Abstract
Structural MRI studies provide evidence for sex differences in the human brain. Differences in surface area and the proportion of gray to white matter volume are observed, particularly in the parietal lobe. To our knowledge, there are no studies examining sex differences of parietal lobe structure in younger populations or in the context of development. The current study evaluated sex difference in the structure of the parietal lobe in children (7-17 years of age). Also, by adding the cohort of previously studied adults (18-50 years of age), sex differences of parietal lobe morphology were examined across the age span of 7-50 years. In the youth sample, we found that, similar to adults, the ratio of parietal lobe cortex to white matter was greater in females. Unlike the adult sample, there were no sex differences in surface area. When examining effects of age, surface area had a significant sex-by-age interaction. Males had essentially no decrease in surfaces area over time, but females had a significant decrease in surface area over time. These findings support the notion of structural sex differences in the parietal lobe, not only in the context of cross sectional assessment, but also in terms of differences of developmental trajectories.
Keywords: development, parietal lobe, sex differences, surface area
Introduction
Male and female brains have been found to display differences in structure, some of them observed as long as 100 years ago [1]. On average, overall cerebral volumes have been shown to be approximately 8-10% larger in males than in females even after accounting for differences in body size [1-4]. In addition, studies have reported that women have a higher proportion of gray matter to white matter volume [5-7]. Moreover, multiple studies have shown that the proportional increase in gray matter (either measured as greater cortical volume or greater cortical depth) in women may be regionally specific to the parietal and posterior temporal lobes [2, 4, 8-10].
Recent research in our laboratory [11] has confirmed and extended the findings of sex differences in the structure of the parietal lobe. As in previous studies, we found that the ratio of parietal lobe gray matter to parietal lobe white matter was higher in women compared to men. Additionally, we found men to have greater parietal surface area compared to women. Moreover, the differences in structure were directly related to performance of a visuospatial task, the Mental Rotations Test (MRT). Men significantly outperformed women on this task and the greater parietal lobe surface area was related to superior performance. For women, the relationship was different as larger proportional gray matter volume in the parietal lobe was associated with poorer performance on the MRT.
Previous work examining sex differences in parietal lobe morphology of children focused on cortical volume and depth. Sowell et al. [4] measured the cortical depth in 176 healthy individuals between 7 and 87 years of age and observed that a subset of younger females had proportionately greater depth of gray matter, independent of differences in brain or stature. Specifically, right inferior parietal lobule gray matter had proportionately greater depth in girls [4]. Furthermore, Caviness et al. [12] provides evidence for sex differences in cortical gray matter volume of healthy adolescents with consistently greater proportions of gray matter than white matter in young females. To the best of our knowledge, no studies to date have specifically evaluated the sex differences of the parietal lobe in a childhood population.
In addition to cross-sectional differences between males and females, there are also important differences between the sexes in the development of brain structure over time [13]. These differences include disparate trajectories of gray matter growth, rates of development and aging, nonlinearity of growth, and heterosynchronous changes over time [14-16].
The rationale for the current study is to better understand the sex differences of the parietal lobe in a childhood population. Whether the sex differences seen in adulthood are also seen in children can potentially shed light on possible etiology. For instance, if there are no differences between the sexes in childhood, then potential factors such as environmental influences may play a role. If the differences seen in adults are also present in childhood then potential biologic mechanisms could be further explored. Moreover, evaluating the developmental trajectory of parietal lobe structure over time can shed light on the change over time (between childhood and adulthood) parietal lobe structure may have. Therefore, the current study was designed: 1) to evaluate parietal lobe structural differences in boys and girls (ages 7-17), and 2) to evaluate the effects of development and aging on parietal lobe morphology by combining this youth sample with our previous sample of adults, creating a sample spanning ages 7-50 years.
Methods
Participants
This is a cross-sectional observational study involving quantitative neuroimaging measures. The two study samples consisted of 76 (n = 38 women, n = 38 men) healthy, normal adult volunteers (ages 18-47), which represent the sample used for our previous publication on parietal lobe morphology [11], and 108 (n = 54 girls, n = 54 boys) healthy, normal children and adolescents (ages 7-17). The youth sample was recruited as a comparison for a study of brain structure and function in children born with oral clefts [17]. The adult sample was recruited as a comparison for a study of brain structure and function in subjects with schizophrenia [18]. Both samples were recruited via newspaper advertising.
For children, medical and psychiatric illness was assessed by parental report in an interview with an experienced research assistant. Children were excluded if parents reported significant (requiring medical intervention) medical, neurological, or psychiatric illness, including alcohol and other substance abuse. Handedness was measured by the Denckla [19] Physical and Neurologic Examination for Subtle Signs (PANESS). The Wechsler Intelligence Scale for Children III (WISC-III) was used to measure the IQ for children ages 7-16 [20]. The Wechsler Adult Intelligence Scale III (WAIS-III) was used to obtain the IQ measures for 17 year-olds. Written informed consent was obtained from one parent and the child, if age 12 or greater, for all participants prior to participation. The study was approved by the University of Iowa Human Subjects Institutional Review Board.
