Summary
Latrophilins (LPHNs) are a small family of G-protein coupled receptors known to mediate the massive synaptic exocytosis caused by the black widow spider venom α-latrotoxin, but their endogenous ligands and function remain unclear. Mutations in LPHN3 are strongly associated with attention deficit hyperactivity disorder (ADHD), suggesting a role for latrophilins in human cognitive function. Using affinity chromatography and mass spectrometry, we identify the FLRT family of leucine-rich repeat transmembrane proteins as endogenous postsynaptic ligands for latrophilins. We demonstrate that the FLRT3 and LPHN3 ectodomains interact with high affinity in trans and that interference with this interaction using soluble recombinant LPHN3, LPHN3 shRNA, or FLRT3 shRNA reduces excitatory synapse density in cultured neurons. In addition, reducing FLRT3 levels with shRNA in vivo decreases afferent input strength and dendritic spine number in dentate granule cells. These observations indicate that LPHN3 and its ligand FLRT3 play an important role in glutamatergic synapse development.
Introduction
Latrophilins have long been known to mediate the potent exocytotic effect that the black widow spider venom α-latrotoxin exerts on synaptic terminals (Krasnoperov et al., 1997; Lelianova et al., 1997). The latrophilin family consists of 3 isoforms, LPHN1-3, encoded by different genes, with Lphn1 and Lphn3 expression largely restricted to the CNS (Ichtchenko et al., 1999; Sugita et al., 1998). All three LPHNs have a similar domain organization, consisting of a GPCR subunit and an unusually large adhesion-like extracellular N-terminal fragment (NTF) with lectin, olfactomedin, and hormone receptor domains. Though much effort has been expended investigating the mechanisms of α-latrotoxin action (Sudhof, 2001), nothing is known about the endogenous function of latrophilins in vertebrates. Further evidence for the importance of latrophilins in the proper functioning of neural circuits comes from recent human genetics studies that have linked LPHN3 mutations to attention deficit hyperactivity disorder (ADHD), a common and highly heritable developmental psychiatric disorder (Arcos-Burgos et al., 2010; Domene et al., 2011; Jain et al., 2011; Ribases et al., 2011).
Here we report the identification of fibronectin leucine-rich repeat transmembrane (FLRT) proteins as endogenous ligands for latrophilins. There are 3 FLRT isoforms encoded by different genes, Flrt1-3, that each encode single pass transmembrane proteins with 10 extracellular leucine-rich repeat (LRR) domains and a juxtamembrane fibronectin type 3 (FN3) domain (Lacy et al., 1999). FLRTs are expressed in many tissues including brain, where the different isoforms have striking cell-type specific expression patterns in the hippocampus and cortex (Allen Mouse Brain Atlas). FLRTs have recently been reported to function in axon guidance and cell migration through an interaction with Unc5 proteins (Yamagishi et al., 2011), but have no known role at synapses.
We report the identification of FLRT3 and LPHN3 as a novel trans-synaptic ligand-receptor pair. This interaction is of high affinity, can occur in trans, and is mediated by the extracellular domains of FLRT3 and LPHN3. Moreover, we present evidence that FLRT3 and LPHN3 regulate excitatory synapse number in vitro and in vivo. These results demonstrate a novel role for LPHN3 and its newly identified ligand FLRT3 in the development of synaptic circuits.
Results
Identification of FLRTs as Candidate LPHN Ligands
To identify candidate LPHN ligands, we used recombinant ecto-LPHN3-Fc protein (Fig. 1A) to identify putative binding proteins from 3-week-old rat synaptosome extracts by affinity chromatography. Proteins bound to ecto-LPHN3-Fc were analyzed by shotgun mass spectrometry (de Wit et al., 2009; Savas et al., 2011). Of the proteins that bound selectively to ecto-LPHN3-Fc, FLRT2 and FLRT3 were amongst the most abundant and of particular interest due to similarities in domain organization with previously identified postsynaptic organizing molecules such as the LRRTMs (de Wit et al., 2011), which were not detected in our purification (Fig. 1B). We also identified proteins in the Teneurin family (also named ODZs), which have recently been reported as ligands for LPHN1 (Silva et al., 2011) (Fig. S1A).
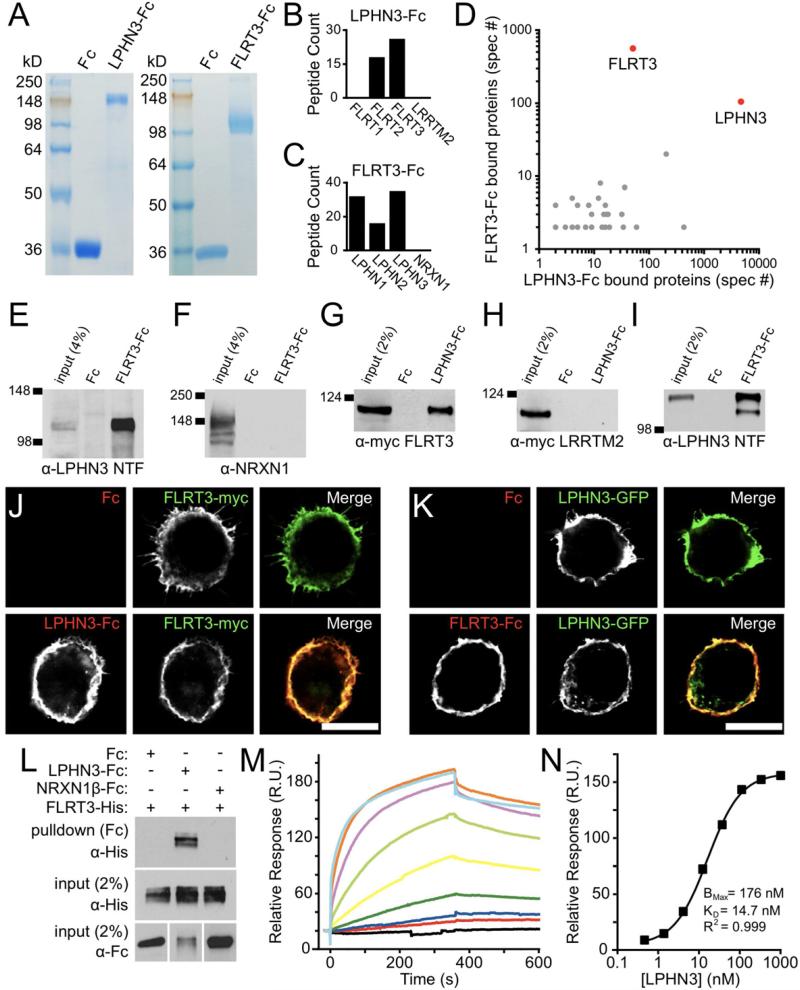
Figure 1. Identification of FLRT3 as a novel endogenous ligand for LPHN3.
