Abstract
Histopathologic findings in arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) are replacement of the normal myocardium with fatty and fibrous elements with preferential involvement of the right ventricle. The right ventricular fibrosis can be visualised by post-gadolinium delayed enhancement inversion recovery imaging (DE imaging). We compared the image quality of three different gradient echo MRI sequences for short axis DE imaging of the right ventricle (RV). We retrospectively analysed MRI scans performed between February 2005 and December 2008 in 97 patients (mean age: 41.2 years, 67% men) suspected of ARVC/D. For DE imaging either a 2D Phase Sensitive (PSIR), a 2D (2D) or a 3D (3D) inversion recovery sequence was used in respectively 38, 32 and 27 MRI-examinations. The RV, divided in 10 segments, was assessed for image quality by two radiologists in random sequence. A consensus reading was performed if results differed between the two readings. Image quality was good in 24% of all segments in the 3D group, 66% in the 2D group and 79% in the PSIR group. Poor image quality was observed in 51% (3D), 10% (2D), and 2% (PSIR) of all segments. Exams were considered suitable for clinical use in 7% of exams in the 3D group, 75% of exams in the 2D group and 90% of exams of the PSIR group. Breathing-artifacts occurred in 22% (3D), 59% (2D) and 53% (PSIR). Motion-artifacts occurred in 56% (3D), 28% (2D) and 29% (PSIR). Post-gadolinium imaging using the PSIR sequence results in better and more consistent image quality of the RV compared to the 2D and 3D sequences.
Keywords: Image quality, Right ventricle, Delayed enhancement, ARVC/D
Introduction
Histopathologic findings in arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) are replacement of the normal myocardium with fatty and fibrous elements [1]. In the classical form of the disease, the right ventricle (RV) is primarily affected, but involvement of the left ventricle can also occur, especially in later stages [1, 2]. The prevalence of ARVC/D is estimated at 1/5,000 and follows an autosomal dominant pattern of inheritance with reduced penetrance and variable clinical expression [3, 4]. At least 12 genetic variances are known, mainly in genes encoding for desmosomal proteins [4]. A positive family history is present in 30–50% of cases [5]. Patients typically present between the second and fourth decade with tachycardias originating from the RV. However, sudden cardiac death (SCD) can occur even at a young age. As many as 10% of all the SCDs before the age of 35 years are associated with ARVC/D [6]. The diagnosis of ARVC/D is made according to the Task Force criteria that were first described by McKenna and were recently modified [7–9]. According to these criteria the diagnosis is based on the detection of certain histological, structural, electrographic, arrhythmic and genetic abnormalities.
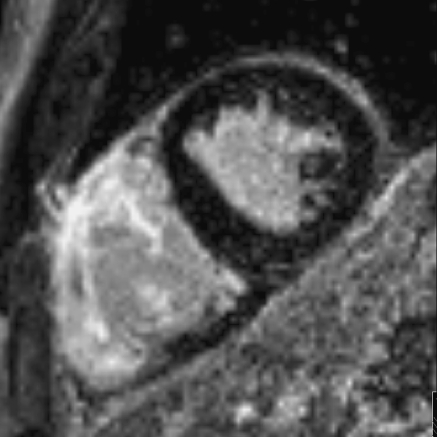
Currently, MRI plays an important role in assessing global or regional dysfunction and structural alternations of the heart in patients suspected of ARVC/D. MRI also has the ability to visualize fibrosis of the myocardium with delayed enhancement inversion recovery (DE) imaging following intravenous injection of a gadolinium contrast agent [10, 11]. The contrast diffuses into the myocardial extracellular space, which is larger in fibrotic myocardium and therefore remains longer in the fibrotic tissue than in healthy myocardium [12]. The gadolinium within the extracellular space can be demonstrated by T1 W gradient echo-inversion recovery (IR) imaging (Fig. 1). The inversion time is chosen to null the signal of the normal myocardium and thereby improve visibility of delayed enhancement in the abnormal myocardium. Although delayed enhancement is not part of the diagnostic criteria for ARVC/D, it can be an important contributor in diagnostics and could guide electrophysiological examination and biopsy [13].
Fig. 1.
Example of delayed enhancement of the lateral and anterior wall of the right ventricle in a 47 year old male with ARVC/D. The diagnosis of ARVC/D was made according to McKenna criteria
DE imaging is most frequently used to demonstrate abnormalities in the left ventricle. Accurate depiction of pathology within the much thinner right ventricle wall often is more challenging. Different IR sequences have been used in our institution in the past years: a 3D, 2D and 2D Phase Sensitive Inversion Recovery (PSIR) sequence. The aim of this study was to investigate which type of sequence (3D, 2D or PSIR) provides the highest quality of post-gadolinium IR images of the right ventricle in patients suspected of ARVC/D.
