Abstract
Context
Saw palmetto fruit extracts are widely used for treating lower urinary tract symptoms attributed to benign prostatic hyperplasia. However, recent clinical trials have questioned their efficacy, at least at standard doses (320 mg daily).
Objective
To determine the effect of a saw palmetto extract at up to three times the standard dose on lower urinary tract symptoms attributed to benign prostatic hyperplasia.
Design
Multicenter placebo-controlled randomized trial conducted from June, 2008 through October, 2010.
Setting
Eleven North American clinical sites.
Participants
Were men at least 45 years old, with a peak urinary flow rate ≥ 4 ml/sec, an AUA Symptom Index (AUASI) score ≥ 8 and ≤ 24, and no exclusions.
Interventions
One, two, and then three 320 mg daily doses of saw palmetto extract or placebo, with dose increases at 24 and 48 weeks.
Main Outcome Measures
Primary outcome was the difference in AUASI score from baseline to 72 weeks. Secondary outcomes were measures of urinary bother; nocturia; uroflow; postvoid residual; prostate-specific antigen; participants’ global assessments; and indices of sexual function, continence, sleep quality, and prostatitis symptoms.
Results
From baseline to 72 weeks, mean AUASI scores decreased from 14.4 to 12.2 points with saw palmetto and from 14.7 to 11.7 points with placebo. The group mean difference in AUASI score change from baseline to 72 weeks between the saw palmetto and placebo groups was 0.79 points favoring placebo (bound of the 95% confidence interval most favorable to saw palmetto was 1.77 points, one-sided P=0.91). Saw palmetto was no more effective than placebo for any secondary outcome. No attributable side effects were identified.
Conclusions
Increasing doses of a saw palmetto fruit extract did not reduce lower urinary tract symptoms more than placebo. (CAMUS study number NCT00603304 http://www.ClinicalTrials.gov)
Introduction
Benign prostatic hyperplasia (BPH) is a common cause of bothersome lower urinary tract symptoms (LUTS) among older men,1 and may be treated with medications, minimally invasive therapies, or surgery.2, 3 Plant extracts are also widely used for LUTS in the United States and Europe. 4 The most common are extracts of the fruit of the saw palmetto dwarf palm tree. In a 2007 U.S. survey, 17.7% of adults reported use of a natural product in the last 30 days, and 5.1% of users had taken saw palmetto5; undoubtedly, the frequency would be higher among older men. A variety of mechanisms for saw palmetto have been proposed including anti-androgenic, anti-inflammatory, and anti-proliferative effects, but none have been conclusively proven. 6–9
In a 2002 Cochrane meta-analysis of the efficacy of saw palmetto extracts for men with LUTS attributed to BPH, 21 clinical trials were identified. Compared to placebo, saw palmetto significantly reduced nocturia, increased self-rated improvement, and improved peak uroflow.10 Adverse effects were infrequent.
However, subsequent more rigorous trials have yielded less positive results. In 2009, an updated Cochrane review identified nine new trials. Though the effect on nocturia remained significant, there was no significant effect on American Urological Association Symptom Index (AUASI) scores or peak uroflow. 11 The most common dose was 160 mg twice daily.
The largest trial was the Saw palmetto Treatment for Enlarged Prostates (STEP) study. Two hundred twenty-five men ≥50 years old with baseline AUASI score ≥8 were randomized at one center to saw palmetto extract 160 mg BID or placebo. No improvement over placebo was found over one year in symptom scores or any secondary endpoints.12 No important toxicity was observed.13
Following publication of STEP we conducted a randomized clinical trial to determine if a standard daily dose of a saw palmetto extract increased to a double and then a triple daily dose over 72 weeks would improve LUTS attributed to BPH. 14
Methods
Trial Design
This study was a randomized, placebo-controlled double-blind multicenter trial of increasing doses of saw palmetto fruit extract. Enrollment began in June, 2008 and follow-up was completed in October, 2010.
Participants
We purposefully recruited a broad spectrum of men into the trial, as in the United States men do not need an evaluation by a health care provider or a prescription to buy and take a saw palmetto extract for lower urinary tract symptoms. Men were eligible for enrollment if they were ≥45 years old, had a peak uroflow rate ≥4 ml/sec, an AUASI score ≥ 8 and ≤ 24 at two screening visits, and signed informed consent. Men were ineligible if they had: prior invasive treatment for BPH; recent alpha blocker (1 month), 5-alpha reductase inhibitor (3 months), or phytotherapy including saw palmetto extract (3 months) treatment; recent treatment with other medications affecting LUTS; creatinine > 2.0 mg/dL; liver function tests more than 3 times normal; coagulopathy or anticoagulation; recent unstable medical conditions; neurologic conditions affecting urination; recent prostatitis or repeated urinary tract infections; prostate or bladder cancer or a prostate-specific antigen level >10 ng/mL; recent or planned genitourinary instrumentation; severe incontinence; recent diuretic initiation or dose change; or medical conditions likely to prevent completion. 14 Participants were non-paid volunteers recruited at 11 North American sites (see Acknowledgments); the study was approved by their and the Data Coordinating Center’s institutional review boards. An independent data and safety monitoring board established by the National Institutes of Health periodically reviewed the progress and safety of the study.
