the IKKα-null cells to a comparable level as the WT IKKα did (Supplementary Figure S1B). These results indicate that IKKγ is not involved in VEGF induction upon UVB exposure, either. Next, we detected VEGF expression in the WT MEFs overexpressing IκBα-AA, an IκBα mutant which can block IκBα degradation and the classical NF-κB pathway activation in response to a variety of stimuli including UVB irradiation (26,29). As shown in Figure 3C, overexpression of IκBα-AA did not noticeably affect UVB-induced upregulation of VEGF expression in the MEFs, indicating that activation of the classical NF-κB pathway is not the major signaling event that controls VEGF expression in the UVB response. To further confirm the above results, we next detected the levels of VEGF induction in WT MEFs stably transfected with the kinase mutant of IKKβ, IKKβ-KM, which can specifically inhibit IKKβ and the downstream classical NF-κB pathway activation as demonstrated in our previous studies (22,26). As shown in Figure 3D, compared with the control vector-transfected WT MEFs, ectopic overexpression of IKKβ-KM did not change the levels of VEGF induction in response to UVB irradiation. Finally, to clarify whether VEGF expression can be affected by changes in the activation status of the alternative NF-κB pathway, we compared the levels of VEGF expression in WT MEFS transfected with p52 siRNA or its control siRNA. As shown in Figure 3E, no obvious changes in the VEGF induction was observed in the presence or absence of p52 expression, which indicate that UVB-induced VEGF expression is unrelated to the activation status of the alternative NF-κB pathway. Taken together, we conclude from these data that the role for IKKα in upregulating VEGF expression during UVB exposure is independent of IKKβ, IKKγ and NF-κB activities.
Figure 3.
The effect of IKKα in regulating UVB-induced VEGF expression is unrelated to IKKβ, IKKγ and NF-κB activities. (A) WT and IKKβ−/– MEFs with a stable transfection of a VEGF promoter-driven luciferase reporter plasmid were treated with UVB (0.5 kJ/m2), and then the VEGF luciferase activities were detected at the indicated time points after UVB exposure. (B) WT MEFs with a stable transfection of a VEGF promoter-driven luciferase reporter plasmid were transfected with the mouse IKKγ siRNA or its control siRNA and then subjected to UVB irradiation 36 h after transfection. The VEGF luciferase activities were detected at the indicated time points after UVB exposure. (C) The expression plasmids containing HA-IκBα-AA or the control vector were transiently transfected into the WT MEFs that were stably transfected with a VEGF promoter-driven luciferase reporter plasmid. The cells were exposed to UVB irradiation (0.5 kJ/m2) 36 h after transfection, and the induction of VEGF luciferase activities were detected at the indicated time points after UVB exposure. (D) A VEGF promoter-driven luciferase reporter plasmid was transiently transfected into WT MEFs that were stably transfected with FLAG-IKKβ-KM. The cells were exposed to UVB irradiation (0.5 kJ/m2) 36 h after transfection, and the induction of VEGF luciferase activities were detected at the indicated time points after UVB exposure. (E) WT MEFs with a stable transfection of a VEGF promoter-driven luciferase reporter plasmid were transfected with the mouse p52 siRNA or its control siRNA. The cells were subjected to UVB irradiation (0.5 kJ/m2) 36 h after transfection, and the VEGF luciferase activities were detected at the indicated time points after UVB exposure.
UVB induces VEGF expression via an AP-1-dependent and a HIF-1α-independent pathway
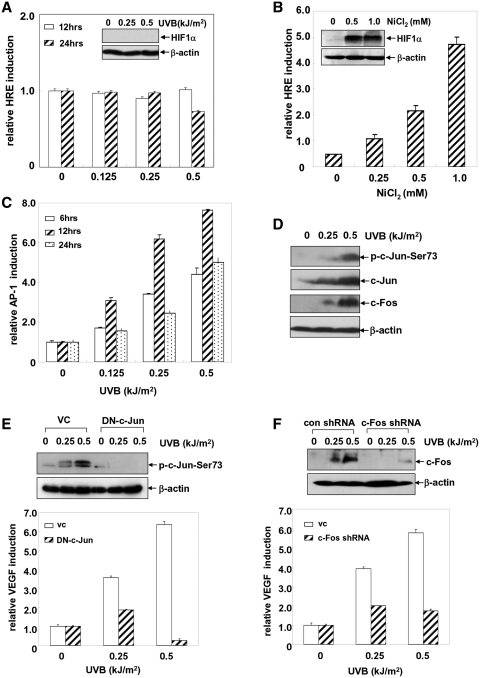
We next investigated the signaling pathway(s) by which IKKα mediated the upregulation of VEGF expression in the UVB response. It is well accepted that HIF1α plays a critical role in inducing the transcription of vegf by binding to the hypoxia response element (HRE) within the vegf promoter in the tumor cells (30). However, whether this type of inducible activity is involved in the UVB-induced VEGF expression in the MEFs is unclear. To answer this question, an HRE luciferase reporter plasmid was introduced into WT MEFs and then the induction of HRE-dependent luciferase activities in response to UVB exposure was detected. As shown in Figure 4A, we did not observe a notable induction of HRE-dependent luciferase activity and the accumulation of HIF1α protein in the WT MEFs exposed to the different doses of UVB irradiation at the indicated time points. As a positive control, NiCl2 effectively caused accumulation of HIF1α and upregulation of vegf transcription in the same cells (Figure 4B). These results suggest that HIF1α is not involved in UVB-induced VEGF expression in the UVB-treated MEFs.
Figure 4.
UVB irradiation induces VEGF expression via an AP-1-dependent and HIF1α-independent pathway. (A and B) An HRE luciferase reporter plasmid was introduced into the WT MEFs and then the stable transfectants were established. The cells were exposed to different doses of UVB (A) or NiCl2 (B), and then the induction of HRE-mediated luciferase activities was detected at 12 or 24 h after stimulation. The levels of HIF1α induction were detected under the same exposure conditions. (C) WT MEFs with the stable transfection of an AP-1 luciferase reporter plasmid were exposed to different doses of UVB and the AP-1 luciferase activities were determined at the indicated time points after exposure. (D) WT MEFs were exposed to different doses of UVB and the activation of c-Jun and the induction of c-Fos expression were detected at 12 h after UVB exposure. (E) WT MEFs that were stably transfected with the dominant-negative mutant of c-Jun (TAM67) or its control vector were exposed to different doses of UVB irradiation, and then the activation of c-Jun was determined at 12 h after UVB exposure. Then the VEGF luciferase reporter plasmid was transiently transfected into WT/TAM67 MEFs and control cells. The cells were exposed to different doses of UVB irradiation 36 h after transfection, and the induction of VEGF luciferase activities was detected at 12 h after exposure. (F) WT MEFs were transfected with the c-Fos shRNA or the control GFP shRNA and then the stable transfectants were established. The cells were exposed to different doses of UVB and the induction of c-Fos was detected at 12 h after UVB stimulation. Then, the VEGF luciferase reporter plasmid was transiently transfected into WT/c-Fos shRNA MEFs or control cells. The cells were exposed to different doses of UVB irradiation 36 h after transfection, and the induction of the VEGF luciferase activities was detected at 12 h after exposure.