Method and materials
Study population
All patients suspected of ARVC/D who underwent a cardiac MRI in the period of September 2005 to December 2008 were included if one of the three studied sequences was utilized. In patients suspected of ARVC/D, the delayed enhancement part of the MRI protocol changed over the years; starting with the 3D sequence in September 2005, followed by the 2D sequence in July 2006 and changing to a PSIR sequence in June 2008.
Acquisition protocol
All cardiac MRI exams were performed on a 1.5T MRI scan (Achieva, Philips Healthcare, the Netherlands) with a SENSE Cardiac coil and ECG triggering. In all patients images were acquired using T1 W Black Blood, cine balanced TFE and DE imaging. DE images were obtained in right 2-chamber, RV outflow tract, 4-chamber and RV short-axis view and acquisition was performed 15 min after administration of gadolinium (gadopentetate dimeglumine, Magnevist, Bayer Health Care Pharmaceuticals, USA) with a dosage of 0.2 ml/kg.
Key 3D, 2D, and PSIR sequence characteristics are shown in Table 1.
Table 1.
Scan sequence characteristics
| 3D | 2D | PSIR | |
|---|---|---|---|
| Type | 3D T1 W-IR TFE post-Gd | 2D T1 W-IR TFE post-Gd | 2D T1 W-IR TFE post-Gd |
| Scan time (average) | 30 s | 150 s | 150 s |
| Average number of breath holds | 2 | 10 | 10 |
| Voxelsize | 1.37/1.43/10.00 | 1.37/1.43/6.00 | 1.37/1.43/6.00 |
| Act. TR (ms) | 4.3 | 7.2 | 7.1 |
| Act. TE (ms) | 1.33 | 3.5 | 3.5 |
| ACQ Matrix M × P | 256 × 195 | 256 × 245 | 256 × 245 |
| TFE shots | 17 | 7 | 7 |
| FOV RL/AP (mm) | 350/279 | 350/350 | 350/350 |
| Slice thickness (mm) | 5 | 6 | 6 |
| Scan mode | 3D | M2D | M2D |
| Technique | GE/FFE | GE/FFE | GE/FFE |
| Fast imaging mode | TFE | TFE | TFE |
| TFE factor | 50 | 35 | 35 |
| Flip angle (deg) | 15 | 25 | 25 |
| Respiratory compensation | Breath hold | Breath hold | Breath hold |
Scan time stated for 3D and 2D is excluding the time needed for choosing the correct inversion time
Data analysis
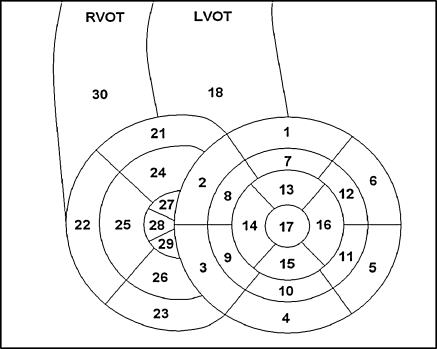
The complete cardiac MRI exams were presented in random sequence to two radiologists experienced in cardiac imaging who were blinded for patient details and type of sequence. The RV was divided into 10 segments as shown in Fig. 2 [14, 15]. Segment 21, 22 and 23 represent the most basal segments and segments 27, 28 and 29 are the most apical segments. First, all short-axis right ventricular segments were visually graded by the radiologists for image quality using the following ordinate scale: good, moderate or poor image quality. Second, the presence of motion and breathing artifacts was assessed, and thirdly, it was noted whether or not there was correct nulling of the signal of the normal myocardium. If data differed between the two readings, a consensus reading was performed.
Fig. 2.
Segmental division of the right ventricle supplemental to the 17-segment model of the left ventricle used by the American Heart Association [14, 15]
To determine which of the three sequences is most suitable for clinical use, the following mathematical function was constructed and applied: segments that were graded as good image quality were given a score of 3 points, moderate segments a score of 2 points and segments with poor image quality a score of 1. This resulted in a maximum score of 30, and a minimum score of 10 per exam. An exam was considered to be clinically suitable if it scored 25 points or higher.
Results
In total 97 patients suspected of ARVC/D underwent MRI. In all 97 exams either a 3D, 2D or a PSIR sequence was performed. Baseline characteristics are shown in Table 2. The highest number of ARVC/D patients is seen in the 2D group. This sequence was used during a period in which all known ARVC/D patients and their relatives were offered genetic investigation and MRI imaging as part of a research study.