Interventions
Participants were randomly assigned equally to receive one, two, and then three 320 mg chocolate-colored gelcaps daily containing a standardized saw palmetto fruit extract with dose escalations at 24 and 48 weeks; or an identical number of placebo gelcaps escalated similarly. The two batches of saw palmetto extract used were standardized to a reference chromatogram (with 85–95% fatty acids as marker substances), 30 mg glycerol, 25 mg sorbitol, 10 mg purified water, and 90 mg gelatin. The placebo contained 375 mg polyethylene glycol, 25 mg glycerol and 75 mg gelatin (matched weight of 475 mg). Participants were asked to take the gelcaps together at a convenient time. Participants with unacceptable side effects could split the dose or be maintained on lower doses. The phytotherapy used in this trial was a proprietary lipidic ethanolic extract of ripe, dried saw palmetto berries, Serenoa repens (W.Bartram) Small (Arecaceae), manufactured by Rottapharm/Madaus, Cologne, Germany and sold as PROSTA-URGENIN UNO capsules (see Appendix). Identification, extraction, and phytochemical content are described in the Saw Palmetto extract monograph published in USP33-NF28 S1 Reissue. 15
Outcomes
The primary outcome was the change in AUASI score from baseline to 72 weeks. The AUASI is a self-administered 7 item index assessing frequency of LUTS (range 0–35 points).16 Secondary analyses on the AUASI were a comparison of the proportion of participants achieving a 3 point score decrease and a repeated measures analysis of scores over time. Secondary outcomes included participants’ global assessments of improvement and satisfaction at end-of-study (both Likert scales) ; as well as change from baseline to 72 weeks in: the BPH Impact Index17, the Quality of Life item from the International Prostate Symptom Score18, the nocturia item from the AUASI 16, peak uroflow, postvoid residual volume, prostate specific antigen (PSA) level, indices of erectile and ejaculatory function19, 20, the ICSmaleIS incontinence scale 21, the Jenkins Sleep Dysfunction Scale22, and the NIH Chronic Prostatitis Symptom Index.23 All questionnaires were available in English and Spanish.
Participants were seen at baseline and at 12, 24, 36, 48, 60, and 72 weeks for outcome assessments. They were assessed for side effects including with blood counts, basic blood chemistries, coagulation tests, electrocardiograms and urinalyses 4 weeks after each dose increase and at end of study, including a query about adverse effects occurring within 30 days of treatment discontinuation. Compliance was estimated by pill counts at each visit, and attendance at protocol-specified visits was tracked.
Sample Size
To detect a hypothetical two-point group mean difference in AUASI score change between saw palmetto extract and placebo groups with a two-sample t-test at a one-sided significance level of 0.05 assuming a common standard deviation of 6 points, a sample size of 157 participants per group was estimated to provide 90% power. A two-point difference approximates the mean drop in AUASI score among men with baseline scores of 8–19 points who report “slight” improvement. 24 To allow for 10% dropouts, a total sample size of 350 participants was planned. During recruitment, the sample size was increased to 369 to allow for dilution of any therapeutic effect among participants unable to take the triple dose. Given that the clinical implications for use of the extract in the “real world” would be the same whether it proved no better or worse than placebo, an a priori decision was made to use one-sided statistical testing. 25
Randomization
Randomization was performed centrally using an internet accessible, password-protected, computer-based system that generated group assignments. Randomization was stratified by baseline AUASI score (8–15 or 16–24 points) and clinical center with randomly permuted blocks in each stratum.
Blinding
Study staff and participants were blinded to treatment assignment. Because of a mild odor of the saw palmetto extract, gelcaps were blister packaged to avoid unblinding during compliance assessments. To test the blind, participants were asked to guess their treatment assignment at end of study.
Statistical Methods
The treatment arms were compared with respect to demographic and baseline measures using Pearson’s chi-square test, the t-test for independent samples and the Wilcoxon rank sum test. The primary analysis was based on the modified intention to treat (MITT) population that included all eligible participants who took at least one dose of study drug and had at least one follow-up assessment. For participants who discontinued prior to 72 weeks, multiple imputations were used to estimate their AUASI at week 72, and other secondary outcome measures. There were 23 participants (12 on saw palmetto and 11 on placebo) who had all secondary outcome measures for week 72 imputed. For an additional 14 participants (4 on saw palmetto and 10 on placebo), 1–2 secondary outcomes at week 72 were imputed. At baseline, secondary measures were missing for 7 participants (2 on saw palmetto, 5 on placebo) and were estimated using multiple imputation. Baseline measures for AUA-SI were obtained from all participants.
Results of the MITT analysis were confirmed in the per-protocol population which included all participants who received treatment for 72 weeks. An unpaired t-test was used to compare the two treatment arms with respect to change in AUASI score from baseline to 72 weeks, using a one-sided P value of 0.05 as the threshold for statistical significance. Prespecified secondary analyses on the primary outcome included a comparison of the proportion of participants achieving at least a 3 point AUASI score decrease at 72 weeks using Fisher’s exact test, and a mixed models repeated measures analysis comparing change in AUASI scores from baseline between the two groups over time. A single prespecified subgroup analysis was based on participants’ race and ethnicity; post hoc subgroup analyses were also conducted by dichotomizing baseline age, AUASI score, BPH Impact Index score, peak uroflow, post-void residual volume, and PSA level at the medians of their distributions; education was dichotomized as college graduate or less. The interaction term of the two-way analysis of variance was used to determine the effect of subgroups on the primary outcome measure. Statistical testing in secondary analyses was not adjusted for multiple comparisons to avoid sacrificing sensitivity for specificity. Analyses were conducted in SAS version 9.2
Finally, to explore for any dose-response, the changes in AUASI score between baseline and 24, 24 and 48, and 48 and 72 weeks were compared, with plans to use the Hochberg step-up method to deal with multiple comparisons, if necessary. Secondary outcomes were assessed using two sample t-tests with one-sided 0.05 significance levels. Rates of occurrence of adverse events and abnormal laboratory values were estimated using the Poisson distribution and compared using a normal approximation.