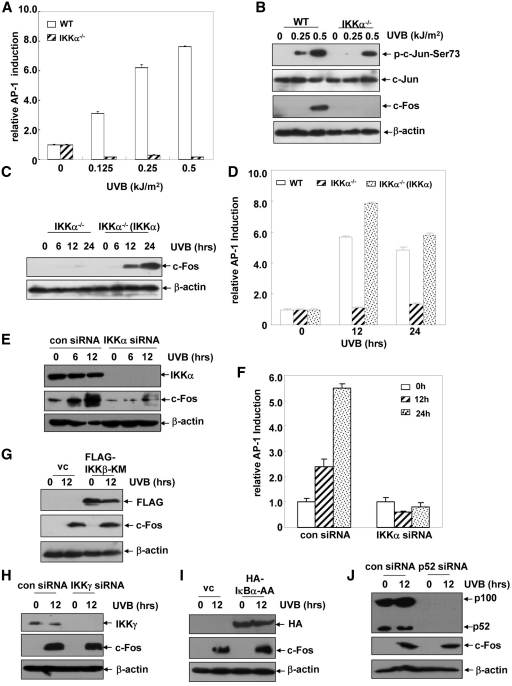
Due to the multiple AP-1-binding sites within the vegf promoter, we next speculated that AP-1 might be involved in regulating UVB-induced VEGF expression. To address this possibility, WT MEFs stably transfected with the AP-1 luciferase reporter plasmid, were exposed to different doses of UVB irradiation, and then the transactivation of AP-1 was detected at the different time points after UVB exposure. As shown in Figure 4C, UVB irradiation could induce both a dose- and time-dependent increase in AP-1 transactivation in the WT MEFs, which was accompanied by a significant phosphorylation of c-Jun and an induction of c-Fos expression under the same conditions (Figure 4D). However, no obvious changes in the activation status or the expression levels of the other AP-1 components (JunD, FosB, Fra-1, ATF2) were observed (data not shown). These results indicate that UVB exposure induces transactivation of AP-1 mainly comprised of c-Jun and c-Fos.
Next, we tested the contribution of AP-1 activation to VEGF expression upon UVB treatment. To this end, the VEGF luciferase reporter plasmid was transiently introduced into WT MEFs overexpressing DN-c-Jun, which were established previously (23). As shown in Figure 4E, DN-c-Jun overexpression significantly inhibited the activation of c-Jun induced by UVB, while VEGF induction was also greatly impaired under the same conditions. Then, a c-Fos shRNA expression plasmid was constructed and transfected into WT MEFs to establish the stable c-Fos shRNA transfectants. We found that UVB-induced c-Fos expression can be almost completely blocked by transfection of c-Fos shRNA into the WT MEFs. Under the same conditions, the inducible expression of VEGF was significantly inhibited compared with that of the control shRNA-transfected cells (Figure 4F). Taken together, these data indicate that UVB irradiation induces VEGF expression through an AP-1(c-Jun/c-Fos)-dependent while a HIF1α-independent manner.
c-Fos functions as the key downstream target of IKKα in regulating VEGF induction upon UVB exposure
To further investigate the functional link between IKKα and AP-1 transactivation in the UVB-induced VEGF expression, we next compared the AP-1-dependent luciferase activity in the WT and IKKα−/– MEFs. We found that UVB-induced AP-1 transactivation was almost completely blocked by ikkα deficiency (Figure 5A). Under the same conditions, c-Jun phosphorylation was slightly reduced, while the induction of c-Fos expression was entirely absent in the IKKα−/– MEFs (Figure 5B). These data suggested that AP-1 functioned as a downstream target of IKKα in the cellular UVB response and that the expression of one of the AP-1 components, c-Fos, is tightly regulated by IKKα. To further confirm these results, we next detected the expression of c-Fos and AP-1 transactivation in the reconstituted IKKα−/–(IKKα) cells. As shown in Figure 5C, reconstitution of IKKα efficiently restored the strong induction of c-Fos expression in response to UVB irradiation, which was accompanied by a recovery of AP-1 transactivation, in the IKKα−/–(IKKα) cells (Figure 5D). These results indicate that c-Fos is the key downstream target of IKKα that comprises the AP-1 heterodimer and regulates VEGF expression in the UVB-treated fibroblasts. We further proved that JNKs were only responsible for UVB-induced c-Jun activation, but not involved in c-Fos induction under the same conditions (Supplementary Figure S2). Therefore, it seems that JNKs and IKKα synergistically mediate AP-1 transactivation via regulating c-Jun activation and c-Fos induction respectively in the cellular UVB response.
Figure 5.
c-Fos functions as the key downstream target of IKKα in regulating VEGF induction upon UVB exposure. (A) WT and IKKα-null cells with a stable transfection of the AP-1 luciferase reporter plasmid were treated with different doses of UVB and then the AP-1 luciferase activities were detected at 12 h after UVB exposure. (B) WT and IKKα-null cells were exposed to different doses of UVB and then the activation of c-Jun and the expression of c-Fos were determined at 12 h after UVB exposure. (C) IKKα−/– and IKKα−/–(IKKα) cells were exposed to UVB (0.5 kJ/m2) and the induction of c-Fos was detected at the indicated time points after exposure. (D) An AP-1 luciferase reporter plasmid was transfected into WT, IKKα−/–and IKKα−/–(IKKα) cells. The cells were subjected to UVB irradiation (0.5 kJ/m2) 36 h after transfection, and then the AP-1 luciferase activities were detected at the indicated time points after UVB exposure. (E) Cl41 cells were transfected with mouse IKKα siRNA or the control siRNA and then subjected to UVB irradiation (0.5 kJ/m2) 36 h after transfection. The induction of c-Fos expression was detected at the indicated time points after UVB exposure. (F) Cl41 cells were transfected with the AP-1 luciferase reporter plasmid in combination with mouse IKKα siRNA or the control siRNA, and then subjected to the UVB irradiation (0.5 kJ/m2) 36 h after transfection. The AP-1 luciferase activities were detected at 12 h after UVB exposure. (G) WT MEFs with a stable transfection of FLAG-IKKβ-KM and the control cells were exposed to UVB (0.5 kJ/m2), and then the induction of c-Fos expression was detected at 12 h after UVB exposure. (H) WT MEFs were transfected with the mouse IKKγ siRNA or its control siRNA and then subjected to UVB irradiation (0.5 kJ/m2) 36 h after transfection. The induction of c-Fos expression was detected at12 h after UVB exposure. (I) The HA-IκBα-AA expression plasmid or the control vector were transiently transfected into the WT MEFs. The cells were exposed to UVB irradiation (0.5 kJ/m2) 36 h after transfection, and then the induction of c-Fos expression was detected at 12 h after UVB exposure. (J) WT MEFs were transfected with a mouse p52 siRNA or its control siRNA and then subjected to UVB irradiation (0.5 kJ/m2) 36 h after transfection. The induction of c-Fos expression was detected at 12 h after UVB exposure.