Table 2.
Baseline characteristics of the total of 97 exams
| 3D | 2D | PSIR | |
|---|---|---|---|
| Exams (n) | 27 | 32 | 38 |
| % Men | 66 | 68 | 65 |
| Age in years (mean ±SD) | 39.3 ± 15.4 | 39.5 ± 17.5 | 43.9 ± 17.9 |
| Assessed segments (n) | 270 | 320 | 380 |
| ARVC/D clinically diagnosed | 1 (4%) | 9 (28%) | 2 (5%) |
Examples of different image quality of the RV inferior wall (segment 29) are shown in Fig. 3.
Fig. 3.
Example of 3 patients with good, moderate and poor image quality (from left to right) of the right ventricular inferior wall (segment 29) in a PSIR exam
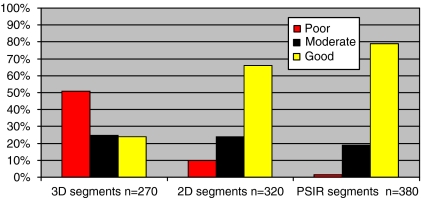
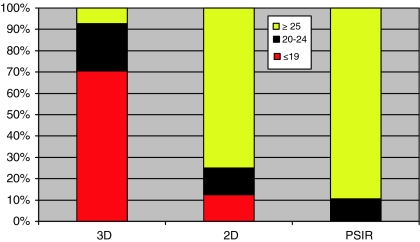
Image quality grading results of all segments per sequence type are shown in Fig. 4. Image quality was considered to be good in 24% of all segments in the 3D group (65/270), 66% in the 2D group (211/320) and in 79% (300/380) in the PSIR group. Image quality was poor in 51% (138/270) of the segments in the 3D group, 10% (31/320) of segments in the 2D group and 2% (6/380) of segments in the PSIR group.
Fig. 4.
Differentiation between image quality of segments by type of sequence
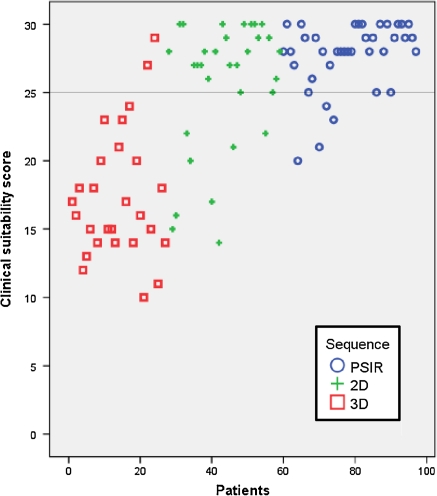
The scores of individual patients, clustered by sequence type, are illustrated in a scatter plot in Fig. 5. The PSIR group showed the least variation in scores. Furthermore a high number of maximum scores (30 points) was noted in the PSIR group (26%) compared to 19% in the 2D group. No maximum scores were reached in the 3D group.
Fig. 5.
Individual patient score sorted by type of sequence
The clinical suitability score was divided in three categories as shown in Fig. 6. Clinically suitable scans (with a score of 25 or higher) were seen in 90% of all exams in the PSIR group, 75% of the 2D group, and in only 7% of the 3D group.
Fig. 6.
Score differentiation by type of sequence
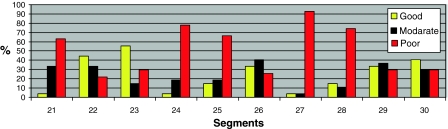
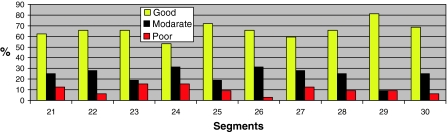
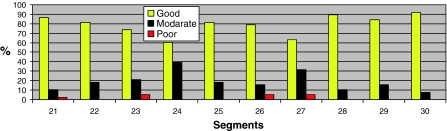
The image quality per segment per type of sequence is shown in Figs. 7, 8 and 9. In the 3D sequence group the anterior RV wall (segments 21, 24, 27) and the lateral RV wall (segments 25 and 28) show the highest percentages of poor image quality (Fig. 7). Consistent scores were seen over all segments in the 2D group (Fig. 8), with the lowest percentage of good image quality (53%) in the anterior RV wall (segment 24). The PSIR group had a high average score of good image quality per segment (Fig. 9). Segments 24 and 27 of the RV anterior wall showed a lower percentage of segments with a good image quality (61 and 63% respectively).
Fig. 7.
