Abstract
In C. elegans males, different subsets of ventral epidermal precursor (Pn.p) cells adopt distinct fates in a position-specific manner: three posterior cells, P(9–11).p, comprise the hook sensillum competence group (HCG) with three potential fates (1°, 2°, or 3°), while eight anterior cells, P(1–8).p, fuse with the hyp7 epidermal syncytium. Here we show that activation of the canonical BAR-1 β-catenin pathway of Wnt signaling alters the competence of P(3–8).p and specifies ectopic HCG-like fates. This fate transformation requires the Hox gene mab-5. In addition, misexpression of mab-5 in P(1–8).p is sufficient to establish HCG competence among these cells, as well as to generate ectopic HCG fates in combination with LIN-12 or EGF signaling. While increased Wnt signaling induces predominantly 1° HCG fates, increased LIN-12 or EGF signaling in combination with MAB-5 overexpression promotes 2° HCG fates in anterior Pn.p cells, suggesting distinctive functions of Wnt, LIN-12, and EGF signaling in specification of HCG fates. Lastly, wild-type mab-5 function is necessary for normal P(9–11).p fate specification, indicating that regulation of ectopic HCG fate formation revealed in anterior Pn.p cells reflect mechanisms of pattern formation during normal hook development.
Keywords: pattern formation, fate transformation, Axin, β-catenin, Hox, WNT, Notch
Introduction
Patterning of cell fates in a position-specific manner is a general feature of development, and ventral epidermal development in the nematode Caenorhabditis elegans has been a useful experimental model to study this fundamental problem. In particular, twelve C. elegans ventral ectodermal precursor cells, Pn.p cells (where n refers to the numbers 1–12), have sexual-dimorphic cell fusion patterns. Pn.p cells that do not fuse to the hyp7 syncytium have distinct developmental fates: six central Pn.p cells, P(3–8).p, become vulval precursor cells (VPCs) in hermaphrodites, and three posterior Pn.p cells, P(9–11).p, form the hook sensillum competence group (HCG) in males (Sulston and Horvitz, 1977; Sulston and White, 1980). Maintenance of Pn.p cells as individual epithelial cells allows them to remain competent to the inductive signals that specify the vulval and hook fates. The fates of both HCG and VPC cells depend on Wnt and EGF signaling; however, it appears that WNT plays the greater role in HCG induction while EGF plays the major role in VPC induction (Yu et al., 2009). LIN-12/Notch signaling promotes both the 2° HCG and VPC fate during development (Greenwald et al., 1983).
The sex-specific fusion pattern of Pn.p cells is established in part by region-specific activities of homeotic complex (HOM-C) genes (Salser et al., 1993). Three members of a HOM-C pseudo-cluster, lin-39, mab-5, and egl-5, are expressed in different subsets of Pn.p cells during early development. lin-39 is expressed in the cells P(3–8).p (Clark et al., 1993; Wang et al., 1993), whereas mab-5 is expressed in P(7–11).p (Salser et al., 1993; Wang et al., 1993). egl-5 is expressed in P12.p and its daughter P12.pa (Jiang and Sternberg, 1998; Ferreira et al., 1999) and represses mab-5 expression in these two cells (Salser et al., 1993). In hermaphrodites, lin-39 activity in P(3–8).p keeps these six cells unfused and competent to form VPCs, while other Pn.p cells fuse with hyp7 in the L1 stage (Clark et al., 1993; Wang et al., 1993). Wnt signaling has been shown to be required for the expression of lin-39 in P(3–6).p (Eisenmann et al., 1998). In males, either lin-39 or mab-5 activity alone prevents cell fusion, but co-expression of lin-39 and mab-5 in the same cell neutralizes each other’s activity (Salser et al., 1993). As a consequence, P(1–2).p cells, which express none of these three HOM-C genes, and P(7–8).p cells, which express both lin-39 and mab-5, fuse in the late L1 stage; while P(3–6).p and P(9–11).p remain unfused at the same stage. The HCG cells, P(9–11).p, correspond to a domain with only mab-5 activity (Salser et al., 1993; Maloof and Kenyon, 1998). A mab-5 loss-of-function mutation completely eliminates this equivalence group by causing the fusion of P(9–11).p cells with hyp7 during the late L1 stage (Kenyon, 1986).
Male P(9–11).p cells each adopt one of the three hook fates (Sulston and White, 1980). In wild-type males, P10.p and P11.p adopt the induced 2° fate and 1° fate, respectively, generating descendants with various epidermal and neuronal fates (Sulston et al., 1980). A 1° fate marker, eat-4::GFP, is expressed in one of the P11.p descendants, the PVV motor neuron (Yu et al., 2009). osm-6::GFP and ceh-26::GFP are two 2° lineage markers: osm-6 is expressed in both hook neurons (HOA and HOB), while ceh-26 is expressed only in HOB (Collet et al., 1998; Yu et al., 2003). Another 2° P10.p descendant generates the hook structure. The P9.p cell adopts the un-induced 3° fate, and usually fuses with the hyp7 epidermal syncytium during the late L2 stage. However, P9.p can express either induced HCG fate when a posterior P10.p and/or P11.p is missing or in the presence of increased EGF or Notch activity (Sulston et al., 1980; Yu et al., 2009). In contrast, male P(3–6).p cells fuse with the surrounding epidermis in the late L2 stage and P(7–8).p cells fuse in the late L1 stage. Unlike the P9.p cell, these anterior Pn.p cells adopt vulval-like fates in response to increased EGF or Notch signaling (Ferguson and Horvitz, 1985; Greenwald et al., 1983), suggesting that male P(3–8).p cells do not have the same developmental potential or competency as male P(9–11).p; in particular, they are competent to make vulval tissue but not hook tissue. In both the VPC and HCG equivalence groups, competence includes the blocking of fusion to the hyp7 epidermis prior to receiving strong EGF, WNT or NOTCH signals.
Here, we present evidence that activated Wnt signaling, as the result of eliminating PRY-1/Axin, not only changes the competence of P(3–8).p but also produces ectopic 1° HCG fates in these Pn.p cells. The Hox gene mab-5 is the determinative element for HCG competence acting downstream of Wnt signaling. Increased MAB-5 activity, in combination with a proliferation signal from either EGF or Notch signaling, mimics the effect of Wnt signaling hyperactivity to induce HCG-like fate transformations in anterior Pn.p cells (P(1–8).p). The requirement of mab-5/Hox for HCG specification revealed in the ectopic conditions is also true for the wild-type HCG, P(9–11).p. We also show that Wnt signaling regulates mab-5 expression in the 1°-fated P11.p cell.
Results
Activated Wnt signaling promotes 1° HCG fates in anterior Pn.p cells
pry-1 encodes an ortholog of Axin, a negative regulator of canonical Wnt signaling (Korswagen et al., 2002). We found that the mu38 reduction-of-function (rf) mutation in the pry-1 gene causes abnormal ventral invaginations in the middle body region of L4 male larvae and multiple small protrusions at similar central positions of adult males. These invaginations and protrusions displayed morphological features resembling structures associated with HCG-like fates: an anchor-shaped structure that looks like the ectopic hooks generated in lin-12 gain-of-function mutant males (Greenwald et al., 1983) was sometimes seen inside the invaginations, and almost all the protrusions had yellow autofluorescence under UV illumination regardless of the shape (Fig. 1A–B). By examining AJM-1–GFP, a marker of cell junctions (Gupta et al., 2003; Sharma-Kishore et al., 1999; Shemer et al., 2000), we noticed that the central Pn.p cells including P(3–6).p and P(7–8).p were often unfused in late L2 or L3 pry-1 mutant males, and some of those Pn.p cells divided multiple times during the late L3 to early L4 stage. Therefore, additional ventral invaginations observed in pry-1 mutant males resulted from inappropriate proliferation of the central Pn.p cells. About 70% of pry-1(mu38) males yielded such a morphological alteration in the P(3–6).p region and about 27% in the P(7–8).p region (Table 1).
Figure 1.
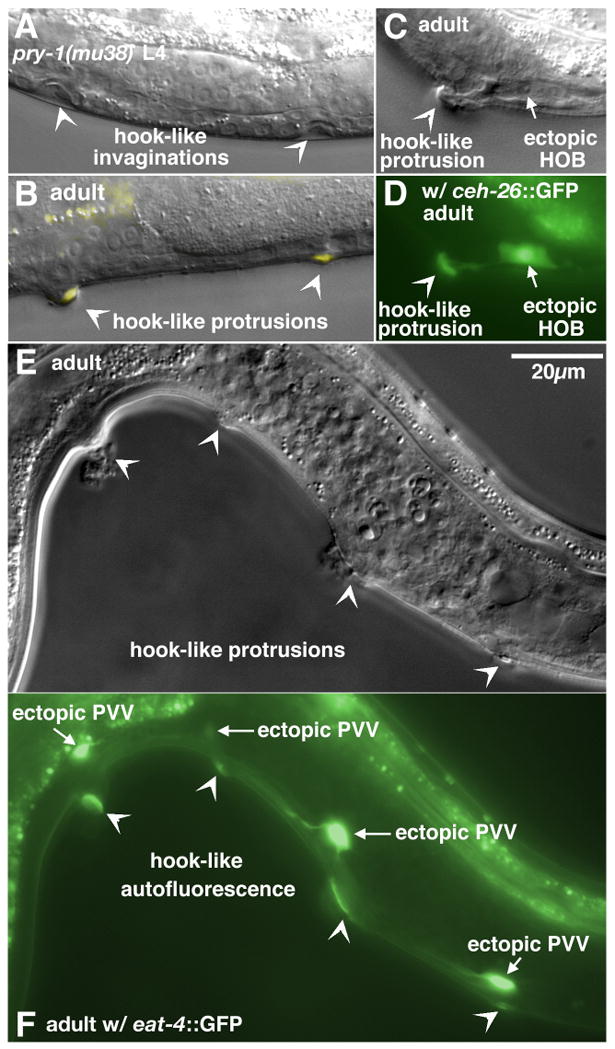
Formation of ectopic HCG fates in pry-1 mutant males. (A) Late L4. Two ventral invaginations with anchor-like shapes inside (arrowheads) were formed in the central region of a pry-1(mu38) male. (B) Overlay of Nomarski and fluorescence images of an adult pry-1(mu38) male. Two ventral protrusions (arrowheads) were observed in the middle body. Autofluorescence could be seen at the tip of the protrusions. (C–D) Nomarski (C) and fluorescence (D) images of an adult pry-1(mu38) male. An autofluorescent ventral protrusion (arrowhead) was associated with an ectopic 2° HOB hook neuron cell (arrow), as indicated by ceh-26::GFP expression. (E–F) Correlation of ectopic 1° PVV-like eat-4::GFP-expressing neurons (arrows) with ventral protrusions (arrowheads) in a pry-1(mu38) male (E, Nomarski; F, fluorescence). Scale bar, 20 μm. Left lateral views.
