Abstract
Background
Ischemic proliferative retinopathy, characterized by pathologic retinal neovascularization, is a major cause of blindness in working age adults and children. Defining the molecular pathways distinguishing pathological neovascularization from normal vessels is critical to controlling these blinding diseases with targeted therapy. Because mutations in Wnt signaling cause defective retinal vasculature in humans with some characteristics of the pathologic vessels in retinopathy, we investigated the potential role of Wnt signaling in pathologic retinal vascular growth in proliferative retinopathy.
Methods and Results
In this study we show that Wnt receptors (Frizzled4 and Lrp5) and activity are significantly increased in pathologic neovascularization in a mouse model of oxygen-induced proliferative retinopathy. Loss of Wnt co-receptor Lrp5 and downstream signaling molecule disheveled2 significantly decreases the formation of pathologic retinal neovascularization in retinopathy. Loss of Lrp5 also affects retinal angiogenesis during development and formation of the blood retinal barrier, which is linked to significant down-regulation of tight junction protein claudin5 (Cln5) in Lrp5−/− vessels. Blocking Cln5 significantly suppresses Wnt-pathway driven endothelial cell sprouting in vitro and developmental and pathologic vascular growth in retinopathy in vivo.
Conclusions
These results demonstrate an important role of Wnt signaling in pathologic vascular development in retinopathy and show a novel function of Cln5 in promoting angiogenesis.
Keywords: angiogenesis, vessels, retinopathy, Wnt
Introduction
Pathologic retinal neovascularization precipitated by vascular loss and hypoxia is the most common cause of blindness in all age groups. Two prominent blinding neovascular eye diseases, retinopathy of prematurity (ROP) in infants and proliferative diabetic retinopathy in working age adults, are both characterized by abnormal proliferation of pathologic vessels. These vessels are distinctly different from normal vasculature morphologically, exhibit increased vascular leakage and are associated with retinal detachment and blindness1, 2. Specifically targeting pathologic neovessels, while sparing normal vessels, would be a major advancement in treating proliferative retinopathy, as well as other vascular diseases with pathologic vessel proliferation, such as cancer. Development of such treatment strategies with selective molecular targeting requires a better understanding of the pathways involved in the regulation of pathologic retinal blood vessel growth.
Wnt signaling may be involved in pathologic vessel growth. Mutations in the Wnt pathway cause several hereditary vascular eye disorders with defective retinal vascular development sharing some characteristics of proliferative retinopathy3-9. The Wnt signaling pathway is fundamentally important in embryonic development and in cancer and cardiovascular diseases10-14. It is essential for cardiac development and differentiation15, 16 and linked to cardiac hypertrophy, cardiac failure and vascular aging17, 18. However, the contribution of Wnt to pathological angiogenesis has not been determined.
Canonical Wnt pathway signaling starts with the binding of a Wnt ligand to the Wnt receptor Frizzled (Fzd) and recruitment of co-receptor low-density lipoprotein receptor-related protein (Lrp5/6), activating and stabilizing β-catenin14. After translocation to the nucleus, β-catenin binds the transcription factor Lef/TCF and activates target gene transcription10, 14. Canonical Wnt signaling is implicated in neuronal development19, cancer 20, 21, regression of embryonic hyaloid vessels in the eye22, and blood brain barrier formation23-25. Mutations in the Wnt receptors Frizzled4 and Lrp5 as well as the Wnt ligand, Norrin, are all linked to hereditary vascular eye diseases in human and in mouse models26-29. Mice deficient in Wnt co-receptor Lrp5 have persistent embryonic hyaloid vessels in the eye9, 28, (as well as low bone density) recapitulating human autosomal-recessive osteoporosis-pseudoglioma syndrome (OPPG), a form of familial exudative vitreoretinopathy (FEVR). Depletion or mutations in Lrp5 result in lack of deeper retinal vessels30, 31, similar to mice lacking Wnt receptor Frizzled4 or Wnt ligand Norrin26, 27, 29. However, the potential role of Wnt signaling in pathologic neovascularization during other post-natally occurring retinal vascular diseases, such as retinopathy of prematurity and diabetic proliferative retinopathy, is not defined and is the focus of this study.
Using a mouse model of oxygen-induced proliferative retinopathy (OIR)32, we investigated the contribution of the Wnt signaling pathway in pathologic vascular growth in retinopathy. We find that components of Wnt signaling are significantly upregulated in pathologic neovessels with induced retinopathy. Loss of Lrp5 and Wnt signaling protein dishevelled2 results in significantly decreased levels of pathologic neovasularization in retinopathy. In addition loss of Lrp5 results in abnormal vascular growing tips and breakdown of the blood retina barrier, which is linked to down-regulation of the tight junction protein claudin5 (Cln5) in retinal vessels. Importantly, inhibition of Cln5 suppresses endothelial sprouting in vitro and retinal vascular growth in vivo during both development and in retinopathy. These results indicate a crucial role of Wnt signaling in promoting pathologic retinal neovascularization in retinopathy beyond its known role in developmental retinal angiogenesis, suggesting that specific targeting of this pathway may lead to treatment options for proliferative retinopathy.
Results
Delayed and incomplete retinal vasculature of Lrp5−/− mice
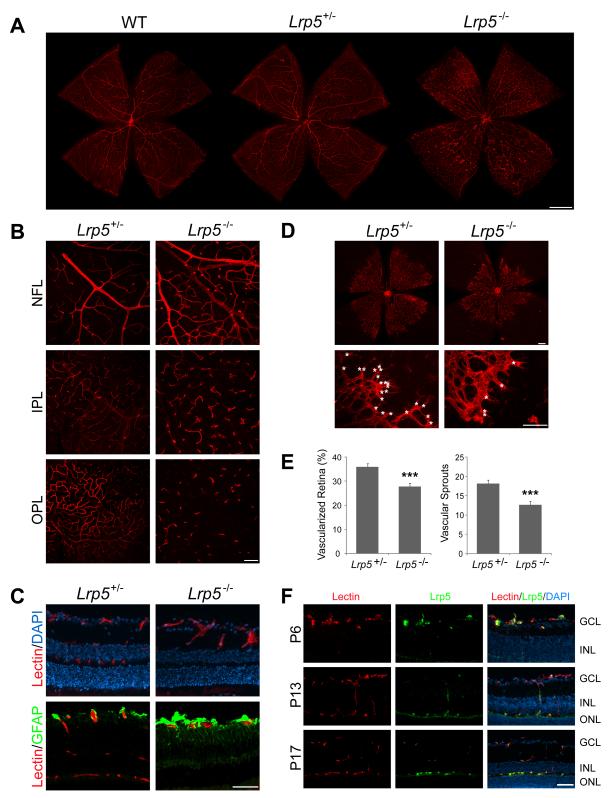
To study the role of Wnt co-receptor Lrp5 in retinal angiogenesis, we first characterized vascular development of the retina in the Lrp5−/− mouse. Murine retinal vascular development starts at birth. The superficial vascular plexus grows radially from the optic disc reaching the periphery by postnatal day (P) 8. The development of the deeper secondary and tertiary network of capillaries follows and is complete approximately 3 weeks after birth33. This stereotyped development is perturbed in adult Lrp5−/− retinas, where vessels are large, dilated and tortuous, with abnormal aggregations of endothelial cells in the mid-periphery of the primary vascular plexus (Fig.1A). Although Lrp5−/− retinas still display vertical vessels extending from the primary vessel plexus toward the deeper vascular layers, these vertical vessel sprouts terminate without forming interconnections, thus failing to create the two deeper layers of capillary networks in the inner and outer plexiform layers (Fig.1B, C). Additionally, glial fibrillary acidic protein (GFAP) levels are increased in astrocytes and Müller cells of Lrp5−/− eyes, indicating increased retinal stress associated with defective retinal vasculature (Fig.1C). The retinal vasculature of heterozygous Lrp5+/− mice appears normal (Fig.1A, B, C).