For the adult sample, previously described by Koscik et al. [11], an experienced research assistant performed a structured interview to assess medical and psychiatric histories using an abbreviated version of the Comprehensive Assessment of Symptoms and History [21]. Participants were excluded if, by self report, they had significant (requiring medical intervention) medical, neurological, or psychiatric illness including alcohol and substance abuse. IQ measures were obtained using the Wechsler Adult Intelligence Scale-Revised (WAIS-R), which was given as part of a cognitive testing battery. Handedness was determined quantitatively by use of the Benton handedness scale. Socioeconomic status was determined for the adult participants and the parents of the youth sample using a modified Hollingshead 5-point scale ranging from 1 to 5 corresponding to higher (1) to lower (5) socioeconomic status. Neuropsychological testing and imaging were completed on the same day, in the morning and afternoon, respectively.
All participants in both the youth and adult samples were Caucasian and were equivalent in age, IQ, socioeconomic status, and handedness across the sex groups. The demographics for the youth sample can be found in Table 1 [see Koscik et al. [11] for the demographics of the adult sample].
Table 1.
Demographics of the Youth Study Group
| Female (N = 54) | Male (N = 54) | P | |||
|---|---|---|---|---|---|
| Mean (S.D.) | Range | Mean (S.D.) | Range | ||
| Age (years) | 12.5 (2.9) | 7.1 - 17.6 | 12.1 (2.8) | 7.8 - 17.9 | 0.450 |
| Full Scale IQ | 108.4 (14.7) | 71.0 - 150.0 | 113.2 (17.2) | 83.0 - 153.0 | 0.116 |
| Height (cm) | 153.2 (14.5) | 121.4 - 181.5 | 152.9 (17.7) | 123.4 - 190.4 | 0.915 |
| Parental SES | 2.3 (0.5) | 1.0 - 4.0 | 2.3 (0.6) | 1.0 - 4.0 | 0.930 |
Mean values, standard deviations, and ranges for demographic variables of the sample separated by sex. P-values from independent samples 2-tailed t-tests to assess differences between men and women are reported. d.f. = 106. Parental SES is measured on a scale from 1-5, where lower values indicate higher SES.
MRI acquisition
The MRI scanner, protocol sequence, and processing were identical for both samples. Images were obtained on a 1.5 Tesla GE Signa MR scanner. Three different sequences were acquired for each participant. T1 weighted images, using a spoiled grass sequence, were acquired with the following parameters: 1.5 mm coronal slices, 40° flip angle, TR = 24ms, TE = 5ms, NEX = 2, FOV = 26cm and a 256 × 192 matrix, a total of 124 contiguous slices. The PD and T2 weighted images were acquired with the following parameters: 3.0 mm coronal slices, TE = 36ms (for PD) or TE = 96ms (for T2), TR = 3000ms, NEX = 1, FOV = 26cm, 256×192 matrix and an echo train length = 8. PD and T2 images, in addition to the T1 image, provides mutli-modal data that allows the greatest accuracy of tissue segmentation. Automatic processing of the images after acquisition was done using Brain Research: Analysis of Images, Networks, and Systems (BRAINS2), a locally developed family of software programs. Details of the image analysis are published elsewhere [22]. Briefly, a three-dimensional data set is created, and the images are realigned, resampled, and the Talairach Atlas is warped onto the brain [23]. Within the stereotactic space, boxes (voxels) were assigned to specific brain regions. Boundaries of the parietal lobe are defined by the central sulcus and above the lateral sulcus extending posteriorly up to the occipital lobe. The boundary between occipital, parietal and temporal lobes are defined as a line drawn on the coronal slice connecting the parieto-occipital sulcus and the occipital notch. The white matter of the parietal lobe includes the white matter directly below the above defined cortical region down to the boundary of the sub-cortical box (which contains the structures of the caudate, putamen and thalamus). Intracranial volume was subdivided into brain tissue and cerebral spinal fluid. The cerebrum was divided into its four lobes; only the parietal lobe was used for the current study. Volumes of tissue were obtained from each region in an automated fashion. Automated measures obtained using a stereotactically based method have been reported by our lab and others to be efficient and accurate for cerebral lobe measures [24, 25]. Quality checks were done to insure accurate segmentation. No data sets were rejected. In addition, this method of measurement has also been validated on pediatric samples in the same age range used in the current study [26].
Surface Anatomy
This method is described in detail in a manuscript by Magnotta et al. [16]. The data were initially segmented using the method described above. The segmented image was then processed using our BRAINSURF program, which extracts a triangle-based polygonal model of an iso-surface, representing the parametric center of the gray matter tissue class. This method opens up sulci to reveal buried cortex through a consistent erosion of the cortical gray matter throughout the entire brain surface. It therefore retains regional information about cortical depth. This computationally efficient and fully automated method provides quantifiable measurements of surface area and depth. Cortical depth is the linear measure that spans the distance from the junction of the white matter and cortex to the surface of the cortex. The average cortical depth measure is calculated for the entire region of the parietal lobe. Surface area is calculated as the sum total of the surface in a given region.
Statistical Analysis
Youth Cross-sectional Assessment of Parietal Lobe Structure
For comparability between samples, our analysis strategy for the youth sample was exactly that of the strategy used by our previous adult analysis. The analyses were completed using SPSS 16.0 for Windows. A priori analyses were performed to ensure that the assumptions of each statistical procedure were not violated. To demonstrate that the demographics between males and females are comparable, independent sample two-tailed t-tests were performed for age, FSIQ, height (cm), and parental SES (measured on a scale from 1-5). Next, mean values and standard deviations were calculated for both males and females for brain morphology measures, including overall cerebral gray and white matter volume, cerebral surface area, and cerebral cortex average depth. Parietal lobe measures included parietal lobe gray and white matter volume, parietal lobe surface area, and parietal cortex average depth. Adjusted means, standard errors, and sex effects are the result of one-way between-groups analysis of covariance (ANCOVA). Covariates were specific for each of the morphometric variables to control for differences between the sexes appropriate for each measure. For overall cerebral measures, differences in stature (i.e., height) may be responsible for some of the differences between men and women in brain size; thus, height and age were used as a covariate for cerebral tissue volume and cerebral surface area [11]. For parietal lobe measures, more precise covariates were used to control for sex differences in overall cerebral size. Hence, the analyses of parietal tissue volume, parietal gray matter volume, and parietal white matter volume were controlled for age and total cerebral tissue volume. Parietal surface area had age and cerebral surface area as covariates. Parietal average depth was considered with age and total cerebral average depth as covariates.
