SUMMARY
Sex hormones such as estrogen and testosterone are essential for sexually dimorphic behaviors in vertebrates. However, the hormone-activated molecular mechanisms that control the development and function of the underlying neural circuits remain poorly defined. We have identified numerous sexually dimorphic gene expression patterns in the adult mouse hypothalamus and amygdala. We find that adult sex hormones regulate these expression patterns in a sex-specific, regionally-restricted manner, suggesting that these genes regulate sex typical behaviors. Indeed, we find that mice with targeted disruptions of each of four of these genes (Brs3, Cckar, Irs4, Sytl4) exhibit extremely specific deficits in sex specific behaviors, with single genes controlling the pattern or extent of male sexual behavior, male aggression, maternal behavior, or female sexual behavior. Taken together, our findings demonstrate that various components of sexually dimorphic behaviors are governed by separable genetic programs.
INTRODUCTION
Sexually reproducing animals display sex-specific social behaviors. Genetic studies have elucidated some of the rules that control such behaviors in mice. These studies show that estrogen sets up the repertoire of sexual and territorial behaviors, and testosterone controls the extent of these displays in males (Wu et al., 2009; Ogawa et al., 2000; Raskin et al., 2009; Juntti et al., 2010; Kudwa and Rissman, 2003; Kudwa et al., 2006; Wu and Shah, 2011). However, the molecular pathways employed by these overarching hormonal mechanisms to influence neural circuits underlying sex-typical behaviors are poorly understood.
Sex steroids can be regarded as master regulators of sex-specific behaviors (Morris et al., 2004; Baum, 2003). The developmental influence (organizational role) of sex hormones can lead to enduring effects on brain and behavior. By contrast, in adults sex steroids elicit reversible changes (activational role) in neural circuits and behavior. Gonadal hormones bind to distinct nuclear hormone receptors that are essential for sex-typical displays (Scordalakes and Rissman, 2003; Raskin et al., 2009; Kudwa and Rissman, 2003; Wersinger et al., 1997; Juntti et al., 2010; Ogawa et al., 2000; Lydon et al., 1995). These receptors directly regulate gene expression by binding DNA (Mangelsdorf et al., 1995), and they can initiate non-transcriptional signaling via mechanisms such as interactions with intracellular kinases and transmembrane receptors (Foradori et al., 2008; Lishko et al., 2011; Micevych and Dominguez, 2009; Revankar et al., 2005; Vasudevan and Pfaff, 2008; McDevitt et al., 2008). Sex hormones or their metabolites can also bind to neurotransmitter receptors to gate their activity (Henderson, 2007). Such non-transcriptional signaling can control neural function at time scales that allow real time modulation of behavior.
Prior work has identified genes downstream of sex hormones that regulate sexually dimorphic behaviors (Kayasuga et al., 2007; Wersinger et al., 2002; Nelson et al., 1995; Winslow and Insel, 2002). The relative paucity of such genes is in contrast to the diversity of these behaviors, and suggests that the underlying neural circuits may be regulated largely by non-transcriptional hormone signaling. These genes may also be difficult to identify because they are expressed at low levels or in a few neurons. We used an unbiased approach to identify genes that are downstream of sex hormones and that control dimorphic behaviors. We reasoned that such genes are expressed dimorphically; using microarrays, we therefore sought to identify sex differences in gene expression in the hypothalamus since this region is essential for dimorphic behaviors and contains sex hormone receptor expressing neurons. We identified 16 genes with sexually dimorphic expression in the hypothalamus and medial amygdala (MeA). Adult sex hormones control the expression of most of these genes, suggesting that they regulate dimorphic behaviors. Indeed, we find that mice singly mutant for four of these genes exhibit deficits in specific components of male mating, intermale aggression, maternal behavior, or female sexual receptivity. Thus, our results show that dimorphic behaviors are modular in the sense that components of these displays are genetically separable.
RESULTS
Identification of sex differences in gene expression in the hypothalamus
We compared gene expression between adult male or female hypothalamus and whole brain using dual color microarrays (Figure 1A) (Verdugo and Medrano, 2006). Our gene profiling and subsequent analysis were devised to identify dimorphic hypothalamus-enriched mRNAs. We disregarded Y-specific genes, X-linked genes with Y paralogs, and genes involved in X-inactivation, because they were not hypothalamus-enriched. Our analysis identified 84 sexually dimorphic candidate transcripts (Table S1).
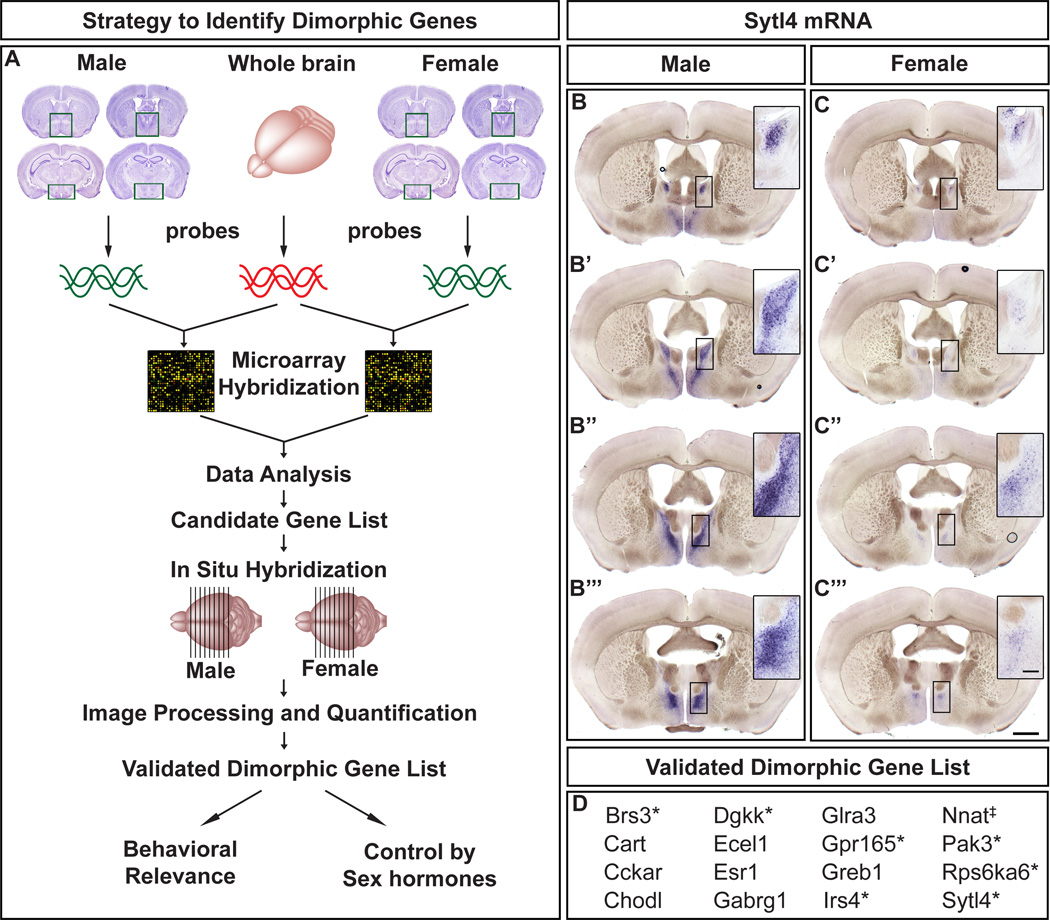
Figure 1. Identification of sexually dimorphic gene expression in the adult mouse brain.
(A) Strategy to identify sex differences in gene expression. Boxed areas in Nissl stained coronal sections depict the dissected regions.
(B–C“’) Expression of Sytl4 mRNA in serial coronal sections through the forebrain, with more rostral sections on top. Upregulated Sytl4 mRNA in the male BNST (insets).
(D) List of genes with sexually dimorphic expression.
*, X-linked gene; ‡, imprinted gene. Scale bar = 1 mm. Inset scale bar = 100 µm.
We screened all 84 genes for sexually dimorphic expression by in situ hybridization (ISH) through the adult forebrain (Figure 1A–C“’). Putative dimorphisms were validated by ISH on ≥ 2 more pairs of males and females. These studies revealed 16 dimorphically expressed genes (Figure 1D). These encode a neuropeptide (CART), GPCRs (Cckar, Brs3, Gpr165), neurotransmitter-gated ion channels (Gabrg1, Glra3), intracellular signaling proteins (Dgkk, Irs4, Pak3, Rps6ka6, Sytl4), a transcription factor (ERα), a protease (Ecel1), and those with poorly understood function (Chodl, Greb1, Nnat). Although the neural function of most of these genes is unknown, they largely encode signaling proteins that could regulate neuronal function and behavior acutely. Many of these genes (Dgkk, Gabrg1, Greb1, Pak3, Rps6ka6) are implicated in various human disorders that occur with sex-skewed ratios (Morrow et al., 2008; Ghosh et al., 2000; Enoch et al., 2009; Yntema et al., 1999; van der Zanden et al., 2011). Seven of these 16 genes are X-linked (Figure 1D), a distribution unlikely to occur by chance (p < 1×10–4). The X-linked genes are not simply female-upregulated because they escape X-inactivation. Rather, with the exception of Brs3, whose expression is upregulated only in females, the expression of other X-linked genes is upregulated only in males or in distinct regions in both sexes (Figures 2–4, S1). Thus, our screen has yielded many dimorphic transcripts with unexpected features and whose functions in dimorphic behaviors are unknown.