Table 1.
Ectopic HCG induction in pry-1 mutants
| Genotypea | HCG-like fate formationb (%)(structure or marker expression) | avg. HCG-like structurec (1° or 2°) | avg. marker expression | nd | ||||
|---|---|---|---|---|---|---|---|---|
| signaling | markers | P(1–2).p | P(3–6).p | P(7–8).p | P(9–11).p | |||
| + | none | 0 | 0 | 0 | 100 | 1.0 | NA | many |
| pry-1 | “ | 0 | 70 | 27 | 39 | 1.8 | NA | 79 |
| + | eat-4::GFP (1°) | 0 | 0 | 0 | 100 | 1.0 | 1.0 (1°) | 117 |
| pry-1 | “ | 0 | 95 | 38 | 5 | 2.2 | 2.0 (1°) | 39 |
| + | ceh-26::GFP (2°) | 0 | 0 | 0 | 100 | 1.0 | 1.0 (2°) | >2000 e |
| pry-1 | “ | 0 | 83 | 17 | 13 | 1.9 | 0.2 (2°) | 30 |
| bar-1 | “ | 0 | 0 | 0 | 86f | 0.8 | 0.7 (2°) | 71 g |
| pry-1; bar-1 | “ | 0 | 0 | 0 | 93f | 1.0 | 0.9 (2°) | 147 |
| + | osm-6::GFP (2°) | 0 | 0 | 0 | 100 | 1.0 | 1.0 (2°) | >200e |
| pry-1 | “ | 0 | 91 | 36 | 27 | 1.7 | 0.4 (2°) | 22 |
| lin-12(gf)/lin-12(lf) | “ | 0 | 0 | 0 | 100 | 2.3 | 2.3 (2°) | 69 |
| pry-1; lin-12(gf)/lin-12(lf) | “ | 26 | 100 | 22 | 4 | 2.8 | 1.4 (2°) | 27 |
Alleles used are: pry-1(mu38), lin-12(n137) as lin-12(gf), and lin-12(n676n909) as lin-12(lf). Integrated arrays are adIs1240 (eat-4::GFP), chIs1200 (ceh-26::GFP), and mnIs17 (osm-6::GFP). All strains contain him-5(e1490) in the background.
Percentage of animals in which Pn.p subgroups adopt HCG-like fates, determined by the presence of HCG-like structure (invagination if scored in L4 or ventral protrusion if scored in adult), and/or ectopic lineage marker expression.
Including HCG-like invagination or ventral protrusion.
Number of animals scored.
Data are from a genetic screen described in Yu et al. (2003).
Only the 2° hook and /or marker expression are scored. Actual HCG induction might be higher if a few animals had the 1° fate only.
Data are also mentioned in Yu et al. (2009).
NA, not available.
It has been shown previously that the pry-1(mu38) mutation causes excessive vulval differentiation in hermaphrodites; specifically, three normally 3° VPCs, P3.p, P4.p, and P8.p, can adopt induced fates and form extra vulval invaginations (Gleason et al. 2002). As the shape of an ectopic hook-like invagination or protrusion can be quite irregular, it is not easily distinguishable from structures generated by a vulval-like fate in some cases. To clarify the nature of the abnormal morphological structures produced by P(3–8).p in pry-1(mu38) males, we examined the expression of vulval and hook lineage-specific markers in pry-1 mutants. Expression of egl-17::CFP, a vulval fate marker, was never detected at the ventral protrusions of pry-1 mutant males. However, pry-1 male mutants displayed many eat-4::GFP-expressing ventral neurons with processes similar to PVV, a descendent of the 1° HCG lineage, each frequently associated with a ventral protrusion (Fig 1E–F; Table 1). The expression of a 1° HCG lineage marker as well as morphological features of ventral structures suggests that a pry-1 mutation induces ectopic HCG fates, rather than abnormal vulval differentiation, among the central Pn.p cells in males. In wild-type males, none of the P(3–8).p cells is a part of the HCG.
The normal 3°-2°-1° pattern of P(9–11).p HCG cell fates is disrupted in pry-1 mutant males as a consequence of P11-to-P12-like fate transformation during the L1 stage (Howard and Sundaram, 2002). In these mutants, the wild-type hook generated by a normal 2° P10.p lineage was absent at the cloacal region, and HCG-like invaginations and protrusions from P(9–10).p were observed at low frequency (Table 1).
We also observed that, based on both marker expression and morphology, ectopic 1° HCG fates were sometimes expressed by adjacent Pn.p cells in pry-1(mu38) males (Fig. 1E–F). In addition, since the 1° HCG fate is required to specify the 2° HCG fate (Yu et al., 2009), we expected that some of the induced Pn.p cells would adopt the 2 fate because of lateral signaling from a nearby 1° Pn.p cell. Indeed, the structures of some protrusions in those pry-1(mu38) males were similar to the 2° hook, and two 2° hook lineage markers, ceh-26::GFP and osm-6::GFP, were occasionally expressed in association with a ventral protrusion (Fig. 1C–D; Table 1). In general, presence of ectopic hook neurons, as indicated by expression of the 2° markers, was less frequent than occurrence of the hook-like structure, indicating that differentiation of hook neurons perhaps requires more intricate interactions and ectopic 2° HCG fate formation is often incomplete in pry-1 mutants. It is also possible that some 1° /2° hybrid fates were formed in the pry-1(mu38) genetic background.
Specification of ectopic HCG fates in pry-1 mutants was fully suppressed by a bar-1/β-catenin mutation (Table 1). No anterior invaginations or protrusions were found in pry-1; bar-1 double mutants. Furthermore, 2° hook structures and neurons were only derived from the normal HCG region (P(9–11).p and P12.p from P12-to-P11 transformation, n= 147). The hook sensillum was completely absent in 7% of double mutants, and was partially lost in another 11% (data not shown), indicating a weak defect in 2° lineage specification similar to what is seen in a bar-1 single mutant (Yu et al., 2009). This suppression suggests that the constitutively activated canonical Wnt signaling pathway results in specification of ectopic 1° HCG fates in P(3–8).p cells of pry-1 mutant males.
Co-activation of Wnt and Notch signaling induces ectopic 2° HCG fates in anterior Pn.p cells
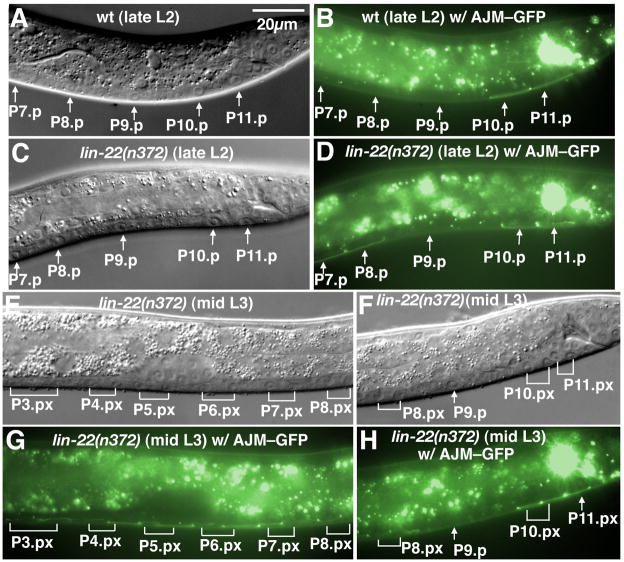
Fusion of P(7–8).p with hyp7 in the late L1 larval stage determines the anterior boundary of the HCG. Although male P(3–6).p remain unfused in the L1 stage as P(9–11).p do, they cannot adopt HCG fates and normally fuse with hyp7 during late L2 (Sulston and White, 1980; Yu et al., 2009) (Fig. 2A–B). One possibility is that the fused P(7–8).p might serve as a physical barrier that prevents any possible inclusion of P(3–6).p into the HCG. In the lin-22(n372) mutant, defective in a hairy homolog, male P(3–8).p remain unfused in late L3, and in some cases divide (Fixsen, 1985; Wrischnik and Kenyon, 1997) (Fig. 2C–H). However, P9.p in lin-22(n372) males still fused with hyp7 normally in the middle-to-late L2 stage and only a single wild-type hook was formed at its normal position anterior to the cloaca, suggesting that just preventing P(7–8).p cell fusion has no effect on HCG patterning (Fig. 2C–D, F and H).
Figure 2.
Alteration of male Pn.p fusion pattern by a lin-22 mutation. (A–D) Late L2. Nomarski (A) and fluorescence (B) images of a wild-type male. In the posterior, a line of AJM-1–GFP expression marked unfused P10.p and P11.p cells. P9.p and more anterior P(7–8).p were fused with hyp7 already. Nomarski (C) and fluorescence (D) images of a lin-22(n371) male in the same developmental stage. P9.p fused as indicated by the loss of AJM-1–GFP expression. However, P(7–8).p, as well as central P(3–6).p (not shown), remained unfused as do P(10–11).p. (E–H) Nomarski (E and F) and fluorescence (G and H) images of a lin-22 male in the middle L3 stage. The unfused central P(3–8).p and posterior P(10–11).p all divided once. Expression of AJM-1–GFP was observed in all those Pn.p descendants. The P11.pa cell was located a little above and thus the AJM-1–GFP expression was not shown in this picture. Scale bar, 20 μm. Left lateral views.