Results
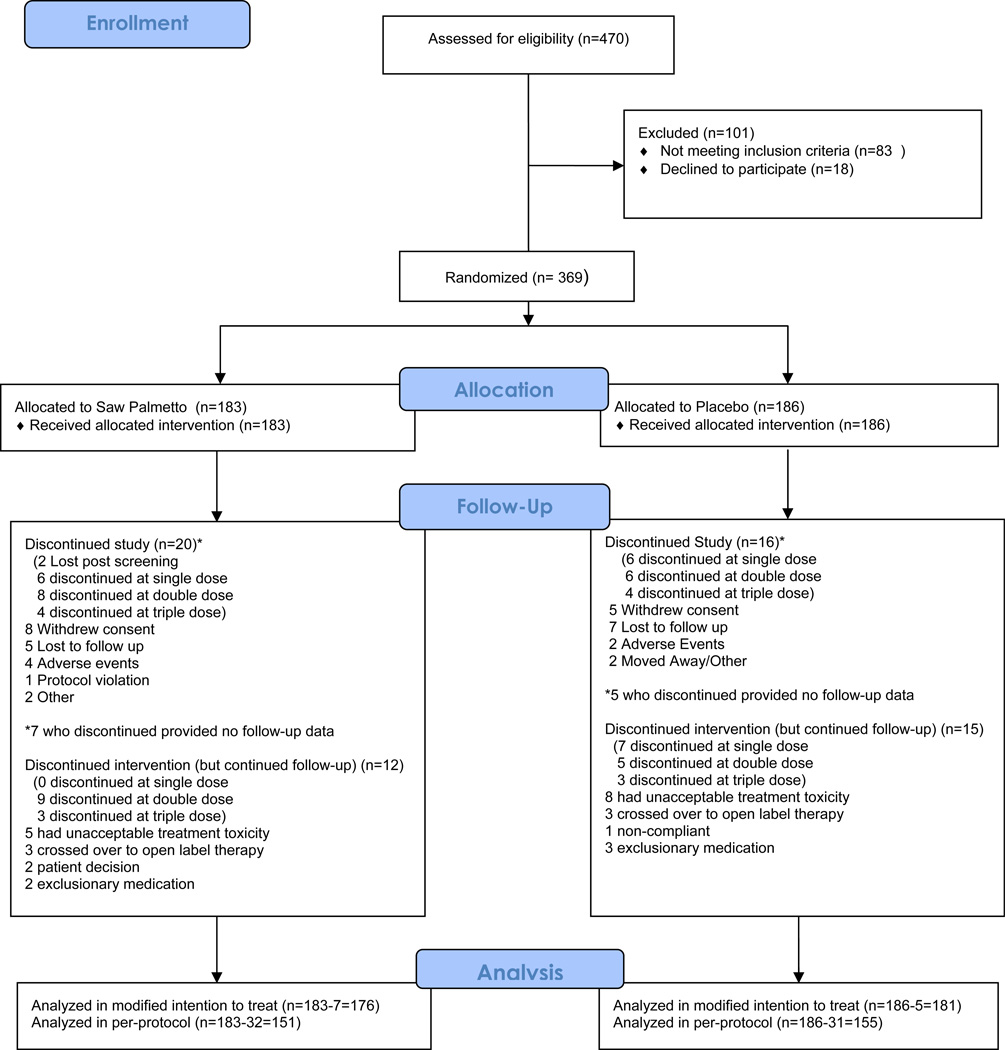
A total of 1032 men were prescreened, usually by telephone; interested and preliminarily eligible men were invited to a screening visit.26 Figure 1 provides a CONSORT diagram for the 470 men attending a first screening visit. A total of 369 men were randomized, between 19 and 52 per site. Table 1 compares the baseline characteristics of the 357 participants randomized and included in the modified intention to treat analysis. Participants had a mean age of about 61 years, and were predominantly well-educated non-Hispanic whites with a mean AUASI score of 14.4 points.
Figure 1.
CONSORT diagram for the trial
Table 1.
Baseline characteristics of participants included in the modified intention to treat analysis. For all scales except as noted higher scores indicate greater dysfunction. (P values from two sample t tests.)
| Variable (units/total score range) |
Total (N=357) |
Saw palmetto (N=176) | Placebo (N=181) |
||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | P value | |
| Age (years) | 60.97 | 8.40 | 61.25 | 8.72 | 60.7 | 8.08 | 0.54 |
| AUASI score (8–24) | 14.55 | 4.52 | 14.42 | 4.29 | 14.69 | 4.75 | 0.58 |
| BPH Impact Index score (0–13) | 3.55 | 2.51 | 3.39 | 2.24 | 3.71 | 2.72 | 0.30 |
| IPSS QOL score (0–6) | 3.21 | 1.20 | 3.2 | 1.2 | 3.23 | 1.21 | 0.83 |
| AUA nocturia item (0–5) | 2.17 | 1.11 | 2.09 | 1.08 | 2.26 | 1.13 | 0.14 |
| Peak uroflow (mL/sec) | 14.90 | 6.92 | 15.03 | 7.15 | 14.78 | 6.71 | 0.74 |
| Post-void residual (mL)** | 41.0 | 13.0, 90.0 | 37.5 | 13.5, 88.0 | 43.0 | 12.0, 92.0 | 0.88 |
| PSA level (ng/mL) | 2.07 | 1.78 | 2.20 | 1.95 | 1.93 | 1.59 | 0.16 |
| IIEF erectile function scale (1–30)* | 19.38 | 9.87 | 18.79 | 10.36 | 19.93 | 9.43 | 0.29 |
| MSHQ-EjD scale (1–20)* | 10.87 | 4.16 | 10.56 | 4.27 | 11.18 | 4.03 | 0.16 |
| ICSmale IS score (0–24) | 3.81 | 2.75 | 3.44 | 2.3 | 4.17 | 3.08 | 0.01* |
| Jenkins Sleep Scale score (0–20) | 7.36 | 4.62 | 6.95 | 4.28 | 7.72 | 4.93 | 0.11 |
| NIH-CPSI | |||||||
| Pain scale (0–21)** | 0 | 0, 2 | 0 | 0, 2 | 0 | 0, 3 | 0.17 |
| Urinary symptom scale (0–10) | 4.15 | 2.20 | 4.02 | 2.31 | 4.27 | 2.08 | 0.28 |
| QOL scale (0–12) | 4.51 | 2.13 | 4.45 | 2.00 | 4.57 | 2.24 | 0.61 |
| Race | N (%) | N (%) | |||||
| Non Hispanic white | 284 (79.6%) | 145 (82.4%) | 139 (76.8%) | 0.42 | |||
| African American | 41 (11.5%) | 17 (9.7%) | 24 (13.3%) | ||||
| Hispanic/Latino/Other | 32 (9.0%) | 14 (8.0%) | 18 (9.9%) | ||||
| Education | |||||||
| Less than high school | 13 (3.6%) | 6 (3.4%) | 7 (3.9%) | 0.64** | |||
| High school graduate | 38 (10.6%) | 20 (11.4%) | 18 (9.9%) | ||||
| Some college | 60 (16.8%) | 26 (14.8%) | 34 (18.8%) | ||||
| College graduate | 99 (27.7%) | 48 (27.3%) | 51 (28.2%) | ||||
| Post college | 142 (39.8%) | 75 (42.6%) | 67 (37.0%) | ||||
| No response | 5 (1.4%) | 1 (0.6%) | 4 (2.2%) | ||||
Higher scores on these scales indicate less dysfunction
Median and interquartile range shown, P-value based on Wilcoxon rank sum test
Compliance with scheduled visits excluding visits after dropouts was 97.0%. Median pill count across attended visits was 98.2%.Of the 306 participants who completed 72 weeks on treatment, all were successfully increased to triple dose and included in the per-protocol analysis. At end of study, of participants randomized to saw palmetto extract who were still on study drug and responded, 45/149 (30.0%) thought they were on saw palmetto, 67/149 (45.0%) thought they were on placebo, and 37/149 (24.8%) said they weren’t sure. Of similar participants randomized to placebo, 66/154 (42.9%) thought they were on placebo, 39/154 (25.3%) thought they were on saw palmetto, and 49/154 (31.8%) said they weren’t sure. The responses were not significantly different (P=0.36).