(A) Coomassie stained SDS-PAGE gels showing ecto-LPHN3-Fc (left) and ecto-FLRT3-Fc (right) purified proteins running as single bands.
(B, C) Number of distinct tryptic peptides for selected proteins detected by mass spectrometry from ecto-LPHN3-Fc (B) or ecto-FLRT3-Fc (C) affinity purifications.
(D) Frequency of detection of all peptides (total spectra count, spec #) for proteins identified in both LPHN3 and FLRT3 affinity purifications.
(E-F) Pull-downs from whole brain extracts with ecto-FLRT3-Fc protein or control Fc protein. Ecto-FLRT3-Fc precipitates LPHN3 NTF (E), but not NRXN1 from brain (F).
(G-I) Pull-downs on lysate of HEK293 cells transfected with FLRT3-myc (G), myc-LRRTM2 (H), or LPHN3-GFP (I) using ecto-Fc or control Fc proteins. Ecto-LPHN3-Fc precipitates FLRT3-myc (G), but not myc-LRRTM2 from HEK cell lysate (H). (I) Ecto-FLRT3-Fc precipitates LPHN3-GFP NTF.
(J, K) Binding of soluble ecto-LPHN3-Fc (J) and ecto-FLRT3-Fc (K) proteins to HEK cells expressing FLRT3 or LPHN3. Scale bar 10 μm.
(L) Direct interaction of FLRT3 and LPHN3 ectodomains. Fc, ecto-LPHN3-Fc, or ecto-NRXN1β(-S4)-Fc was mixed with FLRT3-His, precipitated, and analyzed by western blot. FLRT3-His only binds to ecto-LPHN3-Fc (top).
(M) Surface Plasmon Resonance (SPR)-based measurement of ecto-LPHN3-Fc binding to immobilized ecto-FLRT3. Each colored trace represents the binding response for a different concentration of ecto-LPHN3-Fc, from 1 μM to 0.45 μM in three-fold dilutions, separated by wash and regeneration steps.
(N) Concentration-response function of LPHN3-FLRT3 SPR binding. The KD of the interaction is 14.7 nM.
Since FLRT3 was the most abundant FLRT protein identified in the ecto-LPHN3-Fc pull-down, we carried out complementary experiments with ecto-FLRT3-Fc to confirm this interaction (Fig. 1B, S1A). Affinity chromatography and mass spectrometry using ecto-FLRT3-Fc resulted in the identification of a large number of LPHN1 and LPHN3 peptides, with relatively fewer LPHN2 peptides, but not the abundant presynaptic organizing protein NRXN1 (Fig. 1C). UNC5B (Fig. S1B), a previously reported FLRT3 interactor, was also identified, but at much lower abundance (Karaulanov et al., 2009; Sollner and Wright, 2009; Yamagishi et al., 2011). When total spectra counts from proteins identified in both purifications were compared, LPHN3 and FLRT3 stood out clearly as the proteins most frequently detected in both purifications (with each as bait in one condition and prey in the other) (Fig. 1D). To support our mass spectrometry results, we verified the association of FLRT3 with LPHN3 by western blot in similar ecto-Fc pull-down assays on rat brain extract and transfected heterologous cell lysate (Fig. 1 E-I). Together these findings suggest that FLRTs likely represent novel endogenous ligands for latrophilins.
FLRT3 Can Directly Interact With LPHN3
To test whether FLRT3 and LPHN3 can bind to one another in a cellular context, we expressed FLRT3-myc in HEK293 cells and applied ecto-LPHN3-Fc or control Fc protein. We observed strong binding of ecto-LPHN3-Fc to cells expressing FLRT3-myc, but no binding of Fc (Fig. 1J). Ecto-LPHN3-Fc did not bind to cells expressing myc-LRRTM2 (Fig. S1D), showing that the LPHN3-FLRT3 interaction is specific. Ecto-LPHN3-Fc also bound strongly to the other FLRT isoforms, FLRT1 and FLRT2 (Fig. S1D), and ecto-LPHN1-Fc bound to all FLRT isoforms as well (Fig. S1C). Complementarily, ecto-FLRT3-Fc, but not control Fc, bound strongly to cells expressing LPHN3-GFP (Fig. 1K). Ecto-FLRT3-Fc also bound the previously identified interactors UNC5A, UNC5B, and UNC5C, but did not bind to NRXN1β(+ or -S4)-expressing cells (data not shown). We also confirmed that ecto-LPHN3-Fc, but not ecto-FLRT3-Fc, could bind to cells expressing Teneurin 3, confirming that LPHNs and Teneurins can indeed interact (Fig. S1E). Thus, we find that LPHNs and FLRTs strongly interact with promiscuity between isoforms.
To eliminate the possibility that an unknown co-receptor present in HEK293 cells might mediate the FLRT-LPHN interaction, we performed a cell-free binding assay using purified proteins. Histidine-tagged FLRT3 and ecto-LPHN3-Fc or control Fc proteins were mixed in solution, and the Fc proteins precipitated with bead-coupled Protein A/G and assessed by western blot. We found that FLRT3-His co-precipitated with ecto-LPHN3-Fc, but not with control Fc or NRXN1β(-S4)-Fc (Fig. 1L), confirming a direct interaction between the ectodomains of FLRT3 and LPHN3. To quantitatively characterize the affinity of the FLRT3-LPHN3 interaction, we employed a surface plasmon resonance (SPR) bioassay to measure specific ligand-receptor binding (Fig. 1M). Plotting the maximum relative response versus the ecto-LPHN3-Fc concentrations, we calculated the KD of the LPHN3-FLRT3 interaction to be 14.7 nM (Fig. 1N), indicating a high affinity interaction.
FLRT3 Is a Postsynaptic Protein Expressed by Specific Neuronal Populations
To identify brain regions where FLRT3 is likely to function, we examined Flrt3 expression in the developing brain and found that Flrt3 was highly expressed in specific neuronal populations during the first two postnatal weeks (Fig. 2A, S2A). In the hippocampus, the principal cell layers of the dentate gyrus (DG) and CA3 showed strong signal, whereas Flrt3 expression was not detected in CA1.