Figure 1.
Abnormal and delayed vascular development in Lrp5−/− retina and localization of retinal Lrp5. Retinal vessels were visualized with Isolectin B4 staining (red). A. Retinal flat mounts of age-matched adult wild type, Lrp5+/−, and Lrp5−/− mice (1 month old). B. Flat mounts illustrating the three layers of retinal vascular network in adult littermate Lrp5+/− and Lrp5−/− retina. NFL: nerve fiber layer, IPL: inner plexiform layer, OPL: outer plexiform layer. C. Cross section of adult littermate Lrp5+/− and Lrp5−/− retina stained with isolectin (red, vessels), GFAP (green, astrocytes and Muller cells) and DAPI (blue, nucleus). D. Retinal flat mounts of littermate Lrp5+/− and Lrp5−/− mice at postnatal day (P) 5. Growth fronts of vessels are enlarged in lower panels with vascular sprouts highlighted (*). E. Quantification of the vascularized retinal area and number of vascular sprouts in Lrp5+/− and Lrp5−/− retinas at P5. n=6-9 per group. ***p≤0.001. F: Immunohistochemical localization of Lrp5 protein in retinal cross-sections from P6, P13 and P17 wild type mice. Retinal sections are stained with Lrp5 antibody (green), Isolectin B4 (red, vessels) and DAPI (blue, nucleus). Scale bars: A: 1000μm. B-F: 100μm.
In addition to analyzing the mature retinal phenotype in adult retinas, we assessed retinal vascular development in Lrp5−/− retinas compared to the phenotypically normal Lrp5+/− mice (Fig.1D). At P5, the area of vascularized retina is ~25% less in Lrp5−/− mice compared with heterozygous littermate controls (27±1.9% vs. 36±1.3% of total retinal area; p≤0.001; Fig.1D, E). At the leading edge of the developing vasculature in Lrp5−/− retinas, the vessels appear thickened and the number of vascular sprouting tips is significantly reduced (by ~30%) compared to littermate controls (12.6±1.1 vs. 18.1±1.4 sprouts per view field; p≤0.001; Fig.1D, E). At P8, when the superficial layer of control retinas is almost 100% vascularized, Lrp5−/− retinas are only 70% vascularized. These results suggest that loss of the Wnt co-receptor Lrp5 directly contributes to delayed retinal vessel development.
Localization and expression of Lrp5 in neovessls in developing retina
We next localized Lrp5 protein in the retina to the developing primary vessel plexus (P6) (Fig.1F). At P13 and P17, when deeper layers of retinal vessels are forming and the superficial vascular layer is complete,, Lrp5 staining shifts predominantly to these newly developing vascular layers (Fig. 1F), indicating that Lrp5 is expressed preferentially in newly formed vessels compared to mature blood vessels. This notion is supported by the robust expression of Lrp5 mRNA in whole retina during the first week after birth when rapid vascular growth occurs in the primary vascular plexus. When the retina matures and vascular growth slows, Lrp5 expression gradually declines (Supp. Fig.1A). As expected, Lrp5−/− eyes do not stain for Lrp5 (Supp. Fig.1B).
Localization and expression patterns of Wnt receptors and activity in pathologic retinal neovessels
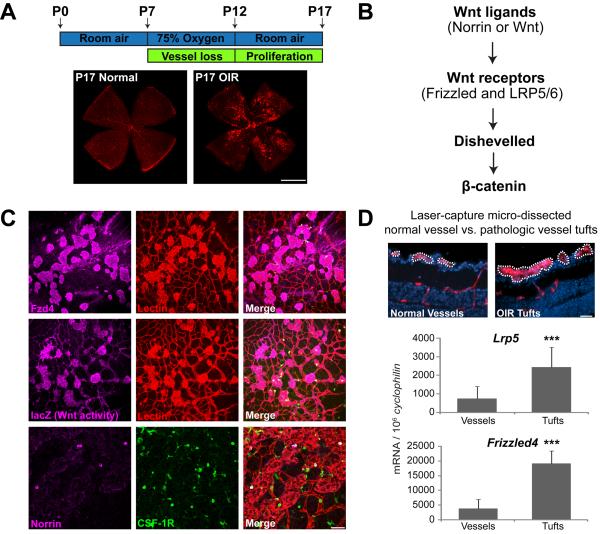
Having established the importance of Wnt signaling and Lrp5 in normal retinal vessel development, we next asked whether the Wnt pathway contributes to the formation of pathologic retinal neovascularization (NV) in oxygen-induced retinopathy (OIR)32. In OIR mouse pups are exposed to 75% oxygen from P7 to P12 followed by room air with a maximum neovascular response at P17 (Fig.2A). We assessed the effects on the pathological neovascular response of modulating the Wnt pathway (ligands and receptors, as well as the downstream signaling protein dishevelled and β-catenin stabilization) (Fig. 2B).
Figure 2.
Localization and expression patterns of Wnt receptors and activity in oxygen-induced retinopathy (OIR). A. Schematic illustration of oxygen-induced retinopathy. Neonatal mice with nursing mothers are exposed to oxygen from postnatal day (P) 7 to P12. Retinas are isolated at P17 and stained with Isolectin B4 to visualize vessels. B. Illustration of Wnt signaling components. Wnt signaling starts with binding of Wnt ligands to Wnt receptors, is mediated by dishevelled protein and results in β-catenin stabilization and target gene transcription. C. Localization of Wnt receptor Frizzled4, Wnt activity and Wnt ligand Norrin in the OIR retina. Retinopathy is induced in wild type mice or TOP-Gal Wnt reporter mice. P17 wild type retinas with OIR are stained with Frizzled4 (magenta) or norrin (magenta), Isolectin B4 (red, vessel) and CSF1-R (green, microglia). P17 retinas of TOP-Gal Wnt reporter mice with OIR are stained with β-Galactosidase (magenta, lacZ expression for Wnt activity) and Isolectin B4 (red, vessel). Both Frizzled4 and Wnt activity (lacZ) show co-locolization with pathologic neovessels (red). Norrin (magenta) colocolizes with a subset of macrophages (green) associated with retinal vessels (red in merged image). D. mRNA expression of Wnt receptor Frizzled4 and Lrp5 in laser-capture microdissected pathologic neovessels (tufts) from OIR wild type mice compared to normal vessels from age-matched control mice raised in room air. Scale bars: A: 1000μm. C: 100μm. D: 50μm.