Figure 2 displays the mean AUASI scores during follow-up. Table 2 provides the group mean changes in AUASI scores from baseline to 72 weeks. The AUASI score decreased a mean of 2.20 points with saw palmetto and 2.99 points with placebo, a group mean difference of 0.79 points favoring placebo (upper bound of the one-sided 95% confidence interval most favorable to saw palmetto was 1.77 points, one-sided P=0.91). The per-protocol analysis comparing the mean decrease in AUASI score among 151 participants on saw palmetto extract to 155 participants on placebo who completed 72 weeks on triple dose yielded a group mean difference of 0.82 points favoring placebo (upper bound of the one-sided 95% confidence interval most favorable to saw palmetto extract was 1.91 points, one-sided P= 0.89). The proportion of participants achieving a 3 point decrease in AUASI score at 72 weeks was 42.6% in the saw palmetto group and 44.2% in the placebo group (one-sided Fisher’s exact test P=0.66). The results of the mixed models repeated measures analysis showed no greater improvement with saw palmetto extract versus placebo (P=0.22). Finally, the analysis of dose response also showed no greater improvement with saw palmetto extract versus placebo at any dose level. Saw palmetto extract was no better than placebo for any secondary outcome (Table 2).
Table 2.
Change in Primary and Secondary Outcome Measures from Baseline to Week 72
| Outcome Measure | Saw Palmetto (N=176) | Placebo (N=181) | |||||
|---|---|---|---|---|---|---|---|
| Baseline Mean |
Week 72 Mean |
Change - Mean (95% CI) |
Baseline Mean |
Week 72 Mean |
Change - Mean (95% CI) |
P-value (1-sided) |
|
| Primary | |||||||
| AUASI score | 14.42 | 12.22 | −2.20 (−3.04, −0.36) |
14.69 | 11.70 | −2.99 (−3.81, −2.17) |
0.91 |
| Secondary | |||||||
| BPH Impact Index | 3.43 | 2.62 | −0.81 (−1.16. −0.46) |
3.70 | 2.47 | −1.23 (−1.60, −0.87) |
0.95 |
| AUASI QOL | 3.20 | 2.86 | −0.34 (−0.52, −0.16) |
3.23 | 2.74 | −0.49 (−0.67, −0.31) |
0.87 |
| AUA Nocturia | 2.09 | 1.84 | −0.36 (−0.72, 0) |
2.26 | 1.78 | −0.15 (−0.44, 0.13) |
0.19 |
| Peak flow rate (mL/sec) | 15.03 | 14.84 | −0.18 (−1.07, 0.70) |
14.78 | 13.99 | −0.79 (−1.58, 0) |
0.84 |
| Post-void residual (mL)* | 37.5 | 44.5 | 4.78 (−30.00, 52.00) |
43.00 | 42.00 | 1.17 (−33.00, 34.00) |
0.31* |
| PSA level (ng/ml) | 2.20 | 2.41 | 0.32 (−0.08, 0.73) |
1.93 | 2.07 | −0.19 (−0.53, 0.14) |
0.97 |
| IIEF erectile scale | 18.81 | 18.29 | −0.52 (−1.63, 0.59) |
19.92 | 18.86 | −1.06 (−2.11, −0.02) |
0.76 |
| MSHQ-EjD scale | 10.56 | 10.18 | −0.38 (−1.04, 0.28) |
11.18 | 11.09 | −0.09 (0.63, 0.45) |
0.25 |
| ICSmaleIS score | 3.44 | 2.96 | −0.48 (−0.80, −0.16) |
4.17 | 3.32 | −0.84 (−1.17, −0.51) |
0.94 |
| Jenkins sleep scale | 6.96 | 6.15 | −0.80 (−1.34, −0.27) |
7.75 | 6.12 | −1.63 (−2.25, −1.01) |
0.98 |
| NIH-CPSI scales | |||||||
| Pain* | 0 | 0 | 0 (−0.08, 0) |
0 | 0 | 0 (−1.00, 0) |
0.20* |
| Urinary symptom | 4.02 | 3.67 | −0.35 (−0.67, −0.03) |
4.27 | 3.41 | −0.86 (−1.22, −0.49) |
0.98 |
| QOL | 4.45 | 3.61 | −0.85 (−1.16, −0.53) |
4.57 | 3.49 | −1.08 (−1.39, −0.77) |
0.85 |
Median and interquartile range shown, P-value based on Wilcoxon rank sum test.