In response to excess LIN-12/Notch or EGF signaling, male P(3–8).p not only escape fusion with hyp7 but also proliferate (Greenwald et al., 1983; Ferguson et al., 1985). However, in both cases, these cells behave like hermaphrodite P(3–8).p and assume vulval-like fates to produce pseudo-vulvae at the ventral side (multivulva or Muv phenotype; WBPhenotype:0000700). In particular, such animals are Muv in the middle of the body but also multi-hook at posterior since P(9–11).p in the same genetic background have inappropriate HCG fates (Ferguson and Horvitz, 1985; Greenwald et al. 1983; Yu et al. 2009). These observations suggest that male P(3–8).p and P(9–11).p have distinct developmental potentials and HCG competence is limited to P(9–11).p.
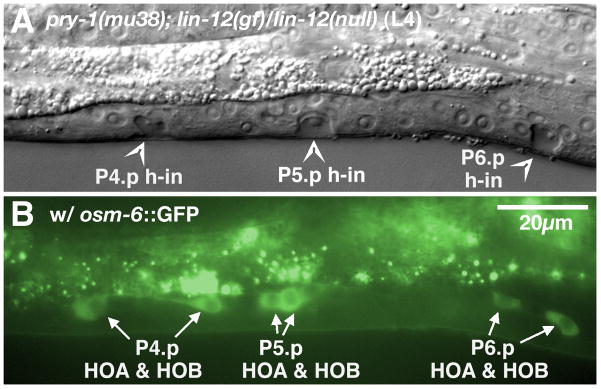
Introduction of a pry-1 mutation into a lin-12(gf) mutant background changed the dimorphic sexual differentiation among different subsets of male Pn.p cells caused by activated Notch signaling. Additional ectopic hook neurons, visualized by osm-6::GFP in the L4 stage, were paired with well-formed ectopic hook invaginations among the central P(3–8).p region of pry-1(mu38); lin-12(gf)/lin-12(null) double mutant males, revealing increased production of 2° HCG fates (Fig. 3, Table 1). Apparently, activated Wnt signaling by a pry-1 mutation causes P(3–8).p to adopt HCG fates, but distinct from the pry-1 single mutants, in which ectopic 1° HCG fates are predominant, extra LIN-12 signaling in the pry-1(mu38); lin-12(gf)/lin-12(null) double mutants promoted 2° HCG fate formation (Fig. 1 and 3; Table 1). Surprisingly, we observed ectopic hook neurons and invaginations from P(1–2).p (Table 1), a phenotype not detected in either of the single mutants. One explanation is that increased Wnt signaling and LIN-12 signaling act together to inhibit P(1–2).p cell fusion: Wnt signaling provides P(1–2).p with HCG competence, while excessive LIN-12 activity leads to the formation of ectopic 2° HCG fates in P(1–2).p.
Figure 3.
Preferential 2° HCG fate transformation in anterior Pn.p cells by activated Wnt and LIN-12 signaling. Nomarski (A) and fluorescence (B) images of an L4 pry-1; lin-12(gf)/lin-12(null) male. Three hook invaginations (arrowheads) were each coupled with a pair of osm-6::GFP-expressing hook neurons (arrows) among the three middle Pn.p cells (P4.p-P6.p). h-in, hook invagination. Scale bar, 20 μm. Left lateral views.
The pry-1 mutation acts through Hox genes to alter anterior Pn.p cell competence
Activities of two Hox genes lin-39 and mab-5 are required to establish the Pn.p fusion pattern in the L1 stage (Salser et al., 1993). The wild-type HCG domain corresponds to a region with only mab-5 expression. Maloof and Kenyon (1998) speculated that generation of HCG competence depends on mab-5 function. To determine whether pry-1 regulates Hox genes to affect P(3–8).p fusion pattern and to alter cell competence, we examined cell fates of male Pn.p defective in both pry-1 and each Hox gene used to determine Pn.p fusion. In most pry-1(mu38); lin-39(n1760) males, P(3–6).p fused with hyp7 and therefore greatly reduced the formation of HCG-like protrusions and ectopic expression of the 1° marker eat-4::GFP among these Pn.p cells. Apparently, in a pry-1 mutant background, lin-39 activity still plays a major role in determination of whether P(3–6).p fuse with the hyp7 epidermal syncytium. However, the occurrence of HCG fates in P(7–10).p or in occasional unfused P(3–6).p was not suppressed by a loss of lin-39 activity (Table 2), suggesting that lin-39 affects Pn.p cell fusion but has no effect on HCG competence.
Table 2.
Hox genes are required for HCG fate transformation induced by Wnt signaling
| genotypea | HCG-like fate formationb (%)(structure or marker expression) | avg. HCG-like structurec (1° or 2°) | avg. 1° marker expression | nd | ||||
|---|---|---|---|---|---|---|---|---|
| Wnt signaling | Hox | markers | P(3–6).p | P(7–8).p | P(9–11).p | |||
| + | + | none | 0 | 0 | 100 | 1.0 | NA | many |
|
| ||||||||
| + | lin-39 | “ | 0 | 0 | 100 | 1.0 | NA | 79 |
|
| ||||||||
| pry-1 | + | “ | 70 | 27 | 39 | 1.8 | NA | 79 |
|
| ||||||||
| pry-1 | lin-39 | “ | 9 | 33 | 14 | 0.6 | NA | 43 |
|
| ||||||||
| + | + | eat-4::GFP (1°) | 0 | 0 | 100 | 1.0 | 1.0 | 117 |
| + | lin-39 | “ | 0 | 0 | 100 | 1.0 | 1.0 | 63 |
| + | mab-5 | “ | 0 | 0 | 0 | 0 | 0 | 84 |
| pry-1 | + | “ | 95 | 38 | 5 | 2.2 | 2.0 | 39 |
| pry-1 | lin-39 | “ | 4 | 56 | 25 | 0.8 | 0.9 | 48 |
| pry-1 | mab-5 | “ | 0 | 0 | 0 | 0 | 0 | 83 |
Alleles used are: pry-1(mu38), mab-5(e1239), lin-39(n1760). Both Hox mutant alleles are probably null. All strains contain him-5(e1490). The eat-4::GFP transgene is adIs1240.
Some of the data in this table are present in Table 1.
Including hook-like invaginations or ventral protrusions.
Number of animals scored.
NA, not available.
The mab-5(e1239lf) mutation causes male P(7–8).p to stay unfused until the late L2 stage as P(3–6).p do, but causes P(9–11).p to fuse with hyp7 during the L1 stage (Kenyon, 1986). In pry-1(mu38); mab-5(e1239) double mutants, male P(9–10).p frequently fused during the L1 stage, while P(3–8).p often remained unfused until the L4 stage and in some cases divided. In contrast to a regional suppression of HCG specification in pry-1; lin-39 double mutant males, the mab-5(lf) mutation fully abolished ectopic expression of HCG-like fates in pry-1 mutants (Table 2). Thus, activated Wnt signaling in pry-1 mutants requires mab-5 activity to express HCG fates in Pn.p cells.
Using an integrated mab-5::gfp array muIs16, we found that expression of mab-5::GFP in pry-1 mutants was not only extended to anterior part of the body but also elevated greatly in general. In such a bright green fluorescent background, we were not able to examine the details of mab-5::GFP expression in Pn.p cells, which could be at a relatively lower level compare to surroundings (data not shown). The pry-1(mu38) mutation probably causes mab-5 misexpression in normally unfused P(3–6).p during the L1 and L2 stages, thereby establishing HCG competence in those cells. The additional mab-5 expression may also shift the balance between antagonistic actions of the two Hox genes lin-39 and mab-5 to block P(7–8).p cell fusion and cause unfused P(7–8).p cells to be HCG competent. In summary, a Pn.p cell acquires HCG competence if it escapes L1 fusion and has MAB-5 activity.
Unfused P(3–6).p were observed in some pry-1(mu38); lin-39(n1760) males, and the fusion of P(9–10).p with the hyp7 syncytium in pry-1(mu38); mab-5(e1239) males was not observed in all mutants either (data not shown), suggesting that activities of lin-39 and mab-5 are likely both increased by the pry-1 mutation and the requirement of one Hox gene by a subset of Pn.p cells to remain unfused could be partially remedied by extra activity of the other. However, up-regulation of mab-5 expression should be favored in pry-1 mutants, as indicated by unfused P(7–8).p, and only mab-5 acts downstream of an activated canonical Wnt pathway to specify HCG competence in P(3–8).p.
Recruitment of anterior Pn.p cells to the HCG by cooperative action of mab-5 overexpression and a proliferation signal
We have shown that a pry-1(rf) mutation not only prevents fusion of the anterior P(3–8).p cells with hyp7 but also changes their competence and promotes ectopic 1° HCG fates among these cells. A mab-5 gain-of-function (gf) promoter mutation e1751 constitutively expresses mab-5 in all Pn.p cells and alters male Pn.p cell fusion pattern in the L1 stage: P(1–2).p remain unfused due to extra mab-5 activity but P(3–8).p fuse with hyp7 because of a functional antagonism between lin-39 and mab-5 (Salser et al., 1993). However, no alteration in hook sensillum lineages was found in a mab-5(e1751gf) mutant (Table 3). Moreover, unlike suppression of the pry-1 mutation by bar-1(ga80lf), the loss-of-function mab-5(e1239) mutation suppressed ectopic HCG fate induction in pry-1 mutants but did not change the scrawny appearance of pry-1 mutant animals (see Materials and Methods), indicating that mab-5 mediates only a part of Wnt signaling outcome. One possible role of Wnt signaling, in addition to establishing HCG competence, is that it can also act as a proliferation signal. To test this hypothesis, we tested whether the combined effect of mab-5 overexpression and a non-Wnt proliferation signal is sufficient to generate ectopic hook fates among P(1–8).p – thereby mimicking the pry-1 mutant phenotype.
Table 3.