During OIR, Wnt receptor Frizzled 4 (Fzd4) is found specifically in pathologic neovascular tufts (Fig. 2C). Wnt activity was localized using the Wnt reporter TOPGAL mice, which express lacZ gene under the control of Tcf promoters and, hence, synthesize lacZ only in cells with active canonical Wnt/β-catenin signaling34, 35. Similar to Frizzled 4, activated Wnt signaling, as evidenced by anti-lacZ staining, is observed specifically in neovascular tufts in OIR retinas (Fig.2C). Moreover, we also localized the Wnt ligand Norrin to a subset of macrophages associated with retinal vessels as seen by co-localization with CSF1-R (Fig.2C). Because in situ hybridization is not quantitative, we used laser capture microdissection to isolate pathologic NV tufts from OIR mice and normal vessels from control mice to measure specific mRNA expression of Wnt receptors in retinal vasculature. Wnt receptor Fzd4 and Lrp5 mRNA levels are ~ 3 to 5 fold higher in neovessels compared to normal vessels (Fig.2D).
Mice lacking Wnt signaling show decreased levels of pathologic neovascularization in retinopathy
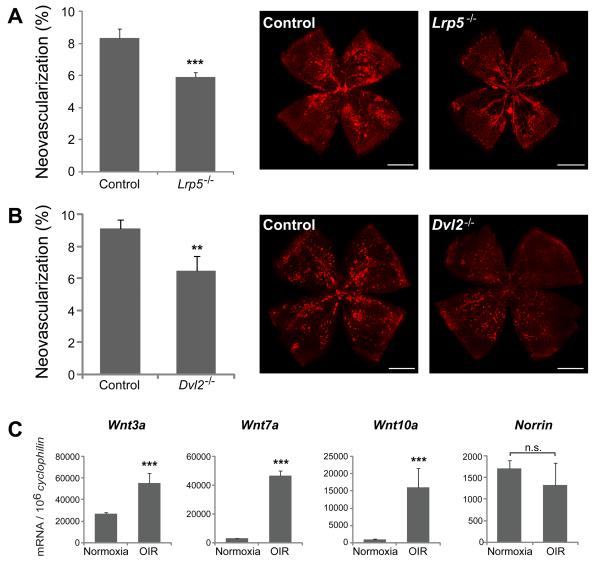
To assess whether Wnt signaling contributes to the formation of pathologic NV, retinopathy (OIR) was induced in Lrp5−/− mice. With loss of Lrp5 there was significantly less pathologic NV compared to wild type controls (5.9±0.3% vs. 8.3±0.6% of total retina area; p≤0.001, Fig.3A). Since Lrp5−/− mice have delayed vascular growth during development which may affect the neovascular response of Lrp5−/− mice in OIR, we examined OIR in another transgenic mouse line Dvl2−/− lacking the Wnt signaling component Dishevelled2 (Dvl2). Dvl2 is a cytoplasmatic phospho-protein that acts directly downstream of Wnt receptors and is required for transmitting Wnt receptor activation signals36 (Fig.2B). We found normal retinal vascular development in Dvl2−/− mice (Supp. Fig.2A, B), potentially reflecting redundant roles of Dvl1, Dvl2 and Dvl3 in development37. However, with OIR, Dvl2−/− retinas develop significantly less pathological NV than littermate controls (6.5±1% vs. 9.1±0.5%; p≤0.01; Fig.3B). These results suggest that Wnt signaling through Lrp5 and Dvl2 is important for formation of pathologic neovascularization in the OIR model independent of abnormalities in normal retinal development, and that blocking the Wnt pathway by genetic depletion of either Lrp5 or Dvl2 suppresses pathologic NV formation.
Figure 3.
Loss of Wnt signaling significantly decreases formation of pathologic neovascularization in OIR. A. Quantification of pathologic neovascularization in P17 retinas from Lrp5−/− mice exposed to OIR compared to wild type controls. Areas of pathologic neovascularization are quantified as percentage of total retinal area. n=16-29 per group; ***p≤0.001. B: Quantification of pathologic neovascularization in P17 retinas from Dishevelled2−/− (Dvl2) mice exposed to OIR compared with heterozygous littermates controls. n=8-24 per group; **p≤0.01. C. mRNA expression of Wnt ligands Wnt3a, Wnt7a, Wnt10a and Norrin in P17 wild type retinas exposed to OIR compared with age matched mice raised in room air. Scale bars: A and B: 1000μm.
Increased expression of Wnt ligands in retinopathy
Having established that loss of Wnt signaling affects pathologic NV in retinopathy, we next asked which Wnt ligands are regulated during pathological neovascularization. Wnt3a, Wnt7a and Wnt10a mRNA expression is significantly up-regulated in OIR retinas at P17 compared with room air controls, with Wnt7a and Wnt10a mRNA increasing up to 7-10 fold (Fig.3C). Expression of the Wnt ligand Norrin is not significantly altered during OIR (Fig.3C). These data suggest that Wnt3a, Wnt7a and Wnt10a are likely Wnt ligands contributing to pathologic neovessel formation in the OIR retina.
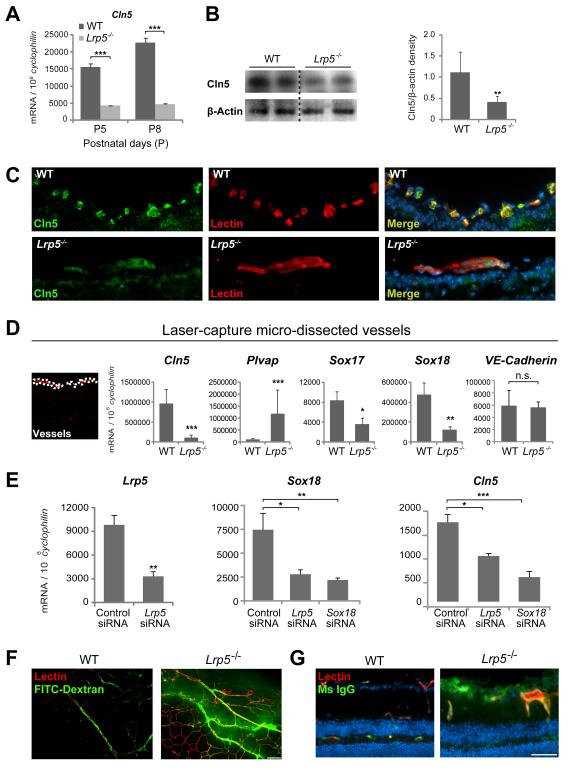
Down-regulation of Cln5 and disruption of blood retina barrier in Lrp5−/− retina
To unravel the molecular mechanisms by which Wnt signaling regulates retinal angiogenesis, we analyzed mRNA expression in wild type and Lrp5−/− retinas at P8 with a microarray. With loss of Lrp5 we identified a significant ~8 fold reduction in expression of the tight junction protein Cln5, confirmed by quantitative RT-PCR (Fig.4A). Protein levels of Cln5 are also markedly reduced in Lrp5−/− retinas (Fig.4B). Cln5 protein is predominantly localized by immunohistochemistry to retinal vessels in both WT and Lrp5−/− retina (with lower staining intensity in Lrp5−/− vessels) (Fig.4C). Since immunohistochemistry is not quantitative we used laser-captured microdissection to isolate retinal vessels from Lrp5−/− and WT mice followed by RT-PCR to analyze gene expression specifically in vessels. We confirmed that Cln5 mRNA is significantly decreased in retinal vessels of Lrp5−/− mice; while Plvap, a marker of vascular permeability, is increased compared to vessels isolated from wild type retinas (Fig.4D). Expression of the endothelial cell marker VE-Cadherin is the same (Fig.4D). Transcription factors Sox17 and Sox18 are also significantly down-regulated in Lrp5−/− vessels compared with wild type vessels by ~2 and 4 fold respectively (Fig.4D). The observation that Sox17 mRNA expression is decreased in Lrp5−/− vessels is in agreement with a previous study reporting decreased Sox17 transcripts in Wnt receptor Frizzled4−/− endothelial cells38. The finding that Sox18 is regulated by Wnt signaling, however, was not reported previously. Since Sox18 is known to control expression of Cln539, we assessed expression of Sox18 and Cln5 in human retinal microvascular endothelial cells (HRMEC) treated with small interference RNA (siRNA) targeting Lrp5 and Sox18. Compared with control siRNA, Lrp5 siRNA significantly inhibited Lrp5 expression by >3 fold (Fig,4E). Lrp5 siRNA treatment significantly inhibited mRNA expression of both Sox18 and Cln5. Sox18 siRNA treatment successfully inhibited Sox18 expression, and importantly inhibited Cln5 mRNA expression by >3 fold (Fig,4E). Together these in vitro data shows that the transcription factor Sox18 mediates down-regulation of Cln5 in Lrp5 deficient endothelial cells.