Figure 3 presents the group mean difference in AUASI score decrease by treatment group stratified by race and ethnicity, as well as the exploratory subgroup analyses for other baseline parameters. These analyses did not reveal any subgroup with a clinically important differential response to saw palmetto compared to placebo. At week 72, the two subjective assessment measures did not differ significantly between the two treatment arms. Participant assessments of urinary symptoms compared to baseline averaged 3.6 and 3.5 for saw palmetto and placebo, respectively, which is between “a little better” and “about the same.” Satisfaction with current status of urinary symptoms averaged 3.1 and 3.0 for saw palmetto and placebo, respectively, which corresponds to “neither satisfied nor dissatisfied.”
Figure 3.
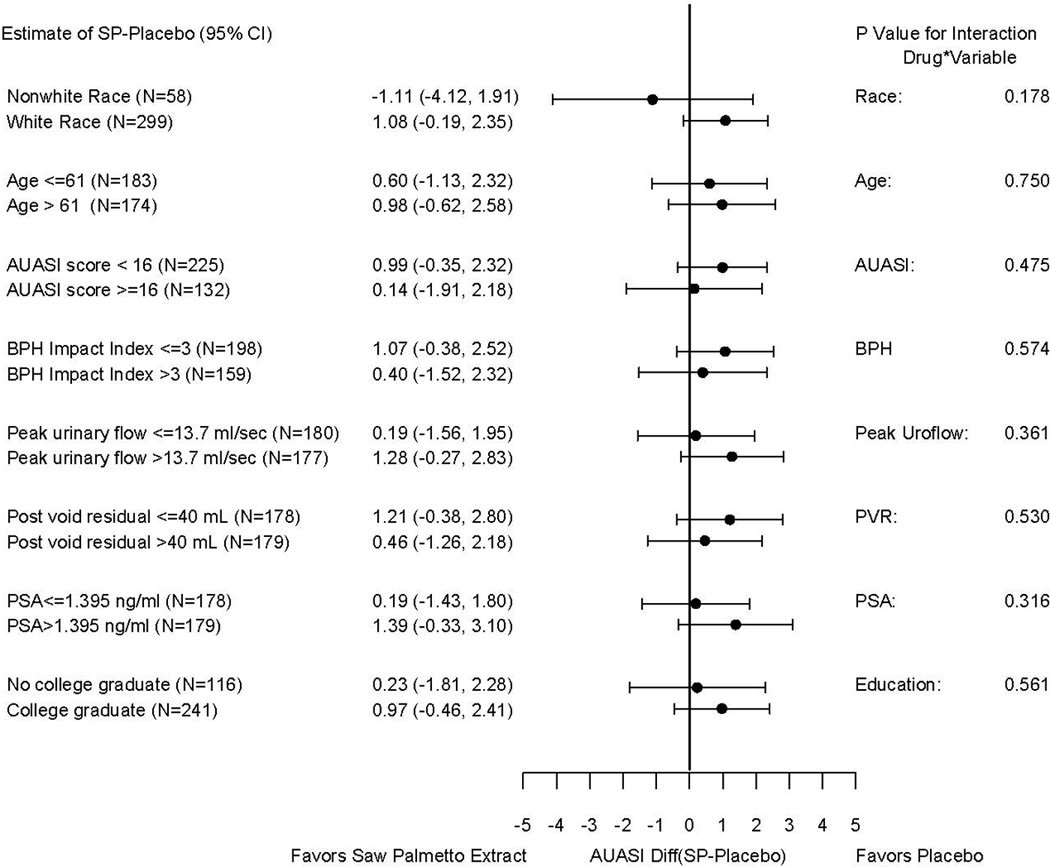
Comparisons of the difference between group mean AUASI score changes from baseline to 72 weeks for the saw palmetto and placebo groups stratified by select baseline variables (continuous variables dichotomized at the median) in the modified intention to treat population. The subgroup analysis by race was prespecified in the study protocol; the rest are exploratory post hoc analyses. (P values based on a test for interaction in the primary analysis.)
Table 3 presents the number of adverse events by treatment group, and Table 4 describes all serious adverse events reported among participants for those adverse events that occurred in at least 5% of study participants. There were no significant differences between groups in the rates of occurrence of any adverse events. The saw palmetto extract appeared to have no attributable side effects, even up to triple doses.
Table 3.
Number of adverse events by treatment group in the modified intention to treat population.
| Type of Adverse Event | No. Adverse Events | No. Participants | ||||
|---|---|---|---|---|---|---|
| Saw Palmetto | Placebo | P-value* | Saw Palmetto | Placebo | P-value** | |
| All Adverse events | 530 | 476 | 0.17 | 136 | 137 | 0.80 |
| Arrythmia | 8 | 10 | 0.72 | 8 | 10 | 0.81 |
| Elevated blood pressure | 14 | 6 | 0.21 | 13 | 6 | 0.10 |
| Upper respiratory | 54 | 60 | 0.72 | 39 | 34 | 0.43 |
| Flu-like symptoms | 19 | 15 | 0.77 | 16 | 12 | 0.43 |
| Ophthalmic | 11 | 11 | 0.95 | 8 | 9 | 1.00 |
| Oral/dental | 26 | 14 | 0.19 | 21 | 12 | 0.10 |
| Musculoskeletal | 81 | 72 | 0.46 | 53 | 46 | 0.35 |
| Genitourinary | 58 | 59 | 0.96 | 41 | 42 | 1.00 |
| Elevated PSA | 15 | 15 | 0.95 | 14 | 13 | 0.84 |
| Gastrointestinal | 52 | 58 | 0.71 | 38 | 39 | 1.00 |
| Dermatologic | 17 | 26 | 0.33 | 12 | 20 | 0.20 |
| Physical injury/trauma | 28 | 11 | 0.11 | 24 | 10 | 0101 |
| Abnormal serum chemistry | 11 | 10 | 0.80 | 11 | 7 | 0.34 |
based on comparison of Poisson rates
based on Fisher’s exact test
Discussion
Saw palmetto extracts have been widely used by men with LUTS, but more recent rigorously conducted trials, particularly the STEP trial12, have not proven superiority to placebo at standard doses of 320 mg daily. We designed this trial to determine whether saw palmetto extract at daily doses up to 960 mg would prove superior to placebo at improving LUTS and other BPH-related outcomes.