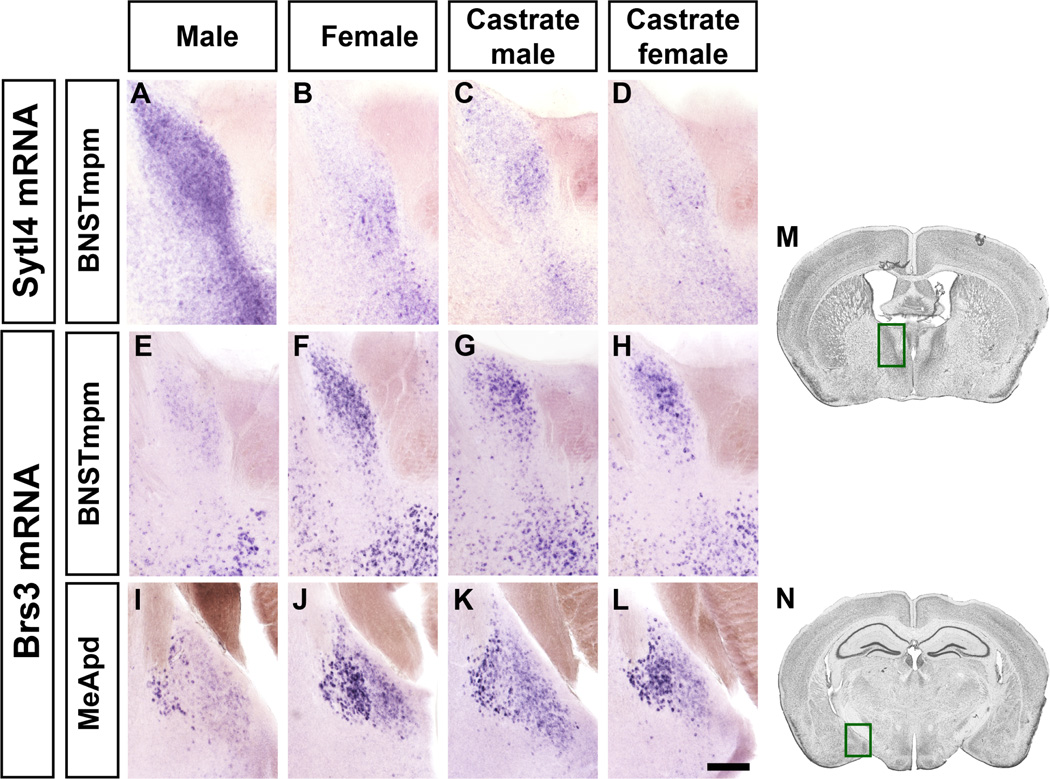
Figure 2. Sexually dimorphic expression of Sytl4 and Brs3.
Sytl4 (A–D) and Brs3 (E–L) mRNA expression in coronal sections. Brains from male and female were processed in parallel whereas those from castrate male or female were processed in separate studies and are shown here (and Figure 3) for comparison purposes.
(A–D) More Sytl4 mRNA in the male BNSTmpm.
(E–L) Less Brs3 mRNA in the male BNSTmpm and MeApd.
(M, N) Boxed areas in Nissl stained sections outline the BNST (M) and MeA (N) regions shown in (A–H) and (I–L), respectively. Scale bar (A–L) = 100 µm.
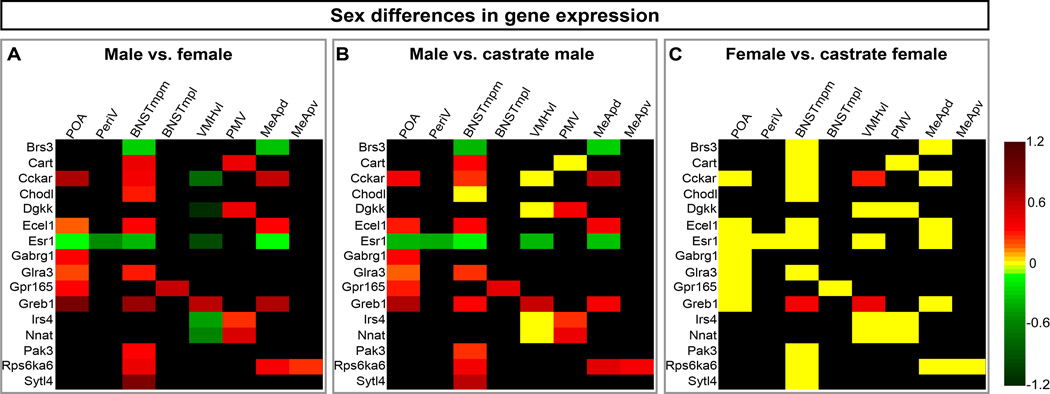
Figure 4. Sexual dimorphism in gene expression and its control by adult sex hormones.
Heat map of log10-transformed fold differences in mRNA expression.
(A) Individual genes are upregulated in ≥1 brain region in one sex or in distinct regions in both sexes. A brain region can show upregulated expression of distinct genes in both sexes. Red = male-upregulated, green = female-upregulated.
(B) Most male-upregulated genes are downregulated after castration. Red = male-upregulated, yellow = no change, green = castrate male-upregulated.
(C) Most genes show similar expression in intact and castrate females. Red = female-upregulated, yellow = no change, green = castrate female-upregulated.
Heat map scale spans from red to green. Black = not sexually dimorphic or not expressed. p < 0.05 for all changes shown in green or red; p > 0.05 for yellow cells.
See also Figures S2, S3.
Complex patterns of sexually dimorphic gene expression
We find dimorphic gene expression in the hypothalamus, the bed nucleus of the stria terminalis (BNST), and MeA (Figures 2–4, S1). The BNST was included in our tissue dissection for gene profiling since it is intermingled with hypothalamic areas, expresses sex hormone receptors, and regulates dimorphic behaviors (Emery and Sachs, 1976; Simerly et al., 1990; Gammie and Nelson, 2001). By contrast, the MeA was not included in our dissection and it is surprising that many of these genes are dimorphic in the MeA. The MeA receives pheromonal input essential for social behaviors and it provides afferents to the BNST and most hypothalamic centers with dimorphic gene expression (Figure 4A) (Canteras et al., 1995; Dulac and Wagner, 2006). Thus, sex differences within the MeA could influence pheromonal information relayed to the BNST and hypothalamus. The sex differences in gene expression within these regions are restricted to specific neuronal pools that are thought to control dimorphic behaviors (Cooke et al., 1998; Simerly, 2002; Blaustein, 2008). These include the BNSTmpm (posteromedial area of the medial BNST), BNSTmpl (posterolateral area of the medial BNST), MeApv (posteroventral MeA), MeApd (posterodorsal MeA), and the POA (preoptic area), VMHvl (ventrolateral area of the ventromedial nucleus), PMV (ventral premamillary nucleus), and periV (rostral periventricular region) in the hypothalamus (Figures 2–4, S1).
Of these 16 genes, 10 are male-upregulated, 2 are female-upregulated, and 4 exhibit a compound dimorphism such that each is upregulated in the female VMHvl and in ≥1 male brain region (Figures 2–4, S1). All 16 transcripts were also expressed in a non-dimorphic pattern in other discrete brain regions (Figure S2B). Microarray studies cannot reveal such complexity in expression patterns, validating the utility of ISH. A microarray study previously identified Sytl4 as being male-upregulated in the brain (Yang et al., 2006), although its dimorphic expression was not confirmed or localized histologically. We find Sytl4 to be upregulated in the male BNSTmpm (Figure 1B–C“’; 2A, B). Male-upregulated POA expression of Gabrg1 has been described in the rat (Nett et al., 1999), and our data extend these findings to the mouse. Some but not other studies have reported sexually dimorphic ERα expression in rodents (Lauber et al., 1991; Simerly et al., 1990; Shughrue et al., 1992; Koch and Ehret, 1989). Our results show unequivocal sex differences in ERα expression (Figures 4A, S1S’–L“, S2A).
These 16 genes are not expressed in white matter, and they label cells that appear to bear a neuronal morphology. We find genes that are upregulated in the female BNSTmpm and MeApd and in the male BNSTmpl even though these regions contain more neurons in the other sex (Figures 2, 4A, S1) (Guillamón et al., 1988; Holmes et al., 2009; Morris et al., 2008a; Shah et al., 2004; Wu et al., 2009). Most sex differences in gene expression also withstand normalization to neuronal number in RT-qPCR studies (Figure S2A). Thus, the sex difference in gene expression cannot be accounted for solely by dimorphic neuronal numbers.
Testicular hormones control sex differences in gene expression
Castration of adult males abrogates sex-typical behaviors (McGill and Tucker, 1964; Beeman, 1947). We tested if these deficits are accompanied by altered dimorphic gene expression by performing ISH in adult male castrates and controls. The expression of most male-upregulated genes is downregulated in castrates and appears feminized (Figures 2, 3, 4B). Brs3 and ERα, which are normally female-upregulated, were upregulated in male castrates (Figures 2, 4B). Thus, testicular hormones enhance, inhibit, or leave unaffected gene expression in a region-specific manner, suggesting that they utilize distinct molecular mechanisms to drive male-typical behaviors. The expression profile in castrate males is plastic, and testosterone provision restores expression of most genes to levels observed in intact males (Figure S3). Thus, testosterone can masculinize expression of most genes we have identified.