In the following experiments, we further found that knocking down IKKα expression in the Cl41 cells can also significantly block c-Fos induction and AP-1 transactivation upon UVB exposure (Figure 5E and F). These results are consistent with the reduced VEGF induction in the IKKα siRNA-transfected Cl41 cells (Figure 2E) and suggest the general effect of IKKα in mediating UVB-induced VEGF expression through a c-Fos/AP-1-dependent pathway.
We next detected the roles for IKKβ and IKKγ in the UVB-induced c-Fos expression. As shown in Figure 5G and H, neither IKKβ-KM overexpression nor IKKγ siRNA transfection could alter the levels of c-Fos induction upon UVB exposure. And the C-terminal deletion mutant of IKKα with the defective IKKγ-binding domain efficiently rescued c-Fos induction in the IKKα-null cells (Supplementary Figure S1A). These data indicate that IKKα-mediated c-Fos expression is unrelated to IKKβ and IKKγ activities. To further address whether NF-κB transactivation is involved in UVB-induced c-Fos expression, we detected the changes in the levels of c-Fos expression after the activation of the classical or alternative NF-κB pathway was inhibited by IκBα-AA or p52 siRNA, respectively. We found that c-Fos induction was not affected by the changes in activation of either NF-κB pathway (Figure 5I and J). Therefore, we conclude that the role for IKKα in regulating c-Fos expression during UVB irradiation is unrelated to the activation status of the IKKβ, IKKγ and NF-κB-dependent pathways, which is consistent with the mechanism of VEGF induction under the same UVB exposure conditions (Figure 2).
IKKα regulates N-terminal phosphorylation and protein stabilization of c-Fos in the cellular UVB response
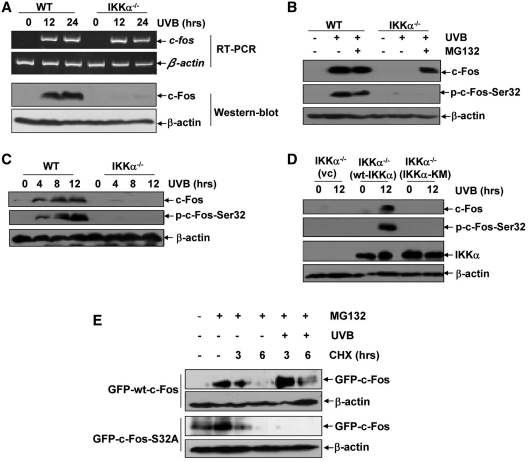
The induction of c-Fos expression can be regulated at multiple levels (31–35). To clarify the mechanism through which IKKα regulates c-Fos induction in the cellular UVB response, we first compared c-fos gene transcription and the induction of c-Fos protein expression in the WT and IKKα−/– cells upon UVB exposure. As shown in Figure 6A, although the increase in c-Fos protein expression was almost completely blocked by ikkα deficiency, the induction of c-fos mRNA transcription did not exhibit detectable differences between WT and IKKα−/– cells under the same UVB exposure conditions. These findings suggest that UVB-induced c-Fos expression might occur at a post-transcriptional level, most possibly results from changes in its protein stability. To test this hypothesis, WT and IKKα−/– cells were pretreated with MG132, an established proteasome inhibitor that disrupts protein degradation, and then exposed to UVB irradiation. As indicated in Figure 6B, pretreatment with MG132 resulted in a significant accumulation of c-Fos protein in the UVB-irradiated IKKα−/– cells. These results demonstrate that IKKα is involved in increasing c-Fos protein stability by actively blocking its degradation via the proteasome in the cellular UVB response.
Figure 6.
IKKα regulates N-terminal phosphorylation and protein stabilization of c-Fos in the UVB response. (A) WT and IKKα−/– cells were exposed to UVB (0.5 kJ/m2), and the induction of c-fos mRNA transcription and protein expression were detected by RT–PCR and immunoblot assay, respectively. (B) WT and IKKα−/– cells were pretreated with MG132 (10 µM) for 4 h followed by exposure to UVB (0.5 kJ/m2). The induction of c-Fos phosphorylation and expression was detected at 6 h after UVB exposure. (C) WT and IKKα−/– cells were exposed to UVB (0.5 kJ/m2), and the induction of c-Fos phosphorylation and expression were detected at the indicated time points after UVB exposure. (D) IKKα-null cells were transfected with the control vector, WT IKKα or IKKα-KM expression plasmids, respectively. The cells were subjected to UVB irradiation (0.5 kJ/m2) 36 h after transfection, and the induction of c-Fos phosphorylation and expression were detected at 12 h after UVB exposure. (E) The expression plasmids containing WT c-Fos and c-Fos S32A (with GFP tags) were constructed and transfected into WT MEFs, respectively. Twenty-four hours after transfection, the cells were pretreated with MG132 (10 µM) for 4 h followed by exposure to CHX (10 µM) at the indicated time periods in the absence or presence of UVB irradiation (0.5 kJ/m2) after MG132 withdrawal. The degradation dynamics of c-Fos was detected with an anti-GFP antibody.