We found that the saw palmetto extract tested in this study had no greater effect than placebo on LUTS attributed to BPH or a broad range of secondary outcomes, though small decreases in AUASI scores were seen in both groups. Superiority to placebo was not demonstrated despite using a saw palmetto preparation prepared with an ethanolic extraction procedure as opposed to the CO2 extraction procedure used in preparing the STEP product, and increasing to three times the standard dose. Increasing to these higher doses was not associated with a greater attributable risk of side effects.
The strengths of our trial, which distinguish it from earlier studies, included the use of a well-characterized saw palmetto extract, an adequate sample size (our one-sided confidence intervals make any clinically important benefit relative to placebo extremely unlikely), recruitment from multiple centers to increase generalizability, an adequate dose of the extract, an adequate duration of treatment (24 weeks at each dose level), excellent compliance with study medication and visits, a comprehensive set of outcome measures, and documentation of adequate blinding of participants.
Do our findings apply to other saw palmetto preparations? We studied just one extract, and because the potential active ingredients and mechanisms are unknown, our findings may not be generalizable. Nevertheless, a recent series of negative trials using different saw palmetto preparations makes it increasingly unlikely a dose of some preparation will be identified that is superior to placebo. 11, 12
The eligibility criteria for this study were intentionally broader than for many previous trials of prescription medications for LUTS attributed to BPH; such as the Medical Therapy of Prostatic Symptoms (MTOPS) study comparing doxazosin, finasteride, and combination therapy to placebo3; in part because of our desire to recruit men who might typically chose to take phytotherapy for LUTS. As a result, participants in this study were slightly younger (mean age 61 versus 63 years), less symptomatic (mean AUASI score 14.5 versus 17 points), with lower PSA levels (mean PSA 2.1 versus 2.4 ng/mL) and substantially higher peak uroflow rates (15 versus 10.5 mL/sec) than men enrolled in MTOPS.3 As a result, a greater percentage of men in this study compared to MTOPS may have had LUTS due to causes other than BPH.27, 28 Nevertheless, the exploratory subgroup analyses did not suggest a differential effect of saw palmetto extract on men more likely to have LUTS due to BPH, such as men with higher PSA levels or lower peak uroflow. Not surprisingly, this study population was demographically and clinically more similar to the STEP population.12
In conclusion, we found that a saw palmetto extract used at up to three times the standard daily dose, while safe and with no attributable side effects we could identify, had no greater effect than placebo on improving lower urinary symptoms or other outcomes related to BPH.
Supplementary Material
Figure 2 (Online only). Mean AUA Symptom Index (AUASI) scores and 95% confidence intervals for Saw Palmetto (SP, red diamonds) and placebo (P, blue diamonds).
Acknowledgements
Funding/Support:
This study was funded by cooperative agreements from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: U01 DK63795, U01 DK63797, U01 DK63825, U01 DK63835, U01 DK63866, U01 DK63833, U01 DK63862, U01 DK63840, U01 DK63883, U01 DK63831, U01 DK63778 and U01 DK63788. Support was also provided by the National Center for Complementary and Alternative Medicine and the Office of Dietary Supplements, NIH. Saw palmetto fruit extract and matching placebo was donated by Rottapharm/Madaus, Cologne, Germany. This study was conducted under an Investigational New Drug Application from the Food and Drug Administration. Rottapharm/Madaus had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation or approval of the manuscript. Rottapaharm/Madaus provided nonbinding comments to the authors on a draft of the manuscript. NIH scientists representing the funding agencies did participate in the design and conduct of the study as well as review and approval of the manuscript, and are listed as authors.
CAMUS Study Group
Steering Committee Chair
Massachusetts General Hospital
Michael J. Barry, MD
Data Coordinating Center
University of Alabama at Birmingham
O. Dale Williams, PhD (Director)
Sreeletha Meleth, PhD (Associate Director)
Alan Cantor, PhD
Clinical Sites
New York University
Andrew McCullough, MD (PI through 12/3/10)
Northern California Kaiser Permanente
Andrew L. Avins, MD, MPH
Harley Goldberg, DO (Co-I)
Luisa Hamilton (Study Coordinator)
Cynthia Huynh (Research Associate)
Northwestern University Feinberg School of Medicine
Kevin T. McVary, MD (PI)
Robert Brannigan, MD (Co-I)
Brian Helfand, MD, PhD (Consultant)
Maria Velez (Study Coordinator)
Nancy Schoenecker, RN, CCRC (Clinical Research Coordinator)
Queens University
J. Curtis Nickel, MD (PI)
Alvaro Morales (Co-I)
D. Robert Siemens, MD (Co-I)
Joe Downey, MSc, CCRP (Study Coordinator)
Janet Clark-Pereira, CCRP (Study Coordinator)
University of Colorado Denver
E. David Crawford, MD (PI)
Shandra S. Wilson, MD (Co-I)
Paul D. Maroni, MD (Co-I)
Patricia DeVore, BS (Clinical Research Coordinator)
Cliff Jones (Clinical Research Coordinator)
University of Iowa
Karl J. Kreder, MD, MBA (PI)
Victoria Sharp, MD, MBA (Co-I)
Diane Meyerholz, RN, BSN (Study Coordinator)
Mary Eno, RN (Study Coordinator)
University of Maryland
Michael J. Naslund, MD (PI)
Ganine Markowitz-Chrystal, MS, CCRC (Study Coordinator)
University of Texas, Southwestern Medical Center
Claus G. Roehrborn, MD (PI)
Brad Hornberger, PA-C (Co-I)
Allison Beaver, RN (Study Coordinator)
Suzie Carter (Data Manager)
Washington University School of Medicine
Gerald L. Andriole, MD (PI)
Vivien Gardner, RN, BSN (Study Coordinator)
Karen Whitmore (Supervisor Patient Services)
Weill Cornell Medical College
Steven A. Kaplan, MD (PI)
Alexis E. Te, MD (Co-I)
Noreen Buckley, NP, CCRC (Study Coordinator)
Maritza Rodriquez (Medical Assistant)
Yale University School of Medicine
Harris E. Foster, Jr., MD (PI)
John W. Colberg, MD (Co-I)
Karen Stavris, RN MSN, CCRC (Study Coordinator)
Biostatistics Consultant
University of Arkansas for Medical Sciences
Jeannette Y. Lee, PhD
National Institutes of Health
National Institute of Diabetes, Digestive & Kidney Diseases
John W. Kusek, PhD
Leroy M. Nyberg, PhD (through 9/2/09)
National Center for Complementary and Alternative Medicine
Catherine M. Meyers, MD
Office of Dietary Supplements
Joseph M. Betz, PhD
Data Safety Monitoring Board
University of Minnesota VA Medical Center
Timothy J. Wilt, MD, MPH (Chair)
University of Illinois at Chicago
Harry H.S. Fong, Ph.D.