Ectopic HCG induction by increased MAB-5 activity and activated LIN-12 or EGF signaling
| genotypea | markers | HCG-like fate formation (%)(structure or marker expression)
|
avg. hook-like structure (1° or 2°) | avg. 2° marker expression | nb | |||
|---|---|---|---|---|---|---|---|---|
| P(1–2).p | P(3–6).p | P(7–8).p | P(9–11).p | |||||
| + | osm-6::GFP (2°) | 0 | 0 | 0 | 100 | 1.0 | 1.0 | >200c |
| mab-5(gf) | “ | 0 | 0 | 0 | 100 | 1.0 | 1.0 | 70 |
| lin-12(gf)/lin-12(lf) | “ | 0 | 0 | 0 | 100 | 2.3 | 2.3 | 69 |
| mab-5(gf) +/+ lin-12(gf) | “ | 64 | 49 | 5 | 100 | 3.7 | 3.4 | 61 |
| mab-5(gf) | none | 0 | 0 | 0 | 100 | 1.0 | NA | 70 |
| lin-15 | “ | 0 | 0 | 0 | 100 | 1.5 | NA | 68 |
| mab-5(gf); lin-15 | 44 | 12 | 0 | 93 | 1.9 | NA | 41 | |
| lin-15 | osm-6::GFP (2°) | 0 | 0 | 0 | 100 | 1.4 | 1.0 | 41 |
| mab-5(gf);lin-15 | “ | 74 | 13 | 6 | 94 | 2.6 | 0.7 | 31 |
| lin-22 | osm-6::GFP (2°) | 0 | 0 | 0 | 100 | 1.0 | 1.0 | 82 |
| lin-12(gf)/lin-12(lf); lin-22 | “ | 5 | 5 | 27 | 100 | 2.5 | 2.2 | 55 |
Alleles used are: mab-5(e1751), a gain-of-function allele; lin-12(n137) as lin-12(gf), a gain-of-function allele; lin-12(n676n909) as lin-12(null), a loss-of-function allele; lin-15(e1763), a severe reduction-of-function allele; lin-22(n372), a reduction-of-function allele. The osm-6::GFP transgene is mnIs17.
Number of animals scored.
Data from Yu et al. (2003).
First, we determined the effects of mab-5(e1751gf) mutation in a constitutively activated LIN-12/NOTCH background (see Materials and Methods). We found that 49% of mab-5(e1751) +/+ lin-12(gf) males had hook sensillum lineages in P(3–6).p and about 64% formed ectopic 2° HCG fates in P(1–2).p (Fig 4A–D; Table 3). Therefore, mab-5 activity and excessive LIN-12 siganling cooperate to promote the 2° HCG fate in P(1–8).p. In addition, no ectopic 1° eat-4::GFP expression was detected in these anterior Pn.p cells in these double mutants (data not shown), reflecting a functional distinction between Wnt and LIN-12 signaling during HCG pattern formation.
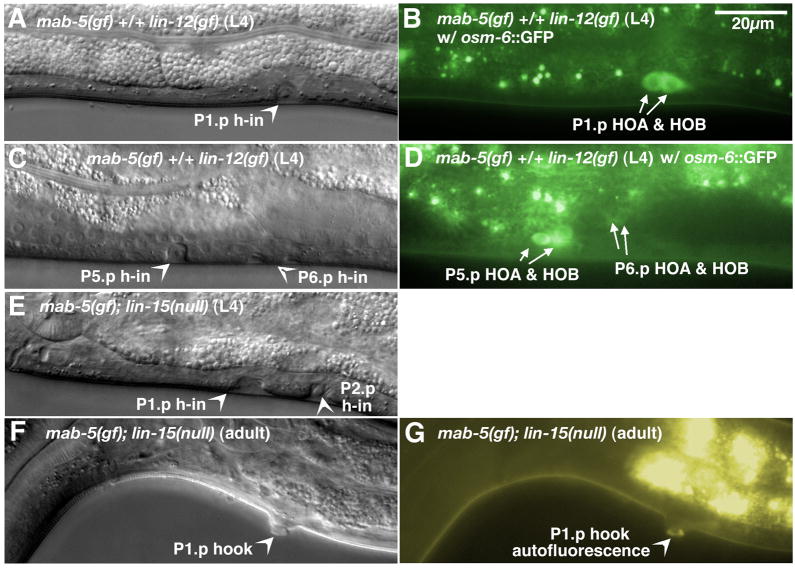
Figure 4.
Induction of ectopic 2° HCG fates by the combined action of constitutive MAB-5 activity and Notch or EGF signaling. (A–D) 2°-like HCG fate transformation in anterior Pn.p cells by increased MAB-5 activity and activated LIN-12 signaling. An L4 mab-5(e1751gf) +/+ lin-12(gf) male had an nice hook invagination and a pair of HOA and HOB hook neurons (marked by osm-6::GFP) derived from the P1.p lineage (A, Nomarski; B, fluorescence). Another L4 mab-5(e1751gf) +/+ lin-12(gf) male generated hook invaginations at the central P5.p and P6.p (arrowheads) (C, Nomarski). Each invagination was associated with a pair of hook neurons (arrows) (D, fluorescence). (EG) Ectopic hook formation in P(1–2).p by extra MAB-5 activity and EGF signaling. (E) An L4 mab-5(e1751gf); lin-15(e1753) male formed two hook-like invaginations at P(1–2).p. Nomarski (F) and fluorescence (G) images of a P1.p hook in an adult mab-5(e1751gf); lin-15(e1753) male. h-in, hook invagination. Scale bar, 20 μm. Left lateral views.
The second combination we tested was the mab-5(e1751) mutation in a lin-15(e1763lf) background. Mutations in lin-15 activate the LET-23(EGFR) pathway (Clark et al., 1994; Huang et al., 1994) likely by allowing inappropriate expression of LIN-3 in the hyp7 epidermis (Cui et al., 2006), the major signaling pathway inducing vulval development. Extra EGF signaling in lin-15(e1763lf) mutants causes P9.p to adopt a 2°-like fate, forming an ectopic rudimentary hook without additional hook neurons (Yu et al., 2009). These lin-15(lf) males also make pseudovulvae at P(3–8).p. We found that the male P(3–8).p Muv phenotype of a lin-15 mutant was fully suppressed by the mab-5(e1751gf) mutation. About 10–20% of mab-5(e1751gf); lin-15(e1763) double mutants had HCG-like fates from male P(3–8).p, usually either P3.p or P8.p (Table 3). In addition, P(1–2).p cells in the majority of double mutants not only divided but also differentiated to make hook-like invaginations and protrusions (Fig. 4E–G; Table 3). Occasionally there was a complete 2° fate transformation as hook neurons were also detected based on ectopic osm-6::GFP expression (data not shown). The generation of P(1–2).p ectopic hooks in mab-5(e1751gf); lin-15 double mutants suggests that additional EGF signaling is capable of inducing HCG fates in anterior Pn.p cells that have acquired HCG competence from mab-5 misexpression.
It has been shown previously that lin-22(+) function inhibits mab-5 expression in the anterior lateral epidermis (Wrischnik and Kenyon, 1997). Therefore, we also tested the effects of lin-22(lf) in an increased LIN-12/NOTCH signaling background. In general, proliferation of male P(3–8).p was enhanced in a lin-12(gf)/lin-12(null); lin-22(n372) strain compared to a lin-12(gf)/lin-12(null) genetic background (Fixsen, 1985; our observations). We observed ectopic hook formation in lin-12(gf)/lin-12(null); lin-22(n372rf) males: P(7–8).p in 27% of the double mutant males produced 2° hook invaginations and hook neurons. However, formation of pseudovulvae was predominant in P(3–6).p (Fig. 5B–C; Table 3). The lin-22 mutation might cause a weak increase of mab-5 expression in anterior Pn.p cells, which allows mab-5 activity to out-compete lin-39 activity in male P(7–8).p and results in unfused P(7–8).p cells in the lin-12(gf)/lin-12(null); lin-22(n372) double mutant males that are competent to adopt HCG fates. Intriguingly, about 5% of the double mutant males formed an ectopic hook and/or hook neurons in P(1–2).p (Fig. 5A; Table 3), although neither of the single mutants had unfused P(1–2).p cells. Unlike in mab-5(e1751gf) mutants, male P(1–2).p in a lin-22 single mutant still fuse normally during the L1 stage. Interaction between the lin-12(gf) and lin-22 reduction-of-function mutations might keep P(1–2).p unfused and cause sufficient ectopic mab-5 expression to provide these two Pn.p cells with HCG competence.
Figure 5.
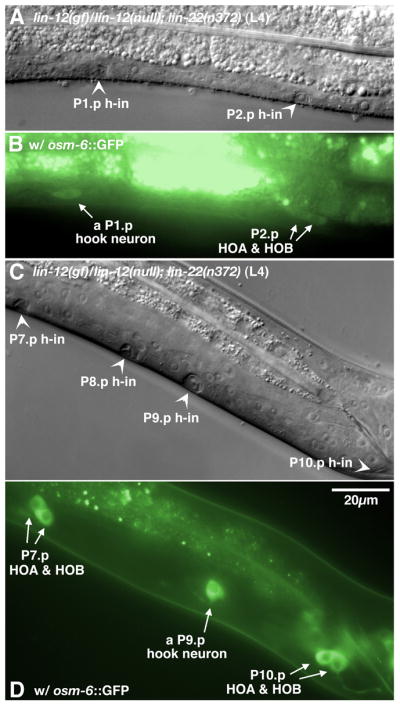
2° HCG fate formation in male Pn.p cells caused by activated LIN-12 signaling in a lin-22 mutant background. (A–B) A L4 lin-12(gf)/lin-12(null); lin-22(n372) male. Nomarski (A) and fluorescence (B) images of two hook-like invaginations at P1.p and P2.p (arrowheads in A) were observed. osm-6::GFP is expressed in both 2° hook neurons. A single osm-6::GFP-expressing hook neuron was seen near the P1.p hook invagination, and the P2.p lineage made a complete hook sensillum with the presence of two osm-6::GFP-expressing cells (arrows in B). (C–D) Nomarski (C) and fluorescence (D) images of (P7-10).p in another L4 lin-12(gf)/lin-12(null); lin-22(n372) male. A P9.p hook sensillum was produced in addition to a P10.p wild-type hook sensillum. Furthermore, P(7–8).p were also induced to form hook invaginations. The P7.p hook invagination was associated with a pair of hook neurons, indicating a complete 2° HCG fate transformation. P8.p only generated a hook invagination, indicating a partial 2° fate transformation. One of the P9.p hook neurons was located in a slightly right focal plane and cannot be seen in the picture. h-in, hook invagination. Scale bar, 20 μm. Left lateral views.