Figure 4.
Down-regulation of tight junction protein claudin5 (Cln5) and disruption of blood retina barrier in Lrp5−/− retina. A. mRNA expression of Cln5 in whole retina isolated from P5 and P8 Lrp5−/− and wild type mice. B. Protein levels of Cln5 in retinal extracts of P8 wild type and Lrp5−/− retinas with Western blot. C. Localization of Cln5 in retinal vessels in cross-section of P8 wild type and Lrp5−/− eyes stained with Cln5 antibody (green), Isolectin B4 (red, vessels) and DAPI (blue, nucleus). D. Quantification of Cln5, Plvap, Sox17, Sox18 and VE-Cadherin mRNA from retinal blood vessels isolated with laser-capture micro-dissection from P8 Lrp5−/− and wild type retinas. n=3. E. Quantification of Lrp5, Sox18 and Cln5 mRNA from human retinal microvascular endothelial cells (HRMEC) treated with siRNA targeting Lrp5 and Sox18 for 48 hours. Data were analyzed with one-way ANOVA with Dunnett’s MCP against control siRNA group. F. Integrity of blood retinal barrier in Lrp5−/− and wild type retinas examined with FITC-dextran perfusion (green) or G. by staining for mouse IgG (green) which is normally confined within blood vessels. Scale bars: C and E: 50μm. F: 100μm. *p≤0.05, **p≤0.01, ***p≤0.001.
Because Cln5 is a known tight junction protein essential for maintaining vessel integrity40, 41 and Plvap is an indicator of vessel permeability42, 43, we assessed the blood-retinal barrier (BRB) function in Lrp5−/− mice and found increased BRB permeability when subjected to fluorescent angiography with FITC-dextran (Fig.4F). In a complementary assay, mouse plasma IgG, which normally is confined to vessels in wild type mice with intact BRB, extravasates into the neuronal tissue of the retina in Lrp5−/− mice, also indicating BRB breakdown (Fig.4G). Together these results suggest that the vasculature of Lrp5−/− retinas is more permeable than WT.
Blocking Cln5 suppresses Wnt-stimulated endothelial spheroid sprouting in vitro and developmental retinal angiogenesis in vivo
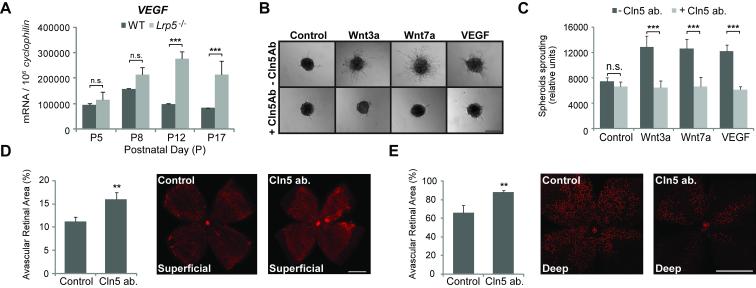
To further investigate the molecular mechanisms that cause impaired angiogenesis in Lrp5−/− mice, we examined vascular endothelial growth factor (VEGF) in Lrp5−/− retinas. Lrp5−/− retinas display similar levels of VEGF mRNA expression at P5 and P8 during vascular development of the superficial vascular layer. After the initial vascular growth there is a breakdown of vascular development after P8 likely leading to inadequate perfusion of the developing retina. Without normal vascular development there is a significantly increased level of VEGF at P12 and P17 compared with WT controls (Fig.5A). This observation suggests that the delay and lack of vascular growth in Lrp5−/− mice is not caused by VEGF deficiency. Other pathways that are critical for angiogenesis such as the angiopoietin-Tie2 pathway44-46 and Notch pathway47, 48 are also not significantly affected in Lrp5−/− retinas (Supp. Fig.3).
Figure 5.
Blocking claudin5 (Cln5) with antibodies suppresses endothelial cell spheroid sprouting in vitro and retinal angiogenesis in vivo. A. Quantification of VEGF mRNA expression in Lrp5−/− retinas at P5, 8, 12 and 17. n=6 per group. ***p≤0.001 between WT and Lrp5−/− retinas. B. Human retinal microvascular endothelial cells (HRMEC) were cultured as multicellular spheroids in the presence of Wnt 3a, Wnt7a and VEGF in combination with Cln5 antibody. Images show spheroidal sprouting after culturing for 24h in collagen matrix. C. Quantification of HRMEC spheroid sprouting with treatment of Wnt3a, Wnt7a and VEGF, in combination with Cln5 antibody. n=10-20 per group. n.s.: not significant, ***p≤0.001 between groups with and without Cln5 antibody. D. Mice injected intravitreally with Cln5 antibody in one eye and rabbit IgG as control in the fellow eye at P2. Retinas are isolated at P7 and percent of avascular retinal area (retinal area without lectin-stained vessels in red) quantified. Cln5 ab. injected eyes show significantly less vascular growth in the superficial retinal layer. n=10. **p≤0.01. E. Mice injected intravitreally with Cln5 antibody in one eye and rabbit IgG as control in the fellow eye at P4. Retinas are isolated at P9 and growth of the deep vascular layer (red) is quantified as percent of avascular retinal area. Cln5 ab. significantly suppresses vascular growth in the deep retinal layer. n=6. **p≤0.01. Scale bars: B: 100μm. D and E: 1000μm.