University of Chicago
Glenn S. Gerber, MD
University of Virginia
Mikel Gray, RN, PhD, CUNP, FAAN
HeteroGeneity LLC
Freddie Ann Hoffman, MD
University of North Carolina
Gary Koch, PhD
University of California at Los Angeles
Mark Litwin, MD, MPH
US Environmental Protection Agency
Warren E. Lux, MD
Harvard Medical School
Michael P. O’Leary, MD, MPH
Intercultural Cancer Council
Col (Ret.) James E. Williams, Jr.
Hines VA Hospital Cooperative Studies Program Coordinating Center
Domenic Reda, PhD
Footnotes
Author Contributions: Drs. Lee and Cantor had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Barry, Avins, Nickel, Roehrborn, Crawford, Foster, Kaplan, Andriole, Naslund, Lee, Williams, Kusek, Betz, McVary
Acquisition of data: Meleth, Kreder, Avins, Nickel, Roehrborn, Crawford, Foster, Kaplan, McCullough, Andriole, Naslund, Williams.
Analysis and interpretation of data: Barry, Meleth, Avins, Nickel, Crawford, Foster, Kaplan, Andriole, Lee, Williams, Kusek, Meyers, McVary, Cantor
Drafting of the manuscript: Barry, Meleth, Nickel, Roehrborn, Crawford, Foster, Kaplan, Lee, Williams, McVary
Critical revision of the manuscript for important intellectual content: Barry, Kreder, Avins, Nickel, Roehrborn, Crawford, Foster, Kaplan, McCullough, Andriole, Naslund, Lee, Williams, Kusek, Meyers, Betz, McVary, Cantor
Statistical analysis: Meleth, Lee, Williams, McVary, Cantor
Obtained funding: Avins, Nickel, Roehrborn, Crawford, Foster, McCullough, Andriole, Lee
Administrative, technical, or material support: Avins, Roehrborn, Crawford, Kaplan, Naslund, Kusek, Meyers, Betz, McVary
Study supervision: Barry, Meleth, Avins, Nickel, Andriole, Naslund, Williams, Kusek, Meyers
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Dr Barry serves on the Board of and receives salary support as President of the not-for-profit (501[3]c) Foundation for Informed Medical Decision Making (http://www.fimdm.org), which develops content for patient education programs. The foundation has an arrangement with a for-profit company, Health Dialog, to coproduce and market these programs to health care organizations.
Dr. Nickel reports receiving consultation funds from GlaxoSmithKline, Pfizer, Watson, Astellas, Ferring, Taris, Triton, Farr Labs, Trillium, Cernelle, and Johnson and Johnson, has provided expert testimony for GlaxoSmithKline and has received payment for development of educational presentations from the Canadian Urological Association.
Dr. Crawford reports receiving payment for lectures from Ferring Pharmaceuticals.
Dr. Andriole reports receiving consultation funds from Amgen, Bayer, Caris, France Foundation, GenProbe, GlaxoSmithKline, Steba Biotech, Ortho-Clinical Diagnostics, and Ferring Pharmaceuticals and has received royalties from “Up to Date”. He reports receiving payment for development of educational presentations from Amgen, and has stock/stock options in: Envisioneering Medical, Viking Medical, Augmenix, and Cambridge Endo. Dr Andriole reports receiving travel/accomodations/meeting expenses from Amgen, Augmenix, Bayer, Cambridge Endo, Caris, France Foundation, GenProbe, Myriad Genetics, Steba Biotech, and Ortho Clinical Diagnostics.
Dr. Naslund reports receiving payment for lectures from Glaxo and Sanofi as well as payment for development of educational presentations for France Foundation.
Dr. Lee reports that funds were paid to her institution for consultancy to Merck.
Dr. McVary reports receiving consultancy funds from Lilly/ICOS, Allergan, NIDDK, Watson Pharm., and Neotract, as well as payment for lectures from GlaxoSmithKline.
No other disclosures were reported.
Appendix (Online only). Supplemental Product Information.