It has been well demonstrated that the turnover of c-Fos proteins is tightly controlled by phosphorylation of the serines and/or threonines located at both the N- and C-termini (Ser32, Thr232 and Ser374) under various conditions (31,32,36,37). Therefore, to address whether IKKα regulates c-Fos protein stability via a phosphorylation-dependent manner in the UVB response, we compared the phosphorylation of c-Fos at the residues indicated above in the WT and IKKα−/– cells by using specific anti-phospho-c-Fos antibodies. As shown in Figure 6C, accompanied by a significant increase in c-Fos expression, UVB exposure could induce a strong phosphorylation at Ser32 in the WT MEFs. However, this response was almost completely blocked by ikkα deficiency under the same conditions. No detectable phosphorylation at the other serine or threonine residues on c-Fos was observed in the UVB-treated WT or IKKα−/– cells (data not shown). Furthermore, we found that although MG132 pretreatment efficiently accumulated c-Fos in the IKKα-null cells, no induction of c-Fos phosphorylation at Ser32 was observed upon UVB irradiation without ikkα gene expression (Figure 6B). In Addition, re-introduction of the WT ikkα gene into the IKKα−/– cells significantly restored the induced phosphorylation of c-Fos at Ser32, which was accompanied by c-Fos expression, upon UVB irradiation. However, these responses were not observed in the IKKα-KM-reconstituted IKKα−/– cells under the same conditions (Figure 6D). These results indicate that IKKα regulates c-Fos protein stability by inducing phosphorylation at its N-terminus.
To further confirm the contribution of Ser32 phosphorylation to the increased c-Fos protein stability in the UVB response, WT c-Fos and its mutant c-Fos S32A expression plasmids were constructed. Then we designed an experimental system to analyze the effect of UVB exposure on the degradation of wt c-Fos and c-Fos S32A. WT cells transfected with wt c-Fos or c-Fos S32A were pre-treated with MG132 and then exposed to the protein synthesis inhibitor, CHX, for the different time periods in the absence or presence of UVB irradiation after MG132 withdrawal. As shown in Figure 6E, MG132 pretreatment can accumulate exogenous wt c-Fos and c-Fos S32A at a comparable level. After withdrawal of MG132 and CHX exposure, the pre-accumulated wt c-Fos and c-Fos S32A showed similar time-dependent degradation dynamics in the absence of UVB exposure. However, upon UVB irradiation, wt c-Fos was more stable and its degradation was significantly inhibited, but c-Fos S32A degraded more rapidly than wt c-Fos under the same UVB irradiation conditions. These data suggest that IKKα-mediated c-Fos phosphorylation at Ser32 protects this protein from degradation via proteasome in the UVB response.
UVB exposure induces phosphorylation of nuclear IKKα, which can associate with c-Fos and recruit to the vegf promoter to trigger the phosphorylation of promoter-bound histone H3
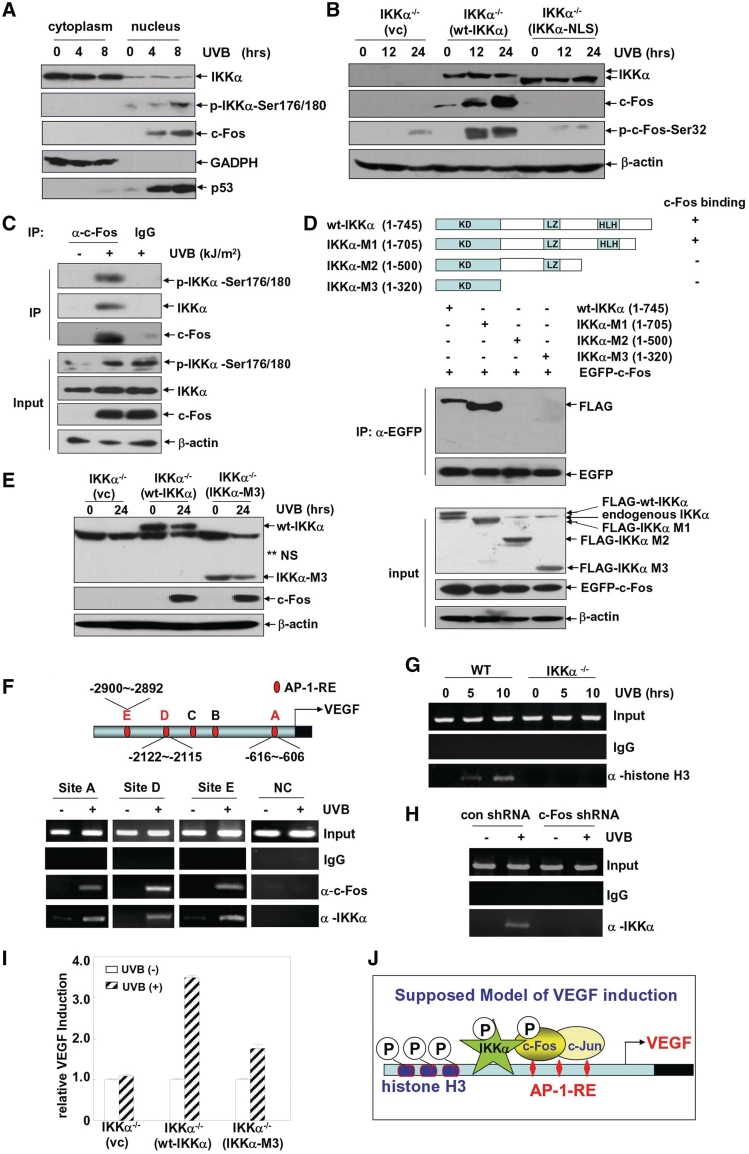
Most of the specific roles for IKKα that are independent of IKKβ in the regulation of gene expression are related to the features of nuclear localization or translocation of IKKα (9,10,38,39). Therefore, in the following experiments, we detected the nuclear/cytoplasmic distribution of IKKα in the UVB-treated MEFs. We found that endogenous IKKα predominantly accumulated in the cytoplasm of the MEFs with no obvious nuclear translocation of IKKα detected after UVB exposure. However, the immunoblotting analysis with the anti-phospho-IKKα antibody revealed that a nuclear pool of IKKα was strongly phosphorylated upon UVB irradiation. Under the same conditions, we also found an accumulation of c-Fos in the nucleus (Figure 7A). Since IKKα kinase activity is critical for mediating c-Fos induction (Figure 2F), we speculated that it might be the activated form of IKKα located within the nucleus that was responsible for the phosphorylation-dependent expression of c-Fos. To test this possibility, IKKα bearing three point mutations within the endogenous NLS (IKKα-NLS) was constructed based upon a previously published report (40), and its ability to mediate c-Fos expression under UVB irradiation was detected. As expected, when WT IKKα and IKKα-NLS were expressed at comparable levels in the IKKα-null cells, IKKα-NLS was only found in the cytoplasm, whereas wt IKKα distributed in both the cytoplasm and nucleus (Supplementary Figure S3A). Under these conditions, we observed a strong recovery of c-Fos phosphorylation and expression in the wt IKKα-reconstituted IKKα−/– cells. All these responses were absent in the IKKα-NLS-transfected IKKα−/– cells (Figure 7B). Accordingly, VEGF induction was also inhibited in the IKKα-NLS-transfected IKKα−/– cells compared to that in the wt IKKα-reconstituted IKKα−/– cells (Supplementary Figure S3B). These findings thus indicate that the nuclear localization of IKKα is critical for mediating c-Fos induction and VEGF expression in the UVB response.