MAB-5 is required for wild-type HCG pattern formation and is regulated by the Wnt receptor LIN-17/Frizzled
Wild-type mab-5 activity represses the fusion fate of male P(9–11).p in the L1 stage (Kenyon, 1986). However, early fusion of P(9–11).p to the hyp7 syncytium caused by mab-5(lf) mutations makes it unclear whether mab-5 function is further required during normal HCG patterning at a later developmental stage. To address this question, we used an eff-1 mutation to block P(9–11).p cell fusion in mab-5(e1239lf) mutants. hy21 is a temperature-sensitive allele of eff-1, an integral membrane protein gene necessary for epithelial cell fusion (Mohler et al., 2002). When grown at 25° C, all hypodermal cells fail to fuse in eff-1(hy21) mutants. We found that eff-1(hy21) males exhibited a weak abnormality in hook morphology, while expression of eat-4::GFP in PVV was generally not affected (48/48), suggesting that HCG fate specification is not altered in these animals. In contrast, neither 1° expression of eat-4::GFP nor presence of the 2 hook structure was observed in eff-1(hy21); mab-5(e1239) males (n=78) even though P10.p and/or P11.p cells remained unfused. Therefore, in the absence of mab-5 activity, the inhibition of cell fusion is insufficient for P(9–11).p to adopt HCG fates, indicating that HCG competence is not just a direct consequence of the prevention of cell fusion. mab-5 activity is necessary for establishment of HCG competence in the normal HCG, P(9–11).p, as well as in anterior Pn.p cells during ectopic HCG fate formation (discussed in the previous sections).
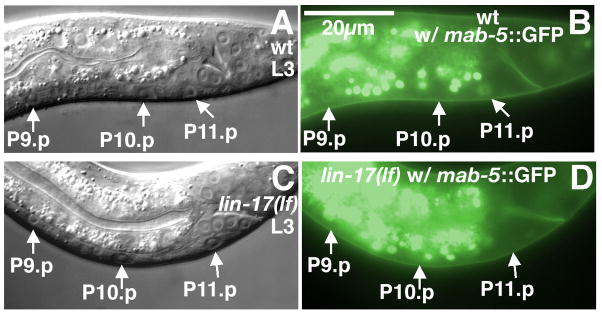
Since we had shown that Wnt signaling is required to specify HCG fates, we investigated whether mab-5/Hox expression was regulated by Wnt signaling. We found that an extrachromosomal translational MAB-5 GFP reporter (Celniker et al., 2009) was expressed in P11.p in all 25 males examined (Fig. 6A–B). However, MAB-5 GFP expression was either abolished or greatly reduced in 7 of 8 lin-17(n671lf) males (Fig. 6C–D). Therefore, Wnt signaling is required for mab-5/Hox expression in P11.p. MAB-5 GFP expression in P10.p at the same stage is barely detectable (Fig. 6A–B). The effect of MAB-5 on 2° fate may be subtle (see below).
Figure 6.
Regulation of MAB-5–GFP expression in P(9–11).p by lin-17. (A–D) Early L3 wild-type male. Nomarski (A) and fluorescence (B) images of the HCG. P10.p and P11.p have moved slightly to the posterior. MAB-5–GFP was expressed in P11.p. Expression in P10.p was hardly detectable in this animal. (C-D) Early L3 lin-17(n671lf) male. MAB-5–GFP expression was absent in P11.p. However, this mutant male still retained some faint GFP expression in P10.p. Scale bar, 20 μm. Left lateral views.
Overexpression of mab-5 by the heat-shock treatment of a hs-mab-5 transgenic line muIs9 during the L2 to early L3 stage did not cause ectopic hook formation in P(9–11).p. Instead, we observed a high percentage of hook abnormalities (about 70%) in adult males, including missing, misshapen, or anteriorly-displaced hooks (Table S1). HOB seemed less affected by heat-shock-induced MAB-5 activity, indicated by ceh-26::gfp expression. About 16% animals had a second cell expressed ceh-26::gfp after the heat shock treatment. An extra osm-6::gfp-expressing cell, in addition to HOA and HOB, was also seen in mab-5(e1751gf); lin-15(e1763) and mab-5(e1751)+/+lin-12(gf) mutants occasionally. This cell is usually located posteriorly next to HOB and it is probably a HOB-like fate transformation within the 2° lineage due to excessive MAB-5 activity. In summary, MAB-5 activity needs to be limited in 2° P10.p; excessive mab-5 activity at a later stage might have a negative effect on the 2° lineage and hook sensillum differentiation.
Discussion
MAB-5/Hox is required for HCG fates
Prior to HCG pattern formation, fusion of male Pn.p cells with the hyp7 epidermal syncytium in the late L1 stage has a direct impact on the existence of P(9–11).p precursor cells. This pattern is regulated by the Hox genes lin-39 and mab-5 (Salser et al., 1993). A mab-5 mutant lacks a hook structure because P(9–11).p fuse to hyp7 during the L1 larval stage. Our analysis of mab-5; eff-1 double mutants indicates that mab-5 is necessary for HCG fate specification in addition to preventing P(9–11).p cell fusion prior to fate specification.
Increased Wnt signaling in a pry-1 mutant extends the boundary of the HCG to anterior Pn.p cells and this action depends on mab-5 activity. Overexpression of mab-5 by a mab-5(e1751gf) mutation makes anterior P(3–8).p cells in lin-12(gf) and lin-15(null) mutants switch from expressing vulval-like fates to HCG fates. Therefore, the Hox gene mab-5 is a determinative component for HCG competence.
Induction of the 1° fate is the key to establish a precise spatial pattern of 3°-2°-1° within the male HCG, given that specification of the 2° fate depends on presence of a 1° fate cell. Our previous work suggested that Wnt signaling acts together with the EGF pathway to induce the HCG fates (Yu et al., 2009). Furthermore, examination of lin-17 and bar-1 expression in P(9–11).p suggests a specific role of Wnt signaling in promoting the 1° HCG fate. Here, we provide further evidence for this function of Wnt signaling by showing that activation of a canonical Wnt pathway is sufficient to produce 1° HCG fates ectopically. We observed adjacent ectopic 1° HCG fate cells in a pry-1 mutant. However, induction of the 2° HCG fate by these 1° fate cells is inefficient, suggesting that an activated canonical Wnt pathway is a potent 1° HCG fate inducer and 1° HCG fate specification of a cell antagonizes 2° fate specification in the same cell, much as is the case for VPCs (Sternberg, 1988; Sternberg, 2005). Supporting a role for mab-5 in HCG specification, particularly 1° fate specification, we found that lin-17-mediated Wnt signaling regulates mab-5 expression in the 1° HCG cell, P11.p.
Our mab-5(gf); lin-12(gf) double mutant analysis also indicates that the requirement for lin-17 function cannot be bypassed during 1° HCG fate specification. In addition, MAB-5 activity seems fine-tuned in the 2° lineage, and unrestricted mab-5 expression by a heat-shock transgene has a detrimental effect on hook formation.
Re-programming Pn.p cells: how to specify a hook
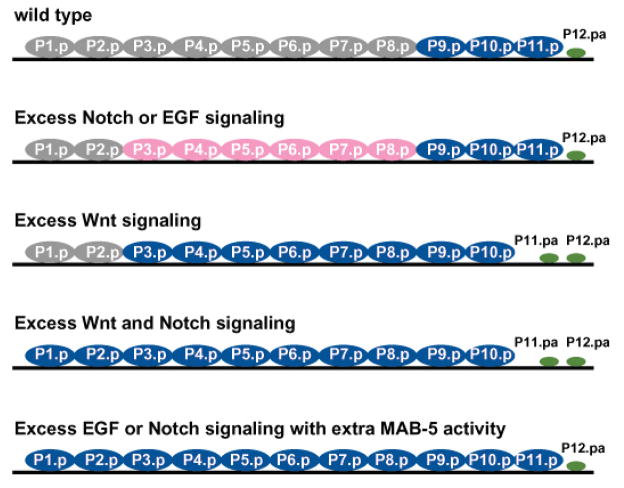
All Pn.p cells anterior to the wild-type HCG, P(9–11).p, can acquire HCG fates depend on the following factors: the type of ectopic signal that is received, which Hox gene is expressed in the cell in the wild type and whether mab-5 activity is present (Fig. 7). The anterior Pn.p cells can be grouped into three categories based on the conditions necessary for ectopic HCG fates.
Figure 7.
Excess Wnt signaling or ectopic MAB-5 activity together with excess EGF or LIN-12/Notch signaling confers hook competence to male Pn.p cells anterior to the HCG. In wild-type males, only P(9–11).p possess HCG competence (blue). All anterior Pn.p cells (P(1–8).p) remain uninduced and fuse with hyp7 (grey). The most posterior cell P12.pa adopts a unique hypodermal fate (dark green). Increased Notch signaling due to a lin-12(gf) mutation or abnormal activation of EGF signaling by a lin-15(null) mutation causes the central Pn.p cells, P(3–8).p, to adopt a vulval-like fate (pink) but does not change the competence of the other Pn.p cells. Extra Wnt signaling caused by the pry-1(mu38) mutation confers HCG competence to P(3–8).p. Combined action of the pry-1(mu38) and lin-12(gf) mutations further extends the anterior boundary of the HCG to include P1.p and P2.p. A similar effect is also observed in conditions of excess MAB-5 activities combined with increased Notch (by a lin-12(gf) mutation) or EGF (by a lin-15(null) mutation) signaling whereby P(1–8).p is recruited into the HCG.
