Abstract
The mortality rate of patients who develop acute kidney injury during sepsis nearly doubles. The effectiveness of therapy is hampered because it is usually initiated only after the onset of symptoms. As renal microvascular failure during sepsis is correlated with the generation of reactive nitrogen species, the therapeutic potential of resveratrol, a polyphenol vasodilator that is also capable of scavenging reactive nitrogen species, was investigated using the cecal ligation and puncture (CLP) murine model of sepsis-induced acute kidney injury. Resveratrol when given at 5.5 h following CLP reversed the decline in cortical capillary perfusion, assessed by intravital microscopy, at 6 h in a dose-dependent manner. Resveratrol produced the greatest improvement in capillary perfusion and increased renal blood flow and the glomerular filtration rate without raising systemic pressure. A single dose at 6 h after CLP was unable to improve renal microcirculation assessed at 18 h; however, a second dose at 12 h significantly improved microcirculation and decreased the levels of reactive nitrogen species in tubules, while improving renal function. Moreover, resveratrol given at 6, 12, and 18 h significantly improved survival. Hence, resveratrol may have a dual mechanism of action to restore the renal microcirculation and scavenge reactive nitrogen species, thus protecting the tubular epithelium even when administered after the onset of sepsis.
Keywords: acute kidney injury, glomerular filtration rate, reactive nitrogen species, renal blood flow, resveratrol, sepsis
Sepsis is a disseminated inflammatory response elicited by a microbial infection1 and is the major cause of death among critically ill patients.2,3 Approximately 750,000 patients in the US2 and 18 million people worldwide are affected annually.4 Unfortunately, the foundation of treatment for septic patients remains nonspecific supportive care.1 Early goal-directed therapy, consisting of fluid resuscitation and supportive treatment to protect organ perfusion, can improve survival;5 however, mortality rates still approach 30% even among adequately resuscitated patients.6,7 It is becoming increasingly clear that restoring the macrocirculation is not always sufficient to adequately restore the microcirculation and preserve organ function. This is a critical issue because the severity of microvascular dysfunction correlates with patient mortality.8,9 Development of acute kidney injury (AKI) is common during severe sepsis and more than doubles the mortality rate to nearly 75%.10 Studies in rodent models have shown that the renal microcirculation is compromised during the development of sepsis.11–15 Thus, there is a pressing need for the development of novel therapeutic approaches to treat sepsis-induced AKI, which can restore perfusion of the renal microcirculation even when initiated after the onset of sepsis.6,16,17
Hallmarks of sepsis include excessive generation of nitric oxide (NO),10 endothelial injury, and a loss of vascular reactivity,6,18 resulting in areas of local microcirculatory hypoperfusion6,17 and areas of tissue hypoxia. This leads to a peritubular pro-oxidant microenvironment favoring the generation of reactive oxygen and reactive nitrogen species (RNS).13,19,20 The reaction of NO with superoxide generates peroxynitrite, a powerful oxidizing RNS that causes protein nitration, DNA damage, and mitochondrial dysfunction, all of which have been suggested to be critical to the progression of AKI during sepsis.10,12,13,21 Thus, RNS generation occurring later in the progression of sepsis could be a suitable therapeutic target for agents administered after the onset of sepsis,12,22 but this has not yet been directly studied.
Resveratrol (RES), a polyphenolic nutraceutical with vasodilatory and antioxidant activities, has been shown to be protective in various disease models,23,24 including septic rats, when administered before or at the very onset of sepsis.25,26 We recently showed that RES is a potent scavenger of peroxynitrite and can protect tubular epithelial cells from the damaging effects of peroxynitrite in vitro.27 To investigate the therapeutic potential of RES, we studied its effects on renal macro- and microcirculation and tubular epithelial RNS generation during sepsis. In the first series of experiments, we determined the acute dose effects of RES on the peritubular microcirculation, as well as its acute effects on renal blood flow (RBF) and glomerular filtration rate (GFR). Thereafter, to more closely mimic the clinical setting,28 we used a delayed treatment regimen initiated after the onset of septic shock to examine the effects of RES on peritubular capillary failure, RNS generation, renal function, and survival.
RESULTS
Acute effects of RES administration on renal cortical microcirculation during sepsis
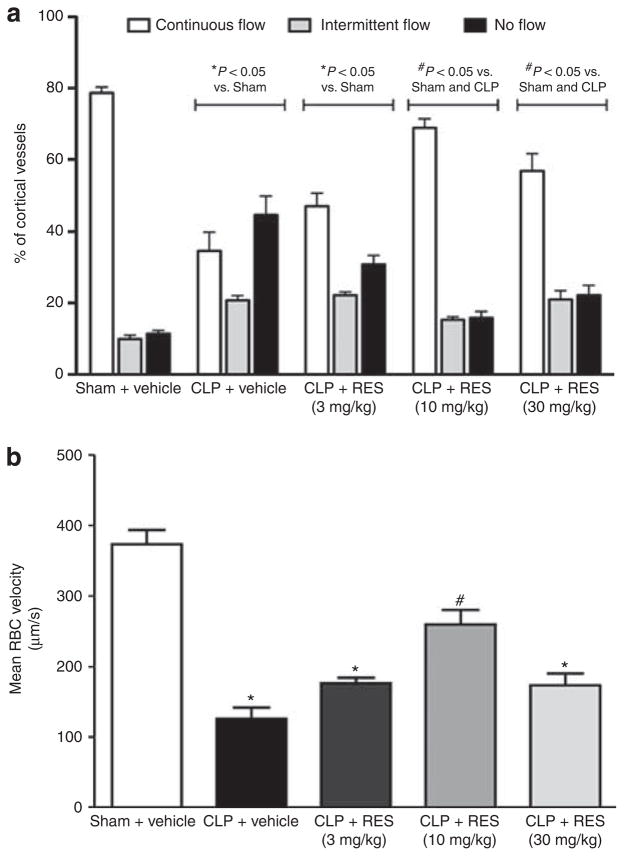
A hallmark of both clinical sepsis and experimental animal models of sepsis is severe microvascular dysfunction.6,29 Using intravital video microscopy (IVVM), we categorized the cortical distribution of peritubular capillary perfusion as continuous, intermittent, or no flow. At 6 h, sham control mice had a high percentage of continuously perfused renal cortical capillaries (79±2%) and only a small percentage of capillaries with no flow (Figure 1). In contrast, at 6 h post cecal ligation and puncture (CLP), the percentage of capillaries with continuous perfusion was reduced to 34±5% and the percentage of capillaries with no perfusion was increased to 45±5%. The acute effects of RES on the renal microcirculation were tested at doses of 3, 10, 30, and 100 mg/kg (intraperitoneal, i.p.) administered at 5.5 h post sham or CLP surgery. Capillary perfusion was then assessed at 6 h. Although the highest dose of RES tested (100 mg/kg) caused no obvious signs of toxicity in sham mice, this dose was lethal in CLP mice within 30 min (100% mortality, n = 3). Therefore, this dose was omitted from all subsequent studies. These categorical data were analyzed using the Hotelling’s T2-test (see Materials and Methods) to compare the distribution between groups. Doses of 10 and 30 mg/kg reversed the decline in capillary perfusion in CLP mice (Figure 1a). As a control, RES (30 mg/kg, i.p.) was administered to sham mice and had no effect on capillary perfusion (data not shown).