Figure 7.
UVB-induced histone H3 phosphorylation at the AP-1-responsive elements within the vegf promoter requires IKKα recruitment and contributes largely to the UVB-induced VEGF expression. (A) WT MEFs were exposed to a single dose (0.5 kJ/m2) of UVB for the time indicated, and then cytoplasmic and nuclear proteins were prepared. The subcellular distribution of c-Fos, IKKα and phosphorylated IKKα were detected by a western blot assay. The expression of GAPDH and p53 were used as markers for cytoplasmic and nuclear extracts, respectively. (B) IKKα-null cells were transfected with a control vector, WT IKKα or IKKα-NLS expression plasmids, respectively. The cells were subjected to UVB irradiation (0.5 kJ/m2) 36 h after transfection, and the induction of c-Fos phosphorylation and expression were detected at the indicated time points after UVB exposure. (C) WT MEFs were left untreated or exposed to UVB (0.5 kJ/m2) for 10 h. Nuclear extracts were prepared and immunoprecipitated with an anti-c-Fos antibody or a rabbit IgG, and then the immunoprecipitants were analyzed by a western blot assay with anti-c-Fos, anti-IKKα and anti-phosphorylated IKKα antibodies. (D) WT IKKα and its deletion mutants were constructed and co-transfected with GFP-c-Fos into 293T cells. Cell lysate was immunoprecipitated with an anti-GFP antibody, and the immunoprecipitants were analyzed by a western blot assay with an anti-FLAG antibody to detect the c-Fos-binding ability of the IKKα mutants. (E) IKKα-null cells were transfected with a control vector, WT IKKα or IKKα-M3 expression plasmids, respectively. The cells were subjected to UVB irradiation (0.5 kJ/m2) 36 h after transfection, and the induction of c-Fos expression was detected at 12 h after UVB exposure. (F) Soluble chromatin was prepared from WT MEFs either untreated or exposed to UVB irradiation (0.5 kJ/m2) for 10 h, and then immunoprecipitated with anti-c-Fos and IKKα antibodies, respectively. The DNA extractions were amplified with primers that covered the putative AP-1-binding sites (A, D and E) within the mouse vegf promoter or the coding sequence in the third exon of the vegf gene, which was used as the negative control in this experiment and marked as NC. (G) Soluble chromatin was prepared from WT and IKKα-null MEFs either untreated or exposed to UVB (0.5 kJ/m2) for 10 h, and then immunoprecipitated with an anti-phospho-histone H3 (Ser10) antibody. The DNA extractions were amplified with primers that covered the putative AP-1-binding site D within the mouse vegf promoter. (H) WT MEFs stably transfected with c-Fos shRNA or the control shRNA were either untreated or exposed to UVB irradiation (0.5 kJ/m2) for 10 h. Then the soluble chromatin was prepared and immunoprecipitated with an anti-IKKα antibody. The DNA extractions were amplified with primers that covered the putative AP-1-binding site D within the mouse vegf promoter. (I) IKKα-null cells were transfected with a VEGF promoter-driven luciferase reporter plasmid in combination with a control vector, WT IKKα or IKKα-M3 expression plasmids, respectively. The cells were subjected to UVB irradiation 36 h after transfection, and the VEGF luciferase activities were detected at 24 h after UVB exposure. (J) The proposed model for the action of IKKα in mediating the UVB-induced VEGF expression. IKKα is required for UVB-induced phosphorylation and accumulation of c-Fos. Moreover, UVB exposure induces the activation of nuclear IKKα, which can recruit to the vegf promoter regions containing AP-1-binding sites by binding with c-Fos and triggers the phosphorylation of promoter-associated histone H3 to facilitate AP-1 transactivation.
Since the activated form of IKKα and the accumulated c-Fos are colocalized within the nucleus, we wondered whether these two proteins might function cooperatively. To address this possibility, we first detected the potential interaction of p-IKKα with c-Fos by co-IP. As shown in Figure 7C, p-IKKα was observed in the UVB-treated c-Fos immunoprecipitates but not in the control IgG and UVB-untreated groups. These results indicate that UVB exposure can induce the accumulation of c-Fos within the nucleus and trigger its interaction with the nuclear IKKα. To clarify whether the interaction of IKKα with c-Fos is required for c-Fos induction, several IKKα C-terminal deletion mutants were constructed (Figure 7D), and their ability to interact with c-Fos was detected (the N-terminal kinase domain and NLS located within this region are required for c-Fos induction, as demonstrated in Figure 6D and Figure 7B). We found that truncation of the C-terminal IKKγ-binding domain within IKKα (IKKα-M1) did not affect its ability to bind c-Fos. However, further deletion of the regions containing the HLH motif (IKKα-M2) and the LZ motif (IKKα-M3) completely destroyed the interaction of IKKα with c-Fos (Figure 7D). Interestingly, although showed a defective c-Fos-binding ability, IKKα-M3 displayed a similar activity in mediating c-Fos expression as the WT IKKα did (Figure 7E). These data indicate that the role for IKKα in mediating c-Fos expression does not require the direct interaction between these two proteins.
So what the functional importance of the interaction between IKKα and c-Fos? As a nuclear protein, IKKα has been demonstrated to act as a chromatin-associated kinase that can phosphorylate histone H3 (Ser10) within the promoter region of NF-κB-responsive genes and contribute largely to the induction of NF-κB transactivation in response to inflammatory stimuli (9,10). Therefore, we proposed that IKKα might also exert a similar function for the AP-1-dependent VEGF expression by binding with c-Fos. To address this hypothesis, a ChIP assay was performed with anti-c-Fos, anti-IKKα and anti-histone H3-p-Ser10 antibodies. We found that among the five motifs homologous to AP-1-reponsive elements within the mouse vegf promoter, c-Fos could recruit to three sites, at positions −616 to −606 (Site A), −2121 to −2115 (Site D) and −2900 to −2892 (Site E), upon UVB exposure. Under the same conditions, a strong association of IKKα to the same chromatin regions was readily observed (Figure 7F), which was accompanied by a significant enrichment of promoter-bound histone H3 Ser10 phosphorylation (Figure 7G). In the absence of ikkα expression, upregulation of histone H3 Ser10 phosphorylation within the vegf promoter was impaired (Figure 7G). More importantly, without the aid of c-Fos, IKKα lost the ability to recruit to the vegf promoter (Figure 7H). In addition, the IKKα deletion mutant (IKKα-M3) with the defective c-Fos-binding ability, can only partially rescue the VEGF expression in the IKKα-null cells compared to its WT counterpart (Figure 7I), even though this mutant possesses a similar ability to induce c-Fos expression (Figure 7E) and AP-1 transactivation (data not shown) as the wt IKKα. These data indicate that IKKα is not only critical for the induction of the AP-1 component, c-Fos, but can also recruit to the vegf promoter by binding with c-Fos and trigger the phosphorylation of promoter-associated histone H3 to facilitate the AP-1 transactivation in the UVB response (Figure 7J).