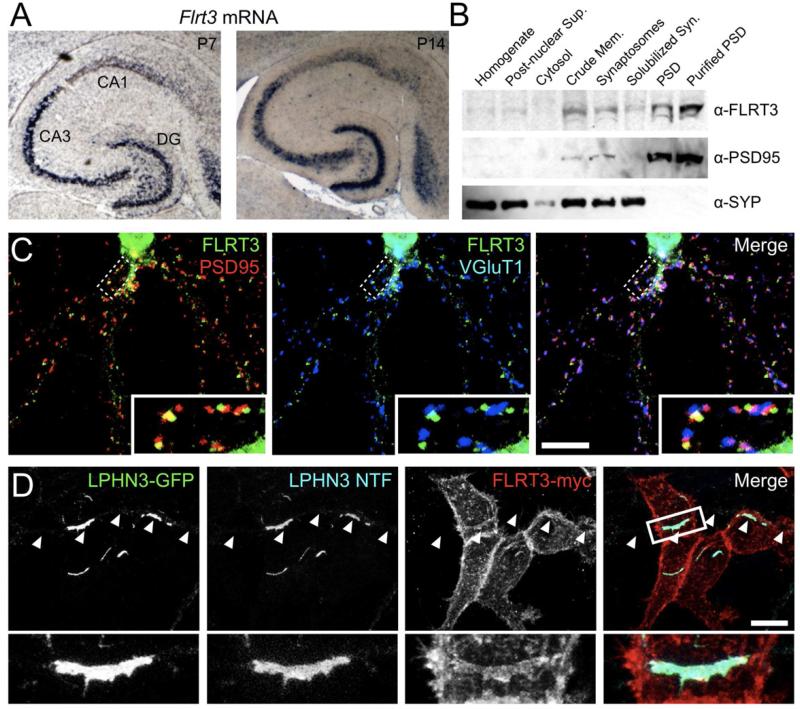
Figure 2. FLRT3 is a postsynaptic protein capable of interaction with LPHN3 in trans.
(A) In situ hybridization with anti-sense Flrt3 probe in horizontal sections from P7 (left) and P14 (right) mouse brain.
(B) Western blot for FLRT3 and known synaptic proteins in fractionated rat brain protein samples. Fractions, from left to right: homogenate, post-nuclear supernatant, cytosol, crude membrane, synaptosome, detergent solubilized synaptosome, postsynaptic density, and purified postsynaptic density.
(C) In dissociated hippocampal neurons, FLRT3-myc (green) localizes to dendrites in a punctate fashion, and partially co-localizes with the postsynaptic marker PSD95 (red) and is closely associated with presynaptic VGluT1 puncta (blue).
(D) Hippocampal neurons transfected with LPHN3-GFP were co-cultured with HEK cells expressing FLRT3-myc and transfected axons (arrowheads) were imaged where they contacted transfected HEK cells. LPHN3-GFP, LPHN3 NTF, and FLRT3-myc were all enriched at axon-HEK cell contacts. Scale bar in (C, D), 10 μm.
Given its interaction with the extracellular domain of LPHNs, we hypothesized that FLRT3 might be a postsynaptic protein. We first employed a subcellular fractionation approach to examine the distribution of FLRT3 across different synaptic fractions (Fig. 2B) and found FLRT3 to be enriched in synaptosome and postsynaptic density (PSD) fractions, mirroring the distribution of PSD95 and in contrast to synaptophysin, which is excluded from PSD fractions. Next, since endogenous FLRT3 could not be detected by immunofluorescence with currently available antibodies, we expressed FLRT3-myc in dissociated hippocampal neurons and examined its subcellular distribution. FLRT3-myc was found in dendrites in puncta that partially co-localized with glutamatergic but not GABAergic synapses (Fig. 2C, S2C). Together these results suggest that FLRT3 is a postsynaptic protein of glutamatergic synapses.
LPHN3 Can Interact with FLRT3 in Trans
As a putative trans-synaptic complex, FLRT3 and LPHN3 must be able to interact across sites of cell-cell contact. We tested whether LPHN3 and FLRT3 can interact in trans by over-expressing LPHN3-GFP in dissociated hippocampal neurons and co-culturing them with HEK293 cells expressing FLRT3-myc or a control construct. . Strong axonal clustering of the GPCR and NTF fragments of LPHN3, as well as enrichment of FLRT3-myc, were observed at sites of contact with FLRT3-myc-expressing HEK293 cells (Fig. 2D). No clustering of LPHN3-GFP was observed when axons contacted control cells (data not shown). The accumulation of FLRT3 at sites of contact with LPHN3-expressing axons (Fig. 2D) demonstrates that FLRT3 is capable of interacting in trans with axonal LPHN3 and that the interaction can mediate mutual recruitment or retention. To test whether FLRT3 and LPHN3 are sufficient to induce pre- and postsynaptic differentiation respectively, we co-cultured neurons with HEK293 cells expressing FLRT3 or LPHN3. We found that FLRT3 was not sufficient to induce clustering of synapsin in axons in this heterologous cell assay (Fig. S2D, E), but that LPHN3-expressing HEK293 cells did cluster PSD95 in contacting dendrites, though less potently than NRXN1β(-S4) (Fig. S2F, G).
FLRT3 and LPHN3 Regulate Glutamatergic Synapse Density In Vitro
After establishing that the latrophilin ligand FLRT3 is expressed in hippocampal neurons and localizes to glutamatergic synapses, we decided to test whether the interaction of endogenous LPHN3 with its ligand is of functional importance. We applied excess soluble ecto-LPHN3-Fc protein to dissociated hippocampal cultures to competitively disrupt endogenous LPHN3 complexes and analyzed glutamatergic synapses on dentate granule cells (GCs) (identified by nuclear Prox1 immunoreactivity: Williams et al., 2011) by immunofluorescence (Fig. 3A). We found that ecto-LPHN3-Fc treatment strongly reduced the density of synaptic puncta (Fig. 3B) without affecting their size (Fig. 3C). These results suggest that reducing the availability of LPHN3 ligands such as FLRT3 by competition with ecto-LPHN3-Fc prevents glutamatergic synapses from developing properly.