Given that Cln5 is significantly down-regulated in Lrp5−/− retinas, we asked whether deficiency in Cln5 might contribute directly to the lack of vessel growth-by affecting endothelial cell adhesion and migration for further vascular development. Using an in vitro spheroid sprouting assay, we found that both Wnt ligands Wnt3a and Wnt7a significantly stimulated HRMEC sprouting, as potently as VEGF (Fig.5B,C). Inhibition of Cln5 with an anti-Cln5 antibody significantly suppressed HRMEC sprouting to basal control levels in Wnt3a and Wnt7a stimulated groups, as well as in VEGF stimulated group (Fig.5B,C), suggesting that Cln5 is essential for proper endothelial cell (EC) sprout formation. These results were confirmed using a second anti-Cln5 antibody (Supp. Fig.4). Toxic effects of the antibody solution were ruled out using sodium azide-containing vehicle control groups (Supp. Fig.4). Moreover, inhibition of Cln5 with intraocular injection of Cln5 antibody at P2 during normal retinal development results in a significant delay of retinal vascular growth in the superficial retinal layer at P7 compared with fellow eyes injected with pre-immune rabbit IgG (superficial retinal area without vessels: 16.1±1.4% vs. 11.1±1.0% of total retina area; p≤0.02 Fig.5D). In addition, to assess the effect of Cln5 on deep vessel layer formation, we injected Cln5 antibody intravitreally at P4 and found that inhibition of Cln5 at P9 also significantly suppresses retinal vascular growth in the deep retinal layer compared with control IgG (deep retinal area without vessels: 88.1±1.8% vs. 66.0±7.9% of total retina area; p≤0.01 Fig.5E).
Inhibition of Cln5 with siRNA suppresses pathologic retinal neovascularization in retinopathy
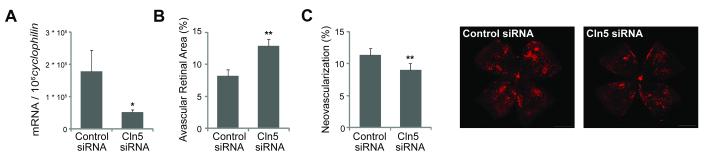
To corroborate these in vivo results obtained with Cln5 antibodies, we assessed the effect of Cln5 on vascular growth in vivo with siRNA targeting Cln5. The specificity and effectiveness of the Cln5 siRNA were previously validated49. We confirmed that intravitreal injection of Cln5 siRNA significantly suppresses retinal Cln5 mRNA expression by more than 3 fold (Fig.6A), and in development significantly inhibits retinal vascular growth compared with contralateral eyes injected with control siRNA (avascular area:12.8±1.1% vs. 8.2±1.0% of total retinal area, p≤0.01, Fig.6B). To assess whether suppression of Cln5 also suppresses pathologic retinal neovessels, we injected intravitreally Cln5 siRNA in OIR mice at P14. At P17, Cln5 siRNA injected eyes had significantly less pathologic neovascularization (9.2±0.7% of total retinal area) compared with contralateral eye injected with control siRNA (11.4±0.7% of total retinal area, p≤0.01, Fig.6C). Together these data suggest that inhibition of Cln5 significantly suppresses angiogenesis both in vitro and in vivo, indicating a novel function of Cln5 in promoting angiogenesis not only during development but also during pathologic neovessel formation.
Figure 6.
Claudin5 (Cln5) siRNA suppresses developmental retinal angiogenesis and formation of pathologic neovascularization in OIR. A. Cln5 siRNA significantly suppresses retinal expression of Cln5. Mice were injected intravitreally with Cln5 siRNA in one eye and control negative siRNA in the other eye at P4. Retinas were isolated at P6 and Cln5 mRNA expression quantified with RT-qPCR. n=3. *p≤0.05. B. Quantification of P7 retinal vascular growth during development with Cln5 siRNA injected intravitreally at P4 and control siRNA injected in contralateral eye. n=10. **p≤0.01. C. Quantification of P17 OIR retina with Cln5 siRNA and control siRNA injected intravitreally at P14 after mice were exposed to 75% oxygen from P7 to P12 to induce retinopathy. n=14. **p≤0.01.
Discussion
In this study we present novel evidence showing that Wnt pathway signaling is important for formation of pathologic neovascularization in retinopathy. It is important to distinguish biochemical pathways differentially involved in pathological neovascularization to consider specific control of pathologic angiogenesis (characterized by abnormal proliferation and increased vascular leakage) versus normal mature vessels. Mutations in Wnt ligand Norrin and receptors Frizzled4 and Lrp5 have been linked previously to several rare human eye diseases such as Norrie disease26 and FEVR5, 8, which both have features of abnormal retinal vessel development and vascular leakage. Recent studies have identified a new binding protein of Wnt receptors, tetraspannin (TSPAN12), which enhances Wnt signaling50, and a transcription factor Sox17 which is upregulated by Wnt signaling38. However, the role of Wnt signaling in pathological proliferative neovascularization remained undefined.
Here we find that Wnt receptors and activity are selectively upregulated in pathologic neovessels in the retina in a mouse model of oxygen-induced proliferative retinopathy (OIR). Reduction of Wnt signaling in Lrp5−/− mice, or dishevelled2−/− mice, significantly suppresses pathologic neovessel formation in OIR. These results are in line with a previous study showing ectopic expression of Wnt ligand Norrin promotes vascular regrowth in OIR51. Together these findings suggest that modulation of Wnt signaling has significant implications not only for congenital hereditary eye diseases with mutations in Wnt pathway, but also for the much more prevalent postnatally-occurring retinal vascular diseases like diabetic retinopathy, ROP and macular degeneration all of which are characterized by pathologic vascular growth. Interestingly in this context, ROP in some severe cases has been linked to mutations of the Wnt ligand Norrin52, and age-related macular degeneration has been associated with gene polymorphisms in the Wnt co-receptor Lrp653.
In addition to defining the role of Wnt signaling in proliferative retinopathy, in this paper we also expanded the characterization of Lrp5 in developmental retinal angiogenesis. Decreased numbers of retinal vascular sprouts and an incomplete deep layer of blood vessels in Lrp5−/− retinas is similar to retinal vascular development abnormalities seen in Norrin−/− and Frizzled4−/− mice26, 27, 30, 38. Frizzled4 expression is same in Lrp5−/− retina compare to WT, however a few other Frizzled receptors (Fzd2, Fzd3, Fzd7) are down-regulated reflecting potential compensatory regulation (Supp. Fig.5). Lrp5 protein is expressed preferentially in newly formed versus mature retinal vessels during development and in pathologic neovessels during OIR. Although expression in other retinal cell types such as neurons and Muller glia cells cannot be excluded31, it is likely that vascular Wnt receptor Fzd4 and Lrp5 are direct regulators of vascular growth in retinopathy, consistent with a previous report showing that conditional loss of Frizzled4 in vascular endothelial cells results in similar retinal vascular defects as systemic Frizzled4 null mice38.
Our study identifies the tight junction protein Cln5 as a direct mediator of retinal angiogenesis regulated by Lrp5 through the transcription factor Sox18. Cln5 is a key component of endothelial cell tight junctions essential in maintaining cell-cell adhesion. Blocking Cln5 significantly suppresses retinal blood vessel during development in both superficial and deep retinal layers, as well as suppresses pathologic neovessel formation in proliferative retinopathy in mice. This effect is corroborated in vitro as blocking Cln5 significantly suppresses Wnt dependent endothelial cell spheroid sprouting. It has been shown previously that blocking a different adhesive protein, R-cadherin, inhibits retinal angiogenesis54. Inhibition of the endothelial cell adhesion protein VE-cadherin also suppresses neovessel sprouting and angiogenesis55-58. Similarly, Cln5 may be necessary for cell-cell adhesion, endothelial cell migration and tube formation and thus would play an essential role for the proper growth of vascular sprouts and vessel growth. It is likely that Cln5 is finely regulated in the retina for optimal tight junction function. Down regulation of Cln5 may contribute, at least in part, to the delayed formation of the primary plexus, and to the complete lack of deeper capillaries in Lrp5−/− retinas. It is important to note that Cln5 is also suppressed in Norrin−/− retinas (seen in a gene expression microarray study59), suggesting that Cln5 may mediate common effects of Wnt signaling in vascular growth.