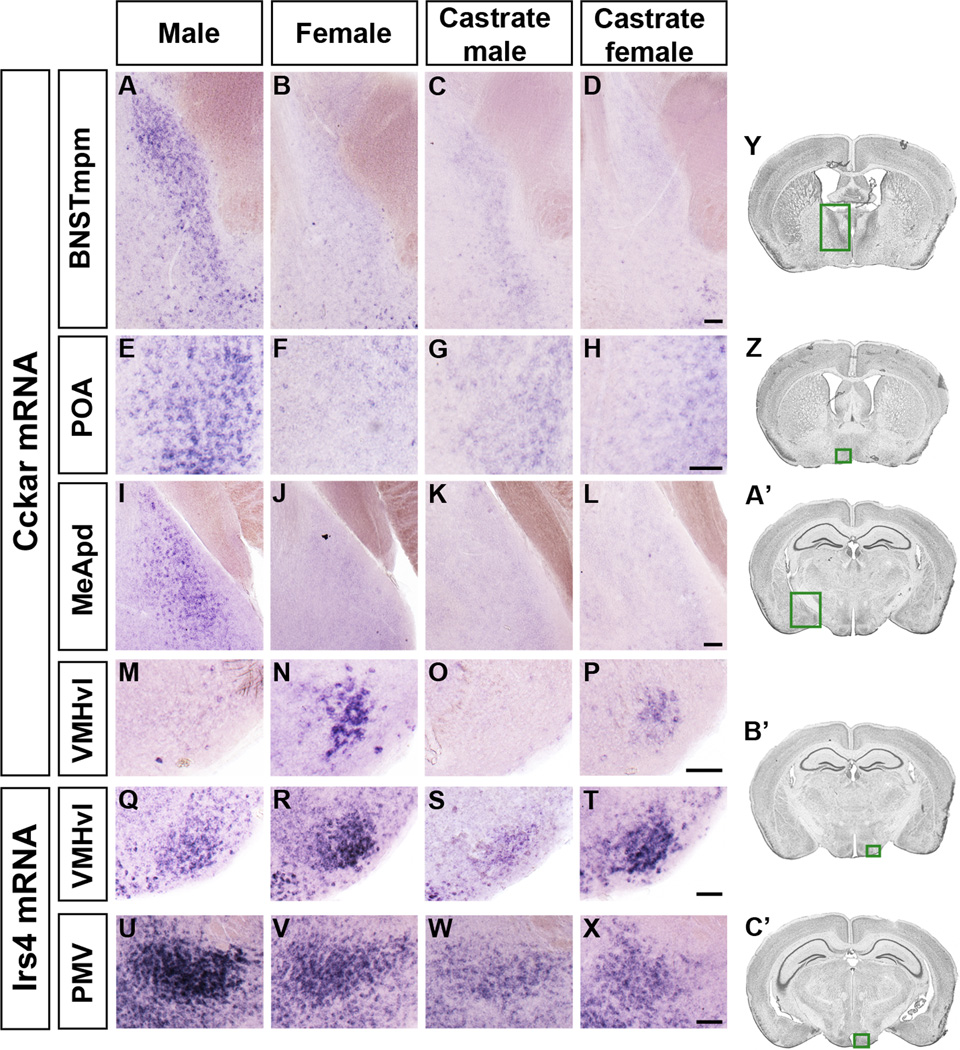
Figure 3. Sexually dimorphic expression of Cckar and Irs4.
Cckar (A–P) and Irs4 (Q–X) mRNA expression in coronal sections.
(A–L) More Cckar mRNA in the male BNSTmpm, POA, and MeApd.
(M–P) More Cckar mRNA in the female VMHvl.
(Q–T) More Irs4 mRNA in the VMHvl of the female and castrate female.
(U–X) More Irs4 mRNA in the male PMV.
(Y, Z, A’–C’) Boxed areas in Nissl stained sections outline the BNST (Y), POA (Z), MeA (A’), VMHvl (B’), and PMV (C’), respectively. Scale bars = 50 µm.
Restricted control of sexual dimorphisms in gene expression by ovarian hormones
Castration rapidly eliminates estrous cycling and female sexual behavior (Allen and Doisy, 1923; Wiesner and Mirskaia, 1930; Ring, 1944), and we compared gene expression between adult castrate females and controls. In contrast to the wholesale changes in gene expression in male castrates, we observed highly circumscribed changes in castrate females. Castration reduced expression of Cckar and Greb1 without affecting other genes (Figures 3, 4C). Our list of genes may underrepresent estrous cycle-regulated transcripts since we prepared hypothalamic mRNA from several males or females. Regardless, sex hormones control dimorphic expression of most genes we have identified in adult males but not females.
Individual genes control discrete components of male-typical behaviors
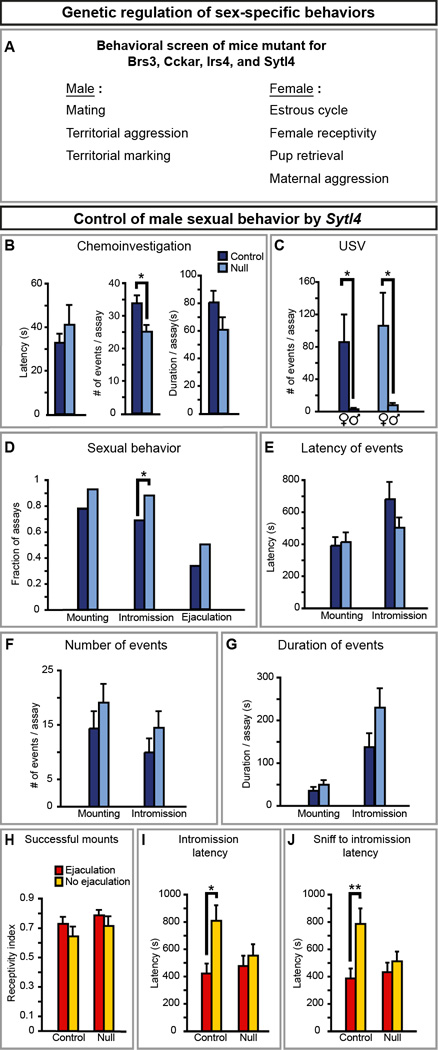
ERα is dimorphically expressed (Figures 4A, S1, S2A) and controls dimorphic behaviors. We sought to determine whether other genes in our list also regulate such behaviors. Male and female mice null for Brs3, Cckar, Irs4, and Sytl4 are fertile but their behavior in standard tests of dimorphic displays is unknown (Ladenheim et al., 2008; Fantin et al., 2000; Gomi et al., 2005; Kopin et al., 1999). We first ascertained that sexual differentiation of brain regions in which these genes are dimorphically expressed is unaffected, at least as revealed by normal ERα expression in the mutant strains (Figure S6). We next examined these mutants for deficits in a range of sex-typical behaviors (Figure 5A).
Figure 5. Sytl4 is required for patterning male sexual behavior.
(A) Mice mutant for Brs3, Cckar, Irs4, or Sytl4 were tested for deficits in various sex- specific displays.
(B) Sytl4−/Y residents (Null) sniff WT female intruders less than Sytl4+/Y residents (Control).
(C) All residents vocalize more to WT female than to WT male intruders.
(D) Sytl4−/Y residents intromit WT female intruders in more tests.
(E–G) The latency, number, and duration of mounts and intromissions are unaffected in Sytl4 mutants.
(H) No difference in fraction of mounts that proceed to intromission between males that ejaculate and males that do not. Receptivity index = (# of mounts with intromission)/(# of all mounts).
(I, J) Control males that ejaculate show a shorter latency to intromit and proceed faster from the first sniff to the first intromission compared to controls who do not ejaculate. These behavioral parameters are decorrelated with ejaculation in null males.
Mean ± SEM; n ≥ 14 animals/genotype; * p < 0.04; ** p < 0.01.
Male mating is elicited with an estrus female, and it consists of chemoinvestigation (sniffing), ultrasonic vocalization (USV), mounting, and intromission (penetration), which can culminate in ejaculation. By contrast, an intruder male is sniffed and attacked by a resident male (Miczek et al., 2001). Male residents also mark territory with many urine spots (Desjardins et al., 1973). Cckar and Irs4 mutant males were similar to WT siblings in these male-typical displays (Figure S4A–T), whereas Brs3 (Table S2) and Sytl4 mutants exhibited behavioral deficits.
Sytl4−/Y mice showed specific changes in some but not all mating parameters (Fig. 5B–G). They sniffed females less but intromitted in more assays, differences that were also confirmed with additional statistical analyses (p < 0.01; data not shown). Although the females allowed intromission, males only ejaculated in a subset of assays as expected (Figure 5D, H). WT males who ejaculate show a reduced latency to intromit and intromit faster after the first sniff (Figure 5I, J). These differences are significant and an indicator of subsequent ejaculation. Although Sytl4−/Y and WT males ejaculated equivalently, loss of Sytl4 function decorrelated mating pattern from ejaculation (Figure 5D, I, J). Sytl4−/Y mice mated in a manner similar to ejaculatory WT males regardless of ejaculation.
Sytl4 mutants do not have pervasive deficits. They attack males and mark urine like WT males (Figure S4U–Z). We also found no deficits in movement, general activity, and social interactions such as grooming (data not shown). Sytl4−/Y mice emit USV to females but not males, indicating that they discriminate between the sexes (Figure 5C). Although Sytl4 regulates insulin release in vitro, Sytl4 mutants have normal insulin titer and a mild decrease in blood glucose (Wang et al., 1999; Gomi et al., 2005). There are also no overt changes in testosterone that could alter mating. The mean and the distribution of serum testosterone titer was similar between Sytl4 null and WT males (WT, 7.2 ± 2.7 nM; Null, 11.6 ± 4.9 nM; n ≥ 13; p > 0.9), with the titer always exceeding the receptor Kd. Testosterone levels were also similar between Sytl4 null and controls at a younger age (Table S3). Thus, Sytl4 controls specific components of male mating.