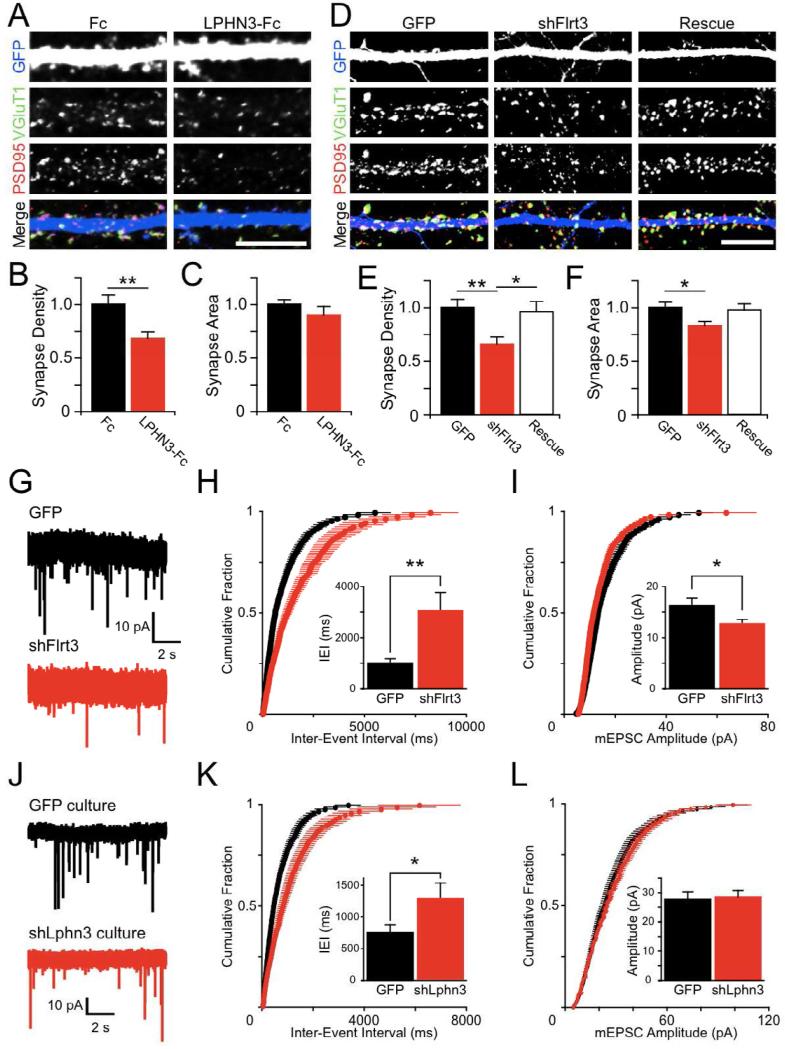
Figure 3. FLRT3 and LPHN3 regulate glutamatergic synapse number.
(A) Competitive disruption of endogenous LPHN interactions with ecto-LPHN3-Fc reduces excitatory synapse density. Hippocampal cultures were fixed and immunostained for VGluT1 (green), PSD95 (red), and GFP (blue) at 14DIV after 6 days of treatment with 10 μg/ml Fc (left) or ecto-LPHN3-Fc (right).
(B) LPHN3-Fc treatment reduces the density of co-localizing VGluT1 and PSD95 puncta on dendrites of identified GCs (Fc 1.00 ± 0.05, n=17; LPHN3-Fc 0.68 ± 0.06, n=14; p=0.01)
(C) Synaptic puncta did not significantly differ in size between Fc- and ecto-LPHN3-Fc-treated neurons (Fc 1.00 ± 0.04, n=17; LPHN3-Fc 0.90 ± 0.08, n=14; n.s.).
(D) Hippocampal neurons were electroporated with GFP control plasmid (GFP), a plasmid encoding shFlrt3 and GFP (shFlrt3), or the shFlrt3 plasmid plus an shFlrt3-resistant FLRT3-myc expression construct (Rescue), and processed for immunofluorescence as in (A).
(E) Synapse density was reduced by shFlrt3 and returned to control levels in the Rescue condition (GFP 1.00 ± 0.08, n=35; shFlrt3 0.66 ± 0.06, n=33; Rescue 0.96 ± 0.09, n=34; ANOVA p<0.01, significant post-hoc comparisons shown on graph).
(F) Synapse area was slightly reduced by shFlrt3 (GFP 1.00 ± 0.05, n=35; shFlrt3 0.83 ± 0.04, n=33; Rescue 0.98 ± 0.06, n=34; ANOVA p<0.05, significant post-hoc comparisons shown on graph).
(G) Example mEPSC traces recorded from cultured hippocampal neurons electroporated with GFP (black) or shFlrt3 (red) plasmids.
(H) Summary of mEPSC frequencies plotted as cumulative probability distributions of inter-event intervals (IEIs) for GFP (black) or shFlrt3 (red) electroporated cells. Inset: Quantification of mean mEPSC IEIs. shFlrt3 cells have longer mean IEIs than control neurons (GFP 1028 ± 147 ms, n=15; shFlrt3 3069 ± 688 ms, n=15; p < 0.01).
(I) Summary of mEPSC amplitude plotted as cumulative probability distributions for GFP (black) or shFlrt3 (red) electroporated cells. Inset: Quantification of mean mEPSC amplitude. shFlrt3 electroporated cells show a small decrease in mean mEPSC amplitude (GFP 16.4 ± 1.2 pA, n=15; shFlrt3 12.8 ± 0.7 pA, n=15; p < 0.05).
(J) mEPSCs were recorded from neurons in hippocampal cultures densely infected with GFP (black) or shLphn3 (red) lentiviruses such that greater than 90% of neurons were transduced.
(K) shLphn3 causes a shift towards longer times in the distribution of IEIs. (GFP 745 ± 125 ms, n=19; shLphn3 1254 ± 219 ms, n=17; p < 0.05). (L) shLphn3 does not affect mEPSC amplitudes (GFP 27.7 ± 2.6 pA, n=19; shLphn3 28.4 ± 2.3 pA, n=17; n.s.). Scale bar in (A, D), 10 μm
If ecto-LPHN3-Fc exerts its effect by disrupting the interaction of LPHN3 with FLRT3, loss of postsynaptic FLRT3 might be expected to result in a similar reduction in synapses. We designed an shRNA to specifically knock down Flrt3 expression (shFlrt3) and verified its efficacy and specificity (Fig. S3A-C). To determine whether endogenous FLRT3 regulates synapse number, we electroporated P0 hippocampal cultures with shFlrt3 or control plasmids to sparsely knock down FLRT3 and analyzed synapses formed onto GC dendrites by immunofluorescence (Fig. 3D). We observed a large decrease in the density of synapses with shFlrt3 (Fig. 3E), an effect similar to that observed with ecto-LPHN3-Fc treatment. This decrease in synapse number was fully rescued by coelectroporation with an shRNA-resistant FLRT3 construct (FLRT3*-myc) (Fig. 3E, S3A). Additionally, we saw a small decrease in the area of synaptic puncta following FLRT3 knockdown (Fig. 3F). To see if the decrease in synapse density assessed by immunofluorescence corresponds to a decrease in functional synapses, we recorded miniature excitatory postsynaptic currents (mEPSCs) from putative GCs in similar cultures (Fig. 3G). Consistent with the reduction in synaptic puncta density, we found a marked decrease in mEPSC frequency (Fig. 3H) and a slight decrease in the mean amplitude of mEPSCs (Fig. 3I) following FLRT3 knockdown. These experiments together suggest that a loss of FLRT3 in postsynaptic GCs reduces the number of excitatory synapses onto those neurons.