This previously undiscovered function of Cln5 in retinal angiogenesis is in addition to its known role in blood brain (or blood retinal) barrier formation. Both FEVR and ROP are characterized by not only inadequate retinal vascularization but also peripheral retinal vascular leakage2, 3, which might be attributable in part to lack of Cln5. In line with our observations that loss of Wnt signaling in Lrp5−/− retinas results in decreased blood-retinal barrier integrity, loss of Wnt ligands Wnt7a and Wnt7b are associated with disruption of tight junctions and loss of blood-brain barrier properties in the central nervous system 23-25. In the brain, deficiency of Cln5 has been linked to blood brain barrier breakdown41 and Cln5 null mice die soon after birth due to defective blood brain barrier formation40 Similarly, in line with our findings that Cln5 is a downstream target of Lrp5 via Sox18, Cln5 has been found to be down-regulated in vascular endothelial cells in the absence of β-catenin24, 47.. Together, these results support the concept that canonical Wnt signaling through Lrp5, β-catenin and Sox18 regulates Cln5 expression in endothelial cells, which in turn mediates not only endothelial barrier function in the retina, but also retinal blood vessel growth in development and in pathology.
In summary, this paper provides direct evidence for an important role of Wnt signaling mediating pathologic neovascularization. Given the selectivity of Wnt signaling for proliferating vessels, the therapeutic implications for modulating components of Wnt/β-catenin pathways in pathologic vessel proliferation are broad. Our identification of the Wnt-dependent angiogenic effects of Cln5 further adds to the potential therapeutic spectrum of Wnt-modulation in angioproliferative diseases. In this respect, modulation of canonical Wnt pathways could be potentially advantageous for treating not only retinopathy, but also other diseases such as tumors where pathological angiogenesis and loss of vascular integrity play a significant role.
Experimental Procedures
Retinal vascular phenotype analysis and oxygen-induced retinopathy
Retinal dissection vascular staining and image analysis were performed using standard published protocols 60, 61. To induce pathological neovascularization in retina, mice with their nursing mother were exposed to 75% oxygen from P7 to P12 followed by room air32. At P17 when the neovascular response is greatest, pathologic retinal neovascularization was evaluated,. To examine the level of gene transcripts, cDNA was prepared from isolated retina at various age followed by RT-qPCR analysis.
Endothelial cell spheroid sprouting assay
Human retinal microvascular endothelial cells (HRMEC) were cultured and prepared for spheroids as described previously62, 63. Sprouting were photographed after treatment of Wnt 3a, Wnt7a, VEGF and Cln5 antibodies for 24 hrs and images were assessed with results expressed as mean ± SEM. n is number of spheroids quantified.
Intravitreal injection
Intravitreal injections with Cln5 antibody or Cln5 siRNA were performed at specific ages following published protocols64. Retinas were isolated for RNA analysis or flatmount for analysis of vascular phenotype during development and in oxygen-induced retinopathy.
Laser capture microdissection of retinal vessels
Retinal vessels were micro-dissected with laser-capture in retinal cross-section from P8 Lrp5−/− and WT mice as described previously64, 65. Enrichment of endothelial cells was confirmed. RNA was extracted and cDNA synthesis for RT-qPCR analysis.
Statistics
Results are presented as mean±SEM for animal studies and mean±SD for non-animal studies. For all statistical analysis, F-Test (for two samples, or Levene’s test for more than two samples) was performed first to assess whether the variance are homogenous. If the variances were homogenous, two sample T-test (or ANOVA if more than two groups of samples) assuming equal variance was performed. If not, two sample T-test (or ANOVA) assuming unequal variance was performed.
Supplementary Material
Acknowledgments
Funding Sources: This work was supported by funding from Knights Templar Eye Foundation, Children’s Hospital Boston Manton Center for Orphan Disease Research Innovation Fund, Juvenile Diabetes Research Foundation International, Charles H. Hood Foundation Child Heath Research Award (to JC), NIH (EY017017, EY017017-04S1), V. Kann Rasmussen Foundation, Children’s Hospital Boston Mental Retardation and Developmental Disabilities Research Center, Research to Prevent Blindness Senior Investigator Award, Alcon Research Institute Award, MacTel Foundation and Roche Foundation for Anemia Research (LEHS). AS is supported by the Deutsche Forschungsgemeinschaft, Freifran von Manendorf Stiftung, Forschungskommission Freiburg. PS is supported by the Canadian Institutes of Health Research and the Charles A. King Trust Award. AM is supported by American Heart Association (AHA) grant 8356180.
Footnotes
Supplemental Data: Supplemental data include five figures and additional experimental procedures.
Disclosures None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lobo CL, Bernardes RC, Figueira JP, de Abreu JR, Cunha-Vaz JG. Three-year follow-up study of blood-retinal barrier and retinal thickness alterations in patients with type 2 diabetes mellitus and mild nonproliferative diabetic retinopathy. Arch Ophthalmol. 2004;122:211–217. doi: 10.1001/archopht.122.2.211. [DOI] [PubMed] [Google Scholar]
- 2.Schulenburg WE, Tsanaktsidis G. Variations in the morphology of retinopathy of prematurity in extremely low birthweight infants. Br J Ophthalmol. 2004;88:1500–1503. doi: 10.1136/bjo.2004.044669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toomes CDL. Familial exudative vitreoretinopathy, autosomal dominant. In: Pagon RA, Bird TC, Dolan CR, Stephens K, editors. GeneReviews [Internet] University of Washington, Seattle; Seattle (WA): 2005. p. 1993. [Google Scholar]
- 4.Berger WR, HH . Norrie disease. In: scriver cr, beaudet al, sly ws, valle d., editors. In the metabolic and molecular bases of inherited disease. 2001. pp. 5977–5985. [Google Scholar]
- 5.Robitaille J, MacDonald ML, Kaykas A, Sheldahl LC, Zeisler J, Dube MP, Zhang LH, Singaraja RR, Guernsey DL, Zheng B, Siebert LF, Hoskin-Mott A, Trese MT, Pimstone SN, Shastry BS, Moon RT, Hayden MR, Goldberg YP, Samuels ME. Mutant frizzled-4 disrupts retinal angiogenesis in familial exudative vitreoretinopathy. Nat Genet. 2002;32:326–330. doi: 10.1038/ng957. [DOI] [PubMed] [Google Scholar]