A gray matter to white matter volume ratio (GM / WM) was also generated for comparison. Proportional tissue composition within the parietal lobe was derived by dividing the parietal gray matter (GM) volume by the parietal white matter (WM) volume, thus larger relative amounts of GM yield a ratio greater than 1 and larger relative WM volumes provide a ratio of less than 1. This well-established method of calculating the ratio of GM / WM is used in several other studies, especially as a means of measuring the relative amounts of GM and WM and age-related change in tissue proportion [11, 27]. The GM / WM was analyzed with an ANCOVA procedure, using age as a covariate. Because this is a proportional measure, covariates for cerebral size differences are unnecessary. All interaction effects were entered into the models, but were excluded if they were not significant using an alpha value of 0.05 for all significance tests.
Assessment of Parietal Lobe Structure Across Age Span
The effects of age on parietal lobe structure were examined by piece-wise exponential decay models using the combined sample of both youth and adult groups. The analysis was limited to only the parietal measures that showed significant sex differences in structure in either the youth or adult group (i.e. parietal lobe surface area and gray/white matter ratio). Piece-wise exponential decay models were used allowing the rates of decay to differ before and after an age cut-off. Whole valued ages were used in finding a cut-off, with the best cut-off point being determined using R-squared. An effect for number of year older than the cut-off age was included if the effect was significant using an alpha value of 0.05. Age and gender interaction effects were also included if significant using an alpha value of 0.05.
Results
Youth Sample
Brain Morphology
Table 2 displays the measures of cerebral and parietal lobe morphology, including tissue volumes (total, GM, and WM), surface area, average depth, and the parietal GM-WM ratio (both combined and in each hemisphere) for the younger participants. There were significant sex differences in cerebral tissue volume after controlling for age and height, with males having overall larger volumes of cerebral tissue [F(1, 106)=32.565, p<0.001]. There was also a significant difference in total cerebral surface area after accounting for age and height [F(1,106)=28.857, p<0.001] with males possessing larger cerebral surface area. There were, however, no significant sex differences in cerebral cortex average depth after controlling for age and height [F(1, 106)=0.007, p=0.936].
Table 2.
Brain Morphology Ages 7 to 17
| Females (N=54) | Males (N=54) | ||||
|---|---|---|---|---|---|
| Mean (S.D.) | Adjusted Mean (S.E.) | Mean (S.D.) | Adjusted Mean (S.E.) | F (P) | |
| Cerebral Tissue Volume (cm3)a | 1119.9 (88.2) | 1122.2 (13.1) | 1230.9 (107.0) | 1228.6 (13.1) | 32.565 (<.001)* |
| Cerebral Surface Area (mm2)a | 185160.7 (13543.2) | 185652.5 (2016.0) | 210523.3 (16527.9) | 201031.6 (2016.0) | 28.857 (<.001)* |
| Cerebral Average Depth (mm)a | 2.330 (0.203) | 2.338 (0.021) | 2.348 (0.179) | 2.340 (0.021) | 0.007 (0.936) |
| Parietal Lobe | |||||
| Tissue Volume (cm3)b | 254.2 (20.6) | 264.7 (1.7) | 277.2 (22.9) | 266.8 (1.7) | 0.649 (0.422) |
| Gray Matter | 161.9 (14.4) | 166.9 (1.4) | 173.8 (14.4) | 168.8 (1.4) | 0.794 (0.375) |
| Right | 81.9 (7.3) | 84.3 (0.7) | 88.0 (6.9) | 85.6 (0.7) | 1.283 (0.260) |
| Left | 80.0 (7.4) | 82.5 (0.7) | 85.8 (7.8) | 83.2 (0.7) | 0.327 (0.568) |
| White Matter | 92.3 (11.9) | 97.8 (1.1) | 103.5 (14.3) | 98.0 (1.1) | 0.016 (0.900) |
| Right | 45.9 (6.1) | 48.5 (0.6) | 51.7 (7.2) | 49.0 (0.6) | 0.274 (0.602) |
| Left | 46.4 (5.9) | 49.2 (0.6) | 51.8 (7.3) | 49.0 (0.6) | 0.087 (0.769) |
| Surface Area (mm2)c | 44608.3 (3848.3) | 46433.1 (324.0) | 47844.7 (4222.8) | 46019.9 (324.0) | 0.720 (0.398) |
| Right | 22645.7 (1918.2) | 23506.5 (178.6) | 24366.4 (2080.0) | 23505.6 (178.6) | 0.000 (0.997) |
| Left | 21962.7 (2011.0) | 22926.6 (166.5) | 23478.2 (2239.1) | 22514.3 (166.5) | 2.713 (0.103) |
| Average Depth (mm)d | 2.277 (0.207) | 2.265 (0.010) | 2.300 (0.191) | 2.290 (0.010) | 3.228 (0.075) |
| Right | 2.268 (0.222) | 2.278 (0.011) | 2.309 (0.201) | 2.299 (0.011) | 2.00 (0.160) |
| Left | 2.242 (0.228) | 2.251 (0.012) | 2.290 (0.190) | 2.280 (0.012) | 3.027 (0.085) |
| Gray/White Matter Ratioe | 1.78 (0.26) | 1.79 (0.03) | 1.71 (0.26) | 1.70 (0.03) | 5.387 (0.022)* |
| Right | 1.81 (0.27) | 1.83 (0.03) | 1.73 (0.26) | 1.72 (0.03) | 5.855 (0.017)* |
| Left | 1.75 (0.26) | 1.76 (0.03) | 1.68 (0.26) | 1.67 (0.03) | 4.451 (0.037)* |
Mean values and standard deviations for both men and women, for brain morphology measures, including overall cerebral volume and surface area and target region, parietal lobe, measures. Adjusted means, standard errors, and sex effects are the result of ANCOVA:
using age and height as covariates (d.f. 1, 106);
using age and cerebral tissue volume as covariates (d.f. 1, 106);
using age and cerebral surface area as covariates (d.f. 1, 106);
using age and cerebral average depth as covariates (d.f. 1, 106);
using age as a covariate (d.f. 1, 106);
p<0.05.