Figure 1. Acute effects of resveratrol (RES) on renal cortical microcirculation at 6 h.
Cecal ligation and puncture (CLP) surgery caused a significant reduction in the categorical perfusion (a) and mean red blood cell (RBC) velocity (b) of the peritubular capillaries at 6 h. Resveratrol administration at 5.5 h acutely improved both categorical perfusion and mean RBC velocity measured at 6 h, with 10 mg/kg (intraperitoneal, i.p.) being the most efficacious dose tested to restore RBC velocity. *P<0.05 compared with sham + vehicle; #P<0.05 compared with sham + vehicle and CLP + vehicle; n = 6–7.
As categorical perfusion data do not address nutritive capillary flow, mean red blood cell (RBC) velocity was measured in continually flowing capillaries from the same videos analyzed in Figure 1a. At 6 h, the measured mean RBC velocity in sham mice was 374±20 μm/s. CLP caused a significant reduction in mean RBC velocity compared with sham (126±16 μm/s; P<0.05). Resveratrol produced a bell-shaped dose–response curve for restoring RBC velocity (Figure 1b), with 10 mg/kg being the most efficacious dose tested.
Acute effects of RES administration on mean arterial pressure (MAP) and heart rate (HR)
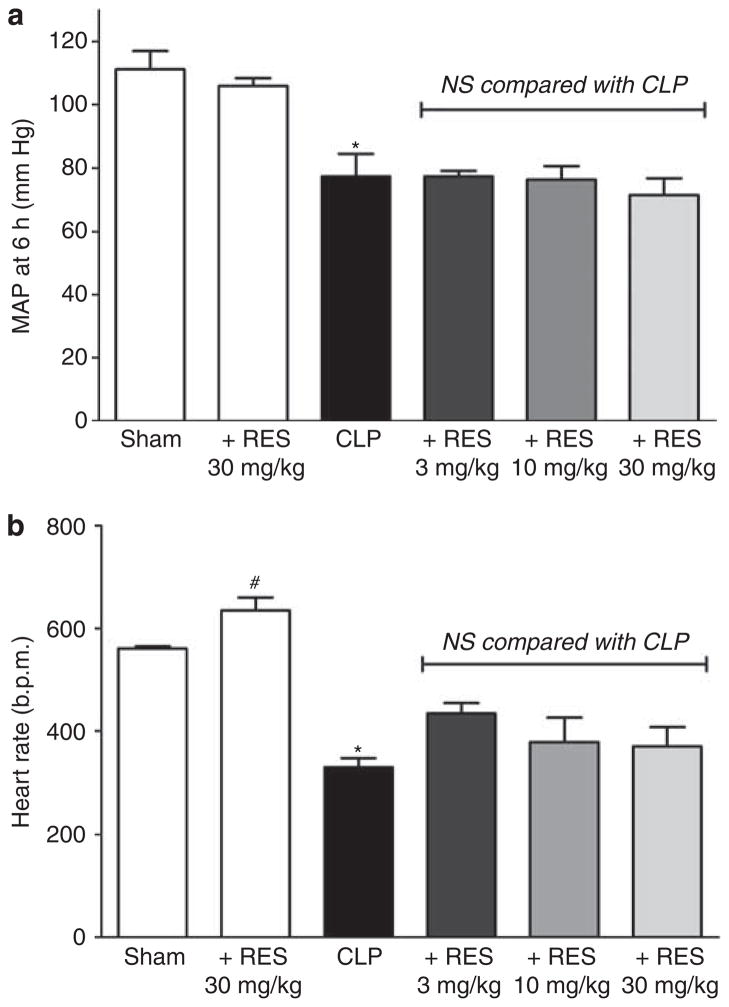
MAP in CLP decreased from 111±7 mm Hg before surgery to 77±7 mm Hg (P<0.05) at 6 h post surgery, whereas MAP in sham did not change significantly (115±7 vs. 111±6 mm Hg). Resveratrol at doses of 3, 10, or 30 mg/kg administered at 5.5 h did not affect MAP at 6 h in CLP, nor did RES at a dose of 30 mg/kg affect MAP in sham (Figure 2a). HR in CLP decreased from 558±49 b.p.m. before surgery to 331±18 b.p.m. (P<0.05) at 6 h, whereas HR in sham did not change significantly (629±27 vs. 561±4 b.p.m.). Resveratrol at doses of 3, 10, or 30 mg/kg administered at 5.5 h did not affect HR at 6 h in CLP, but 30 mg/kg did increase HR slightly in sham (P<0.05; Figure 2b). These data suggested that the increase in microcirculatory perfusion in CLP mice observed following RES administration was not the result of an increase in MAP or HR.
Figure 2. Acute effects of resveratrol (RES) on mean arterial pressure (MAP) and heart rate (HR) at 6 h.
Cecal ligation and puncture (CLP) surgery resulted in a significant decrease in MAP (a) and HR (b) at 6 h. Resveratrol at any of the tested doses had no significant (NS) effect on MAP or HR in CLP mice. However, RES (30 mg/kg, intraperitoneal, i.p.) slightly increased HR in sham. *P<0.05 compared with sham by analysis of variance; #P<0.05 compared with sham by Student’s t-test; n = 3–7.