Individual genes control discrete components of female-typical behaviors
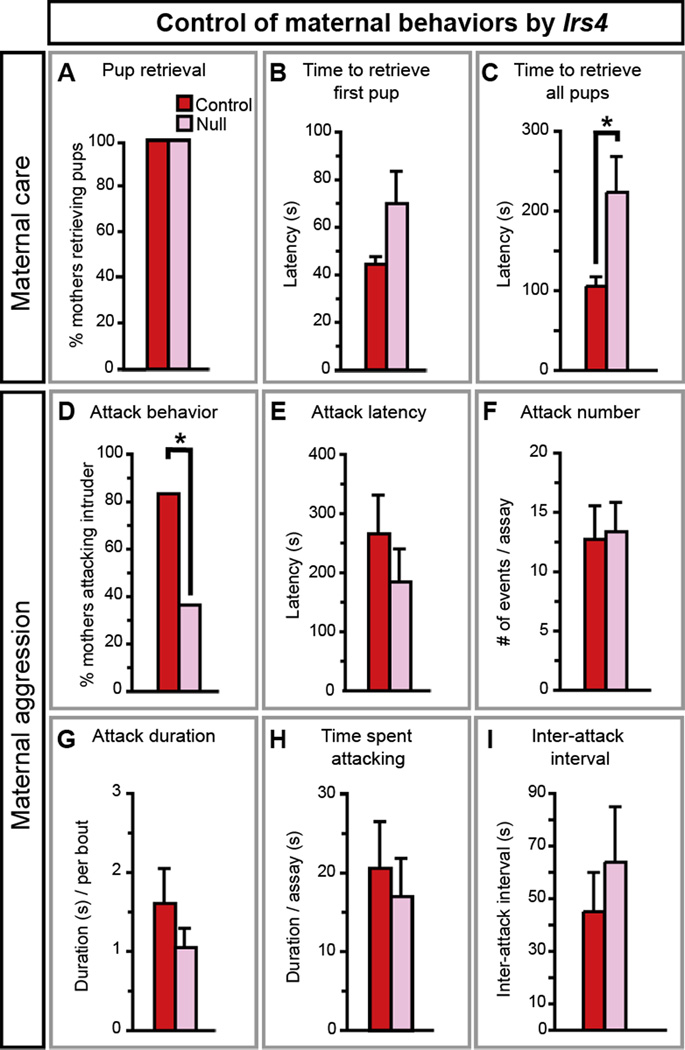
We tested females null for Brs3, Cckar, Irs4, or Sytl4 for deficits in female-typical behaviors. Females reject male mating attempts except during a peri-ovulatory period (estrus) when they are sexually receptive. Nursing females retrieve pups that wander from the nest and attack intruders in the cage (Gandelman, 1972). The mouse estrous cycle is ~5 days. Mutants and controls of each strain had 1 cycle within a 5 day period (n = 3–8/genotype/strain; p ≥ 0.5), indicating that these genes are not essential for estrous cyclicity. Brs3 or Sytl4 null females behaved similar to WT siblings (Figure S5). By contrast, Irs4 and Cckar mutants exhibited deficits in female-typical behaviors.
Irs4 mutants mated, delivered litters, and weaned them in a WT manner (Figure S5, Table S4). In tests of pup retrieval, control and mutant mothers retrieved pups and they did so with similar latencies (Figure 6A, B), but Irs4−/− mothers took longer to retrieve all pups (Figure 6C). Irs4 mutants were also impaired in maternal aggression such that fewer Irs4−/− mothers attacked intruders (Figure 6D, Movies S1, S2). However, when Irs4 mutants attacked, they did so in a WT fashion (Figure 6E–I). Thus, the circuit for maternal aggression appears intact in Irs4−/− females, but it may be activated less frequently than in WT.
Figure 6. Irs4 is essential for maternal behaviors.
(A, B) Irs4−/− (Null) and Irs4−/+ (Control) mothers retrieve pups (A), and the latency to retrieve the first pup is similar between the two groups (B).
(C) Irs4 null mothers take longer to retrieve all pups.
(D) Fewer Irs4 null mothers attack intruder males.
(E–I) When Irs4 null mothers attack intruders they do so similar to control mothers.
Mean ± SEM; n ≥11/genotype; * p < 0.04.
Irs4 mutants do not have systemic deficits. Irs4 is homologous to intracellular adaptor proteins essential for insulin receptor signaling (Burks and White, 2001). However, Irs4−/− females maintain normal weight and blood glucose and insulin titers (Fantin et al., 2000). The mutants also showed WT activity in social interaction and motor performance (Table S4). Thus, Irs4 is specifically required for maternal behaviors that may be essential for pup survival in nature because mouse pups are altricial and adults are infanticidal toward young of other mice.
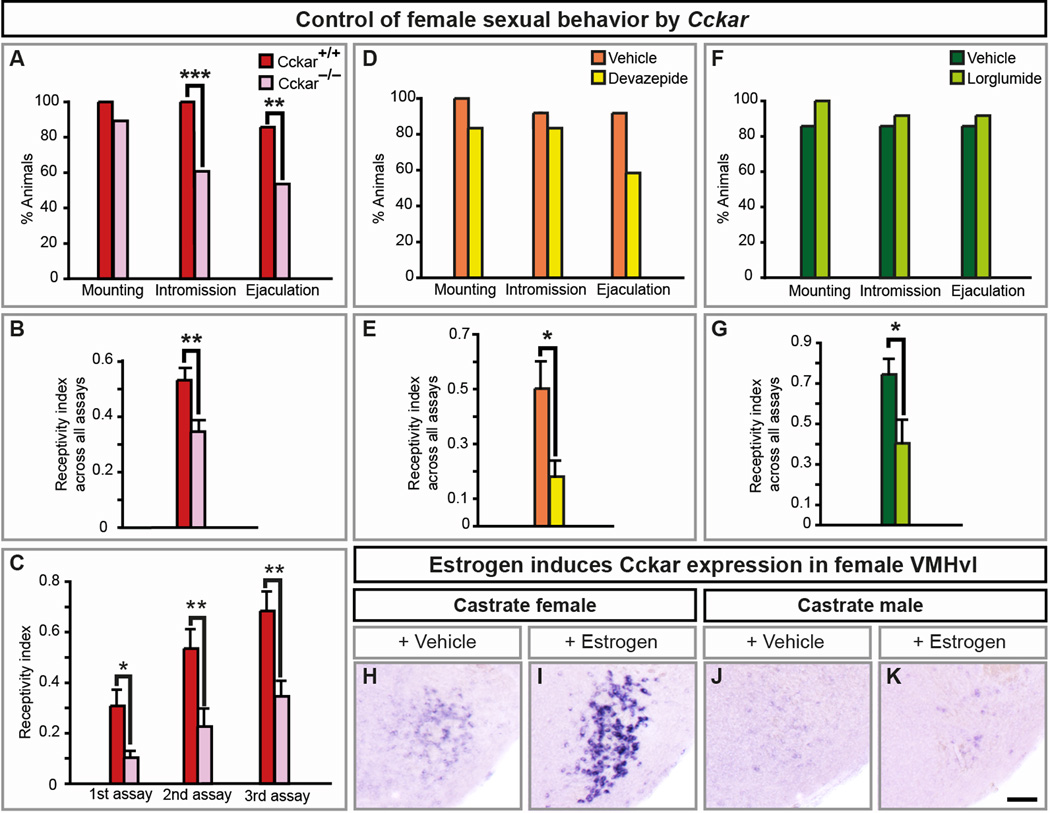
Although Cckar, a cholecystokinin receptor, can control feeding and metabolism (Pirnik et al., 2010), Cckar null mice are normoglycemic and maintain normal body weight (Whited et al., 2006). Studies in rats have been inconclusive on the role of CCK and Cckar in the estrous cycle and female receptivity (Akesson et al., 1987; Hilke et al., 2007; Oro et al., 1988; Dornan et al., 1989; Babcock et al., 1988; Bloch et al., 1987; Holland et al., 1997; Mendelson and Gorzalka, 1984). We find a diminution in receptivity in Cckar−/− females such that they do not readily permit intromission or ejaculation (Figure 7A). This deficit is not due to reduced interest by WT males who mounted all females equivalently (Figure 7A). Even in assays with intromission, Cckar mutants allowed fewer mounts to progress to intromission (Figure 7B, Movies S3, S4). Sexually experienced WT females permit more intromissions; although experienced Cckar−/− females allowed more intromissions, they were always less receptive than controls (Figure 7C).
Figure 7. Cckar is essential for female sexual behavior.
(A) WT males mount Cckar null or WT females equivalently, but fewer mutant females allow males to intromit or ejaculate.
(B) Lower receptivity index in Cckar−/− females.
(C) Lower receptivity index in sexually experienced Cckar−/− females.
(D, F) WT males mount, intromit or ejaculate equivalently with females treated with vehicle or Cckar antagonists.
(E, G) Both Cckar antagonists reduce sexual receptivity of females.
(H–K) Estrogen increases Cckar mRNA in the VMHvl of castrate females.