P(7–8).p
In wild-type males, antagonism between the Hox genes lin-39 and mab-5 prevents both P7.p and P8.p from responding to Wnt signals because they fuse early on in the L1 stage. However, P(7–8).p will express 1° and/or 2° HCG fates after the following genetic manipulations: 1) excessive Wnt signaling, 2) mab-5 overexpression in combination with increased EGF signaling, 3) mab-5 overexpression in combination with increased Notch signaling, or 4) increased Notch signaling together with reduced mab-5 inhibition.
Male P(7–8).p cells are likely sensitive to the relative ratios of lin-39 and mab-5 activities for the L1 fusion (Salser et al., 1993). Unfused P(7–8).p cells in pry-1(mu38) males demonstrate that excess Wnt signaling breaks the antagonistic balance between Hox genes lin-39 and mab-5. Two observations lead us to propose that mab-5 is probably a preferred downstream target of Wnt signaling, as seen in specification of Q neuroblast lineages (Maloof et al., 1999; Korswagen et al., 2000). First, we found that mab-5 is regulated in the HCG by lin-17-mediated Wnt signaling. Second, P7.p and P8.p do not acquire HCG fates in pry-1(lf); mab-5(lf) mutants. Preferential upregulation of mab-5 in P7.p and P8.p in pry-1 mutants bypasses fusion in the L1 and causes the expression of ectopic HCG fates.
Either increased Notch or EGF signaling can also prevent P7.p and P8.p from fusing to hyp7 in the L1, possibly by favoring the upregulation of lin-39/Hox. However, unfused P(7–8).p behave like more anterior P(3–6).p and adopt vulval-like fates in the two mutant backgrounds, while posterior P(9–11).p have an overinduced HCG phenotype and form multi-hooks (Greenwald et al., 1983; Yu et al., 2009). Additional mab-5 activity enables unfused P(7–8).p in the increased Notch or EGF signaling background to assume HCG fates.
P(3–6).p
Although central P(3–6).p only express lin-39 in the wild type, they express HCG fates under similar conditions as P(7–8).p. Increased Wnt signaling in a pry-1 mutant affects the L1 fusion decision of P(7–8).p cells but is not sufficient to significantly interfere with lin-39 function to cause frequent abnormal fusion of P(3–6).p in the L1 stage as seen in mab-5(e1751gf) mutants. Unfused P(3–6).p become HCG competent in response to ectopic mab-5 activity either by activation of Wnt signaling or a mab-5(e1751gf) mutation. The difference in lin-39 and mab-5 levels between P(7–8).p and P(3–6).p are reflected only in the penetrance of the expression of ectopic HCG fates by both groups as a consequence of escaping the L1 fusion and HCG induction.
P(1–2).p
Because both P1.p and P2.p express neither lin-39/Hox nor mab-5/Hox and fuse in the L1 stage in the wild type, we can identify what the minimum requirements for hook fate specification by examining the conditions under which P(1–2).p adopt HCG fates. Increased Wnt, EGF or Notch signaling alone do not change P(1–2).p cell fusion pattern; mab-5 overexpression causes P(1–2).p to remain unfused but is not insufficient to generate ectopic HCG lineages. However, excessive Wnt signaling together with Notch signaling, excessive Notch or EGF signaling in a mab-5(gf) background, or increased Notch signaling in a lin-22 mutant background result in P(1–2).p acquiring HCG fates, suggesting that both a proliferation signal and a threshold of mab-5 activity are required to generate a HCG fate.
Stepwise specification of male HCG fates
Specification of cell fates in the male hook sensillum competence group (HCG) comprises multiple steps, a process similar to hermaphrodite vulval development. The first step is to distinguish HCG fates from non-HCG fates among male Pn.p cells; the second step is to choose between induced (1° or 2°) HCG fates and a non-induced (3°) HCG fate; the third step is to determine the 1° or 2° HCG fate. Mutation of the Axin homolog pry-1 leads to pleiotropic effects on male Pn.p cells and the consequent formation of ectopic HCG fates in anterior Pn.p cells indicate multiple roles of Wnt signaling during HCG patterning. We propose the following scheme for male HCG fate specification (Fig. 8). Male P(9–11).p cells remain unfused in the late L1 stage due to mab-5 activity, and thereby are potentially responsive to an inductive signal. A WNT signal from the posterior regulates mab-5 activity thereby determining HCG competence. Since our reporter gene assay suggests that mab-5 levels might be higher in P11.p than in P10.p, the levels of mab-5 expression might help establish the pattern of cell fates among the P(9–11).p cells. In P11.p, activated LIN-17 receptor signals downstream via components such as BAR-1 to promote the 1 fate, which produces ligands for the LIN-12/Notch receptor to promote the 2° fate in the adjacent P10.p cell. The stepwise determination progressively restricts P(9–11).p cells into one of the three HCG fates. Once the cell fates are established, mab-5 activity is further fine tuned among HCG sub-lineages to ensure precise differentiation of the male hook sensillum.
Figure 8.
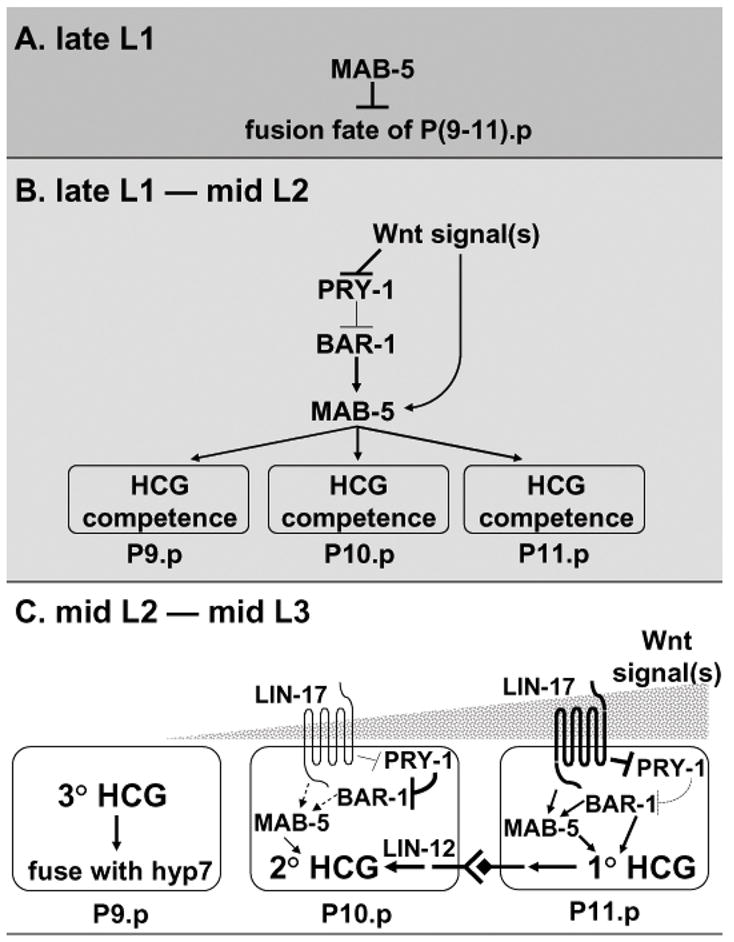
Model for development of the male HCG. (A) Regional MAB-5 activity preset during embryonic development keeps P(9–11).p unfused in the late L1 stage. (B) During the late L1 to mid-L2 stage, continuing presence of mab-5 activity in P(9–11).p, which is regulated by Wnt and probably other graded signals from the posterior tail region, makes these cells able to generate hook sensillum tissue (HCG competence). (C) During the mid-L2 to mid-L3 stage, a strong Wnt signal induces the 1° HCG fate in P11.p since it is closest to the posterior. The posterior signal(s) make P10.p assume an induced fate as well. With the presence of a 1° P11.p cell, the P10.p cell is locked into the 2° HCG fate by activated LIN-12 signaling. The P9.p cell, which has minimum mab-5 activity and receives the lowest level of inductive signals, adopts the uninduced 3° fate and finally fuses with hyp7.
Comparing Competence and Fate specification in the VPCs and HCG
Overall, VPC and HCG patterning are quite similar: the precise cell fate is generated by progressive restriction through competence, induction, and lateral inhibition mediated by multiple signal integration at different steps, representing a general scenario of complex pattern formation.
Specifically, both VPC and HCG competence are established by Wnt signaling (Eisenmann et al., 1998; this work) and one of the two Hox genes, lin-39 and mab-5, respectively (Clandinin et al., 1997; this work). Expression patterns of both Hox genes are the same in both hermaphrodite and male, with lin-39 expression in P(3–8).p and mab-5 expression in P(7–11).p (Clark et al., 1993; Salser et al., 1993; Wang et al., 1993). However, sex-specific utilization of these two Hox genes, lin-39 and mab-5, determines whether a hermaphrodite vulva or a male hook, respectively, is formed (Maloof and Kenyon, 1998; this work). In hermaphrodites, lin-39 function is favored in the central Pn.p cells, and the ability of mab-5 to prevent P(9–11).p fusion with hyp7 is somehow blocked (Salser et al., 1993). As a transcription factor, mab-5 regulates target gene expression. One possibility is that a negative regulator in hermaphrodites sequesters mab-5 from its targets. Alternatively, mab-5 may act with a co-regulator that is missing in hermaphrodites. The Hox genes appear to play a permissive role in VPC and HCG induction because neither multi-vulvae (Maloof and Kenyon, 1998) nor multi-hooks (this work) are observed when lin-39 or mab-5, respectively, are overexpressed.