Acute effect of RES administration on RBF and GFR
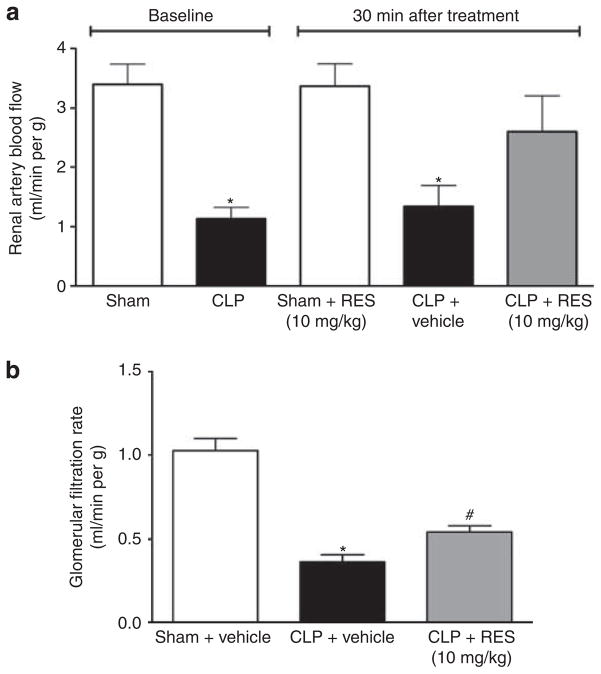
As RBF is a major determinant of renal microcirculatory perfusion, the effects of RES on RBF were determined. At 5.5 h after CLP or sham surgery, a baseline measurement of RBF was obtained and RES (10 mg/kg) or vehicle was administered via the penile vein. A second measurement of RBF was obtained at 6 h. At 5.5 h, RBF was significantly lower in CLP mice compared with sham (1.1±0.2 versus 3.4±0.3 ml/min per g, respectively; P<0.05; Figure 3a). At 30 min post treatment, vehicle had no effect on RBF in CLP mice and RES had no effect on RBF in sham mice. In contrast, RES significantly raised RBF to 2.6±0.6 ml/min per g (P<0.05 compared with CLP), a value not different from that seen in sham.
Figure 3. Acute effects of resveratrol (RES) on renal blood flow (RBF) and glomerular filtration rate (GFR) at 6 h.
Cecal ligation and puncture (CLP) caused a significant reduction in both RBF (a) and GFR (b) at 6 h. Resveratrol (10 mg/kg, i.v.) administered at 5.5 h restored RBF and improved GFR at 6 h. *P<0.05 compared with sham and #P<0.05 compared with sham and CLP; n = 7–11 for (a) and n = 4–5 for (b).
To assess the acute effects of RES administration on renal function, GFR was measured in conscious mice beginning at 6 h after CLP or sham surgery. CLP mice showed a significantly lower GFR at 6 h compared with sham (0.36±0.04 versus 1.03±0.07 ml/min per g; P<0.05). Administration of RES 30 min before GFR measurement in CLP mice (Figure 3b) resulted in an acute improvement in GFR (0.54±0.04 ml/min per g; P<0.05 compared with CLP).
Effects of delayed administration of RES on renal microcirculation at 18 h
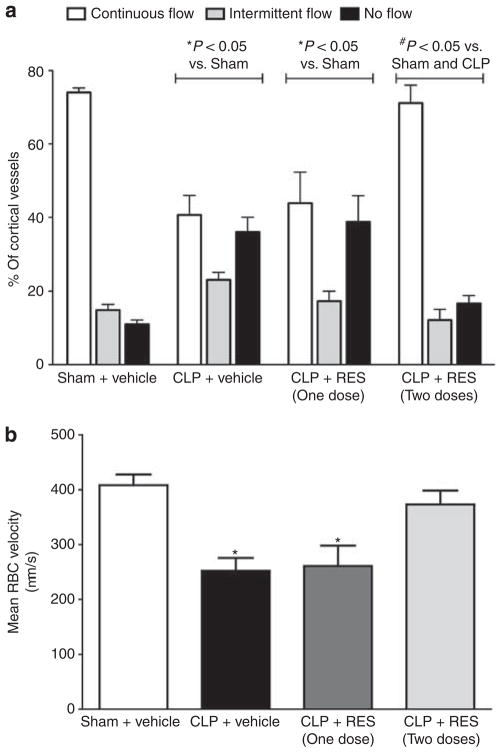
At 18 h following CLP, capillary perfusion and mean RBC velocity were still low relative to sham (Figure 4). A single dose of RES (10 mg/kg, i.p.) administered at 6 h was unable to maintain perfusion through 18 h. However, a second dose administered at 12 h prevented the change in the distribution of cortical perfusion and the decline in RBC velocity (Figure 4a and b).
Figure 4. Effect of delayed resveratrol (RES) administration on renal cortical microcirculation at 18 h.
At 18 h post cecal ligation and puncture (CLP), categorical perfusion (a) and mean red blood cell (RBC) velocity (b) remained low compared with 6 h (Figure 1). A single dose of RES (10 mg/kg, intraperitoneal, i.p.) administered at 6 h did not improve the renal microcirculation at 18 h. However, administration of an additional dose of RES (10 mg/kg, i.p.) at 12 h resulted in a complete restoration of both categorical capillary perfusion (a) and mean RBC velocity (b) at 18 h. *P<0.05 compared with sham and #P<0.05 compared with sham and CLP; n = 6–7.
Effects of delayed administration of RES on RNS generation in the renal tubules
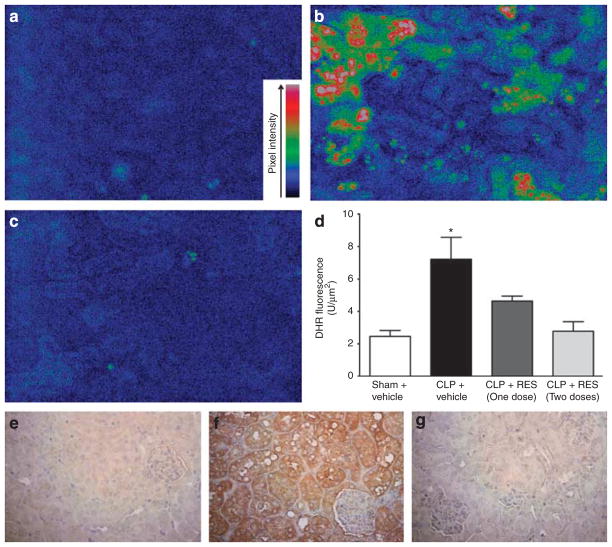
As oxidation of dihydrorhodamine-1,2,3 is not absolutely selective for the RNS peroxynitrite,30,31 two complementary approaches were used to detect the generation of peroxynitrite: (1) oxidation of dihydrorhodamine-1,2,3 to rhodamine and (2) detection of immunoreactive nitrotyrosine–protein adducts.32 We previously reported that rhodamine fluorescence appears in tubules bordered by capillaries with no flow, and that both rhodamine fluorescence and nitrotyrosine—protein adducts appear in the vacuoles of injured tubules.12 Representative pseudocolored images of rhodamine fluorescence from the sham, CLP, and CLP + RES (two doses) groups were captured from the cortices of live mice using IVVM and are presented in Figure 5a–c, respectively, along with the quantification of pixel intensity in Figure 5d. Only weak rhodamine fluorescence was observed in sham at 18 h (Figure 5a), but rhodamine fluorescence was increased in tubules from CLP mice (Figure 5b). A single dose of RES (10 mg/kg) administered at 6 h significantly reduced rhodamine fluorescence in CLP mice, as did two doses (Figure 5d).
Figure 5. Effects of delayed resveratrol (RES) administration on reactive nitrogen species (RNS) generation in the renal tubules at 18 h.