- 6.Toomes C, Bottomley HM, Jackson RM, Towns KV, Scott S, Mackey DA, Craig JE, Jiang L, Yang Z, Trembath R, Woodruff G, Gregory-Evans CY, Gregory-Evans K, Parker MJ, Black GC, Downey LM, Zhang K, Inglehearn CF. Mutations in lrp5 or fzd4 underlie the common familial exudative vitreoretinopathy locus on chromosome 11q. Am J Hum Genet. 2004;74:721–730. doi: 10.1086/383202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen ZY, Battinelli EM, Fielder A, Bundey S, Sims K, Breakefield XO, Craig IW. A mutation in the norrie disease gene (ndp) associated with x-linked familial exudative vitreoretinopathy. Nat Genet. 1993;5:180–183. doi: 10.1038/ng1093-180. [DOI] [PubMed] [Google Scholar]
- 8.Jiao X, Ventruto V, Trese MT, Shastry BS, Hejtmancik JF. Autosomal recessive familial exudative vitreoretinopathy is associated with mutations in lrp5. Am J Hum Genet. 2004;75:878–884. doi: 10.1086/425080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepe A, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Juppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, van den Boogaard MJ, Van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML. Ldl receptor-related protein 5 (lrp5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 10.Moon RT. Wnt/beta-catenin pathway. Sci STKE. 2005;2005:cm1. doi: 10.1126/stke.2712005cm1. [DOI] [PubMed] [Google Scholar]
- 11.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. Wnt and beta-catenin signalling: Diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 12.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 13.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Logan CN, R The wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 15.Gessert S, Kuhl M. The multiple phases and faces of wnt signaling during cardiac differentiation and development. Circ Res. 2010;107:186–199. doi: 10.1161/CIRCRESAHA.110.221531. [DOI] [PubMed] [Google Scholar]
- 16.Bergmann MW. Wnt signaling in adult cardiac hypertrophy and remodeling: Lessons learned from cardiac development. Circ Res. 2010;107:1198–1208. doi: 10.1161/CIRCRESAHA.110.223768. [DOI] [PubMed] [Google Scholar]
- 17.Rao TP, Kuhl M. An updated overview on wnt signaling pathways: A prelude for more. Circ Res. 2010;106:1798–1806. doi: 10.1161/CIRCRESAHA.110.219840. [DOI] [PubMed] [Google Scholar]
- 18.Naito AT, Shiojima I, Komuro I. Wnt signaling and aging-related heart disorders. Circ Res. 2010;107:1295–1303. doi: 10.1161/CIRCRESAHA.110.223776. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Huso D, Cahill H, Ryugo D, Nathans J. Progressive cerebellar, auditory, and esophageal dysfunction caused by targeted disruption of the frizzled-4 gene. J Neurosci. 2001;21:4761–4771. doi: 10.1523/JNEUROSCI.21-13-04761.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 21.Barker N, Clevers H. Mining the wnt pathway for cancer therapeutics. Nat Rev Drug Discov. 2006;5:997–1014. doi: 10.1038/nrd2154. [DOI] [PubMed] [Google Scholar]
- 22.Lobov IB, Rao S, Carroll TJ, Vallance JE, Ito M, Ondr JK, Kurup S, Glass DA, Patel MS, Shu W, Morrisey EE, McMahon AP, Karsenty G, Lang RA. Wnt7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature. 2005;437:417–421. doi: 10.1038/nature03928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for cns, but not non-cns, angiogenesis. Proc Natl Acad Sci U S A. 2009;106:641–646. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, Taketo MM, von Melchner H, Plate KH, Gerhardt H, Dejana E. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical wnt signaling regulates organ-specific assembly and differentiation of cns vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- 26.Xu Q, Wang Y, Dabdoub A, Smallwood PM, Williams J, Woods C, Kelley MW, Jiang L, Tasman W, Zhang K, Nathans J. Vascular development in the retina and inner ear: Control by norrin and frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116:883–895. doi: 10.1016/s0092-8674(04)00216-8. [DOI] [PubMed] [Google Scholar]
- 27.Rehm HL, Zhang DS, Brown MC, Burgess B, Halpin C, Berger W, Morton CC, Corey DP, Chen ZY. Vascular defects and sensorineural deafness in a mouse model of norrie disease. J Neurosci. 2002;22:4286–4292. doi: 10.1523/JNEUROSCI.22-11-04286.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kato M, Patel MS, Levasseur R, Lobov I, Chang BH, Glass DA, 2nd, Hartmann C, Li L, Hwang TH, Brayton CF, Lang RA, Karsenty G, Chan L. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in lrp5, a wnt coreceptor. J Cell Biol. 2002;157:303–314. doi: 10.1083/jcb.200201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richter M, Gottanka J, May CA, Welge-Lussen U, Berger W, Lutjen-Drecoll E. Retinal vasculature changes in norrie disease mice. Invest Ophthalmol Vis Sci. 1998;39:2450–2457. [PubMed] [Google Scholar]
- 30.Xia CH, Liu H, Cheung D, Wang M, Cheng C, Du X, Chang B, Beutler B, Gong X. A model for familial exudative vitreoretinopathy caused by lpr5 mutations. Hum Mol Genet. 2008;17:1605–1612. doi: 10.1093/hmg/ddn047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia CH, Yablonka-Reuveni Z, Gong X. Lrp5 is required for vascular development in deeper layers of the retina. PLoS One. 2010;5:e11676. doi: 10.1371/journal.pone.0011676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith LE, Wesolowski E, McLellan A, Kostyk SK, D’Amato R, Sullivan R, D’Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 33.Stahl A, Connor KM, Sapieha P, Chen J, Dennison RJ, Krah NM, Seaward MR, Willett KL, Aderman CM, Guerin KI, Hua J, Lofqvist C, Hellstrom A, Smith LE. The mouse retina as an angiogenesis model. Invest Ophthalmol Vis Sci. 2010;51:2813–2826. doi: 10.1167/iovs.10-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hens JR, Wilson KM, Dann P, Chen X, Horowitz MC, Wysolmerski JJ. Topgal mice show that the canonical wnt signaling pathway is active during bone development and growth and is activated by mechanical loading in vitro. J Bone Miner Res. 2005;20:1103–1113. doi: 10.1359/JBMR.050210. [DOI] [PubMed] [Google Scholar]
- 35.DasGupta R, Fuchs E. Multiple roles for activated lef/tcf transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- 36.Hamblet NS, Lijam N, Ruiz-Lozano P, Wang J, Yang Y, Luo Z, Mei L, Chien KR, Sussman DJ, Wynshaw-Boris A. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development. 2002;129:5827–5838. doi: 10.1242/dev.00164. [DOI] [PubMed] [Google Scholar]
- 37.Etheridge SL, Ray S, Li S, Hamblet NS, Lijam N, Tsang M, Greer J, Kardos N, Wang J, Sussman DJ, Chen P, Wynshaw-Boris A. Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development. PLoS Genet. 2008;4:e1000259. doi: 10.1371/journal.pgen.1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ye X, Wang Y, Cahill H, Yu M, Badea TC, Smallwood PM, Peachey NS, Nathans J. Norrin, frizzled-4, and lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell. 2009;139:285–298. doi: 10.1016/j.cell.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fontijn RD, Volger OL, Fledderus JO, Reijerkerk A, de Vries HE, Horrevoets AJ. Sox-18 controls endothelial-specific claudin-5 gene expression and barrier function. Am J Physiol Heart Circ Physiol. 2008;294:H891–900. doi: 10.1152/ajpheart.01248.2007. [DOI] [PubMed] [Google Scholar]