DISCUSSION
UVB irradiation is an important environmental threat to the skin because it can induce permanent genetic changes as well as angiogenesis-related skin cancers (1–3). Among the various angiogenic growth factors, VEGF is considered to be key in triggering photodamage-related angiogenesis and tumorigenesis (4,5). In the current study, we have focused on the elucidation of the molecular mechanisms and the signaling pathway responsible for UVB-induced VEGF expression in mouse fibroblasts, one of the major types of stromal cells in the skin. Our results indicate that IKKα plays a critical role in mediating VEGF induction via the activation of an AP-1-dependent pathway in UVB-treated fibroblasts, which is unrelated to NF-κB and HIF1α activities (Figures 2–5). In addition, we have also observed the action of IKKα in mediating AP-1 transactivation and VEGF expression in UVB-treated mouse epidermal cells (Figures 2D, 5E and F) and human keratinocytes (data not shown). Therefore, IKKα might exert a general effect in regulating VEGF expression through an AP-1-dependent manner.
IKKα is a multi-functional signaling protein whose activities can be exerted through a variety of its downstream substrates, including NF-κB (9–19). Most of the recent studies have focused on elucidating the implications of IKKα, which are unrelated to IKKβ, in the control of the cellular growth properties of both normal and tumor cells under physiological and pathological conditions. And the results indicate that IKKα exerts a dual role in the cell growth control by either promoting cell survival and proliferation or by mediating growth arrest under different conditions. Therefore, IKKα is regarded as both a tumor promoter and a tumor suppressor depending on the cellular growth properties and the downstream targets that are regulated (7). Aside from these functions, IKKα is also reported to independently function in restricting inflammation by triggering the turnover of p65 and c-Rel or suppressing the DNA-binding ability of p65 in response to inflammatory stimuli (14,15). In the current study, we have disclosed the biological significance of IKKα in mediating the inflammatory response by upregulating VEGF expression upon UVB exposure. Most importantly, this novel function of IKKα is unrelated to the activation status of NF-κB. Taken together, we anticipate that IKKα might have a more extensive IKKβ-independent role, from cancer to immunity, and may also function as a dual-regulator for the cellular inflammatory response under different stress conditions.
The specific functions of IKKα in mediating UVB-induced cell damage effects have been investigated in our previous studies. The results indicate that IKKα can mediate both growth arrest and apoptotic response upon UVB irradiation by regulating cyclin D1 expression (26) and p53 transactivity (Li, Y. et al., unpublished data), respectively. These data suggest that IKKα plays an important role in protecting the cells from UVB-induced photodamage. In the current study, we have disclosed the novel function of IKKα in mediating the inflammatory response by modulating VEGF induction. Moreover, the critical role for IKKα in regulating the production of another inflammatory cytokine, TNFα, upon UVB irradiation was also observed during our recent studies (Li, Y. et al. unpublished data). Therefore, IKKα seems to also function as a mediator of UVB-induced inflammatory injury. Based on these data, we conclude that IKKα can exert multiple roles during the UVB response, including control of cell growth and survival and mediation of the inflammatory response.
In the study to investigate the molecular mechanisms of IKKα in mediating UVB-induced VEGF induction, we found that IKKα-dependent c-Fos induction, transactivation of AP-1, recruitment of IKKα to the vegf promoter and phosphorylation of the promoter-associated histone H3 (Figures 5–7) are the key sequential signaling events for mediating VEGF expression upon UVB irradiation. One of the most important steps, the regulation of c-Fos induction by IKKα, attracted our interest. c-Fos is a well-known oncoprotein whose expression can be regulated via transcriptional, post-transcriptional, translational and post-translational mechanisms (31–35). The observation that c-fos mRNA transcription was significantly upregulated but its protein expression was impaired in the UVB-treated IKKα-null cells excluded the possible role for IKKα in regulating c-fos transcription and mRNA stability (Figure 6A). Then the accumulation of c-Fos in the UVB-irradiated IKKα-null cells with MG132 pretreatment demonstrated the critical role for IKKα in preventing proteasome-dependent degradation of c-Fos in the UVB response (Figure 6B). It is known that c-Fos protein stability is greatly increased upon phosphorylation of the serines and/or threonines located at its N- and C-termini under various conditions (31,32,36,37). Based on this information, we found action of IKKα in triggering N-terminal phosphorylation of c-Fos at Ser32, which contributes to the increase in c-Fos protein stability upon UVB irradiation (Figure 6C, D and E). Under the same conditions, we did not observe phosphorylation of c-Fos at the C-terminus (data not shown), which had been shown to contribute largely to the stabilization of c-Fos by growth factors-induced signals (32). Most importantly, c-Fos phosphorylation and induction required IKKα kinase activity and nuclear localization (Figures 6D and 7B) but did not depend on the direct interaction between IKKα and c-Fos (Figure 7D and E). This conclusion was based on the IKKα deletion mutant, which has an intact kinase domain and functional NLS (such as IKKα-M3), can efficiently induce c-Fos expression but has an impaired ability to bind c-Fos (Figure 7D). According to these results, we propose that IKKα might indirectly interact with c-Fos through an unidentified signaling molecule, whose activity can be regulated by IKKα and is responsible for the phosphorylation and expression of c-Fos in the UVB response. We have noticed that a previously published report has demonstrated the involvement of ERK5 in c-Fos Ser32 phosphorylation and stabilization by gp130-dependent signals (31). Whether ERK5 also participates in IKKα-mediated c-Fos expression in the cellular UVB response deserves further investigation.