Representative pseudocolored images of rhodamine fluorescence (active generation during the imaging period) for sham, cecal ligation and puncture (CLP), and CLP + RES (two doses) are shown in (a–c), respectively (×200 original magnification). The quantification of rhodamine fluorescence is shown in (d). A single dose of RES at 6 h (10 mg/kg, i.p.) partially, but significantly, reduced the increase in rhodamine fluorescence caused by CLP, whereas a second dose given at 12 h completely blocked rhodamine fluorescence (d). Representative images of immunoreactive nitrotyrosine–protein adducts (cumulative generation of peroxynitrite over 18 h) are shown in (e) and (f) (×400 original magnification). Preincubation of the anti-nitrotyrosine antibody with 10 mmol/l nitrotyrosine was used as the nonspecific binding control (not shown). Tissue from sham mice (e) showed faint specific staining, whereas tissue from CLP mice showed strong specific staining localized to the tubules (f). Tissue from CLP mice treated with two doses of RES showed little specific staining (g) at 18 h. *P<0.05 compared with all other groups; n = 6–7. DHR, dihydrorhodamine-1,2,3.
Representative images of nitrotyrosine staining from the sham, CLP, and CLP + RES (two doses) groups are presented in Figure 5e–g, respectively. Sham tissue displayed only weak staining for nitrotyrosine–protein adducts. In contrast, at 18 h after CLP, nitrotyrosine staining was intense in renal tubules but not in glomeruli. No staining was observed in the nonspecific binding control (not shown), where the anti-nitrotyrosine antibody was preincubated with 10 mmol/l nitrotyrosine before use. Two doses of RES blocked the formation of nitrotyrosine–protein adducts. Both independent methods of detection (ongoing generation with rhodamine and cumulative generation with nitrotyrosine immunohistochemistry) indicated that RES reduced renal levels of RNS.
Effects of delayed administration of RES on systemic NO generation
The systemic inflammatory response following CLP is associated with early release of cytokines such as tumor necrosis factor-α22,33 and increased generation of NO.13 To help address whether RES decreased RNS levels by decreasing NO generation, the effects of RES on serum NO levels were determined by measuring serum nitrate + nitrite concentration. At 18 h, levels in CLP were significantly higher than that in sham (166±16 versus 60±5 μmol/l; P<0.05). Two doses of RES (10 mg/kg) administered at 6 and 12 h did not affect nitrate + nitrite concentration (181±20 μmol/l; P>0.05 compared with CLP).
Effects of delayed administration of RES on renal morphological damage at 18 h
At 18 h, morphological changes in the CLP group were characterized by mild brush border loss, tubular degeneration, and vacuolization in the early segments of proximal tubules (Figure 6b). The two-dose RES treatment regimen reduced the morphological damage and the tubular injury score (Figure 6c and d).
Figure 6. Effects of delayed resveratrol (RES) administration on renal morphology at 18 h.
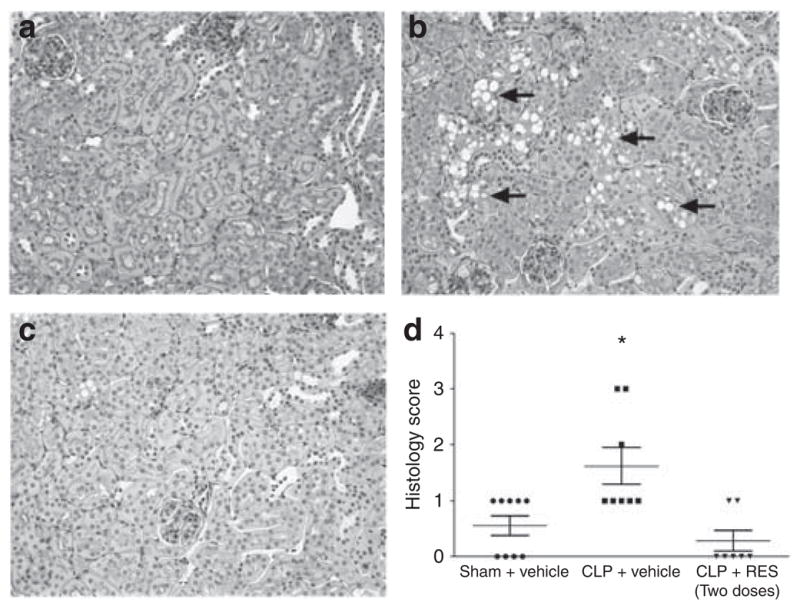
Representative periodic acid–Schiff-stained sections for sham, cecal ligation and puncture (CLP), and CLP + RES (two doses) are shown in (a–c), respectively (×200 original magnification), and tissue injury scores are shown in d. CLP caused mild morphological changes characterized by loss of brush border, cast formation, and vacuolization (indicated by arrows). Administration of two doses of RES significantly reduced the tubular injury score. *P<0.05 compared with sham; n = 6–9.
Effects of delayed administration of RES on renal function at 18 h
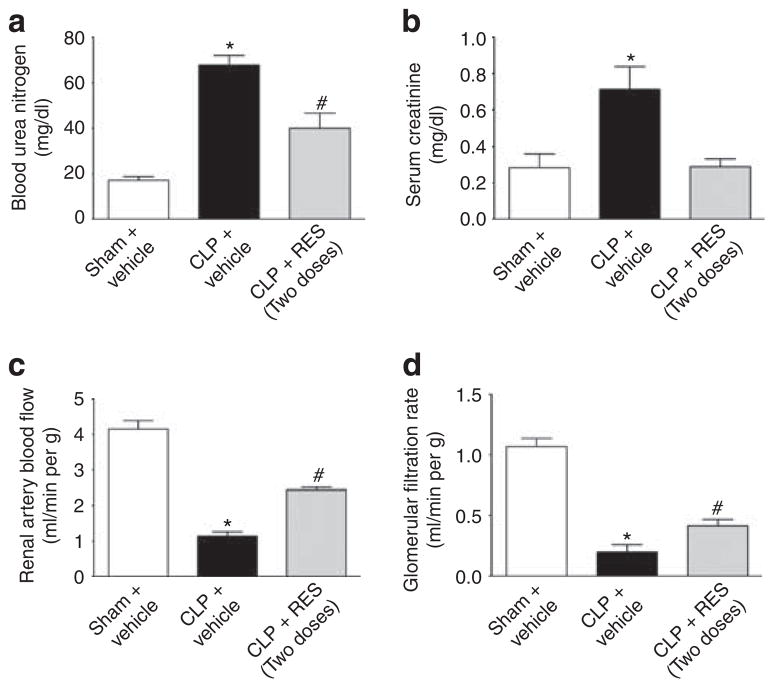
As RES was shown to improve renal microcirculation, RNS generation, and tubular morphology, we investigated the ability of RES to protect against the development of AKI. At 18 h post CLP, two clinically used markers of AKI, blood urea nitrogen, and creatinine, were increased in CLP mice (Figure 7a and b). Although the single dose of RES (10 mg/kg) administered at 6 h reduced only serum creatinine levels (data not shown), two doses of RES administered at 6 and 12 h post CLP significantly reduced both markers (Figure 7a and b). As serum creatinine is no longer considered a reliable marker of GFR and renal function in mice,34 GFR and RBF were measured at 18 h. CLP mice showed a significant reduction in both GFR and RBF compared with sham at 18 h (Figure 7c and d). Administration of two doses of RES significantly improved (doubled) GFR from 0.20±0.06 ml/min per g in CLP to 0.41±0.05 ml/min per g in CLP + RES (P<0.05) and significantly improved (doubled) RBF from 1.1±0.1 ml/min per g in CLP to 2.4±0.1 ml/min per g in CLP + RES (P<0.05). However, RES did not restore RBF or GFR to sham levels.