If LPHN3 is involved in promoting the number of glutamatergic synapses, as suggested by our dominant negative experiment with ecto-LPHN3-Fc (Fig. 3A-C), then loss of presynaptic LPHN3 should also reduce synapse number. To test this predication, we infected hippocampal cultures with a lentivirus expressing an shRNA against LPHN3 (shLphn3: Fig. S3D) such that the preponderance (>90%) of neurons in the culture was transduced and recorded mEPSCs from neurons in LPHN3 knockdown and control cultures (Fig. 3J). Culture-wide knockdown of LPHN3 reduced the frequency of mEPSCs (Fig. 3K) without affecting mEPSC amplitude (Fig. 3L), suggesting that loss of LPHN3 decreases the number of excitatory synapses in these cultures.
These experiments in dissociated hippocampal cultures show that three distinct manipulations designed to perturb LPHN3-FLRT3 complexes – competition with ecto-LPHN3-Fc (Fig. 3A-C), shRNA knockdown of FLRT3 (Fig. 3D-I), and shRNA knockdown of LPHN3 (Fig. 3J-L) – all lead to a reduction in the number of glutamatergic synapses, strongly suggesting that FLRT3 and LPHN3 serve to positively regulate synapse number.
Postsynaptic FLRT3 Regulates the Function of Glutamatergic Synapses In Vivo
To test whether endogenous FLRT3 contributes to synapse development in vivo, we first used in utero electroporation to sparsely label and manipulate DG GCs for anatomical analysis. In fixed slices from P14 electroporated mice, GFP-filled GC dendrites in the middle molecular layer were imaged on a confocal microscope and dendritic spines counted (Fig. 4A). Knockdown of FLRT3 resulted in a highly significant reduction in dendritic protrusion density relative to controls (Fig. 4B). The density of spines on the apical dendrites of electroporated CA1 pyramidal neurons, which do not express Flrt3 (Fig. 2A), did not differ between shFlrt3 and control cells (Fig. 4C,D), functionally confirming the specificity of the shRNA used.
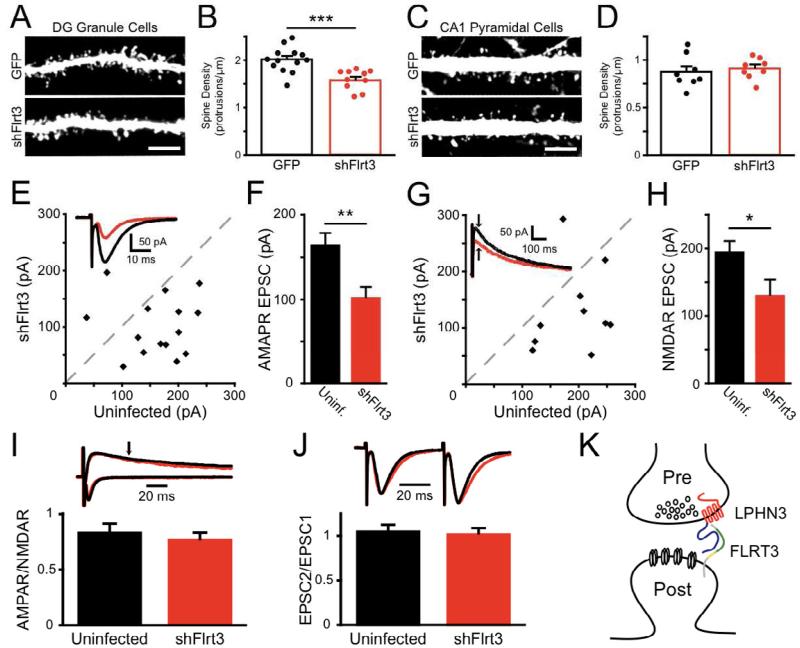
Figure 4. FLRT3 regulates perforant path synapses onto dentate granule cells in vivo.
(A-D) Mouse embryos were electroporated in utero with GFP or shFlrt3 plasmids at E15 and GFP-expressing dendrites imaged at P14-16. (A) Dendrites of electroporated GCs were imaged in the middle molecular layer (MML) of the DG.
(B) Flrt3 shRNA significantly reduces the density of dendritic protrusions (GFP 2.01 ± 0.07 protrusions/μm, n=13; shFlrt3 1.60 ± 0.07 protrusions/μm, n=10; p<0.001).
(C) Dendrites of electroporated CA1 pyramidal neurons, which do not express Flrt3, were imaged in the stratum radiatum.
(D) Flrt3 shRNA does not affect the density of dendritic protrusions in CA1 (GFP 0.87 ± 0.06 protrusions/μm, n=8; shFlrt3 0.91 ± 0.04 protrusions/μm, n=8; n.s.). (E-J) P5 rat pups were stereotaxically injected with shFlrt3 lentivirus and acute slices cut at P13-16 for recording.
(E) Simultaneous whole-cell voltage clamp recordings of evoked perforant path AMPAR-mediated EPSCs onto shFlrt3 infected and uninfected DG GCs. Scatter plot shows mean EPSC amplitude from individual experiments. Inset: example average evoked AMPAR-EPSCs recorded simultaneously from shFlrt3 infected (red) and uninfected (black) GCs.
(F) shFlrt3 significantly reduces the amplitude of AMPAR-EPSCs (shFlrt3 101.1 ± 13.4 pA, uninfected 162.8 ± 15.0 pA, n=15, p<0.01).
(G) Evoked NMDAR-EPSCs were measured simultaneously in shFlrt3 infected (red) and uninfected (black) GCs. Inset: example average evoked NMDAREPSCs. The NMDAR-EPSC measurement was taken at the time indicated by the arrow.
(H) shFlrt3 significantly reduces the amplitude of NMDAR-mediated EPSCs (shFlrt3 129.6 ± 23.9 pA, uninfected 193.7 ± 16.9 pA, n= 0, p<0.05).
(I) shFlrt3 does not affect the ratio of AMPAR-EPSCs to NMDAR-EPSCs at single inputs (shFlrt3 0.76 ± 0.07, uninfected 0.83 ± 0.09, n=10, n.s.).