Parietal Tissue Volume
After controlling for age and cerebral tissue volume, there was no observed main effect of sex on total parietal tissue volume [ F(1, 106)=0.649, p=0.422].
Parietal Gray Matter Volume
There were no observed sex differences in parietal GM volume after controlling for age and cerebral tissue volume [F(1,106)=0.794, p=0.375]. Similarly, there were no sex differences in the left hemisphere [F(1,106)=0.327, p=0.568] or the right hemisphere [F(1,106)=1.283, p=0.260] parietal GM volume. Thus, the volume of parietal lobe cortex, with respect to the rest of the cerebrum, is not sexually dimorphic.
Parietal White Matter Volume
There were no observed differences in parietal WM volume after controlling for age and cerebral tissue volume [F(1,106)=0.016, p=0.900]. This was true as well of both hemispheres individually [right—F(1,106)=0.247, p=0.602 & left—F(1,106)=0.087, p=0.769].
Parietal Surface Area
In the younger participants, there were no significant sex differences observed in total parietal surface area after controlling for age and cerebral surface area [F(1,106)=0.720, p=0.398]. Even after controlling for overall differences in brain size, by accounting for cerebral surface area, the younger males and females did not differ in their parietal surface areas in either hemisphere [right—F(1,106)=0.000, p=0.997 & left—F(1,106)=2.713, p=0.103].
Parietal Average Depth
There were no observed differences in parietal average depth after controlling for age and cerebral average depth [F(1,106)=3.228, p=0.075]. This was true of both hemispheres individually as well [right—F(1,106)=2.000, p=0.160 & left—F(1,106)=3.027, p=0.085].
Parietal Gray Matter-White Matter Ratio
Sex differences in tissue composition were observed in the parietal lobe in the younger sample [F(1,106)=5.387 (0.022)] with females having a higher GM / WM compared to males. This increase in the GM / WM in females compared to males was observed bilaterally [right—F(1,106)=5.855, p=0.017 and left—F(1,106)=4.451, p=0.037].
Comparison to Adult Sample
In regard to sex differences, the previously evaluated adult sample showed that males had proportionately greater parietal lobe surface area compared to women [11]. As described above, this was not seen in the youth sample as parietal lobe surface area was found to be no different between girls and boys. In the adult sample, the GM / WM within the parietal lobe was found to be increased in women. This sex difference was also seen in the youth sample with girls having greater GM / WM ratios in the parietal lobes compared to boys.
Parietal Lobe Structure Across Age Span: Results of Exponential Decay Models
Tables 3 and 4 present the results of the exponential decay models for particular brain measures and age. For parietal lobe surface area (Table 3) the effect for number of year older than the cut-off age was not significant regardless of the cut-off employed (p >0.1) and so an exponential decay model with a rate of decay encompassing all ages was used. The effect of age in modeling parietal lobe surface area differs between males and females yielding an interaction effect p = .0394. The rate of parietal surface area decline is steeper and more significant in females than in males as shown in Figure 1. Parietal surface area declining by a factor of 0.9975 per year (or 0.9757 for a 10 year period) for females and only a factor of 0.9993 per year (or 0.9933 for a 10-year period) for males.
Table 3.
Results of Exponential Decay Model for Change Over Time in Parietal Lobe Surface Area
| Baseline Rate of Change from Female to Male (95% Confidence Interval) | Rate of Change for 1 year increase of age for males (95% Confidence Interval) | Rate of Change for 1 year increase of age for females (95% Confidence Interval) | p-value of Gender Age Interaction | |
|---|---|---|---|---|
| Parietal Lobe Surface Area | 0.9749 (0.9425, 1.0073) | 0.9993 (0.9982, 1.0005) | 0.9975 (0.9961, 0.9989) | 0.0394 |
| Right | 0.9807 (0.9445, 1.0168) | 0.9994(0.9981, 1.0007) | 0.9977 (0.9961, 0.9992) | 0.0798 |
| Left | 0.9689 (0.9352, 1.0026) | 0.9993 (0.9981, 1.0005) | 0.9974 (0.9959, 0.9989) | 0.0370 |
Table 4.