Due to its nuclear localization and ability to associate with chromatin, IKKα is regarded as a novel regulator for gene expression (9,10). It has been well documented that the nuclear pool of IKKα can trigger the phosphorylation of the promoter-bound histone H3, which is critical for the optimal induction of various genes including NF-κB-responsive candidates and others, such as c-Fos in EGF-stimulated cells (9,10,38). However, whether the recruitment of IKKα to a specific promoter region can occur independently or is dependent on multiple promoter-bound factors, including components of transcriptional factors or coactivators, has not been fully clarified. Previous reports have demonstrated that interactions with the coactivator CBP or SRC3 is required for the recruitment of IKKα to NF-κB- or hormone-responsive promoters, respectively (11,39). In the current study, we have observed the interaction of IKKα with c-Fos within the nucleus (Figure 7C), which is critical for the binding of IKKα to the vegf promoter in response to UVB irradiation (Figure 7H). Notably, the efficiency of IKKα-M3, which has defective c-Fos-binding abilities, in mediating VEGF expression was partially decreased (Figure 7I). Thus, these data have provided novel evidence for the chromatin-associated kinase activity of IKKα in regulating VEGF expression in the UVB response.
In summary, the data in this study have demonstrated a novel role and accompanying mechanism of IKKα in regulating VEGF induction in the cellular UVB response. Our findings may help in the understanding of signaling events that regulate skin angiogenesis following UVB irradiation and provide a potential clue for preventing angiogenesis-related skin damage by targeting IKKα.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online: Supplementary Figures 1–3.
FUNDING
National Natural Science Foundation of China (No. 30871277, 30970594, 31171342); Beijing Natural Science Foundation (5092022, 5102035); National Key Research and Development Program on Fundamental Sciences (973 Project, 2011CB503803) to L.S. Funding for open access charge: National Natural Science Foundation of China (No. 31171342).
Conflict of interest statement. None declared.
Supplementary Material
REFERENCES
- 1.Lee E, Koo J, Berger T. UVB phototherapy and skin cancer risk: a review of the literature. Int. J. Dermatol. 2005;44:355–360. doi: 10.1111/j.1365-4632.2004.02186.x. [DOI] [PubMed] [Google Scholar]
- 2.Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, Voorhees JJ. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002;138:1462–1470. doi: 10.1001/archderm.138.11.1462. [DOI] [PubMed] [Google Scholar]
- 3.Ichihashi M, Ueda M, Budiyanto A, Bito T, Oka M, Fukunaga M, Tsuru K, Horikawa T. UV-induced skin damage. Toxicology. 2003;189:21–39. doi: 10.1016/s0300-483x(03)00150-1. [DOI] [PubMed] [Google Scholar]
- 4.Hirakawa S, Fujii S, Kajiya K, Yano K, Detmar M. Vascular endothelial growth factor promotes sensitivity to ultraviolet B-induced cutaneous photodamage. Blood. 2005;105:2392–2399. doi: 10.1182/blood-2004-06-2435. [DOI] [PubMed] [Google Scholar]
- 5.Clydesdale GJ, Dandie GW, Muller HK. Ultraviolet light induced injury: immunological and inflammatory effects. Immunol. Cell Biol. 2001;79:547–568. doi: 10.1046/j.1440-1711.2001.01047.x. [DOI] [PubMed] [Google Scholar]
- 6.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 7.Chariot A. The NF-kappaB-independent functions of IKK subunits in immunity and cancer. Trends Cell. Biol. 2009;19:404–413. doi: 10.1016/j.tcb.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat. Rev. Mol. Cell. Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto Y, Verma UN, Prajapati S, Kwak YT, Gaynor RB. Histone H3 phosphorylation by IKK-alpha is critical for cytokine-induced gene expression. Nature. 2003;423:655–659. doi: 10.1038/nature01576. [DOI] [PubMed] [Google Scholar]
- 10.Anest V, Hanson JL, Cogswell PC, Steinbrecher KA, Strahl BD, Baldwin AS. A nucleosomal function for IkappaB kinase-alpha in NF-kappaB-dependent gene expression. Nature. 2003;423:659–663. doi: 10.1038/nature01648. [DOI] [PubMed] [Google Scholar]
- 11.Huang WC, Ju TK, Hung MC, Chen CC. Phosphorylation of CBP by IKKalpha promotes cell growth by switching the binding preference of CBP from p53 to NF-kappaB. Mol. Cell. 2007;26:75–87. doi: 10.1016/j.molcel.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoberg JE, Popko AE, Ramsey CS, Mayo MW. IkappaB kinase alpha-mediated derepression of SMRT potentiates acetylation of RelA/p65 by p300. Mol. Cell. Biol. 2006;26:457–471. doi: 10.1128/MCB.26.2.457-471.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoberg JE, Yeung F, Mayo MW. SMRT derepression by the IkappaB kinase alpha: a prerequisite to NF-kappaB transcription and survival. Mol. Cell. 2004;16:245–255. doi: 10.1016/j.molcel.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Liu B, Yang Y, Chernishof V, Loo RR, Jang H, Tahk S, Yang R, Mink S, Shultz D, Bellone CJ, et al. Proinflammatory stimuli induce IKKalpha-mediated phosphorylation of PIAS1 to restrict inflammation and immunity. Cell. 2007;129:903–914. doi: 10.1016/j.cell.2007.03.056. [DOI] [PubMed] [Google Scholar]
- 15.Lawrence T, Bebien M, Liu GY, Nizet V, Karin M. IKKalpha limits macrophage NF-kappaB activation and contributes to the resolution of inflammation. Nature. 2005;434:1138–1143. doi: 10.1038/nature03491. [DOI] [PubMed] [Google Scholar]
- 16.Descargues P, Sil AK, Karin M. IKKalpha, a critical regulator of epidermal differentiation and a suppressor of skin cancer. EMBO J. 2008;27:2639–2647. doi: 10.1038/emboj.2008.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu F, Park E, Liu B, Xia X, Fischer SM, Hu Y. Critical role of IkappaB kinase alpha in embryonic skin development and skin carcinogenesis. Histol. Histopathol. 2009;24:265–271. doi: 10.14670/HH-24.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park E, Zhu F, Liu B, Xia X, Shen J, Bustos T, Fischer SM, Hu Y. Reduction in IkappaB kinase alpha expression promotes the development of skin papillomas and carcinomas. Cancer Res. 2007;67:9158–9168. doi: 10.1158/0008-5472.CAN-07-0590. [DOI] [PubMed] [Google Scholar]