Figure 7. Effects of delayed resveratrol (RES) administration on renal function at 18 h.
At 18 h post cecal ligation and puncture (CLP), mice showed elevated levels of blood urea nitrogen (a) and serum creatinine (b), as well as significantly reduced renal blood flow (RBF) (c) and glomerular filtration rate (GFR) (d). Administration of two doses of RES significantly decreased blood urea nitrogen and serum creatinine levels, while improving RBF and GFR at 18 h (a–d). *P<0.05 compared with sham and #P<0.05 compared with sham and CLP; n = 4–5 for a and b, n = 6–8 for c and d.
Effects of delayed administration of RES on survival
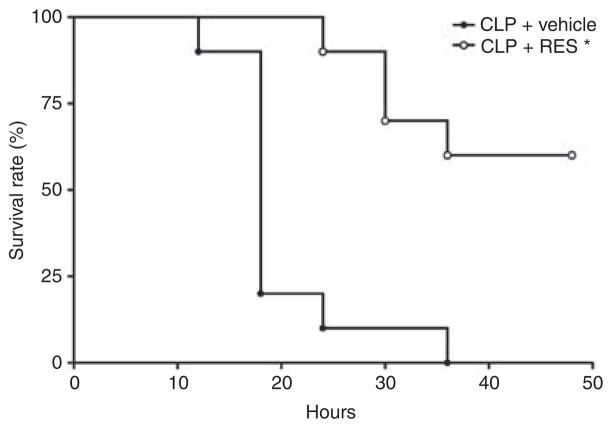
The ability of RES to improve renal microcirculation and restore renal function led us to investigate the potential of RES to increase survival following CLP using a clinically relevant dosing protocol starting 6 h after the induction of sepsis (CLP). Two groups of mice were subjected to CLP. Beginning at 6 h, one group received vehicle at 6, 12, and 18 h, whereas the other group received RES (10 mg/kg, i.p.) at 6, 12, and 18 h. Using a defined criteria of impending mortality (core temperature of <28 °C35), RES significantly improved survival (Figure 8; P<0.001 using the Mantel–Cox log rank test).
Figure 8. Effects of delayed resveratrol (RES) administration on survival.
Administration of RES (10 mg/kg, i.p) to cecal ligation and puncture (CLP) mice at 6, 12, and 18 h resulted in a significant improvement in survival compared with vehicle-treated mice (*P<0.01; Mantel–Cox log-rank test; n = 10/group).
DISCUSSION
Microvascular dysfunction is a strong predictor of death among septic patients.29 In both lipopolysaccharide20,36 and CLP12–14 models of sepsis-induced AKI, peritubular capillary hypoperfusion occurs rapidly and is associated with subsequent generation of peroxynitrite in the renal epithelium. As we recently showed that RES is a potent scavenger of peroxynitrite and can protect tubular epithelial cells from the damaging effects of peroxynitrite in vitro,27 we studied the therapeutic potential of RES using a murine model of sepsis and a clinically relevant treatment regimen.
It is becoming increasingly clear that early goal-directed therapy has the greatest chance of improving the outcome in patients with severe sepsis.37,38 Moreover, targeting the microcirculation to preserve/restore perfusion would not only lessen injury but also promote organ recovery.39,40 Unfortunately, effective therapy in the septic patient is hampered because therapy usually begun only after the onset of symptoms (i.e., systemic inflammatory response syndrome).28 In this study, we examined the acute effects of RES on CLP-induced AKI using a clinically relevant course of therapy that began after the development of septic shock and the decline in renal microcirculatory perfusion.
We chose to examine the effects of RES at 6 h post CLP, a time when systemic inflammation had already begun22 and peritubular capillary perfusion was severely reduced.13 Within 30 min of administration, RES restored both measures of peritubular capillary perfusion: categorical perfusion (an index of overall perfusion) and RBC velocity. Surprisingly, in CLP mice, RES exhibited a bell-shaped dose–response curve. Doses higher than 10 mg/kg were less effective in restoring velocity or, in the case of the 100 mg/kg dose, lethal within 30 min. It is important to note that RES was administered i.p. rather than orally, where extremely high oral doses exhibit little signs of toxicity, presumably because of the low bioavailability of oral RES.41 The mechanisms of such a rapid lethal toxicity in CLP mice at the 100 mg/kg dose is unknown but likely related to the fact that the mice were in septic shock as this dose of RES did not produce signs of toxicity in sham mice.
CLP also induced a rapid decline in MAP within the first 6 h, approaching the lower limit of the reported renal autoregulatory pressure in the mouse necessary to maintain RBF and GFR.42 The state of RBF during sepsis-induced AKI is controversial. For example, in a sheep model of Escherichia coli infusion, RBF increases over time,43 whereas autologous fecal peritonitis in pigs, a model more closely related to CLP, showed a slow decline in RBF as MAP decreased.44 We found that RBF and GFR were significantly reduced at 6 h, paralleling the decline in MAP. It was striking how rapidly RES increased both RBF and GFR in our model. This effect on RBF was likely due to a decrease in renal vascular resistance as RES did not raise MAP or HR and had no effect on RBF in sham mice with normal MAP. One possible mechanism for the observed rapid increase in RBF was the activation of potassium channels45,46 present in both afferent and efferent arterioles.47 Further studies are needed to directly address the mechanism of decreased RBF in septic mice and the ability of RES to restore RBF. Although RES was able to acutely restore RBF, it only partially improved GFR, suggesting that RBF was not the only determinant of GFR. CLP causes renal capillary leakage by 6 h14 and this could have contributed to the reduction in GFR1 and reduced efficacy of RES. Nevertheless, these findings support the notion that agents that reduce renal vascular resistance during sepsis could be beneficial in this hemodynamic (prerenal) form of AKI.48
When sepsis is associated with hypotension and decreased RBF, an increase in renal vascular resistance is a compensatory mechanism to help maintain GFR, albeit at the potential cost of peritubular capillary perfusion.49 The decline in capillary perfusion appeared to be highly dependent on RBF as restoring RBF increased both the number of capillaries perfused and the quality of nutritive flow, despite only a modest improvement in GFR. On the basis of the acute dose–response effects of RES, the dose of 10 mg/kg was evaluated for its ability to prevent AKI, which develops between 18 and 24 h in this model.11,22 Against measures of capillary perfusion, RNS generation, and serum blood urea nitrogen/creatinine, a single dose of RES administered at 6 h improved only some of these markers of injury at 18 h. However, when a second dose was administered at 12 h, there was significant improvement in all markers of renal injury; when mice were dosed through 18 h, 48-h survival was significantly improved, although dosing was delayed until after the onset of renal hemodynamic failure. The ineffectiveness of the single dose to restore perfusion at 18 h may be because of the extremely short half-life of RES (~30 min) in rodents,50,51 which supports the finding that multiple dosing with RES was necessary to prevent renal injury. These findings also raise the possibility that continuous infusion may allow for a lower effective dose and thus lessen the chance for toxicity.