(J) shFlrt3 does not affect the ratio of EPSC amplitudes evoked by pairs of stimuli delivered at 20 Hz (EPSC2/EPSC1) (shFlrt3 1.01 ± 0.07, uninfected 1.04 ± 0.08, n=13, n.s.).
(K) Schematic of proposed FLRT3-LPHN3 synaptic interaction. FLRT3 is located at postsynaptic sites and interacts trans-synaptically with the NTF of LPHN3 via its extracellular domain. This interaction promotes synaptic development and its disruption reduces synapse number. Scale bar in (A, C), 5 μm.
To determine whether the reduction in GC dendritic spine density reflects a decrease in the strength of synaptic input onto these cells, we stereotaxically injected shFlrt3 or control lentivirus into the hippocampus of postnatal day 5 (P5) rat pups and cut acute slices for electrophysiology between P13 and P16. Infected GCs were identified by GFP epifluorescence, and simultaneous whole-cell voltage-clamp recordings were made from nearby infected and uninfected cells while perforant path synaptic inputs were evoked from the middle molecular layer. We observed that AMPAR-mediated EPSCs onto shFlrt3-infected neurons were strongly reduced in amplitude relative to simultaneously recorded uninfected control cells (Fig. 4E-F). NMDAR-mediated EPSCs, measured 50 ms after the stimulus at a holding potential of +40 mV, were similarly reduced by FLRT3 knockdown (Fig. 4G-H). This reduction was proportional, as the ratio of AMPAR EPSC to NMDAR EPSC for each input was not affected by FLRT3 knockdown (Fig. 4I), consistent with a reduction in number of synapses rather than a selective loss of certain glutamate receptors. GCs infected with a control lentivirus did not differ from uninfected cells by any of these measures (Fig. S4AD and de Wit et al., 2009). When we recorded mEPSCs from infected GCs we found no difference in mEPSC amplitudes and a trend towards reduction in frequency that did not reach significance (Fig. S4E-G), also suggesting that loss of FLRT3 does not affect the strength of single synapses. Furthermore, the paired-pulse ratio of EPSCs evoked at 20 Hz was unaffected (Fig. 4J). These results indicate that loss of FLRT3 leads to an attenuation of the strength of glutamatergic transmission from the perforant path onto GCs and a reduction in the number of GC dendritic spines, further supporting a role for FLRT3 in regulating synapse formation onto GCs.
Discussion
Latrophilins have garnered much interest because of their role in αlatrotoxin-stimulated neurotransmitter release, but their endogenous functions have until now remained unknown. Here we report that the single-pass transmembrane proteins of the FLRT family, FLRT1-3, are endogenous ligands for LPHN1 and LPHN3. These interactions are mediated by the N-terminal fragment of LPHNs and the extracellular domain of FLRTs, and are promiscuous amongst isoforms. The high-affinity interaction between LPHN3 and FLRT3, together with the postsynaptic enrichment of FLRT3, suggest that LPHN3 and FLRT3 form a trans-synaptic complex (Fig. 4K).
The resemblance of FLRTs to characterized LRR-containing synaptic organizers (de Wit et al., 2011) suggested to us that the trans-synaptic interaction of LPHN with FLRT might regulate synaptic development and function. Consistent with this hypothesis, we observed that three separate manipulations targeting the LPHN3-FLRT3 complex reduce excitatory synapse number in cultured neurons (Fig. 3). We further show that loss of FLRT3 in vivo by lentivirus-mediated shRNA knockdown reduces the strength of evoked perforant path synaptic inputs onto dentate GCs and the number of dendritic spines. Our results suggest that FLRT3 may primarily regulate synapse number, whereas LRRTM2 may regulate synapse function by controlling AMPAR recruitment (de Wit et al., 2009; and see Soler-Llavina et al, 2011). Thus FLRTLPHN and LRRTM-NRXN complexes, along with others, may regulate distinct aspects of synapses.
How FLRTs signal postsynaptically is not known, but cis interactions of FLRTs have been reported in other systems. FLRT3 has been implicated in modulation of FGF signaling by interacting with FGFRs (Bottcher et al., 2004; Wheldon et al., 2010) and regulation of cadherin- and protocadherin-mediated cell adhesion by signaling intracellularly through the small GTPase Rnd1 (Chen et al., 2009; Karaulanov et al., 2009). Both FGF signaling (Umemori et al., 2004; Terauchi et al., 2010) and cadherin adhesion (Takeichi, 2007; Williams et al., 2011) are known to influence synapse development, making them two possible effectors for the postsynaptic action of FLRT3.
The immediate presynaptic molecular and cell biological consequences of FLRT binding to LPHN remain unknown. Binding may activate downstream signaling or serve primarily to stabilize LPHN at appropriate presynaptic sites. Ligand binding to LPHNs may result in Ca2+ elevations through G-protein signaling and IP3-mediated calcium release from the endoplasmic reticulum (ER), as has been shown to occur in response to α-LTX binding (Davletov et al., 1998; Ichtchenko et al., 1998). That we observe FLRT-induced accumulation of both the LPHN3 NTF and GPCR domains (Fig. 2D) may also provide a hint of mechanism. Latrophilins are constitutively cleaved into two subunits (Krasnoperov et al., 2002; Silva et al., 2009), and it has been suggested that the association between subunits may be dynamically regulated by ligand binding to modulate latrophilin G-protein signaling. This raises the possibility that FLRT binding to the LPHN NTF may lead to re-association of the LPHN subunits and engender subsequent G-protein signaling. Whether binding of FLRTs and Teneurins to LPHNs induce similar signaling or have different functional consequences remains to be explored.
Interestingly, FLRTs have also recently been shown to function in axon guidance during embryonic development by interacting with axonal Unc5 proteins (Yamagishi et al., 2011). This function is potentially non-cell autonomous, given that it is proposed to occur between proteolytically cleaved, soluble FLRT ectodomains acting as diffusible cues. Our manipulations of FLRT3 in vivo were sparse and, for viral experiments, began at a developmental stage at which axon guidance was complete, suggesting that the effects we see of FLRT3 on synapses are cell autonomous and not the result of axon guidance defects. Thus an early, non-cell autonomous FLRT-Unc5 interaction may mediate axon guidance, and a later cell autonomous FLRT-LPHN interaction may regulate synaptic maturation and function. This dual function is reminiscent of the manner in which semaphorins (Pasterkamp and Giger, 2009) and Ephs/Ephrins (Klein, 2009) function in both axon guidance and synaptogenesis.