Mean ± SEM; n ≥ 21 (A–C); n ≥ 12/treatment (D–G); n ≥ 3/treatment (H–K); * p < 0.05, ** p < 0.01, *** p ≤ 0.001. Scale bar = 100 µm.
See also Figures S5, S6, and Movies S3, S4.
Cckar expression in the VMHvl requires ovarian hormones (Figures 3, 4C). Estrogen, which elicits receptivity via ERα in the VMHvl (Musatov et al., 2006), induces Cckar in the VMHvl (Figure 7H, I). By contrast, estrogen, which does not elicit receptivity in males, did not induce Cckar in the male VMHvl (Figure 7J, K). We do not yet know if Cckar expression in the VMHvl drives receptivity. Nevertheless, Cckar is induced in the female VMHvl by hormones that drive estrus and receptivity, and Cckar is essential for normal receptivity.
We next tested whether Cckar regulates receptivity in adults. We induced estrus in castrate WT females and injected devazepide or lorglumide, structurally distinct, specific, competitive Cckar antagonists (Berna et al., 2007). Strikingly, these antagonists reduced sexual receptivity (Figure 7E, G). In contrast to our findings with Cckar mutants, WT males intromitted and ejaculated normally with antagonist-treated females (Figure 7A, D, F). This difference may reflect a developmental role of Cckar in the underlying circuit. The behavioral deficits observed in Cckar mutants or antagonist-treated females do not reflect sensorimotor obtundation because they displayed normal general mobility and social interactions (data not shown). Cckar mutant females also exhibited normal maternal behaviors (Figure S5P–U). Thus, our results show that Cckar functions in adult females to control sexual behavior.
DISCUSSION
Our studies reveal a cellular and molecular representation of gender of inordinate complexity in the hypothalamus and amygdala. The genes we have identified provide an entry point for understanding the physiology of dimorphic neural circuits. Our findings also provide evidence for separable genetic programs that control particular components of sexually dimorphic behaviors.
Sex differences in gene expression in the brain
In contrast to the rich array of dimorphisms in mammalian behaviors and neuroanatomy, few dimorphisms in gene expression have been identified in the brain, and the dimorphic function of most of these genes is unknown (De Vries, 1990; Simerly, 2002; McCarthy, 2008; Cahill, 2006; Gagnidze et al., 2010; Dewing et al., 2003). Since most neural functions are common to both sexes, molecular dimorphisms are likely embedded in shared neural circuits. This has made it difficult to prospectively identify dimorphisms in gene expression beyond genes such as those unique to the Y chromosome or genes involved in X-inactivation (Rinn and Snyder, 2005; Rinn et al., 2004). Many dimorphisms in gene expression have been observed in more homogenous tissues such as liver (van Nas et al., 2009; Clodfelter et al., 2006; Yang et al., 2006; Rinn et al., 2004). The Allen Institute of Brain Science (http://mousediversity.alleninstitute.org/) have examined expression of ~70 genes by ISH to reveal additional sexual dimorphisms in the adult mouse brain; this approach, while powerful, would require enormous resources if conducted with all genes. By contrast, we used microarrays to identify potential sex differences in gene expression followed by ISH validation; this approach has yielded a new set of sexually dimorphic genes. One drawback of our approach is the limited sensitivity of microarrays in detecting transcripts present at low abundance. Indeed, our list of dimorphic mRNAs does not include other dimorphically expressed genes such as the androgen receptor, aromatase, or ERβ (Shah et al., 2004; Wu et al., 2009; Roselli and Resko, 1987; Wolfe et al., 2005). Nevertheless, our results provide a general strategy to identify genes controlling dimorphic behaviors by first identifying genes expressed in a sexually dimorphic manner.
Many dimorphic genes we have identified harbor estrogen responsive elements (EREs; data not shown), and ERs may directly regulate transcription of such genes. Indeed, ERα is found on some of these EREs in breast cancer cells (Carroll et al., 2006). Sex chromosome linked genes can influence sexual differentiation of the brain independent of sex hormones (Arnold et al., 2003). All X-linked genes we have identified are regulated by sex hormones, suggesting that these represent a distinct set of X-linked genes. There are many imprinted genes in the mouse brain, with a subset being dimorphically imprinted (Gregg et al., 2010b, 2010a). One of these, Nnat, is expressed from the paternal allele (Kagitani et al., 1997; Gregg et al., 2010b). We show that Nnat is dimorphically upregulated in distinct hypothalamic areas in the two sexes, indicating significant complexity in the control of Nnat expression.
Adult castrates lose behaviors typical of their sex but do not behave like the opposite sex. Thus, a castrate male mouse does not attack males, but it does not display female-typical receptivity. We find that some, but not all, genes switch their sex-typical expression pattern following castration, thereby providing a molecular correlate in the brain of the intermediate behavioral state of castrates. These dimorphic genes may also control morphological plasticity controlled by adult sex hormones in regions such as the MeApd, VMHvl, and POA (Morris et al., 2008b; Cooke, 2006; Dugger et al., 2008, 2007; Balthazart et al., 2010; Konishi, 1989).
Genetic control of sexually dimorphic behaviors
Our findings, in conjunction with previous work (Wu and Shah, 2011), suggest a model in which sex hormones govern a sexually dimorphic gene expression program such that individual genes regulate specific components of dimorphic behaviors. Such a model may be premature since we understand very little about how these genes influence behavior. For example, Sytl4 is upregulated in the male BNSTmpm and Sytl4 mutants, similar to male rats following surgical BNST lesions, mate aberrantly (Emery and Sachs, 1976). Nevertheless, Sytl4 expression in a small set of non-BNST neurons makes it difficult to conclude that Sytl4 functions in the BNST to control mating. In future studies, conditional genetic manipulations will permit a better understanding of gene function in discrete neuronal pools. The genes we have identified can also be used to engineer such conditional genetic manipulations. Thus, genes expressed in the VMHvl could potentially be used to identify neurons that control attacks in mice (Lin et al., 2011).
Individual genes can control complex behaviors as well as reflexive displays (Winslow et al., 1993; Brown et al., 1996; Scheller et al., 1983; Nishimori et al., 1996; Osborne et al., 1997; de Bono and Bargmann, 1998; Bendesky et al., 2011; Liu et al., 2011). We have identified many genes that control sex-specific behaviors. Mice mutant for these genes show deficits in specific behavioral parameters such that the sex-typical repertoire of behaviors is retained and other dimorphic interactions are unaffected. Our findings suggest that it may be possible to deconstruct all sex-typical displays into genetically separable behavioral components. Analogous genetic wiring of dimorphic behaviors may also operate in fruitflies and worms (Von Schilcher, 1976; Garcia et al., 2001), which employ non-hormonal mechanisms of sexual differentiation (Cline and Meyer, 1996; Manoli et al., 2006; Dickson, 2008). The notion that a behavior can be deconstructed into a suite of genetically encoded behavioral modules is similar to findings that complex neuropsychiatric conditions may also consist of discretely heritable traits (Kellendonk et al., 2009). Such studies have also revealed that different mutations in a gene can lead to distinct phenotypes (Zoghbi and Warren, 2010). It will be interesting to determine if mice mutant for the genes we have identified also exhibit other behavioral phenotypes.
Genetic wiring of components of dimorphic behaviors has intuitive appeal since these behaviors are subject to stringent selection. Such wiring allows evolutionary modulation of a reproductive behavior without disrupting it entirely. Social experience can modify sex-specific behaviors (Insel and Fernald, 2004), and it will be interesting to test if it alters sexually dimorphic expression of genes that control these behaviors. In summary, our findings suggest that sexually dimorphic displays may be a composite of behavioral routines that are genetically separable. It is possible that all innate social behaviors can be deconstructed similarly. In the case of sexually dimorphic displays, it will be important to identify all the underlying genes, and to understand how these genes act within neural circuits to influence social interactions.
EXPERIMENTAL PROCEDURES
Animals
Adult mice were used for all studies. Mice null for Brs3, Cckar, Irs4, and Sytl4 have been described previously (Kopin et al., 1999; Ladenheim et al., 2008; Fantin et al., 2000; Gomi et al., 2005). All studies were in accordance with IACUC protocols at UCSF.
Histology
We performed ISH on serial coronal sections spanning the rostrocaudal extent of the hypothalamus and MeA. To identify sex differences in gene expression, we performed ISH from male and female brains in parallel. This procedure precludes variability of signal:noise arising from lot-to-lot changes in reagents and permits direct sex comparison of expression.
Behavioral Assays
Testing was initiated, recorded, and analyzed as described previously (Juntti et al., 2010; Wu et al., 2009). We tested the role of Cckar in sexual receptivity once with sexually experienced females. Estrus was induced and, 20 min prior to being introduced to a sexually experienced WT male resident for 30 min, females were injected intraperitoneally with 50 µL of Devazepide (500 µg; Tocris) or Lorglumide (500 µg; Sigma) resuspended in DMSO and saline, respectively. In preliminary studies we failed to observe any effect of Lorglumide at a low dose of 50 µg/female. The antagonist doses we chose were based primarily on studies of gastric emptying and feeding with doses up to 250 µg/mouse. We increased this higher dose 2-fold to permit more antagonist to reach neurons and used 500 µg/adult mouse for further studies.
Statistical Analysis
Quantitation of data was performed blind to relevant variables, including sex, genotype, and drug treatment. Unless otherwise specified, we performed the following statistical tests. Categorical data was analyzed by a Fisher’s exact test. For other comparisons, we first tested the distribution of the data with Lilliefors’ goodness-of-fit normality test. Data not violating the test were analyzed with parametric tests (Student’s t test) and other data was analyzed with the non-parametric Wilcoxon rank sum test.