Results of Exponential Decay Model for Change Over Time in Parietal Lobe Gray/White Matter Ratio
| Rate of Change from Female to Male (95% Confidence Interval) | Rate of Change for 1 year increase of age when Age<23 (95% Confidence Interval) | Rate of Change for 1 year increase of age when Age>23 (95% Confidence Interval) | p-value of Gender Age Interaction | p-value of Gender Age Interaction for years over 23 | |
|---|---|---|---|---|---|
| Gray/White Matter Ratio | 0.9432 (0.9120, 0.9745) | 0.9649 (0.9613, 0.9685) | 0.9998 (0.9946, 1.0051) | 0.7010 | 0.8222 |
| Right | 0.9417 (0.9097, 0.9737) | 0.9650 (0.9613, 0.9687) | 0.9987 (0.9933, 1.0042) | 0.6361 | 0.7284 |
| Left | 0.9450 (0.9128, 0.9773) | 0.9647 (0.9611, 0.9684) | 1.0009 (0.9955, 1.0062) | 0.7605 | 0.9081 |
Figure 1.
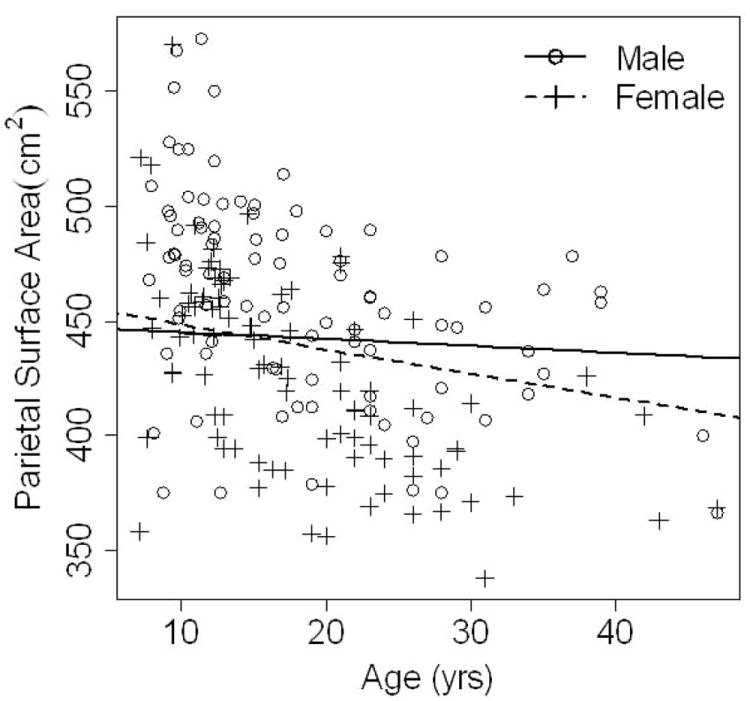
Parietal surface area is shown on the y axis, plotted against age on the x axis. Exponential decay curves are plotted for males (solid line) and females (dotted line) at the mean value of total cerebral surface area. The rate of change in parietal surface area over time for males is very slight (not significantly different from a factor of 1) indicating little change in surface area over time. However, the rate of change for females is smaller than 1 indicating more significant decrement in surface area over time resulting in a lower parietal surface area compared to males by the adult years.
In contrast to the sex by age interaction for the parietal lobe surface area, there are similar trajectories for both males and females over time in the GM to WM ratio (Table 4). Effect of age on GM/WM is different for older ages than younger ages with a significant decline prior to age 23 followed by stabilization after age 23. These effects are represented in Figure 2. It is important to mention that this GM / WM, despite having a similar decline in males and females, is still greater in females in both the younger and older participants with males have 0.9432 times the GM/WM of males. This same pattern is seen in each hemisphere as well.
Figure 2.
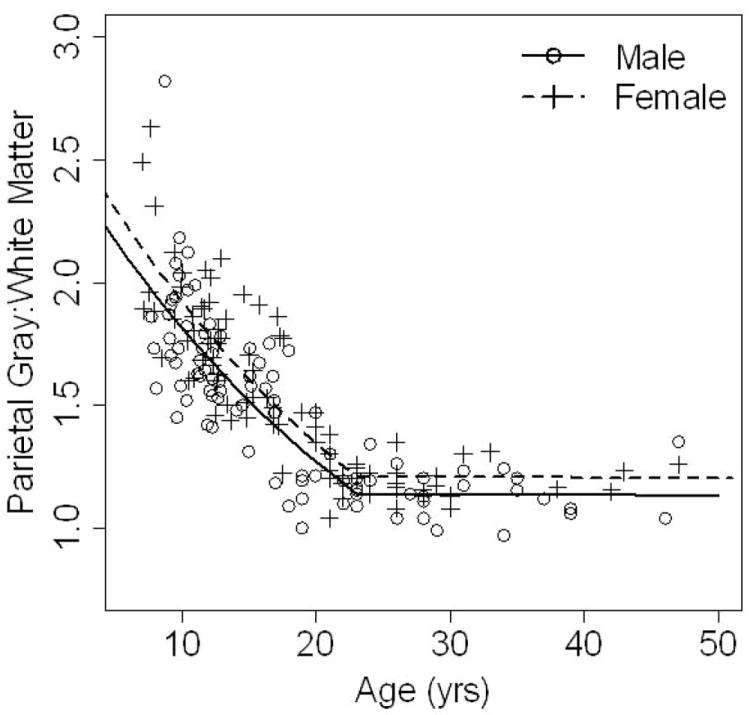
Mean parietal gray-to-white matter ratio is shown on the y axis, plotted against age on the x axis. Males (solid line) and females (dotted line) have the same rate of decline (there is no sex by age interaction) throughout the entire age span. In the younger ages, the change over time is quite rapid with a steep decline in ratio values. However, after age 23, the parietal gray-to-white matter ratio essentially stabilizes and comes to a plateau. The females (dotted line) have higher measures of parietal lobe gray-to-white matter ratios throughout the wide age range measured here (7-50 years).