While Lphn1 and Lphn3 are broadly expressed in the brain, Flrt2 and Flrt3 show striking cell-type specific expression patterns, with complementary and non-overlapping expression in the hippocampus. Thus, while binding is possible between all LPHNs and all FLRTs, it may be that only a certain combination of LPHNs and FLRTs is present at any given synapse. Due to a lack of suitable antibodies, we do not know if FLRTs are present at all synapses on cells that express them, or if only a subset of synapses is FLRT-positive. Similarly, whether FLRT2 and FLRT3 exert the same effect on synapses that contain them, and how FLRT2 and FLRT3 are allocated to synapses in cells that express both (e.g. L2/3 cortical pyramidal neurons) are questions that will require further investigation. Based on their expression patterns, we propose that latrophilins may be ubiquitous presynaptic components capable of interacting with different postsynaptic ligands, namely FLRTs and Teneurins, to regulate synaptic connectivity. This pattern would follow the precedent set by neurexins for widely-expressed presynaptic regulators interacting with structurally unrelated postsynaptic ligands at different types of synapses (Williams, de Wit, and Ghosh, 2010). Understanding how the brain assembles specific types of synapses between the correct partner cells will require consideration of multiple parallel trans-synaptic signaling complexes, and the latrophilin-FLRT complex is poised to be an important unit in accomplishing this task. Given the genetic association of LPHN3 mutations (Arcos-Burgos et al., 2010; Arcos-Burgos and Muenke, 2010; Domene et al., 2011; Jain et al., 2011; Martinez et al., 2011; Ribases et al., 2011) and FLRT3 copy number variations (Lionel et al., 2011) with ADHD, further characterization of the FLRT3-LPHN3 complex may lead to a better understanding of the pathology and etiology of this disorder.
Experimental Procedures
Please see Supplemental Information.
Supplementary Material
Acknowledgements
We thank Katie Tiglio, Joseph Antonios, Christine Wu, and Tim Young for assistance with virus injections, virus and recombinant protein production, and antibody testing. This work was supported by the Brain and Behavior Research Foundation (formerly NARSAD) (JdW), Autism Speaks grant #2617 (DC), NIH fellowship F32AG039127 (JNS), and NIH grants NS067216 (AG), NS064124 (AG), P41 RR011823 (JRY) and R01 MH067880 (JRY).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen Mouse Brain Atlas [Internet] Allen Institute for Brain Science. ©2009. Available from: http://mouse.brain-map.org.
- Arcos-Burgos M, Jain M, Acosta MT, Shively S, Stanescu H, Wallis D, Domene S, Velez JI, Karkera JD, Balog J, et al. A common variant of the latrophilin 3 gene, LPHN3, confers susceptibility to ADHD and predicts effectiveness of stimulant medication. Mol Psychiatry. 2010;15:1053–1066. doi: 10.1038/mp.2010.6. [DOI] [PubMed] [Google Scholar]
- Bottcher RT, Pollet N, Delius H, Niehrs C. The transmembrane protein XFLRT3 forms a complex with FGF receptors and promotes FGF signalling. Nat Cell Biol. 2004;6:38–44. doi: 10.1038/ncb1082. [DOI] [PubMed] [Google Scholar]
- Chen X, Koh E, Yoder M, Gumbiner BM. A protocadherincadherin-FLRT3 complex controls cell adhesion and morphogenesis. PLoS One. 2009;4:e8411. doi: 10.1371/journal.pone.0008411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davletov BA, Meunier FA, Ashton AC, Matsushita H, Hirst WD, Lelianova VG, Wilkin GP, Dolly JO, Ushkaryov YA. Vesicle exocytosis stimulated by alpha-latrotoxin is mediated by latrophilin and requires both external and stored Ca2+. EMBO J. 1998;17:3909–3920. doi: 10.1093/emboj/17.14.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit J, Sylwestrak E, O'Sullivan ML, Otto S, Tiglio K, Savas JN, Yates JR, 3rd, Comoletti D, Taylor P, Ghosh A. LRRTM2 interacts with Neurexin1 and regulates excitatory synapse formation. Neuron. 2009;64:799–806. doi: 10.1016/j.neuron.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit J, Hong W, Luo L, Ghosh A. Role of leucine-rich repeat proteins in the development and function of neural circuits. Annu Rev Cell Dev Biol. 2011;27:697–729. doi: 10.1146/annurev-cellbio-092910-154111. [DOI] [PubMed] [Google Scholar]
- Domene S, Stanescu H, Wallis D, Tinloy B, Pineda DE, Kleta R, Arcos-Burgos M, Roessler E, Muenke M. Screening of human LPHN3 for variants with a potential impact on ADHD susceptibility. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:11–18. doi: 10.1002/ajmg.b.31141. [DOI] [PubMed] [Google Scholar]
- Ichtchenko K, Bittner MA, Krasnoperov V, Little AR, Chepurny O, Holz RW, Petrenko AG. A novel ubiquitously expressed alpha-latrotoxin receptor is a member of the CIRL family of G-protein-coupled receptors. J Biol Chem. 1999;274:5491–5498. doi: 10.1074/jbc.274.9.5491. [DOI] [PubMed] [Google Scholar]
- Ichtchenko K, Khvotchev M, Kiyatkin N, Simpson L, Sugita S, Sudhof TC. Alpha-latrotoxin action probed with recombinant toxin: receptors recruit alpha-latrotoxin but do not transduce an exocytotic signal. EMBO J. 1998;17:6188–6199. doi: 10.1093/emboj/17.21.6188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain M, Velez JI, Acosta MT, Palacio LG, Balog J, Roessler E, Pineda D, Londono AC, Palacio JD, Arbelaez A, et al. A cooperative interaction between LPHN3 and 11q doubles the risk for ADHD. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.59. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaulanov E, Bottcher RT, Stannek P, Wu W, Rau M, Ogata S, Cho KW, Niehrs C. Unc5B interacts with FLRT3 and Rnd1 to modulate cell adhesion in Xenopus embryos. PLoS One. 2009;4:e5742. doi: 10.1371/journal.pone.0005742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. Bidirectional modulation of synaptic functions by Eph/ephrin signaling. Nat Neurosci. 2009;12:15–20. doi: 10.1038/nn.2231. [DOI] [PubMed] [Google Scholar]