Microarray
MEEBO arrays (Verdugo and Medrano, 2006) were used for all hybridizations and the data has been deposited in the NIH GEO database (accession #: GSE33307). Different normalization schemes for microarray analysis make distinct assumptions about the data. We performed various normalizations to generate a list-of-list of dimorphic genes that were robust to different normalizations and enriched in the hypothalamus compared to whole brain.
Supplementary Material
ACKNOWLEDGMENTS
We thank R. Axel, H. Baier, H. Bourne, and S. Lomvardas for comments on the manuscript; D. Anderson, C. Bargmann, D. Julius, H. Zoghbi, and Shahlab members for discussions; and A. Barczak, P. Nittler, and E. Tumer for help with microarrays. This work was supported by NSF graduate fellowship (OMA); Sandler postdoctoral fellowship (XX); Genentech graduate fellowship (CFY, JKC); ARCS award (JKC); Edward Mallinckrodt, Jr. Foundation, NARSAD, and NIH (NMS; R01NS049488, DP1OD006425).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akesson TR, Mantyh PW, Mantyh CR, Matt DW, Micevych PE. Estrous cyclicity of 125I-cholecystokinin octapeptide binding in the ventromedial hypothalamic nucleus. Evidence for downmodulation by estrogen. Neuroendocrinology. 1987;45:257–262. doi: 10.1159/000124737. [DOI] [PubMed] [Google Scholar]
- Allen E, Doisy EA. An ovarian hormone. Preliminary report on its localization, extraction and partial purification, and action in test animals. Journal of the American Medical Association. 1923;81:819–821. doi: 10.1001/jama.250.19.2681. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Rissman EF, De Vries GJ. Two perspectives on the origin of sex differences in the brain. Ann. N. Y. Acad. Sci. 2003;1007:176–188. doi: 10.1196/annals.1286.018. [DOI] [PubMed] [Google Scholar]
- Babcock AM, Block GJ, Micevych PE. Injections of cholecystokinin into the ventromedial hypothalamic nucleus inhibit lordosis behavior in the rat. Physiol. Behav. 1988;43:195–199. doi: 10.1016/0031-9384(88)90237-5. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Charlier TD, Barker JM, Yamamura T, Ball GF. Sex steroid-induced neuroplasticity and behavioral activation in birds. Eur. J. Neurosci. 2010;32:2116–2132. doi: 10.1111/j.1460-9568.2010.07518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum MJ. Activational and organizational effects of estradiol on male behavioral neuroendocrine function. Scand J Psychol. 2003;44:213–220. doi: 10.1111/1467-9450.00338. [DOI] [PubMed] [Google Scholar]
- Beeman EA. The effect of male hormone on aggressive behavior in mice. Physiol. Zool. 1947;20:373–405. doi: 10.1086/physzool.20.4.30151969. [DOI] [PubMed] [Google Scholar]
- Bendesky A, Tsunozaki M, Rockman MV, Kruglyak L, Bargmann CI. Catecholamine receptor polymorphisms affect decision-making in C. elegans. [[Accessed April 17, 2011]];Nature. 2011 doi: 10.1038/nature09821. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21412235. [DOI] [PMC free article] [PubMed]
- Berna MJ, Tapia JA, Sancho V, Jensen RT. Progress in developing cholecystokinin (CCK)/gastrin receptor ligands that have therapeutic potential. Curr Opin Pharmacol. 2007;7:583–592. doi: 10.1016/j.coph.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein JD. Neuroendocrine regulation of feminine sexual behavior: lessons from rodent models and thoughts about humans. Annu Rev Psychol. 2008;59:93–118. doi: 10.1146/annurev.psych.59.103006.093556. [DOI] [PubMed] [Google Scholar]
- Bloch GJ, Babcock AM, Gorski RA, Micevych PE. Cholecystokinin stimulates and inhibits lordosis behavior in female rats. Physiol. Behav. 1987;39:217–224. doi: 10.1016/0031-9384(87)90012-6. [DOI] [PubMed] [Google Scholar]
- de Bono M, Bargmann CI. Natural variation in a neuropeptide Y receptor homolog modifies social behavior and food response in C. elegans. Cell. 1998;94:679–689. doi: 10.1016/s0092-8674(00)81609-8. [DOI] [PubMed] [Google Scholar]
- Brown JR, Ye H, Bronson RT, Dikkes P, Greenberg ME. A defect in nurturing in mice lacking the immediate early gene fosB. Cell. 1996;86:297–309. doi: 10.1016/s0092-8674(00)80101-4. [DOI] [PubMed] [Google Scholar]
- Burks DJ, White MF. IRS proteins and beta-cell function. Diabetes. 2001;50 Suppl 1:S140–S145. doi: 10.2337/diabetes.50.2007.s140. [DOI] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Organization of projections from the medial nucleus of the amygdala: a PHAL study in the rat. J. Comp. Neurol. 1995;360:213–245. doi: 10.1002/cne.903600203. [DOI] [PubMed] [Google Scholar]
- Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, et al. Genome-wide analysis of estrogen receptor binding sites. Nat. Genet. 2006;38:1289–1297. doi: 10.1038/ng1901. [DOI] [PubMed] [Google Scholar]
- Cline TW, Meyer BJ. Vive la différence: males vs females in flies vs worms. Annu. Rev. Genet. 1996;30:637–702. doi: 10.1146/annurev.genet.30.1.637. [DOI] [PubMed] [Google Scholar]
- Clodfelter KH, Holloway MG, Hodor P, Park S-H, Ray WJ, Waxman DJ. Sex-dependent liver gene expression is extensive and largely dependent upon signal transducer and activator of transcription 5b (STAT5b): STAT5b-dependent activation of male genes and repression of female genes revealed by microarray analysis. Mol. Endocrinol. 2006;20:1333–1351. doi: 10.1210/me.2005-0489. [DOI] [PubMed] [Google Scholar]
- Cooke BM. Steroid-dependent plasticity in the medial amygdala. Neuroscience. 2006;138:997–1005. doi: 10.1016/j.neuroscience.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Cooke B, Hegstrom CD, Villeneuve LS, Breedlove SM. Sexual differentiation of the vertebrate brain: principles and mechanisms. Front Neuroendocrinol. 1998;19:323–362. doi: 10.1006/frne.1998.0171. [DOI] [PubMed] [Google Scholar]
- Desjardins C, Maruniak JA, Bronson FH. Social rank in house mice: differentiation revealed by ultraviolet visualization of urinary marking patterns. Science. 1973;182:939–941. doi: 10.1126/science.182.4115.939. [DOI] [PubMed] [Google Scholar]
- Dewing P, Shi T, Horvath S, Vilain E. Sexually dimorphic gene expression in mouse brain precedes gonadal differentiation. Brain Res. Mol. Brain Res. 2003;118:82–90. doi: 10.1016/s0169-328x(03)00339-5. [DOI] [PubMed] [Google Scholar]
- Dickson BJ. Wired for sex: the neurobiology of Drosophila mating decisions. Science. 2008;322:904–909. doi: 10.1126/science.1159276. [DOI] [PubMed] [Google Scholar]
- Dornan WA, Bloch GJ, Priest CA, Micevych PE. Microinjection of cholecystokinin into the medial preoptic nucleus facilitates lordosis behavior in the female rat. Physiol. Behav. 1989;45:969–974. doi: 10.1016/0031-9384(89)90223-0. [DOI] [PubMed] [Google Scholar]
- Dugger BN, Morris JA, Jordan CL, Breedlove SM. Androgen receptors are required for full masculinization of the ventromedial hypothalamus (VMH) in rats. Horm Behav. 2007;51:195–201. doi: 10.1016/j.yhbeh.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugger BN, Morris JA, Jordan CL, Breedlove SM. Gonadal steroids regulate neural plasticity in the sexually dimorphic nucleus of the preoptic area of adult male and female rats. Neuroendocrinology. 2008;88:17–24. doi: 10.1159/000119740. [DOI] [PubMed] [Google Scholar]
- Dulac C, Wagner S. Genetic analysis of brain circuits underlying pheromone signaling. Annu. Rev. Genet. 2006;40:449–467. doi: 10.1146/annurev.genet.39.073003.093937. [DOI] [PubMed] [Google Scholar]
- Emery DE, Sachs BD. Copulatory behavior in male rats with lesions in the bed nucleus of the stria terminalis. Physiol. Behav. 1976;17:803–806. doi: 10.1016/0031-9384(76)90044-5. [DOI] [PubMed] [Google Scholar]
- Enoch M-A, Hodgkinson CA, Yuan Q, Albaugh B, Virkkunen M, Goldman D. GABRG1 and GABRA2 as independent predictors for alcoholism in two populations. Neuropsychopharmacology. 2009;34:1245–1254. doi: 10.1038/npp.2008.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantin VR, Wang Q, Lienhard GE, Keller SR. Mice lacking insulin receptor substrate 4 exhibit mild defects in growth, reproduction, and glucose homeostasis. Am. J. Physiol. Endocrinol. Metab. 2000;278:E127–E133. doi: 10.1152/ajpendo.2000.278.1.E127. [DOI] [PubMed] [Google Scholar]
- Foradori CD, Weiser MJ, Handa RJ. Non-genomic actions of androgens. Front Neuroendocrinol. 2008;29:169–181. doi: 10.1016/j.yfrne.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnidze K, Pfaff DW, Mong JA. Gene expression in neuroendocrine cells during the critical period for sexual differentiation of the brain. Prog. Brain Res. 2010;186:97–111. doi: 10.1016/B978-0-444-53630-3.00007-5. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Nelson RJ. cFOS and pCREB activation and maternal aggression in mice. Brain Res. 2001;898:232–241. doi: 10.1016/s0006-8993(01)02189-8. [DOI] [PubMed] [Google Scholar]