A major difference between VPC and HCG development is the major inductive signal used to specify the 1° fate: the EGF pathway induces the 1° VPC fate (Hill and Sternberg, 1992) while Wnt signaling promotes the 1° HCG fate (Yu et al., 2009; this work). However, both EGF and Wnt act to induce HCG as well as VPC fates, and it has been observed that excessive Wnt signaling can at least partially substitute for EGF signaling in VPC induction (Gleason et al., 2002), and vice versa in HCG specification (Yu et al., 2009). The local abundance of the signal could explain why different inductive signals are utilized in VPC and HCG patterning. The availability of the Wnt and EGF inductive signals differ spatially in hermaphrodites and males, contributing further to the sex-specific bias of Hox gene expression. Although Wnts are present in the central region of the body (Eisenmann et al., 1998; Inoue et al., 2004) and the EGF ligand is produced in the tail (Chamberlin and Sternberg, 1994; Jiang and Sternberg, 1998), the EGF signal emanates from a concentrated source, the gonadal anchor cell, only in the hermaphrodite, while Wnt signaling is more abundant in the tail region as elucidated by extensive tail defects caused by deficient Wnt signaling (Sternberg and Horvitz, 1988; Herman and Horvitz, 1994; Chamberlin and Sternberg, 1995; Herman et al., 1995; Jiang and Sternberg, 1998). As such, only the required Hox gene is promoted in each region in a sex-specific manner. E.g. lin-39 activity in males is not reinforced due to lack of a strong extrinsic signal in the central region. Therefore, different signaling pathways may not be the direct cause of sexually dimorphic organogenesis. The specificity of signaling relies on Hox genes to direct sex-specific pattern formation among competent precursor cells.
Materials and Methods
General methods, nomenclature and strains
Methods for handling and culturing of C. elegans are described by Brenner (1974). Unless otherwise noted, all experiments were performed at 20°C. Alleles and transgenes that were used in this work are listed in Table S2. The detailed strain information is included in Table S3.
In the text, we refer to the lin-12 gain-of-function mutation lin-12(n137) as “lin-12(gf)”, and the null mutation lin-12(n676 n909) as “lin-12(null)”. Unlinked double mutant strains were constructed according to standard methods (Huang and Sternberg, 1995). During some strain constructions, genetic markers within same linkage group of one allele were used in trans to that allele to facilitate selection.
The original eat-4::GFP transgene integrated on chromosome X is linked to n765, a temperature-sensitive mutant allele of lin-15, raising the possibility that additional PVV-like cells might be due to an interactions between the pry-1 and lin-15 mutations. To remove the lin-15(n765) allele linked to the adIs1240 (eat-4::GFP) transgene, him-5 (e1490) V; adIs1240 lin-15(n765ts) X males were crossed into dpy-6(e14) unc-9(n101) X hermaphrodites and F2 Unc-non-Dpy animals expressing eat-4::GFP were selected. We re-assayed eat-4::GFP expression in pry-1 males grown at 15 C, a permissive temperature for the lin-15(n765) mutation, and in pry-1 mutants after removal of the lin-15(n765) mutation from background by genetic recombination. In both conditions, pry-1 mutants displayed significant ectopic eat-4::GFP expression, associated with ventral protrusions. These results suggest that pry-1(mu38) induces formation of ectopic PVVs from P(3–10).p cells, although we cannot rule out some minor effect from lin-15.
The strain carrying the pry-1(mu38) mutation and syIs78 (AJM-1–GFP) transgene, which are both located on linkage group I, was obtained by picking pry-1(mu38) homozygous hermaphrodites with AJM-1–GFP expression in a F2 population after cross. pry-1(mu38); bar-1(ga80) double mutant males had a Bar-1-like gross morphology, and were much healthier than pry-1 single mutant males, suggesting a complete suppression of the pry-1(mu38) phenotype by the bar-1(ga80) mutation. In this double mutant, the presence of pry-1(mu38) allele in the strain was verified by strain deconstruction. By contrast, in pry-1(mu38); mab-5(e1239) double mutants, loss of mab-5 function did not change the scrawny appearance of pry-1 mutant animals, indicating that mab-5 only participated in some aspects of Wnt signaling. Although small bumps were sporadically formed at the ventral side as a result of occasional proliferation of Pn.p cells, neither hook-like structure nor yellowish autofluorescence was seen in pry-1(mu38); mab-5(e1239) males. About one-third of pry-1; mab-5 double mutant males had an additional neuronal cell expressing eat-4::GFP in the preanal ganglion region. By comparing them with mab-5(e1239) single mutants, we deduced that this neuron is not likely from the Pn.p lineages and is probably differentiated from a Pn.a progeny that fails to die in the mab-5(e1239lf) mutant background (Kenyon, 1986). Unlike what is seen in mab-5 single mutants, the fusion of P(9–10).p to the hyp7 epidermal syncytium is not complete in pry-1(mu38); mab-5(e1239) males. This could be caused by additional lin-39 activity, if excess Wnt signaling in pry-1 mutants increased activities of both Hox genes, but with a preference for mab-5.
To generate a double mutant containing both lin-12(n137gf) and mab-5(e1751gf) alleles, we made a heterozygous strain since mab-5 and lin-12 are closely linked on linkage group III, with one copy of chromosome III containing a mab-5(e1751) allele and a wild-type lin-12(+) allele, and the other copy a mab-5(+) allele and a lin-12(gf) allele. In this compound heterozygous background, mab-5(e1751) single males can be distinguished by fused rays 1 and 2 with a wild-type hook. Males with more than one hook anterior to the cloaca, indicating the presence of lin-12(gf), were examined for HCG-like fate formation in anterior Pn.p.
Despite their hookless phenotype and abnormal HCG lineages, lin-17 mutations often do not completely abolish cell divisions of P10.p and P11.p, raising a question of whether mab-5 overexpression is able to rescue the lineage defects in lin-17 mutants. The lin-17(n671); mab-5(e1751) animals were very sick and the hermaphrodites usually rupture before production of any progeny. In the double mutant males, the presence of mab-5(e1751) mutation was verified based on gaps in their lateral alae (cuticular ridges). The lin-17(n671); mab-5(e1751gf) double mutant males showed a Lin-17-like tail phenotype: 27/28 animals were hookless and only 1/28 had a rudimentary hook-like protrusion. It is unclear whether the mab-5(e1751) mutation causes extra MAB-5 activity in P(9–11).p and whether the extra MAB-5 activity, if any, is independent of lin-17 regulation. However, production of ectopic 2° HCG fates in anterior Pn.p cells of mab-5(e1751) +/+ lin-12(gf) males was suppressed by introduction of a lin-17(n671) mutation to the background (n=30). Of the 30 mutants examined, at least two lin-17 (n671); mab-5(e1751) +/+ lin-12(gf) males displayed a Lin-17-like hookless phenotype at the tail but formed rudimentary hook-like protrusions among anterior Pn.p cells, indicating the presence of extra LIN-17-independent MAB-5 activity in anterior Pn.p cells in this triple mutant background. Therefore, additional MAB-5 activity is not sufficient to bypass the requirement of LIN-17-mediated Wnt signaling for HCG fate specification, consistent with a role of lin-17 in the lineages generated by the HCG cells once their fates are specified.
Microscopy
Cell anatomy and lineages were observed in living animals using Nomarski optics as described (Sulston and Horvitz, 1977). We took advantage of B cell development as an internal reference for the developmental timing. The male-specific B blast cell divides in the late L1 stage, just after the generation of Pn.p cells. The second cell division of B lineage is in middle L2. By the late L2 stage, there are ten B progeny. These ten cells then adjust their positions and form a characteristic assembly around the proctoderm in the early L3 stage. Cells of the B lineage further divide just before P(10–11).p divisions in about the middle L3 stage. The appropriate position of the developing male gonad was another temporal indicator. For viewing GFP expression, a Chroma Technology High Q GFP long pass filter set [450 nm excitation, 505 nm emission] was used in conventional fluorescence microscopy (Zeiss Axioskop). The same filter was also used for visualizing autofluorescence.
Supplementary Material
Acknowledgments
We thank Cynthia Kenyon for the muIs9 and muIs16 strains, John Murray and Rob Waterston for the Ex [MAB-5 GFP] strain (RW10320), Takao Inoue, Mihoko Kato, Gary Schindelman and Amir Sapir for helpful discussions and critical reading of this manuscript. We thank the Caenorhabditis Genetic Center for providing some strains used in this study. This work was supported by the HHMI, with which P.W. S. is an Investigator, and USPHS grant #1P50DK57325 to G. Germino (co-PI, P.W.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Hui Yu, Email: hui.yu@rice.edu.
Adeline Seah, Email: seah@caltech.edu.