Segmental differentiation of quality in the 3D group
Fig. 8.
Segmental differentiation of quality in the 2D group
Fig. 9.
Segmental differentiation of quality in the PSIR group
Breathing artifacts were more frequent in the 2D (59%) and PSIR (53%) sequence compared to the 3D sequence (22%). Motion artifacts occurred most frequently in the 3D group (55%) as compared to the PSIR 29% and 2D 28% group. Nulling of the normal myocardium was considered to be correct in 96% of the 3D scans, 95% of the PSIR scans and 91% of the 2D scans.
Discussion
DE imaging has shown to be an accurate and useful diagnostic tool in ischaemic and non-ischaemic cardiomyopathies, but with a primary focus on the left ventricle [11, 12]. DE imaging of the right ventricle has been studied to a much lesser extent.
The use of a 3D acquisition technique has shown to be both accurate and fast in obtaining information on pathology of the left ventricle wall [16–18]. When DE imaging was included in our MRI study protocol for patients suspected of ARVC/D, we initially chose a 3D acquisition technique. In our experience though, such a 3D technique often proved insufficient to accurately depict the much thinner right ventricle wall. We therefore changed our scanning protocol to a 2D sequence. This was a non-phase-sensitive 2D sequence.
Later the scanning protocol was changed to a 2D PSIR sequence. In non-phase-sensitive sequences the inversion time has to be chosen manually using a look locker technique. In PSIR, a standard inversion time can be chosen and there is a lower risk of obtaining DE images with an incorrect inversion time compared to a non-phase-sensitive sequence. PSIR imaging shows the full negative-to-positive value range of magnetization and therefore delivers better contrast between normal myocardium and fibrotic tissue over a range of different inversion times [19]. In other ways our PSIR and 2D sequence are technically similar.
The aim of this study was to investigate which type of delayed enhancement inversion-recovery sequence provides the highest image quality for the right ventricle in patients suspected of ARVC/D. To our knowledge, this is the first article which describes the differences of image quality of the right ventricle between three post-gadolinium sequences. This study shows better and more consistent image quality obtained with the PSIR sequence compared to the 3D and 2D sequences.
Image quality of both the PSIR and 2D sequence was substantially better compared to the 3D sequence. This could be largely due to the 3D sequence having an almost twice as big voxel size in the Z axis compared to the PSIR and 2D sequence (Table 1). Image quality may have been better for the 3D sequence if a voxel size similar to that of the PSIR and 2D sequences was used. This would also result in a longer scanning time of about 45 s with 3 breathholds reducing the main advantage, shorter scanning time, of a 3D over a 2D sequence. No images and data were acquired concerning scanning with a smaller voxelsize using the 3D sequence.
A secondary finding of this study was the difficulty in visualizing the anterior wall of the right ventricle. This was most pronounced in the 3D sequence. There are several reasons why the anterior wall of the right ventricle is difficult to visualize. Not only is the anterior wall much more mobile, but it is also more oblique to the short axis scanning plane compared to the septum and inferior wall. Therefore partial volume and motion artifacts are expected to be of greater influence on the image quality of the segments of the anterior wall, as visualized in the short axis images analysed in this study. A RV vertical long axis (2 chamber) view (although not investigated by this study) may provide better image quality of the anterior wall.
The way in which the artifacts are distributed over the sequences can be explained by the way the image data is acquired. The 3D sequence acquires its data from 2 breath-holds while the two other sequences acquire data from 10 breath-holds. Most breathing artifacts occurred in the 2D and PSIR sequence at the end of those 10 breath holds, when patients apparently experienced some shortness of breath due to frequent breath-holds. The 3D sequence however acquires data from a larger part of the R-R interval of the ECG and, as mentioned before, has a larger voxel size. It is therefore more sensitive to (cardiac) motion artifacts.
Furthermore, this was a retrospective study in which the radiologists could not be completely blinded for type of sequence because of their experience with all three sequences. The decision to change the MRI protocol in our institution for patients suspected with ARVC/D from the 3D to the 2D and then to the PSIR sequences was partly based on those experiences. There may have been some bias as a result of this.
Remarks can also be made on the relatively small group size, the heterogeneity between the groups and the assessment of only the short axis images.
In conclusion the PSIR sequence produces a better and more consistent image quality of delayed enhancement imaging of the right ventricle compared to the 3D and 2D sequences.