- Gandelman R. Mice: postpartum aggression elicited by the presence of an intruder. Horm Behav. 1972;3:23–28. doi: 10.1016/0018-506x(72)90003-7. [DOI] [PubMed] [Google Scholar]
- Garcia LR, Mehta P, Sternberg PW. Regulation of distinct muscle behaviors controls the C. elegans male’s copulatory spicules during mating. Cell. 2001;107:777–788. doi: 10.1016/s0092-8674(01)00600-6. [DOI] [PubMed] [Google Scholar]
- Ghosh MG, Thompson DA, Weigel RJ. PDZK1 and GREB1 are estrogen-regulated genes expressed in hormone-responsive breast cancer. Cancer Res. 2000;60:6367–6375. [PubMed] [Google Scholar]
- Gomi H, Mizutani S, Kasai K, Itohara S, Izumi T. Granuphilin molecularly docks insulin granules to the fusion machinery. J. Cell Biol. 2005;171:99–109. doi: 10.1083/jcb.200505179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg C, Zhang J, Butler JE, Haig D, Dulac C. Sex-specific parent-of-origin allelic expression in the mouse brain. Science. 2010a;329:682–685. doi: 10.1126/science.1190831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg C, Zhang J, Weissbourd B, Luo S, Schroth GP, Haig D, Dulac C. High-resolution analysis of parent-of-origin allelic expression in the mouse brain. Science. 2010b;329:643–648. doi: 10.1126/science.1190830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillamón A, Segovia S, del Abril A. Early effects of gonadal steroids on the neuron number in the medial posterior region and the lateral division of the bed nucleus of the stria terminalis in the rat. Brain Res. Dev. Brain Res. 1988;44:281–290. doi: 10.1016/0165-3806(88)90226-x. [DOI] [PubMed] [Google Scholar]
- Henderson LP. Steroid modulation of GABAA receptor-mediated transmission in the hypothalamus: effects on reproductive function. Neuropharmacology. 2007;52:1439–1453. doi: 10.1016/j.neuropharm.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilke S, Hökfelt T, Darwish M, Theodorsson E. Cholecystokinin levels in the rat brain during the estrous cycle. Brain Res. 2007;1144:70–73. doi: 10.1016/j.brainres.2007.01.107. [DOI] [PubMed] [Google Scholar]
- Holland KL, Popper P, Micevych PE. Infusion of CCK-A receptor mRNA antisense oligodeoxynucleotides inhibits lordosis behavior. Physiol. Behav. 1997;62:537–543. doi: 10.1016/s0031-9384(97)80331-9. [DOI] [PubMed] [Google Scholar]
- Holmes MM, McCutcheon J, Forger NG. Sex differences in NeuN- and androgen receptor-positive cells in the bed nucleus of the stria terminalis are due to Bax-dependent cell death. Neuroscience. 2009;158:1251–1256. doi: 10.1016/j.neuroscience.2008.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Fernald RD. How the brain processes social information: searching for the social brain. Annu. Rev. Neurosci. 2004;27:697–722. doi: 10.1146/annurev.neuro.27.070203.144148. [DOI] [PubMed] [Google Scholar]
- Juntti SA, Tollkuhn J, Wu MV, Fraser EJ, Soderborg T, Tan S, Honda S-I, Harada N, Shah NM. The androgen receptor governs the execution, but not programming, of male sexual and territorial behaviors. Neuron. 2010;66:260–272. doi: 10.1016/j.neuron.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagitani F, Kuroiwa Y, Wakana S, Shiroishi T, Miyoshi N, Kobayashi S, Nishida M, Kohda T, Kaneko-Ishino T, Ishino F. Peg5/Neuronatin is an imprinted gene located on sub-distal chromosome 2 in the mouse. Nucleic Acids Res. 1997;25:3428–3432. doi: 10.1093/nar/25.17.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayasuga Y, Chiba S, Suzuki M, Kikusui T, Matsuwaki T, Yamanouchi K, Kotaki H, Horai R, Iwakura Y, Nishihara M. Alteration of behavioural phenotype in mice by targeted disruption of the progranulin gene. Behav. Brain Res. 2007;185:110–118. doi: 10.1016/j.bbr.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Kellendonk C, Simpson EH, Kandel ER. Modeling cognitive endophenotypes of schizophrenia in mice. Trends Neurosci. 2009;32:347–358. doi: 10.1016/j.tins.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, Ehret G. Immunocytochemical localization and quantitation of estrogen-binding cells in the male and female (virgin, pregnant, lactating) mouse brain. Brain Res. 1989;489:101–112. doi: 10.1016/0006-8993(89)90012-7. [DOI] [PubMed] [Google Scholar]
- Konishi M. Birdsong for neurobiologists. Neuron. 1989;3:541–549. doi: 10.1016/0896-6273(89)90264-x. [DOI] [PubMed] [Google Scholar]
- Kopin AS, Mathes WF, McBride EW, Nguyen M, Al-Haider W, Schmitz F, Bonner-Weir S, Kanarek R, Beinborn M. The cholecystokinin-A receptor mediates inhibition of food intake yet is not essential for the maintenance of body weight. J. Clin. Invest. 1999;103:383–391. doi: 10.1172/JCI4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudwa AE, Rissman EF. Double oestrogen receptor alpha and beta knockout mice reveal differences in neural oestrogen-mediated progestin receptor induction and female sexual behaviour. J. Neuroendocrinol. 2003;15:978–983. doi: 10.1046/j.1365-2826.2003.01089.x. [DOI] [PubMed] [Google Scholar]
- Kudwa AE, Michopoulos V, Gatewood JD, Rissman EF. Roles of estrogen receptors alpha and beta in differentiation of mouse sexual behavior. Neuroscience. 2006;138:921–928. doi: 10.1016/j.neuroscience.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Ladenheim EE, Hamilton NL, Behles RR, Bi S, Hampton LL, Battey JF, Moran TH. Factors contributing to obesity in bombesin receptor subtype-3-deficient mice. Endocrinology. 2008;149:971–978. doi: 10.1210/en.2007-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauber AH, Mobbs CV, Muramatsu M, Pfaff DW. Estrogen receptor messenger RNA expression in rat hypothalamus as a function of genetic sex and estrogen dose. Endocrinology. 1991;129:3180–3186. doi: 10.1210/endo-129-6-3180. [DOI] [PubMed] [Google Scholar]
- Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P, Anderson DJ. Functional identification of an aggression locus in the mouse hypothalamus. Nature. 2011;470:221–226. doi: 10.1038/nature09736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lishko PV, Botchkina IL, Kirichok Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature. 2011;471:387–391. doi: 10.1038/nature09767. [DOI] [PubMed] [Google Scholar]
- Lin Y, Jiang Y, Si Y, Kim J-Y, Chen Z-F, Rao Y. Molecular regulation of sexual preference revealed by genetic studies of 5-HT in the brains of male mice. Nature. 2011;472:95–99. doi: 10.1038/nature09822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O’Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoli DS, Meissner GW, Baker BS. Blueprints for behavior: genetic specification of neural circuitry for innate behaviors. Trends Neurosci. 2006;29:444–451. doi: 10.1016/j.tins.2006.06.006. [DOI] [PubMed] [Google Scholar]
- McCarthy MM. Estradiol and the developing brain. Physiol. Rev. 2008;88:91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt MA, Glidewell-Kenney C, Jimenez MA, Ahearn PC, Weiss J, Jameson JL, Levine JE. New insights into the classical and non-classical actions of estrogen: evidence from estrogen receptor knock-out and knock-in mice. Mol. Cell. Endocrinol. 2008;290:24–30. doi: 10.1016/j.mce.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill TE, Tucker GR. Genotype and sex drive in intact and in castrated male mice. Science. 1964;145:514–515. doi: 10.1126/science.145.3631.514. [DOI] [PubMed] [Google Scholar]
- Mendelson SD, Gorzalka BB. Cholecystokinin-octapeptide produces inhibition of lordosis in the female rat. Pharmacol. Biochem. Behav. 1984;21:755–759. doi: 10.1016/s0091-3057(84)80015-5. [DOI] [PubMed] [Google Scholar]
- Micevych P, Dominguez R. Membrane estradiol signaling in the brain. Front Neuroendocrinol. 2009;30:315–327. doi: 10.1016/j.yfrne.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Maxson SC, Fish EW, Faccidomo S. Aggressive behavioral phenotypes in mice. Behav. Brain Res. 2001;125:167–181. doi: 10.1016/s0166-4328(01)00298-4. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat. Neurosci. 2004;7:1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual dimorphism in neuronal number of the posterodorsal medial amygdala is independent of circulating androgens and regional volume in adult rats. J. Comp. Neurol. 2008a;506:851–859. doi: 10.1002/cne.21536. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, King ZA, Northcutt KV, Breedlove SM. Sexual dimorphism and steroid responsiveness of the posterodorsal medial amygdala in adult mice. Brain Res. 2008b;1190:115–121. doi: 10.1016/j.brainres.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow EM, Kane A, Goff DC, Walsh CA. Sequence analysis of P21-activated kinase 3 (PAK3) in chronic schizophrenia with cognitive impairment. Schizophr. Res. 2008;106:265–267. doi: 10.1016/j.schres.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Kaplitt MG, Ogawa S. RNAi-mediated silencing of estrogen receptor {alpha} in the ventromedial nucleus of hypothalamus abolishes female sexual behaviors. Proc. Natl. Acad. Sci. U.S.A. 2006;103:10456–10460. doi: 10.1073/pnas.0603045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nas A, Guhathakurta D, Wang SS, Yehya N, Horvath S, Zhang B, Ingram-Drake L, Chaudhuri G, Schadt EE, Drake TA, et al. Elucidating the role of gonadal hormones in sexually dimorphic gene coexpression networks. Endocrinology. 2009;150:1235–1249. doi: 10.1210/en.2008-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RJ, Demas GE, Huang PL, Fishman MC, Dawson VL, Dawson TM, Snyder SH. Behavioural abnormalities in male mice lacking neuronal nitric oxide synthase. Nature. 1995;378:383–386. doi: 10.1038/378383a0. [DOI] [PubMed] [Google Scholar]