Discussion
The parietal lobe has sex differences in structure within children and adolescents; girls have larger parietal lobe gray matter to white matter ratios (GM / WM) compared to boys. This is a finding reported for adult populations by our group [2, 11] and others [4-10]. There are relatively few studies examining this proportion in the parietal lobe of children; however, it is consistent with previous work by Sowell et al. [4], which found proportionately thicker gray matter in girls, as well as the previous reports by Caviness et al. [12] that detail a proportionately thicker cortex in female adolescents. The fact that this finding is present early in childhood and present through development is supported by the results of our age-effects analysis showing that the GM / WM ratio in both men and women is quite dynamic, with significant changes from age 7-50; however, the trajectory of those changes are the same for both men and women such that the sex difference is consistent and unchanging over time.
In contrast to the stable sex differences of the parietal lobe GM / WM ratio, the parietal surface area measure showed a different pattern. In the youth sample, we found no significant differences between males and females in the parietal lobe surface area. This is in contrast to what we had seen previously in adults where males had proportionately larger parietal lobe surface areas compared to females. The results of our effects of age analysis on the combined youth and adult sample showed a significant age by sex interaction in which the rate of gray matter surface area decreased substantially over time in females, but was relatively stable in males from childhood into middle age. This differential trajectory in development explained the findings of no difference in parietal surface area in the youth sample, but sex differences in the adult sample.
Previous studies have shown that other measures of cortical morphology (volume, cortical depth) have similar age changes over time (decreased volume, cortical thinning). These changes have been speculated to represent both synaptic pruning and myelination progressing into the peripheral gray matter [28-30]. As unnecessary synapses are eliminated and axonal myelination increases, efficiency of neurotransmission improves and the volume of the cortex decreases. More importantly, these processes may be different between the sexes, resulting in differential trajectories of cortical morphology.
In our previous study of parietal lobe morphology in adults, we found that adult females not only had a greater parietal GM / WM ratio than adult males, but that this difference was also related to poorer performance on a visuospatial task, the Mental Rotation Task (MRT). That is, the greater the ratio of GM / WM in the parietal lobe, the worse women performed on the MRT. In contrast, adult males compared to adult females had proportionately greater parietal lobe surface area, and this morphologic difference was associated with a performance advantage for adult males on MRT: the greater the parietal lobe surface area, the greater the MRT performance. In children and adolescents, the presence and consistency of the sex difference in MRT spatial ability found in adults is less clear. Some studies find that young males perform better than young females in visuospatial tasks, including variations of the MRT, as early as age four; it appears to be a stable difference that does not disappear over time [31-34]. Alternatively, other studies find that there are no differences in visuospatial ability in early adolescence and that both boys and girls improve equally on MRT visuospatial tasks with age and with training interventions to enhance their performance on the given tasks [35-37]. These latter studies suggest that differential environments during development may potentially contribute to the differences in performance of these tasks in adults.
The fact that the sex differences of the parietal lobe surface area are absent in the youth sample and are later detectable in an adult sample, however, opens the door to the possibility that this particular measure may be more influenced by environment. White et al. [38] conducted a study that examined structural differences in monozygotic twins compared with matched controls and found that volume measures of the cerebrum (gray matter, white matter, and lobar volumes), cerebellum, caudate, and thalamus were highly correlated, indicating a high degree of heritability in these measures. In addition, cortical depth was also highly correlated between monozygotic twins. Cortical surface measures, though, were the least correlated within twin pairs thus, raising the possibility of environmental influence uniquely for this measure. The fact that more young boys, compared to young girls, play longer and more frequently with toys that require visuospatial skills, such as building blocks, Erector Sets, or Legos, may be one of many environmental influences on the development of their parietal lobe surface area [39]. In support of this, a recent animal study shows region-specific changes in brain structure related to differential types of learning experiences [40].
In contrast, a more recent study has shown that both cortical depth and cortical surface area are both highly heritable (0.81 and 0.89 respectively) [41]. More importantly, these two measures were found to have distinct genetic components, suggesting that differential biologic factors (genetic make-up) may be just as likely as differential environments to contribute to sex difference in cortical surface area changes over time.
In addition, sex differences in developmental trajectories of the structure of the cortex are well documented and parallel the sex differences in the timing of puberty. Geidd et al. [42] has shown that not only are the changes in the cerebral cortex heterosyncronous across regions of the cerebrum, but that males show clear differences in the timing of these developmental changes compared to females with cortical volumes peaking in girls up to 2 years prior to that of boys. Other studies have shown that these cortical changes and sex differences are directly related to hormonal levels, underscoring the notion that these structural changes may be more closely related to biological factors than different environmental factors [14].