Acknowledgments
Conflicts of interest
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Basso C, Thiene G, Corrado D, et al. Arrhythmogenic right ventricular cardiomyopathy. Dysplasia, dystrophy, or myocarditis? Circulation. 1996;94(5):983–991. doi: 10.1161/01.cir.94.5.983. [DOI] [PubMed] [Google Scholar]
- 2.Sen-Chowdhry S, Syrris P, Ward D, et al. Clinical and genetic characterization of families with arrhythmogenic right ventricular dysplasia/cardiomyopathy provides novel insights into patterns of disease expression. Circulation. 2007;115(13):1710–1720. doi: 10.1161/CIRCULATIONAHA.106.660241. [DOI] [PubMed] [Google Scholar]
- 3.Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113(14):1807–1816. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 4.Muthappan P, Calkins H. Arrhythmogenic right ventricular dysplasia. Prog Cardiovasc Dis. 2008;51(1):31–43. doi: 10.1016/j.pcad.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Corrado D, Fontaine G, Marcus FI, McKenna WJ, et al. Arrhythmogenic right ventricular dysplasia/cardiomyopathy: need for an international registry. Study Group on Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy of the Working Groups on Myocardial and Pericardial Disease and Arrhythmias of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the World Heart Federation. Circulation. 2000;101(11):e101–e106. doi: 10.1161/01.cir.101.11.e101. [DOI] [PubMed] [Google Scholar]
- 6.Basso C, Corrado D, Thiene G. Cardiovascular causes of sudden death in young individuals including athletes. Cardiol Rev. 1999;7(3):127–135. doi: 10.1097/00045415-199905000-00009. [DOI] [PubMed] [Google Scholar]
- 7.McKenna WJ, Thiene G, Nava A, et al. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology. Br Heart J. 1994;71(3):215–218. doi: 10.1136/hrt.71.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria. Eur Heart J. 2010;31(7):806–814. doi: 10.1093/eurheartj/ehq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010;121(13):1533–1541. doi: 10.1161/CIRCULATIONAHA.108.840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sen-Chowdhry S, Prasad SK, Syrris P, et al. Cardiovascular magnetic resonance in arrhythmogenic right ventricular cardiomyopathy revisited: comparison with task force criteria and genotype. J Am Coll Cardiol. 2006;48(10):2132–2140. doi: 10.1016/j.jacc.2006.07.045. [DOI] [PubMed] [Google Scholar]
- 11.Bohl S, Wassmuth R, Abdel-Aty H, et al. Delayed enhancement cardiac magnetic resonance imaging reveals typical patterns of myocardial injury in patients with various forms of non-ischemic heart disease. Int J Cardiovasc Imaging. 2008;24(6):597–607. doi: 10.1007/s10554-008-9300-x. [DOI] [PubMed] [Google Scholar]
- 12.Dulce MC, Duerinckx AJ, Hartiala J, et al. MR imaging of the myocardium using nonionic contrast medium: signal- intensity changes in patients with sub-acute myocardial infarction. Am J Roentgenol. 1996;160:963–970. doi: 10.2214/ajr.160.5.8470611. [DOI] [PubMed] [Google Scholar]
- 13.Tandri H, Saranathan M, Rodriguez ER, et al. Noninvasive detection of myocardial fibrosis in arrhythmogenic right ventricular cardiomyopathy using delayed-enhancement magnetic resonance imaging. J Am Coll Cardiol. 2005;45(1):98–103. doi: 10.1016/j.jacc.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 14.Prakken NH, Velthuis BK, Cramer MJM (2008) UMC Utrecht, The Netherlands (unpublished data)
- 15.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association, American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging. Circulation. 2002;105:539. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 16.Peukert D, Laule M, Taupitz M, et al. 3D and 2D delayed-enhancement magnetic resonance imaging for detection of myocardial infarction: preclinical and clinical results. Acad Radiol. 2007;14(7):788–794. doi: 10.1016/j.acra.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Kino A, Zuehlsdorff S, Sheehan JJ, et al. Three-dimensional phase-sensitive inversion-recovery turbo FLASH sequence for the evaluation of left ventricular myocardial scar. Am J Roentgenol. 2009;193(5):W381–W388. doi: 10.2214/AJR.08.1952. [DOI] [PubMed] [Google Scholar]
- 18.van den Bosch HC, Westenberg JJ, Post JC, et al. Free-breathing MRI for the assessment of myocardial infarction: clinical validation. Am J Roentgenol. 2009;192(6):W277–W281. doi: 10.2214/AJR.08.1580. [DOI] [PubMed] [Google Scholar]
- 19.Kellman P, Arai AE, McVeigh ER, et al. Phase-sensitive inversion recovery for detecting myocardial infarction using gadolinium-delayed hyperenhancement. Magn Reson Med. 2002;47(2):373–383. doi: 10.1002/mrm.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]