- Nett ST, Jorge-Rivera JC, Myers M, Clark AS, Henderson LP. Properties and sex-specific differences of GABAA receptors in neurons expressing gamma1 subunit mRNA in the preoptic area of the rat. J. Neurophysiol. 1999;81:192–203. doi: 10.1152/jn.1999.81.1.192. [DOI] [PubMed] [Google Scholar]
- Nishimori K, Young LJ, Guo Q, Wang Z, Insel TR, Matzuk MM. Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc. Natl. Acad. Sci. U.S.A. 1996;93:11699–11704. doi: 10.1073/pnas.93.21.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Chester AE, Hewitt SC, Walker VR, Gustafsson JA, Smithies O, Korach KS, Pfaff DW. Abolition of male sexual behaviors in mice lacking estrogen receptors alpha and beta (alpha beta ERKO) Proc. Natl. Acad. Sci. U.S.A. 2000;97:14737–14741. doi: 10.1073/pnas.250473597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oro AE, Simerly RB, Swanson LW. Estrous cycle variations in levels of cholecystokinin immunoreactivity within cells of three interconnected sexually dimorphic forebrain nuclei. Evidence for a regulatory role for estrogen. Neuroendocrinology. 1988;47:225–235. doi: 10.1159/000124916. [DOI] [PubMed] [Google Scholar]
- Osborne KA, Robichon A, Burgess E, Butland S, Shaw RA, Coulthard A, Pereira HS, Greenspan RJ, Sokolowski MB. Natural behavior polymorphism due to a cGMP-dependent protein kinase of Drosophila. Science. 1997;277:834–836. doi: 10.1126/science.277.5327.834. [DOI] [PubMed] [Google Scholar]
- Pirnik Z, Maixnerová J, Matysková R, Koutová D, Zelezná B, Maletínská L, Kiss A. Effect of anorexinergic peptides, cholecystokinin (CCK) and cocaine and amphetamine regulated transcript (CART) peptide, on the activity of neurons in hypothalamic structures of C57Bl/6 mice involved in the food intake regulation. Peptides. 2010;31:139–144. doi: 10.1016/j.peptides.2009.09.035. [DOI] [PubMed] [Google Scholar]
- Raskin K, de Gendt K, Duittoz A, Liere P, Verhoeven G, Tronche F, Mhaouty-Kodja S. Conditional inactivation of androgen receptor gene in the nervous system: effects on male behavioral and neuroendocrine responses. J. Neurosci. 2009;29:4461–4470. doi: 10.1523/JNEUROSCI.0296-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- Ring JR. The estrogen-progesterone induction of sexual receptivity in the spayed female mouse. Endocrinology. 1944;34:269–275. [Google Scholar]
- Rinn JL, Snyder M. Sexual dimorphism in mammalian gene expression. Trends Genet. 2005;21:298–305. doi: 10.1016/j.tig.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Rinn JL, Rozowsky JS, Laurenzi IJ, Petersen PH, Zou K, Zhong W, Gerstein M, Snyder M. Major molecular differences between mammalian sexes are involved in drug metabolism and renal function. Dev. Cell. 2004;6:791–800. doi: 10.1016/j.devcel.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Resko JA. The distribution and regulation of aromatase activity in the central nervous system. Steroids. 1987;50:495–508. doi: 10.1016/0039-128x(87)90034-1. [DOI] [PubMed] [Google Scholar]
- Scheller RH, Jackson JF, McAllister LB, Rothman BS, Mayeri E, Axel R. A single gene encodes multiple neuropeptides mediating a stereotyped behavior. Cell. 1983;32:7–22. doi: 10.1016/0092-8674(83)90492-0. [DOI] [PubMed] [Google Scholar]
- Von Schilcher F. The behavior of cacophony, a courtship song mutant in Drosophila melanogaster. Behav Biol. 1976;17:187–196. doi: 10.1016/s0091-6773(76)90444-2. [DOI] [PubMed] [Google Scholar]
- Scordalakes EM, Rissman EF. Aggression in male mice lacking functional estrogen receptor alpha. Behav. Neurosci. 2003;117:38–45. [PubMed] [Google Scholar]
- Shah NM, Pisapia DJ, Maniatis S, Mendelsohn MM, Nemes A, Axel R. Visualizing sexual dimorphism in the brain. Neuron. 2004;43:313–319. doi: 10.1016/j.neuron.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Bushnell CD, Dorsa DM. Estrogen receptor messenger ribonucleic acid in female rat brain during the estrous cycle: a comparison with ovariectomized females and intact males. Endocrinology. 1992;131:381–388. doi: 10.1210/endo.131.1.1612018. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu. Rev. Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J. Comp. Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Pfaff DW. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front Neuroendocrinol. 2008;29:238–257. doi: 10.1016/j.yfrne.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Verdugo RA, Medrano JF. Comparison of gene coverage of mouse oligonucleotide microarray platforms. BMC Genomics. 2006;7:58. doi: 10.1186/1471-2164-7-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries GJ. Sex Differences in Neurotransmitter Systems. Journal of Neuroendocrinology. 1990;2:1–13. doi: 10.1111/j.1365-2826.1990.tb00385.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Takeuchi T, Yokota H, Izumi T. Novel rabphilin-3-like protein associates with insulin-containing granules in pancreatic beta cells. J. Biol. Chem. 1999;274:28542–28548. doi: 10.1074/jbc.274.40.28542. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Ginns EI, O’Carroll A-M, Lolait SJ, Young WS., 3rd Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol. Psychiatry. 2002;7:975–984. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Sannen K, Villalba C, Lubahn DB, Rissman EF, De Vries GJ. Masculine sexual behavior is disrupted in male and female mice lacking a functional estrogen receptor alpha gene. Horm Behav. 1997;32:176–183. doi: 10.1006/hbeh.1997.1419. [DOI] [PubMed] [Google Scholar]
- Whited KL, Thao D, Lloyd KCK, Kopin AS, Raybould HE. Targeted disruption of the murine CCK1 receptor gene reduces intestinal lipid-induced feedback inhibition of gastric function. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;291:G156–G162. doi: 10.1152/ajpgi.00569.2005. [DOI] [PubMed] [Google Scholar]
- Wiesner BP, Mirskaia L. On the endocrine basis of mating in the mouse. Experimental Physiology. 1930;20:273–279. [Google Scholar]
- Winslow JT, Insel TR. The social deficits of the oxytocin knockout mouse. Neuropeptides. 2002;36:221–229. doi: 10.1054/npep.2002.0909. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Wolfe CA, Van Doren M, Walker HJ, Seney ML, McClellan KM, Tobet SA. Sex differences in the location of immunochemically defined cell populations in the mouse preoptic area/anterior hypothalamus. Developmental Brain Research. 2005;157:34–41. doi: 10.1016/j.devbrainres.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Wu MV, Shah NM. Control of masculinization of the brain and behavior. Curr. Opin. Neurobiol. 2011;21:116–123. doi: 10.1016/j.conb.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MV, Manoli DS, Fraser EJ, Coats JK, Tollkuhn J, Honda S-I, Harada N, Shah NM. Estrogen masculinizes neural pathways and sex-specific behaviors. Cell. 2009;139:61–72. doi: 10.1016/j.cell.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Schadt EE, Wang S, Wang H, Arnold AP, Ingram-Drake L, Drake TA, Lusis AJ. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 2006;16:995–1004. doi: 10.1101/gr.5217506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yntema HG, van den Helm B, Kissing J, van Duijnhoven G, Poppelaars F, Chelly J, Moraine C, Fryns JP, Hamel BC, Heilbronner H, et al. A novel ribosomal S6-kinase (RSK4; RPS6KA6) is commonly deleted in patients with complex X-linked mental retardation. Genomics. 1999;62:332–343. doi: 10.1006/geno.1999.6004. [DOI] [PubMed] [Google Scholar]
- van der Zanden LFM, van Rooij IALM, Feitz WFJ, Knight J, Donders ART, Renkema KY, Bongers EMHF, Vermeulen SHHM, Kiemeney LALM, Veltman JA, et al. Common variants in DGKK are strongly associated with risk of hypospadias. Nat. Genet. 2011;43:48–50. doi: 10.1038/ng.721. [DOI] [PubMed] [Google Scholar]
- Zoghbi HY, Warren ST. Neurogenetics: advancing the “next-generation” of brain research. Neuron. 2010;68:165–173. doi: 10.1016/j.neuron.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.