To interpret our data in relation to previous studies, it is important to note a significant difference in methodology from some of the studies examining gray matter developmental trajectories into early adulthood that found an inverted parabolic curve best fit the age-related change in the parietal lobe [15, 42]. These studies used a mixed model including longitudinal data on gray matter volumes for each cerebral lobe. With only cross-sectional data for 184 participants from youth to adulthood, the pre-adolescent increase in cortical gray matter and nonlinear developmental trajectory was most likely obscured by inter-individual variation [42]. Because our sample is cross-sectional, subtle nonlinearity, particularly prepubertal increases in parietal lobe gray matter volume, may not be demonstrated. Our study population extended up to age 47, but, with fewer participants in the adult age range, detection of nonlinearity in mid-adulthood may be limited as well. Cohort effects across this wide age span may also obscure subtle nonlinearity. Large longitudinal studies may be better suited to eliminate cohort effects and refine models of developmental trajectories [15]. Despite our study’s limitations, however, these data clarify our findings in our previous studies and emphasize the importance of considering sex, age and developmental trajectories when investigating cerebral structure-function relationships.
In the current study, evaluation of morphology was at a very ‘gross regional’ level, evaluating the entire parietal lobe. The cortex within the parietal lobe is made up of multiple structurally and functionally distinct sub-regions. Future studies that hone in on a more fine-grained region of interest method may locate specific areas within the parietal lobe that may manifest the findings reported in the current, more global analysis.
The effects of sex hormones on the developing maturing brain is of vital importance, and the foundation on which much of the structural and functional sex differences in the brain are based. The effects of sex hormones during brain development are well studied in both the non-human and human samples [5, 43, 44]. However, the effects of sex hormones on maturation of the brain during puberty is an area in which little is known in humans [45]. Although many neuroimaging studies (including the current one) evaluate changes in brain structure over time and relate these changes to age, these measures are only a proxy to puberty and are a limitation in our ability to disentangle the effects of sex hormones from the effects of age in these changes.
In summary, the current study found that the sex differences in parietal lobe structure seen in adults (ratio of parietal lobe cortex to white matter being greater in females), is also seen in a childhood sample of 7-17 years. When examining effects of age, surface area had a significant sex-by-age interaction. Males had no decrease in surface area over time, but females had a significant decrease over time. These findings support the notion of structural sex differences in the parietal lobe, not only in the context of cross sectional assessment, but also in terms of differences of developmental trajectories.
Acknowledgments
This research was supported in part by the following grants:
NIDCR Brain Structure and Function in Children with Oral Clefts 1 RO1 DE01 14399 01 A1.
General Clinical Research Centers Program Grant RR00059, National Center for Research Resources, National Institutes of Health.
NIMH Grants MH31593, MH40856 and MH43271
Joel Salinas was supported by the Doris Duke Clinical Research Fellowship Program
We would also like to thank Ellen van der Plas and Eric Axelson for their assistance and technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marshall J. Relations between the Weight of the Brain and its Parts, and the Stature and Mass of the Body, in Man. J Anat Physiol. 1892;26(Pt 4):445–500. [PMC free article] [PubMed] [Google Scholar]
- 2.Nopoulos P, et al. Sexual dimorphism in the human brain: evaluation of tissue volume, tissue composition and surface anatomy using magnetic resonance imaging. Psychiatry Res. 2000;98(1):1–13. doi: 10.1016/s0925-4927(99)00044-x. [DOI] [PubMed] [Google Scholar]
- 3.Raz N, et al. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: replicability of regional differences in volume. Neurobiol Aging. 2004;25(3):377–96. doi: 10.1016/S0197-4580(03)00118-0. [DOI] [PubMed] [Google Scholar]
- 4.Sowell ER, et al. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex. 2007;17(7):1550–60. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldstein JM, et al. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 2001;11(6):490–7. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- 6.Gur RC, et al. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. J Neurosci. 1999;19(10):4065–72. doi: 10.1523/JNEUROSCI.19-10-04065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luders E, et al. Mapping cortical gray matter in the young adult brain: effects of gender. Neuroimage. 2005;26(2):493–501. doi: 10.1016/j.neuroimage.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Im K, et al. Gender difference analysis of cortical thickness in healthy young adults with surface-based methods. Neuroimage. 2006;31(1):31–8. doi: 10.1016/j.neuroimage.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 9.Luders E, et al. Gender effects on cortical thickness and the influence of scaling. Hum Brain Mapp. 2006;27(4):314–24. doi: 10.1002/hbm.20187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schlaepfer TE, et al. Structural differences in the cerebral cortex of healthy female and male subjects: a magnetic resonance imaging study. Psychiatry Res. 1995;61(3):129–35. doi: 10.1016/0925-4927(95)02634-a. [DOI] [PubMed] [Google Scholar]