- 19.Luo JL, Tan W, Ricono JM, Korchynskyi O, Zhang M, Gonias SL, Cheresh DA, Karin M. Nuclear cytokine-activated IKKalpha controls prostate cancer metastasis by repressing Maspin. Nature. 2007;446:690–694. doi: 10.1038/nature05656. [DOI] [PubMed] [Google Scholar]
- 20.Liu B, Park E, Zhu F, Bustos T, Liu J, Shen J, Fischer SM, Hu Y. A critical role for I kappaB kinase alpha in the development of human and mouse squamous cell carcinomas. Proc. Natl Acad. Sci. USA. 2006;103:17202–17207. doi: 10.1073/pnas.0604481103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreno-Maldonado R, Ramirez A, Navarro M, Fernandez-Acenero MJ, Villanueva C, Page A, Jorcano JL, Bravo A, Llanos Casanova M. IKKalpha enhances human keratinocyte differentiation and determines the histological variant of epidermal squamous cell carcinomas. Cell Cycle. 2008;7:2021–2029. doi: 10.4161/cc.7.13.6147. [DOI] [PubMed] [Google Scholar]
- 22.Song L, Li J, Zhang D, Liu ZG, Ye J, Zhan Q, Shen HM, Whiteman M, Huang C. IKKbeta programs to turn on the GADD45alpha-MKK4-JNK apoptotic cascade specifically via p50 NF-kappaB in arsenite response. J. Cell. Biol. 2006;175:607–617. doi: 10.1083/jcb.200602149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song L, Li J, Hu M, Huang C. Both IKKalpha and IKKbeta are implicated in the arsenite-induced AP-1 transactivation correlating with cell apoptosis through NF-kappaB activity-independent manner. Exp. Cell Res. 2008;314:2187–2198. doi: 10.1016/j.yexcr.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao M, Dong W, Hu M, Yu M, Guo L, Qian L, Guo N, Song L. GADD45alpha mediates arsenite-induced cell apoptotic effect in human hepatoma cells via JNKs/AP-1-dependent pathway. J. Cell Biochem. 2010;109:1264–1273. doi: 10.1002/jcb.22509. [DOI] [PubMed] [Google Scholar]
- 25.Song L, Li J, Ye J, Yu G, Ding J, Zhang D, Ouyang W, Dong Z, Kim SO, Huang C. p85alpha acts as a novel signal transducer for mediation of cellular apoptotic response to UV radiation. Mol. Cell. Biol. 2007;27:2713–2731. doi: 10.1128/MCB.00657-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song L, Dong W, Gao M, Li J, Hu M, Guo N, Huang C. A novel role of IKKalpha in the mediation of UVB-induced G0/G1 cell cycle arrest response by suppressing Cyclin D1 expression. Biochim. Biophys. Acta. 2010;1803:323–332. doi: 10.1016/j.bbamcr.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song L, Gao M, Dong W, Hu M, Li J, Shi X, Hao Y, Li Y, Huang C. p85alpha mediates p53 K370 acetylation by p300 and regulates its promoter-specific transactivity in the cellular UVB response. Oncogene. 2011;30:1360–1371. doi: 10.1038/onc.2010.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haigh JJ. Role of VEGF in organogenesis. Organogenesis. 2008;4:247–256. doi: 10.4161/org.4.4.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Ju W, Renouard J, Aden J, Belinsky SA, Lin Y. 17-allylamino-17-demethoxygeldanamycin synergistically potentiates tumor necrosis factor-induced lung cancer cell death by blocking the nuclear factor-kappaB pathway. Cancer Res. 2006;66:1089–1095. doi: 10.1158/0008-5472.CAN-05-2698. [DOI] [PubMed] [Google Scholar]
- 30.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sasaki T, Kojima H, Kishimoto R, Ikeda A, Kunimoto H, Nakajima K. Spatiotemporal regulation of c-Fos by ERK5 and the E3 ubiquitin ligase UBR1, and its biological role. Mol. Cell. 2006;24:63–75. doi: 10.1016/j.molcel.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 32.Pellegrino MJ, Stork PJS. Sustained activation of extracellular signal-regulated kinase by nerve growth factor regulates c-fos protein stabilization and transactivation in PC12 cells. J. Neurochem. 2006;99:1480–1493. doi: 10.1111/j.1471-4159.2006.04250.x. [DOI] [PubMed] [Google Scholar]
- 33.Stancovski I, Gonen H, Orian A, Schwartz AL, Ciechanover A. Degradation of the proto-oncogene product c-Fos by the ubiquitin proteolytic system in vivo and in vitro: identification and characterization of the conjugating enzymes. Mol. Cell. Biol. 1995;15:7106–7116. doi: 10.1128/mcb.15.12.7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takeuchi K, Shibamoto S, Nagamine K, Shigemori I, Omura S, Kitamura N, Ito F. Signaling pathways leading to transcription and translation cooperatively regulate the transient increase in expression of c-Fos protein. J. Biol. Chem. 2001;276:26077–26083. doi: 10.1074/jbc.M102704200. [DOI] [PubMed] [Google Scholar]
- 35.Jariel-Encontre I, Salvat C, Steff AM, Pariat M, Acquaviva C, Furstoss O, Piechaczyk M. Complex mechanisms for c-fos and c-jun degradation. Mol. Biol. Rep. 1997;24:51–56. doi: 10.1023/a:1006804723722. [DOI] [PubMed] [Google Scholar]
- 36.Ferrara P, Andermarcher E, Bossis G, Acquaviva C, Brockly F, Jariel-Encontre I, Piechaczyk M. The structural determinants responsible for c-Fos protein proteasomal degradation differ according to the conditions of expression. Oncogene. 22:1461–1474. doi: 10.1038/sj.onc.1206266. [DOI] [PubMed] [Google Scholar]
- 37.Coronella-Wood J, Terrand J, Sun H, Chen QM. c-Fos phosphorylation induced by H2O2 prevents proteasomal degradation of c-fos in cardiomyocytes. J. Biol. Chem. 2004;279:33567–33574. doi: 10.1074/jbc.M404013200. [DOI] [PubMed] [Google Scholar]
- 38.Anest V, Cogswell PC, Baldwin AS., Jr IkappaB kinase alpha and p65/RelA contribute to optimal epidermal growth factor-induced c-fos gene expression independent of IkappaBalpha degradation. J. Biol. Chem. 2004;279:31183–31189. doi: 10.1074/jbc.M404380200. [DOI] [PubMed] [Google Scholar]
- 39.Wu RC, Qin J, Hashimoto Y, Wong J, Xu J, Tsai SY, Tsai MJ, O'Malley BW. Regulation of SRC-3 (pCIP/ACTR/AIB-1/RAC-3/TRAM-1) Coactivator activity by I kappa B kinase. Mol. Cell. Biol. 2002;22:3549–3561. doi: 10.1128/MCB.22.10.3549-3561.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marinari B, Moretti F, Botti E, Giustizieri ML, Descargues P, Giunta A, Stolfi C, Ballaro C, Papoutsaki M, Alema S, et al. The tumor suppressor activity of IKKa in stratified epithelia is exerted in part via the TGF-b antiproliferative pathway. Proc. Natl Acad. Sci. USA. 2008;105:17091–17096. doi: 10.1073/pnas.0809288105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.