Peritubular capillary hypoperfusion can lead to a hypoxic pro-oxidant microenvironment favoring the generation of RNS.12,20 This can lead to epithelial injury (swelling, cast formation, cell sloughing), which can disrupt tubular–glomerular feedback regulation of GFR52 and perpetuate microcirculatory failure.53 The ability of RES to scavenge peroxynitrite in renal tubular epithelial cells27 is likely another mechanism of protection; however, the potential for RES to reduce peroxynitrite levels indirectly by scavenging superoxide cannot be ruled out.54 Interestingly, even the single dose of RES administered at 6 h reduced the levels of RNS generation by the tubular epithelium at 18 h. This finding supports the notion that RES metabolites (glucuronides and sulfates) retain a degree of RNS-scavenging activity.23,55 Resveratrol may have also upregulated endogenous antioxidant defenses;25,26,56 however, this effect is less likely given the short treatment time and rapid response. Recently, RES was shown to increase mitochondrial biogenesis and/or protect the mitochondria through activation of the protein deacetylase, Sirtuin 1.57,58 This potential additional mechanism warrants further investigation because of the critical role of mitochondrial dysfunction in the development of multiple organ failure during sepsis.59
Although these studies cannot distinguish between the relative roles of hypoperfusion and oxidant generation, the data suggest that interrupting the cycle of renal injury by restoring perfusion as early as possible or by scavenging oxidants may allow the tubular epithelium to recover, lessening AKI and improving survival. Drugs such as RES with this dual mechanism of action (restoration of perfusion and RNS scavenging) may provide a novel therapeutic option for the treatment of sepsis-induced AKI because protection may be possible even when administered after the onset of sepsis.
MATERIALS AND METHODS
CLP model of sepsis
All animals were housed and handled in accordance with the National Institute of Health Guide for the Care of Laboratory Animals with approval from an internal animal care and use committee. CLP was performed on male C57/BL6 mice (Harlan, Indianapolis, IN) aged 39–40 weeks as described previously.12,13 Under isoflurane anesthesia, 1.5 cm of the cecal tip was ligated using a 4-0 silk suture and punctured twice with a 21-gauge needle. An approximately 1-mm column of fecal material was expressed. In sham-operated mice (sham), the cecum was isolated but neither ligated nor punctured. Following surgery all mice received 1 ml of prewarmed saline and were placed in individual cages on a heating pad. Mice studied at time points longer than 6 h were given imipenem/cilastatin (14 mg/kg) and 1.5 ml of normal saline (40 ml/kg, subcutaneous) at 6 h.
Administration of RES
Fresh solutions of trans-resveratrol (Cayman Chemical Company, Ann Arbor, MI) were prepared in dimethyl sulfoxide (vehicle), kept in the dark, and diluted in normal saline just before use. To assess the very acute effects of RES, mice were administered RES at 5.5 h and studied at 6 h. When mice were studied at 18 h, RES was administered at 6 h post CLP and then again at 12 h because of the relative short half-life of RES in rodents (~30 min50,51). For the 48-h survival study, RES was administered through the development of renal injury by dosing at 6, 12, and 18 h.12
Intravital video microscopy
IVVM was performed as described previously.12,13,20 Briefly, following anesthesia, FITC-labeled dextran (500 kDa, Sigma, St Louis, MO) and dihydrorhodamine-1,2,3 (DHR, Invitrogen, Carlsbad, CA) were administered through the penile vein to visualize the capillary vascular space and detect RNS generation, respectively. The doses of FITC-dextran and DHR were 1.4 μmol/kg and 0.8 mg/kg, respectively, in 2.1 ml/kg normal saline. The left kidney was exposed by a flank incision and positioned on a glass stage above an inverted Zeiss Axiovert 200M fluorescent microscope equipped with an Axiocam HSm camera (Zeiss, Jena, Germany). Video images of 10 s (approximately 30 frames/s) at ×200 magnification were acquired from five randomly selected, nonoverlapping fields of view. Body temperature was maintained at 36–37 °C with a warming lamp.
Assessment of renal microcirculation
Capillaries were randomly selected from each of the video images collected during IVVM and categorized as ‘continuous flow’ when RBC movement was continuous; ‘intermittent flow’ when RBC movement stopped or reversed; or ‘no flow’ when no RBC movement was observed. Approximately 150 capillaries were analyzed for each animal. Data were expressed as the percentage of vessels in each of the three categories. RBC velocity was calculated in continuously flowing capillaries by measuring the distance traveled by a single RBC over time. Data were expressed as μm/s.
Detection of RNS generation using IVVM
DHR is preferentially oxidized to fluorescent rhodamine by peroxynitrite and perhaps other reactive oxygen species/RNS species but not by superoxide.30,31 Rhodamine fluorescence was visualized at 535 nm excitation and 590 nm emission. Still images exposed for 500 ms were captured from the fields of view used to determine capillary perfusion. Fluorescence intensity was measured by ImageJ (NIH, Bethesda, MD) after first subtracting background fluorescence intensity. Data were expressed as arbitrary units/μm2.
Measurement of MAP and HR in conscious mice
MAP and HR were monitored continuously in conscious mice using biotelemetry. Transmitters (Data Sciences International, Minneapolis, MN) were implanted into the carotid artery under isoflurane anesthesia and the animals were allowed to recover for 48 h. Cardiovascular parameters were recorded for 10 s every 5 min. At 5.5 h following surgery, mice were administered RES or vehicle.