References
- Celniker SE, Dillon LA, Gerstein MB, Gunsalus KC, Henikoff S, Karpen GH, Kellis M, Lai EC, Lieb JD, MacAlpine DM, Micklem G, Piano F, Snyder M, Stein L, White KP, Waterston RH modENCODE Consortium. Unlocking the secrets of the genome. Nature. 2009;459:927–30. doi: 10.1038/459927a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin HM, Sternberg PW. The lin-3/let-23 pathway mediates inductive signalling during male spicule development in Caenorhabditis elegans. Development. 1994;120:2713–2721. doi: 10.1242/dev.120.10.2713. [DOI] [PubMed] [Google Scholar]
- Chamberlin HM, Sternberg PW. Mutations in the Caenorhabditis elegans gene vab-3 reveal distinct roles in fate specification and unequal cytokinesis in an asymmetric cell division. Dev Biol. 1995;170:679–689. doi: 10.1006/dbio.1995.1246. [DOI] [PubMed] [Google Scholar]
- Clark SG, Chisholm AD, Horvitz HR. Control of cell fates in the central body region of C. elegans by the homeobox gene lin-39. Cell. 1993;74:43–55. doi: 10.1016/0092-8674(93)90293-y. [DOI] [PubMed] [Google Scholar]
- Clark SG, Lu WX, Horvitz HR. The Caenorhabditis elegans locus lin-15, a negative regulator of a tyrosine kinase signaling pathway, encodes two different proteins. Genetics. 1994;137:987–997. doi: 10.1093/genetics/137.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clandinin TR, Katz WS, Sternberg PW. Caenorhabditis elegans HOM-C genes regulate the response of vulval precursor cells to inductive signal. Dev Biol. 1997;182:150–161. doi: 10.1006/dbio.1996.8471. [DOI] [PubMed] [Google Scholar]
- Collet J, Spike CA, Lundquist EA, Shaw JE, Herman RK. Analysis of osm-6, a gene that affects sensory cilium structure and sensory neuron function in Caenorhabditis elegans. Genetics. 1998;148:187–200. doi: 10.1093/genetics/148.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M, Chen J, Myers TR, Hwang BJ, Sternberg PW, Greenwald I, Han M. SynMuv genes redundantly inhibit lin-3/EGF expression to prevent inappropriate vulval induction in C. elegans. Dev Cell. 2006;10:667–672. doi: 10.1016/j.devcel.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Eisenmann DM, Maloof JN, Simske JS, Kenyon C, Kim SK. The b-catenin homolog BAR-1 and LET-60 Ras coordinately regulate the Hox gene lin-39 during Caenorhabditis elegans vulval development. Development. 1998;125:3667–3680. doi: 10.1242/dev.125.18.3667. [DOI] [PubMed] [Google Scholar]
- Ferguson EL, Horvitz HR. Identification and characterization of 22 genes that affect the vulval cell lineages of the nematode C. elegans. Genetics. 1985;110:17–72. doi: 10.1093/genetics/110.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira HB, Zhang Y, Zhao C, Emmons SW. Patterning of Caenorhabditis elegans posterior structures by the Abdominal-B homolog, egl-5. Dev Biol. 1999;207:215–228. doi: 10.1006/dbio.1998.9124. [DOI] [PubMed] [Google Scholar]
- Fixsen WD. Massachusetts Institute of Technology . Ph D Thesis. 1985. The Genetic Control of Hypodermal Cell Lineages during Nematode Development. [Google Scholar]
- Gleason JE, Korswagen HC, Eisenmann DM. Activation of Wnt signaling bypasses the requirement for RTK/Ras signaling during C. elegans vulval induction. Genes Dev. 2002;16:1281–1290. doi: 10.1101/gad.981602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald IS, Sternberg PW, Horvitz HR. The lin-12 locus specifies cell fates in C. elegans. Cell. 1983;34:435–444. doi: 10.1016/0092-8674(83)90377-x. [DOI] [PubMed] [Google Scholar]
- Gupta BP, Wang M, Sternberg PW. The C. elegans LIM homeobox gene lin-11 specifies multiple cell fates during vulval development. Development. 2003;130:2589–2601. doi: 10.1242/dev.00500. [DOI] [PubMed] [Google Scholar]
- Hedgecock EM, Culotti JG, Hall DH, Stern BD. Genetics of cell and axon migrations in C. elegans. Development. 1987;100:365–382. doi: 10.1242/dev.100.3.365. [DOI] [PubMed] [Google Scholar]
- Herman MA, Horvitz HR. The Caenorhabditis elegans gene lin-44 controls the polarity of asymmetric cell divisions. Development. 1994;120:1035–1047. doi: 10.1242/dev.120.5.1035. [DOI] [PubMed] [Google Scholar]
- Herman MA, Vassilieva LL, Horvitz HR, Shaw JE, Herman RK. The C. elegans gene lin-44, which controls the polarity of certain asymmetric cell divisions, encodes a Wnt protein and acts cell nonautonomously. Cell. 1995;83:101–110. doi: 10.1016/0092-8674(95)90238-4. [DOI] [PubMed] [Google Scholar]
- Hodgkin J. Male phenotypes and mating efficiency in C. elegans. Genetics. 1983;103:43–64. doi: 10.1093/genetics/103.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin J, Horvitz HR, Brenner S. Nondisjunction mutants of the nematode C. elegans. Genetics. 1979;91:67–94. doi: 10.1093/genetics/91.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz HR, Sternberg PW, Greenwald IS, Fixsen W, Ellis HM. Mutations that affect neural cell lineages and cell fates during the development of the nematode C. elegans. Cold Spring Harb Symp Quant Biol. 1983;48:453–463. doi: 10.1101/sqb.1983.048.01.050. [DOI] [PubMed] [Google Scholar]
- Howard RM, Sundaram MV. C. elegans EOR-1/PLZF and EOR-2 positively regulate Ras and Wnt signaling and function redundantly with LIN-25 and the SUR-2 Mediator component. Genes & Dev. 2002;16:1815–1827. doi: 10.1101/gad.998402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LS, Sternberg PW. Genetic dissection of developmental pathways. Methods in Cell Biology. In: Epstein HF, Shakes DC, editors. Caenorhabditis elegans: Modern biological analysis of an organism. Vol. 48. San Diego: Academic Press, Inc; 1995. pp. 97–122. [DOI] [PubMed] [Google Scholar]
- Huang LS, Tzou P, Sternberg PW. The lin-15 locus encodes two negative regulators of Caenorhabditis elegans vulval development. Mol Biol Cell. 1994;5:395–411. doi: 10.1091/mbc.5.4.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Oz HS, Wiland D, Gharib S, Deshpande R, Hill RJ, Katz WS, Sternberg PW. C. elegans LIN-18 Is a Ryk Ortholog and Functions in Parallel to LIN-17/Frizzled in Wnt Signaling. Cell. 2004;118:795–806. doi: 10.1016/j.cell.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Jiang L, Sternberg PW. Interactions of EGF, Wnt and Hom-C genes specify the P12 neuroectoblast fate in C. elegans. Development. 1998;125:2337–2347. doi: 10.1242/dev.125.12.2337. [DOI] [PubMed] [Google Scholar]
- Kenyon C. A gene involved in the development of the posterior body region of C. elegans. Cell. 1986;46:477–487. doi: 10.1016/0092-8674(86)90668-9. [DOI] [PubMed] [Google Scholar]
- Korswagen HC, Herman MA, Clevers HC. Distinct beta-catenins mediate adhesion and signalling functions in C. elegans. Nature. 2000;406:527–532. doi: 10.1038/35020099. [DOI] [PubMed] [Google Scholar]
- Korswagen HC, Coudreuse DY, Betist MC, van de Water S, Zivkovic D, Clevers HC. The Axin-like protein PRY-1 is a negative regulator of a canonical Wnt pathway in C. elegans. Genes & Dev. 2002;16:1291–1302. doi: 10.1101/gad.981802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RY, Sawin ER, Chalfie M, Horvitz HR, Avery L. EAT-4, a homolog of a mammalian sodium-dependent inorganic phosphate cotransporter, is necessary for glutamatergic neurotransmission in Caenorhabditis elegans. J Neurosci. 1999;19:159–167. doi: 10.1523/JNEUROSCI.19-01-00159.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloof JN, Kenyon C. The Hox gene lin-39 is required during C. elegans vulval induction to select the outcome of Ras signaling. Development. 1998;125:181–190. doi: 10.1242/dev.125.2.181. [DOI] [PubMed] [Google Scholar]
- Maloof JN, Whangbo J, Harris JM, Jongeward GD, Kenyon C. A Wnt signaling pathway controls Hox gene expression and neuroblast migration in C. elegans. Development. 1999;126:37–49. doi: 10.1242/dev.126.1.37. [DOI] [PubMed] [Google Scholar]
- Mohler WA, Shemer G, del Campo JJ, Valansi C, Opoku-Serebuoh E, Scranton V, Assaf N, White JG, Podbilewicz B. The type I membrane protein EFF-1 is essential for developmental cell fusion. Dev Cell. 2002;2:355–362. doi: 10.1016/s1534-5807(02)00129-6. [DOI] [PubMed] [Google Scholar]
- Salser SJ, Kenyon C. Activation of a C. elegans Antennapedia homolog in migrating cells controls their direction of migration. Nature. 1992;355:255–258. doi: 10.1038/355255a0. [DOI] [PubMed] [Google Scholar]
- Salser SJ, Loer CM, Kenyon C. Multiple HOM-C gene interactions specify cell fates in the nematode central nervous system. Genes & Dev. 1993;7:1714–1724. doi: 10.1101/gad.7.9.1714. [DOI] [PubMed] [Google Scholar]
- Sternberg PW, Horvitz HR. lin-17 mutations of Caenorhabditis elegans disrupt certain asymmetric cell divisions. Dev Biol. 1988;130:67–73. doi: 10.1016/0012-1606(88)90414-9. [DOI] [PubMed] [Google Scholar]
- Sulston JE, Horvitz HR. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol. 1977;56:110–156. doi: 10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
- Sulston JE, White JG. Regulation and cell autonomy during postembryonic development of C. elegans. Dev Biol. 1980;78:577–597. doi: 10.1016/0012-1606(80)90353-x. [DOI] [PubMed] [Google Scholar]
- Wang BB, Muller-Immergluck MM, Austin J, Robinson NT, Chisholm A, Kenyon C. A homeotic gene-cluster patterns the anteroposterior body axis of C. elegans. Cell. 1993;74:29–42. doi: 10.1016/0092-8674(93)90292-x. [DOI] [PubMed] [Google Scholar]
- Wrischnik LA, Kenyon CJ. The role of lin-22, a hairy/Enhancer of split homolog, in patterning the peripheral nervous system of C. elegans. Development. 1997;124:2875–2888. doi: 10.1242/dev.124.15.2875. [DOI] [PubMed] [Google Scholar]
- Yoo AS, Bais C, Greenwald I. Crosstalk between the EGFR and LIN-12/Notch pathways in C. elegans vulval development. Science. 2004;303:663–666. doi: 10.1126/science.1091639. [DOI] [PubMed] [Google Scholar]
- Yu H, Pretot RF, Burglin TR, Sternberg PW. Distinct roles of transcription factors EGL-46 and DAF-19 in specifying the functionality of a polycystin-expressing sensory neuron necessary for C. elegans male vulva location behavior. Development. 2003;130:5217–5227. doi: 10.1242/dev.00678. [DOI] [PubMed] [Google Scholar]
- Yu H, Seah A, Herman MA, Ferguson EL, Horvitz HR, Sternberg PW. Wnt and EGF pathways act together to induce C. elegans male hook development. Dev Biol. 2009;327:419–432. doi: 10.1016/j.ydbio.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.