References
- 1.Jacobsen SJ, Girman CJ, Guess HA, Oesterling JE, Lieber MM. New diagnostic and treatment guidelines for benign prostatic hyperplasia. Potential impact in the United States. Arch Intern Med. 1995 Mar 13;155(5):477–481. [PubMed] [Google Scholar]
- 2.Committee AUAPG. AUA guideline on management of benign prostatic hyperplasia (2003). Chapter 1: Diagnosis and treatment recommendations. J Urol. 2003 Aug;170(2 Pt 1):530–547. doi: 10.1097/01.ju.0000078083.38675.79. [DOI] [PubMed] [Google Scholar]
- 3.McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003 Dec 18;349(25):2387–2398. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 4.Dedhia RC, McVary KT. Phytotherapy for lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2008 Jun;179(6):2119–2125. doi: 10.1016/j.juro.2008.01.094. [DOI] [PubMed] [Google Scholar]
- 5.Barnes P, Bloom B, Nahin R. Complementary and alternative medicine use among adults and children: United States, 2007. 2008 December 10; 2008. [PubMed] [Google Scholar]
- 6.Gerber GS. Saw palmetto for the treatment of men with lower urinary tract symptoms. J Urol. 2000 May;163(5):1408–1412. [PubMed] [Google Scholar]
- 7.Buck AC. Is there a scientific basis for the therapeutic effects of serenoa repens in benign prostatic hyperplasia? Mechanisms of action. J Urol. 2004 Nov;172(5 Pt 1):1792–1799. doi: 10.1097/01.ju.0000140503.11467.8e. [DOI] [PubMed] [Google Scholar]
- 8.Maccagnano C, Salonia A, Briganti A, et al. A Critical Analysis of Permixon™ in the Treatment of Lower Urinary Tract Symptoms Due to Benign Prostatic Enlargement. European Urology Supplement. 2006;5(4):430–440. [Google Scholar]
- 9.Pais P. Potency of a novel saw palmetto ethanol extract, SPET-085, for inhibition of 5alpha-reductase II. Adv Ther. 2010 Aug;27(8):555–563. doi: 10.1007/s12325-010-0041-6. [DOI] [PubMed] [Google Scholar]
- 10.Wilt T, Ishani A, Mac Donald R. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2002;(3):CD001423. doi: 10.1002/14651858.CD001423. [DOI] [PubMed] [Google Scholar]
- 11.Tacklind J, MacDonald R, Rutks I, Wilt TJ. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2009;(2):CD001423. doi: 10.1002/14651858.CD001423.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bent S, Kane C, Shinohara K, et al. Saw palmetto for benign prostatic hyperplasia. N Engl J Med. 2006 Feb 9;354(6):557–566. doi: 10.1056/NEJMoa053085. [DOI] [PubMed] [Google Scholar]
- 13.Avins AL, Bent S, Staccone S, et al. A detailed safety assessment of a saw palmetto extract. Complement Ther Med. 2008 Jun;16(3):147–154. doi: 10.1016/j.ctim.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee J, Andriole G, Avins A, et al. Redesigning a large-scale clinical trial in response to negative external trial results: the CAMUS study of phytotherapy for benign prostatic hyperplasia. Clin Trials. 2009 Dec;6(6):628–636. doi: 10.1177/1740774509352199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.United States Pharmacopeial Convention. The Official Compendia of Standards USP 33/NF28 S1 Reissue. Rockville, MD: United States Pharmacopeial Convention; 2010. United States Pharmacopeia, Saw Plametto Extract. [Google Scholar]
- 16.Barry MJ, Fowler FJ, Jr, O'Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992 Nov;148(5):1549–1557. doi: 10.1016/s0022-5347(17)36966-5. discussion 1564. [DOI] [PubMed] [Google Scholar]
- 17.Barry MJ, Fowler FJ, Jr, O'Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK. Measuring disease-specific health status in men with benign prostatic hyperplasia. Measurement Committee of The American Urological Association. Med Care. 1995 Apr;33(4 Suppl):AS145–AS155. [PubMed] [Google Scholar]
- 18.O'Leary MP, Wei JT, Roehrborn CG, Miner M, Registry BPH, Patient Survey Steering C. Correlation of the International Prostate Symptom Score bother question with the Benign Prostatic Hyperplasia Impact Index in a clinical practice setting. BJU Int. 2008 Jun;101(12):1531–1535. doi: 10.1111/j.1464-410X.2008.07574.x. [DOI] [PubMed] [Google Scholar]
- 19.Rosen RC, Catania JA, Althof SE, et al. Development and validation of four-item version of Male Sexual Health Questionnaire to assess ejaculatory dysfunction. Urology. 2007 May;69(5):805–809. doi: 10.1016/j.urology.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 20.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997 Jun;49(6):822–830. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 21.Donovan JL, Peters TJ, Abrams P, Brookes ST, de aa Rosette JJ, Schafer W. Scoring the short form ICSmaleSF questionnaire. International Continence Society. J Urol. 2000 Dec;164(6):1948–1955. [PubMed] [Google Scholar]
- 22.Jenkins CD, Stanton BA, Niemcryk SJ, Rose RM. A scale for the estimation of sleep problems in clinical research. J Clin Epidemiol. 1988;41(4):313–321. doi: 10.1016/0895-4356(88)90138-2. [DOI] [PubMed] [Google Scholar]
- 23.Litwin MS, McNaughton-Collins M, Fowler FJ, Jr, et al. The National Institutes of Health chronic prostatitis symptom index: development and validation of a new outcome measure. Chronic Prostatitis Collaborative Research Network. J Urol. 1999 Aug;162(2):369–375. doi: 10.1016/s0022-5347(05)68562-x. [DOI] [PubMed] [Google Scholar]
- 24.Barry MJ, Williford WO, Chang Y, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J Urol. 1995 Nov;154(5):1770–1774. doi: 10.1016/s0022-5347(01)66780-6. [DOI] [PubMed] [Google Scholar]
- 25.Knottnerus JA, Bouter LM. The ethics of sample size: two-sided testing and one-sided thinking. J Clin Epidemiol. 2001 Feb;54(2):109–110. doi: 10.1016/s0895-4356(00)00276-6. [DOI] [PubMed] [Google Scholar]
- 26.Lee JY, Foster HE, McVary KT, et al. Recruitment of Participants to a Clinical Trial of Botanical Therapy for Benign Prostatic Hyperplasia. J Altern Complement Med. 2011 May 9; doi: 10.1089/acm.2010.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chapple CR, Roehrborn CG. A shifted paradigm for the further understanding, evaluation, and treatment of lower urinary tract symptoms in men: focus on the bladder. Eur Urol. 2006 Apr;49(4):651–658. doi: 10.1016/j.eururo.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 28.Nickel JC. The overlapping lower urinary tract symptoms of benign prostatic hyperplasia and prostatitis. Curr Opin Urol. 2006 Jan;16(1):5–10. doi: 10.1097/01.mou.0000193365.46081.cd. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 2 (Online only). Mean AUA Symptom Index (AUASI) scores and 95% confidence intervals for Saw Palmetto (SP, red diamonds) and placebo (P, blue diamonds).