Measurement of RBF
Under isoflurane anesthesia, the right renal artery was isolated from the vein and a Transonic Systems (Ithaca, NY)-calibrated 0.5 PSL renal artery Doppler flow probe was positioned around the renal artery. RBF was recorded after the flow stabilized (approximately 10 min after placement of the probe) using PowerLab and LabChart software (AD Instruments, Dunedin, New Zealand). Resveratrol (10 mg/kg) or vehicle was administered through the penile vein. Body temperature was maintained at 36–37 °C with a heating lamp. Data were expressed in ml/min per g kidney weight.
Measurement of GFR
GFR was measured using the single bolus FITC-inulin clearance method.60 Briefly, a 5% solution was prepared by dissolving FITC-inulin (Sigma, St Louis, MO) in normal saline. The solution was injected through the penile vein at a dose of 3.74 μl/g. Blood (25 μl) was collected into heparinized capillary tubes at 3, 7, 10, 15, 35, 55, 75, 90, and 120 min post injection. FITC-inulin in serum was measured at 485 nm excitation and 538 emission and quantified against a known concentration. Inulin clearance was calculated using a two-phase decay nonlinear regression analysis. GFR was calculated using the fast and slow phases of inulin clearance after normalizing to the combined weight of both kidneys.
Measurement of total serum NO levels
Serum nitrate + nitrite levels were determined using the Total Nitric Oxide Assay Kit (Assay Designs, Ann Arbor, MI) as directed by the manufacturer and concentration expressed as in μmol/l.
Measurement of serum creatinine and blood urea nitrogen concentration
Serum creatinine levels and blood urea nitrogen were measured using the QuantiChrom Creatinine Assay kit and Urea Assay kit, respectively (BioAssay Systems, Hayward, CA).
Immunohistochemistry staining for nitrotyrosine
Nitrotyrosine–protein adducts were detected using a polyclonal anti-nitrotyrosine antibody (Millipore, Billerica, MA) diluted 1:1200 in 1% bovine serum albumin, 0.5% milk in 1 × Tris-buffered saline, pH 7.6, as described previously.12 Preincubation of the anti-nitrotyrosine antibody with 10 mmol/l nitrotyrosine was used as the nonspecific binding control.
Histology
The periodic acid–Schiff-stained sections were scored in a blinded, semiquantitative manner. For each animal, at least 10 high-power (×400) fields were examined. The percentage of tubules that displayed cellular necrosis, loss of brush border, cast formation, vacuolization, and tubule dilation was scored as follows: 0 = none, 1 = <10%, 2 = 11–25%, 3 = 26–45%, 4 = 46–75%, and 5 = >76%.
Survival study
Mice subjected to CLP surgery were administered either RES (10 mg/kg, i.p.) or vehicle at 6, 12, and 18 h following CLP and monitored for 48 h. Core body temperature was used as an indicator of pending mortality35 and was measured every 6 h using a rectal probe. Mice were considered as non-survivors if they died or had to be killed because of two consecutive readings of core temperature below 28 °C.
Statistical analysis
Data, presented as mean±s.e.m., were analyzed using Prism 5.0 (GraphPad Software, San Diego, CA). Before analysis of categorical perfusion, the data were transformed using isometric log ratio transformations. Hotelling’s T2-test was then used to calculate test statistics between pairs of groups, and permutation tests were used to calculate P-values for each comparison (Figure 1a and 4a). Renal tubular injury scores were analyzed using the nonparametric Kruskal–Wallis test, followed by the Dunn multiple comparison test (Figure 6d). Survival curves (Figure 8) were analyzed using a Mantel–Cox log rank test. For all other data, Student’s t-test was used when two groups were compared, and a one-way analysis of variance followed by the Newman–Keuls post-hoc test was used when three or more groups were compared. A P-value <0.05 was considered significant.
Acknowledgments
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases grants F30 DK085705 (JHH), 10PRE4140065 (ZW), R01 DK075991-S1 (to support KAS), and R01 DK075991 (PRM). Statistical support was provided by HJ Spencer III of the UAMS Translational Institute supported by the National Institutes of Health National Center for Research Resources grant UL1 RR029884.
Footnotes
A portion of this work was presented as an abstract at the Experimental Biology 2011 Meeting.
DISCLOSURE
All the authors declared no competing interests.
References
- 1.Lee WL, Slutsky AS. Sepsis and endothelial permeability. N Engl J Med. 2010;363:689–691. doi: 10.1056/NEJMcibr1007320. [DOI] [PubMed] [Google Scholar]
- 2.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 3.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Marshall JC, Vincent JL, Guyatt G, et al. Outcome measures for clinical research in sepsis: a report of the 2nd Cambridge Colloquium of the International Sepsis Forum. Crit Care Med. 2005;33:1708–1716. doi: 10.1097/01.ccm.0000174478.70338.03. [DOI] [PubMed] [Google Scholar]
- 5.Rivers EP, Coba V, Whitmill M. Early goal-directed therapy in severe sepsis and septic shock: a contemporary review of the literature. Curr Opin Anesthesiol. 2008;21:128–140. doi: 10.1097/ACO.0b013e3282f4db7a. [DOI] [PubMed] [Google Scholar]
- 6.Lundy DJ, Trzeciak S. Microcirculatory dysfunction in sepsis. Crit Care Clin. 2009;25:721–731. viii. doi: 10.1016/j.ccc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Otero RM, Nguyen HB, Huang DT, et al. Early goal-directed therapy in severe sepsis and septic shock revisited: concepts, controversies, and contemporary findings. Chest. 2006;130:1579–1595. doi: 10.1378/chest.130.5.1579. [DOI] [PubMed] [Google Scholar]
- 8.Sakr Y, Dubois MJ, De Backer D, et al. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med. 2004;32:1825–1831. doi: 10.1097/01.ccm.0000138558.16257.3f. [DOI] [PubMed] [Google Scholar]
- 9.Vincent JL, De Backer D. Microvascular dysfunction as a cause of organ dysfunction in severe sepsis. Crit Care. 2005;9(Suppl 4):S9–S12. doi: 10.1186/cc3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heemskerk S, Masereeuw R, Russel FG, et al. Selective iNOS inhibition for the treatment of sepsis-induced acute kidney injury. Nat Rev Nephrol. 2009;5:629–640. doi: 10.1038/nrneph.2009.155. [DOI] [PubMed] [Google Scholar]