- Krasnoperov V, Lu Y, Buryanovsky L, Neubert TA, Ichtchenko K, Petrenko AG. Post-translational proteolytic processing of the calcium-independent receptor of alpha-latrotoxin (CIRL), a natural chimera of the cell adhesion protein and the G protein-coupled receptor. Role of the G protein-coupled receptor proteolysis site (GPS) motif. J Biol Chem. 2002;277:46518–46526. doi: 10.1074/jbc.M206415200. [DOI] [PubMed] [Google Scholar]
- Krasnoperov VG, Bittner MA, Beavis R, Kuang Y, Salnikow KV, Chepurny OG, Little AR, Plotnikov AN, Wu D, Holz RW, et al. alpha-Latrotoxin stimulates exocytosis by the interaction with a neuronal G-protein-coupled receptor. Neuron. 1997;18:925–937. doi: 10.1016/s0896-6273(00)80332-3. [DOI] [PubMed] [Google Scholar]
- Lacy SE, Bonnemann CG, Buzney EA, Kunkel LM. Identification of FLRT1, FLRT2, and FLRT3: a novel family of transmembrane leucine-rich repeat proteins. Genomics. 1999;62:417–426. doi: 10.1006/geno.1999.6033. [DOI] [PubMed] [Google Scholar]
- Lelianova VG, Davletov BA, Sterling A, Rahman MA, Grishin EV, Totty NF, Ushkaryov YA. Alpha-latrotoxin receptor, latrophilin, is a novel member of the secretin family of G protein-coupled receptors. J Biol Chem. 1997;272:21504–21508. doi: 10.1074/jbc.272.34.21504. [DOI] [PubMed] [Google Scholar]
- Lionel AC, Crosbie J, Barbosa N, Goodale T, Thiruvahindrapuram B, Rickaby J, Gazzellone M, Carson AR, Howe JL, Wang Z, et al. Rare copy number variation discovery and cross-disorder comparisons identify risk genes for ADHD. Sci Transl Med. 2011;3:95ra75. doi: 10.1126/scitranslmed.3002464. [DOI] [PubMed] [Google Scholar]
- Pasterkamp RJ, Giger RJ. Semaphorin function in neural plasticity and disease. Curr Opin Neurobiol. 2009;19:263–274. doi: 10.1016/j.conb.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribases M, Ramos-Quiroga JA, Sanchez-Mora C, Bosch R, Richarte V, Palomar G, Gastaminza X, Bielsa A, Arcos-Burgos M, Muenke M, et al. Contribution of LPHN3 to the genetic susceptibility to ADHD in adulthood: a replication study. Genes Brain Behav. 2011;10:149–157. doi: 10.1111/j.1601-183X.2010.00649.x. [DOI] [PubMed] [Google Scholar]
- Savas JN, Stein BD, Wu CC, Yates JR., 3rd Mass spectrometry accelerates membrane protein analysis. Trends Biochem Sci. 2011;36:388–396. doi: 10.1016/j.tibs.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JP, Lelianova V, Hopkins C, Volynski KE, Ushkaryov Y. Functional cross-interaction of the fragments produced by the cleavage of distinct adhesion G-protein-coupled receptors. J Biol Chem. 2009;284:6495–6506. doi: 10.1074/jbc.M806979200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JP, Lelianova VG, Ermolyuk YS, Vysokov N, Hitchen PG, Berninghausen O, Rahman MA, Zangrandi A, Fidalgo S, Tonevitsky AG, Dell A, Volynski KE, Ushkaryov YA. Latrophilin 1 and its endogenous ligand Lasso/teneurin-2 form a high-affinity transsynaptic receptor pair with signaling capabilities. Proc Natl Acad Sci U S A. 2011;108:12113–12118. doi: 10.1073/pnas.1019434108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler-Llavina GJ, Fuccillo MV, Ko J, Sudhof TC, Malenka RC. The neurexin ligands, neuroligins and leucine-rich repeat transmembrane proteins, perform convergent and divergent synaptic functions in vivo. Proc Natl Acad Sci U S A. 2011;108:16502–16509. doi: 10.1073/pnas.1114028108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollner C, Wright GJ. A cell surface interaction network of neural leucine-rich repeat receptors. Genome Biol. 2009;10:R99. doi: 10.1186/gb-2009-10-9-r99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC. alpha-Latrotoxin and its receptors: neurexins and CIRL/latrophilins. Annu Rev Neurosci. 2001;24:933–962. doi: 10.1146/annurev.neuro.24.1.933. [DOI] [PubMed] [Google Scholar]
- Sugita S, Ichtchenko K, Khvotchev M, Sudhof TC. alpha-Latrotoxin receptor CIRL/latrophilin 1 (CL1) defines an unusual family of ubiquitous G-protein-linked receptors. G-protein coupling not required for triggering exocytosis. J Biol Chem. 1998;273:32715–32724. doi: 10.1074/jbc.273.49.32715. [DOI] [PubMed] [Google Scholar]
- Takeichi M. The cadherin superfamily in neuronal connections and interactions. Nat Rev Neurosci. 2007;8:11–20. doi: 10.1038/nrn2043. [DOI] [PubMed] [Google Scholar]
- Terauchi A, Johnson-Venkatesh EM, Toth AB, Javed D, Sutton MA, Umemori H. Distinct FGFs promote differentiation of excitatory and inhibitory synapses. Nature. 2010;465:783–787. doi: 10.1038/nature09041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemori H, Linhoff MW, Ornitz DM, Sanes JR. FGF22 and its close relatives are presynaptic organizing molecules in the mammalian brain. Cell. 2004;118:257–70. doi: 10.1016/j.cell.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Wheldon LM, Haines BP, Rajappa R, Mason I, Rigby PW, Heath JK. Critical role of FLRT1 phosphorylation in the interdependent regulation of FLRT1 function and FGF receptor signalling. PLoS One. 2010;5:e10264. doi: 10.1371/journal.pone.0010264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ME, de Wit J, Ghosh A. Molecular mechanisms of synaptic specificity in developing neural circuits. Neuron. 2010;68:9–18. doi: 10.1016/j.neuron.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ME, Wilke SA, Dagget A, Davis E, Otto S, Ravi D, Ripley B, Bushong EA, Ellisman MH, Klein G, Ghosh A. Cadherin-9 regulates synapse-specific differentiation in the developing hippocampus. Neuron. 2011;25:640–655. doi: 10.1016/j.neuron.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi S, Hampel F, Hata K, Del Toro D, Schwark M, Kvachnina E, Bastmeyer M, Yamashita T, Tarabykin V, Klein R, Egea J. FLRT2 and FLRT3 act as repulsive guidance cues for Unc5-positive neurons. EMBO J. 2011;14:2920–2933. doi: 10.1038/emboj.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.