- 11.Koscik T, et al. Sex differences in parietal lobe morphology: relationship to mental rotation performance. Brain Cogn. 2009;69(3):451–9. doi: 10.1016/j.bandc.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caviness VS, Jr, et al. The human brain age 7-11 years: a volumetric analysis based on magnetic resonance images. Cerebral cortex. 1996;6(5):726–36. doi: 10.1093/cercor/6.5.726. [DOI] [PubMed] [Google Scholar]
- 13.Sowell ER, et al. Mapping cortical change across the human life span. Nat Neurosci. 2003;6(3):309–15. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- 14.Giedd JN, et al. Puberty-related influences on brain development. Mol Cell Endocrinol. 2006;254-255:154–62. doi: 10.1016/j.mce.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 15.Lenroot RK, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36(4):1065–73. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magnotta VA, et al. Quantitative in vivo measurement of gyrification in the human brain: changes associated with aging. Cereb Cortex. 1999;9(2):151–60. doi: 10.1093/cercor/9.2.151. [DOI] [PubMed] [Google Scholar]
- 17.Nopoulos P, et al. Abnormal brain structure in children with isolated clefts of the lip or palate. Arch Pediatr Adolesc Med. 2007;161(8):753–8. doi: 10.1001/archpedi.161.8.753. [DOI] [PubMed] [Google Scholar]
- 18.Flaum MA, Andreasen NC, Arndt S. The Iowa prospective longitudinal study of recent-onset psychoses. Schizophr Bull. 1992;18(3):481–90. doi: 10.1093/schbul/18.3.481. [DOI] [PubMed] [Google Scholar]
- 19.Denckla MB. Revised Neurological Examination for Subtle Signs (1985) Psychopharmacol Bull. 1985;21(4):773–800. [PubMed] [Google Scholar]
- 20.Wechsler D. Wechsler Intelligence Scale for Children, 3rd Edition, Manual. The Psychological Corporation; Washington, DC: 1991. [Google Scholar]
- 21.Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH). An instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry. 1992;49(8):615–23. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- 22.Magnotta VA, et al. Structural MR image processing using the BRAINS2 toolbox. Computerized medical imaging and graphics: the official journal of the Computerized Medical Imaging Society. 2002;26(4):251–64. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- 23.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: An approach to cerebral imaging. New York: Thieme; 1988. [Google Scholar]
- 24.Andreasen NC, et al. Automatic atlas-based volume estimation of human brain regions from MR images. J Comput Assist Tomogr. 1996;20(1):98–106. doi: 10.1097/00004728-199601000-00018. [DOI] [PubMed] [Google Scholar]
- 25.Collins DL, et al. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18(2):192–205. [PubMed] [Google Scholar]
- 26.Kates WR, et al. Automated Talairach atlas-based parcellation and measurement of cerebral lobes in children. Psych Res. 1999;91(1):11–30. doi: 10.1016/s0925-4927(99)00011-6. [DOI] [PubMed] [Google Scholar]
- 27.Salat DH, Kaye JA, Janowsky JS. Prefrontal gray and white matter volumes in healthy aging and Alzheimer disease. Arch Neurol. 1999;56(3):338–44. doi: 10.1001/archneur.56.3.338. [DOI] [PubMed] [Google Scholar]
- 28.Reiss AL, et al. Brain development, gender and IQ in children. A volumetric imaging study. Brain. 1996;119(Pt 5):1763–74. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- 29.Sowell ER, et al. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24(38):8223–31. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sowell ER, et al. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21(22):8819–29. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brosnan MJ. Spatial ability in children’s play with Lego blocks. Percept Mot Skills. 1998;87(1):19–28. doi: 10.2466/pms.1998.87.1.19. [DOI] [PubMed] [Google Scholar]
- 32.Kerns KA, Berenbaum SA. Sex differences in spatial ability in children. Behav Genet. 1991;21(4):383–96. doi: 10.1007/BF01065974. [DOI] [PubMed] [Google Scholar]
- 33.Levine SC, et al. Early sex differences in spatial skill. Dev Psychol. 1999;35(4):940–9. doi: 10.1037//0012-1649.35.4.940. [DOI] [PubMed] [Google Scholar]
- 34.Masters MS, Sanders B. Is the gender difference in mental rotation disappearing? Behav Genet. 1993;23(4):337–41. doi: 10.1007/BF01067434. [DOI] [PubMed] [Google Scholar]
- 35.De Lisi R, Wolford JL. Improving children’s mental rotation accuracy with computer game playing. J Genet Psychol. 2002;163(3):272–82. doi: 10.1080/00221320209598683. [DOI] [PubMed] [Google Scholar]
- 36.Sanz de Acedo Lizarraga M. Improvement of mental rotation in girls and boys. Sex Roles. 2003;49(5):277–286. [Google Scholar]
- 37.Waber DP, Carlson D, Mann M. Developmental and differential aspects of mental rotation in early adolescence. Child Dev. 1982;53(6):1614–21. [PubMed] [Google Scholar]
- 38.White T, Andreasen NC, Nopoulos P. Brain volumes and surface morphology in monozygotic twins. Cerebral cortex. 2002;12(5):486–93. doi: 10.1093/cercor/12.5.486. [DOI] [PubMed] [Google Scholar]
- 39.Baldizar J. Assessing sex typing and androgyny in children: the children’s sex role inventory. Developmental Psychology. 1991;27(3):505–515. [Google Scholar]
- 40.Lerch JP, et al. Maze training in mice induces MRI-detectable brain shape changes specific to the type of learning. Neuroimage. 2010;54(3):2086–95. doi: 10.1016/j.neuroimage.2010.09.086. [DOI] [PubMed] [Google Scholar]
- 41.Panizzon MS, et al. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 2009;19(11):2728–35. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giedd JN, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 43.McCarthy MM, et al. Mechanisms mediating oestradiol modulation of the developing brain. J Neuroendocrinol. 2008;20(6):777–83. doi: 10.1111/j.1365-2826.2008.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nature neuroscience. 2004;7(10):1040–7. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- 45.Blakemore SJ, Burnett S, Dahl RE. The role of puberty in the developing adolescent brain. Human brain mapping. 2010;31(6):926–33. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]