- 40.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR. Vegf-mediated disruption of endothelial cln-5 promotes blood-brain barrier breakdown. Proc Natl Acad Sci U S A. 2009;106:1977–1982. doi: 10.1073/pnas.0808698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shue EH, Carson-Walter EB, Liu Y, Winans BN, Ali ZS, Chen J, Walter KA. Plasmalemmal vesicle associated protein-1 (pv-1) is a marker of blood-brain barrier disruption in rodent models. BMC Neurosci. 2008;9:29. doi: 10.1186/1471-2202-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keuschnigg J, Henttinen T, Auvinen K, Karikoski M, Salmi M, Jalkanen S. The prototype endothelial marker pal-e is a leukocyte trafficking molecule. Blood. 2009;114:478–484. doi: 10.1182/blood-2008-11-188763. [DOI] [PubMed] [Google Scholar]
- 44.Hackett SF, Ozaki H, Strauss RW, Wahlin K, Suri C, Maisonpierre P, Yancopoulos G, Campochiaro PA. Angiopoietin 2 expression in the retina: Upregulation during physiologic and pathologic neovascularization. J Cell Physiol. 2000;184:275–284. doi: 10.1002/1097-4652(200009)184:3<275::AID-JCP1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 45.Hackett SF, Wiegand S, Yancopoulos G, Campochiaro PA. Angiopoietin-2 plays an important role in retinal angiogenesis. J Cell Physiol. 2002;192:182–187. doi: 10.1002/jcp.10128. [DOI] [PubMed] [Google Scholar]
- 46.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 47.Phng LK, Potente M, Leslie JD, Babbage J, Nyqvist D, Lobov I, Ondr JK, Rao S, Lang RA, Thurston G, Gerhardt H. Nrarp coordinates endothelial notch and wnt signaling to control vessel density in angiogenesis. Dev Cell. 2009;16:70–82. doi: 10.1016/j.devcel.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalen M, Gerhardt H, Betsholtz C. Dll4 signalling through notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 49.Campbell M, Nguyen AT, Kiang AS, Tam LC, Gobbo OL, Kerskens C, Ni Dhubhghaill S, Humphries MM, Farrar GJ, Kenna PF, Humphries P. An experimental platform for systemic drug delivery to the retina. Proc Natl Acad Sci U S A. 2009;106:17817–17822. doi: 10.1073/pnas.0908561106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Junge HJ, Yang S, Burton JB, Paes K, Shu X, French DM, Costa M, Rice DS, Ye W. Tspan12 regulates retinal vascular development by promoting norrin-but not wnt-induced fzd4/beta-catenin signaling. Cell. 2009;139:299–311. doi: 10.1016/j.cell.2009.07.048. [DOI] [PubMed] [Google Scholar]
- 51.Ohlmann A, Seitz R, Braunger B, Seitz D, Bosl MR, Tamm ER. Norrin promotes vascular regrowth after oxygen-induced retinal vessel loss and suppresses retinopathy in mice. J Neurosci. 2010;30:183–193. doi: 10.1523/JNEUROSCI.3210-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dickinson JL, Sale MM, Passmore A, FitzGerald LM, Wheatley CM, Burdon KP, Craig JE, Tengtrisorn S, Carden SM, Maclean H, Mackey DA. Mutations in the ndp gene: Contribution to norrie disease, familial exudative vitreoretinopathy and retinopathy of prematurity. Clin Experiment Ophthalmol. 2006;34:682–688. doi: 10.1111/j.1442-9071.2006.01314.x. [DOI] [PubMed] [Google Scholar]
- 53.Haines JL, Schnetz-Boutaud N, Schmidt S, Scott WK, Agarwal A, Postel EA, Olson L, Kenealy SJ, Hauser M, Gilbert JR, Pericak-Vance MA. Functional candidate genes in age-related macular degeneration: Significant association with vegf, vldlr, and lrp6. Invest Ophthalmol Vis Sci. 2006;47:329–335. doi: 10.1167/iovs.05-0116. [DOI] [PubMed] [Google Scholar]
- 54.Dorrell MI, Aguilar E, Friedlander M. Retinal vascular development is mediated by endothelial filopodia, a preexisting astrocytic template and specific r-cadherin adhesion. Invest Ophthalmol Vis Sci. 2002;43:3500–3510. [PubMed] [Google Scholar]
- 55.Navaratna D, Maestas J, McGuire PG, Das A. Suppression of retinal neovascularization with an antagonist to vascular endothelial cadherin. Arch Ophthalmol. 2008;126:1082–1088. doi: 10.1001/archopht.126.8.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vestweber D. Ve-cadherin: The major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler Thromb Vasc Biol. 2008;28:223–232. doi: 10.1161/ATVBAHA.107.158014. [DOI] [PubMed] [Google Scholar]
- 57.Liao F, Doody JF, Overholser J, Finnerty B, Bassi R, Wu Y, Dejana E, Kussie P, Bohlen P, Hicklin DJ. Selective targeting of angiogenic tumor vasculature by vascular endothelial-cadherin antibody inhibits tumor growth without affecting vascular permeability. Cancer Res. 2002;62:2567–2575. [PubMed] [Google Scholar]
- 58.Liao F, Li Y, O’Connor W, Zanetta L, Bassi R, Santiago A, Overholser J, Hooper A, Mignatti P, Dejana E, Hicklin DJ, Bohlen P. Monoclonal antibody to vascular endothelial-cadherin is a potent inhibitor of angiogenesis, tumor growth, and metastasis. Cancer Res. 2000;60:6805–6810. [PubMed] [Google Scholar]
- 59.Schafer NF, Luhmann UF, Feil S, Berger W. Differential gene expression in ndph-knockout mice in retinal development. Invest Ophthalmol Vis Sci. 2009;50:906–916. doi: 10.1167/iovs.08-1731. [DOI] [PubMed] [Google Scholar]
- 60.Connor KM, Krah NM, Dennison RJ, Aderman CM, Chen J, Guerin KI, Sapieha P, Stahl A, Willett KL, Smith LE. Quantification of oxygen-induced retinopathy in the mouse: A model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protoc. 2009;4:1565–1573. doi: 10.1038/nprot.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stahl A, Connor KM, Sapieha P, Willett KL, Krah NM, Dennison RJ, Chen J, Guerin KI, Smith LE. Computer-aided quantification of retinal neovascularization. Angiogenesis. 2009;12:297–301. doi: 10.1007/s10456-009-9155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stahl A, Paschek L, Martin G, Gross NJ, Feltgen N, Hansen LL, Agostini HT. Rapamycin reduces vegf expression in retinal pigment epithelium (rpe) and inhibits rpe-induced sprouting angiogenesis in vitro. FEBS Lett. 2008;582:3097–3102. doi: 10.1016/j.febslet.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 63.Korff T, Augustin HG. Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differentiation. J Cell Biol. 1998;143:1341–1352. doi: 10.1083/jcb.143.5.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen J, Connor KM, Aderman CM, Willett KL, Aspegren OP, Smith LE. Suppression of retinal neovascularization by erythropoietin sirna in a mouse model of proliferative retinopathy. Invest Ophthalmol Vis Sci. 2009;50:1329–1335. doi: 10.1167/iovs.08-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen J, Connor KM, Aderman CM, Smith LE. Erythropoietin deficiency decreases vascular stability in mice. J Clin Invest. 2008;118:526–533. doi: 10.1172/JCI33813. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.