- 11.Wu L, Tiwari MM, Messer KJ, et al. Peritubular capillary dysfunction and renal tubular epithelial cell stress following lipopolysaccharide administration in mice. Am J Physiol Renal Physiol. 2007;292:F261–F268. doi: 10.1152/ajprenal.00263.2006. [DOI] [PubMed] [Google Scholar]
- 12.Wu L, Gokden N, Mayeux PR. Evidence for the role of reactive nitrogen species in polymicrobial sepsis-induced renal peritubular capillary dysfunction and tubular injury. J Am Soc Nephrol. 2007;18:1807–1815. doi: 10.1681/ASN.2006121402. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, Herzog C, Kaushal GP, et al. Actinonin, a meprin A inhibitor, protects the renal microcirculation during sepsis. Shock. 2011;35:141–147. doi: 10.1097/SHK.0b013e3181ec39cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yasuda H, Yuen PS, Hu X, et al. Simvastatin improves sepsis-induced mortality and acute kidney injury via renal vascular effects. Kidney Int. 2006;69:1535–1542. doi: 10.1038/sj.ki.5000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seely KA, Holthoff JH, Burns ST, et al. Hemodynamic changes in the kidney in a pediatric rat model of sepsis-induced acute kidney injury. Am J Physiol Renal Physiol. 2011;301:F209–F217. doi: 10.1152/ajprenal.00687.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trzeciak S, Cinel I, Phillip Dellinger R, et al. Resuscitating the microcirculation in sepsis: the central role of nitric oxide, emerging concepts for novel therapies, and challenges for clinical trials. Acad Emerg Med. 2008;15:399–413. doi: 10.1111/j.1553-2712.2008.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hollenberg SM. Think locally: evaluation of the microcirculation in sepsis. Intensive Care Med. 2010;36:1807–1809. doi: 10.1007/s00134-010-1973-7. [DOI] [PubMed] [Google Scholar]
- 18.Zanotti-Cavazzoni SL, Hollenberg SM. Cardiac dysfunction in severe sepsis and septic shock. Curr Opin Crit Care. 2009;15:392–397. doi: 10.1097/MCC.0b013e3283307a4e. [DOI] [PubMed] [Google Scholar]
- 19.Walker LM, York JL, Imam SZ, et al. Oxidative stress and reactive nitrogen species generation during renal ischemia. Toxicol Sci. 2001;63:143–148. doi: 10.1093/toxsci/63.1.143. [DOI] [PubMed] [Google Scholar]
- 20.Wu L, Mayeux PR. Effects of the inducible nitric oxide synthase inhibitor L-N6-(1-iminoethyl)-lysine on microcirculation and reactive nitrogen species generation in the kidney following lipopolysaccharide administration in mice. J Pharmacol Exp Ther. 2007;320:1061–1067. doi: 10.1124/jpet.106.117184. [DOI] [PubMed] [Google Scholar]
- 21.Guo R, Wang Y, Minto AW, et al. Acute renal failure in endotoxemia is dependent on caspase activation. J Am Soc Nephrol. 2004;15:3093–3102. doi: 10.1097/01.ASN.0000145530.73247.F5. [DOI] [PubMed] [Google Scholar]
- 22.Miyaji T, Hu X, Yuen PS, et al. Ethyl pyruvate decreases sepsis-induced acute renal failure and multiple organ damage in aged mice. Kidney Int. 2003;64:1620–1631. doi: 10.1046/j.1523-1755.2003.00268.x. [DOI] [PubMed] [Google Scholar]
- 23.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 24.Gresele P, Cerletti C, Guglielmini G, et al. Effects of resveratrol and other wine polyphenols on vascular function: an update. J Nutr Biochem. 2011;22:201–211. doi: 10.1016/j.jnutbio.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Kolgazi M, Sener G, Cetinel S, et al. Resveratrol reduces renal and lung injury caused by sepsis in rats. J Surg Res. 2006;134:315–321. doi: 10.1016/j.jss.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 26.Sebai H, Ben-Attia M, Sani M, et al. Protective effect of resveratrol on acute endotoxemia-induced nephrotoxicity in rat through nitric oxide independent mechanism. Free Radic Res. 2008;42:913–920. doi: 10.1080/10715760802555577. [DOI] [PubMed] [Google Scholar]
- 27.Holthoff JH, Woodling KA, Doerge DR, et al. Resveratrol, a dietary polyphenolic phytoalexin, is a functional scavenger of peroxynitrite. Biochem Pharmacol. 2010;80:1260–1265. doi: 10.1016/j.bcp.2010.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russell JA. Management of sepsis. N Engl J Med. 2006;355:1699–1713. doi: 10.1056/NEJMra043632. [DOI] [PubMed] [Google Scholar]
- 29.Cepinskas G, Wilson JX. Inflammatory response in microvascular endothelium in sepsis: role of oxidants. J Clin Biochem Nutr. 2008;42:175–184. doi: 10.3164/jcbn.2008026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crow JP. Peroxynitrite scavenging by metalloporphyrins and thiolates. Free Rad Biol Med. 2000;28:1487–1494. doi: 10.1016/s0891-5849(00)00249-5. [DOI] [PubMed] [Google Scholar]
- 31.Gomes A, Fernandes E, Lima JL. Use of fluorescence probes for detection of reactive nitrogen species: a review. J Fluoresc. 2006;16:119–139. doi: 10.1007/s10895-005-0030-3. [DOI] [PubMed] [Google Scholar]
- 32.Beckman JS. Oxidative damage and tyrosine nitration from peroxynitrite. Chem Res Tox. 1996;9:836–844. doi: 10.1021/tx9501445. [DOI] [PubMed] [Google Scholar]
- 33.Wang Z, Rabb H, Haq M, et al. A possible molecular basis of natriuresis during ischemic-reperfusion injury in the kidney. J Am Soc Nephrol. 1998;9:605–613. doi: 10.1681/ASN.V94605. [DOI] [PubMed] [Google Scholar]
- 34.Doi K, Yuen PS, Eisner C, et al. Reduced production of creatinine limits its use as marker of kidney injury in sepsis. J Am Soc Nephrol. 2009;20:1217–1221. doi: 10.1681/ASN.2008060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warn PA, Brampton MW, Sharp A, et al. Infrared body temperature measurement of mice as an early predictor of death in experimental fungal infections. Lab Anim. 2003;37:126–131. doi: 10.1258/00236770360563769. [DOI] [PubMed] [Google Scholar]
- 36.Tiwari MM, Brock RW, Kaushal GP, et al. Disruption of renal peritubular blood flow in lipopolysaccharide-induced renal failure: role of nitric oxide and caspases. Am J Physiol Renal Physiol. 2005;289:F1324–F1332. doi: 10.1152/ajprenal.00124.2005. [DOI] [PubMed] [Google Scholar]
- 37.Dudley C. Maximizing renal preservation in acute renal failure. BJU Int. 2004;94:1202–1206. doi: 10.1046/j.1464-410x.2004.05129.x. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen HB, Corbett SW, Menes K, et al. Early goal-directed therapy, corticosteroid, and recombinant human activated protein C for the treatment of severe sepsis and septic shock in the emergency department. Acad Emerg Med. 2006;13:109–113. doi: 10.1197/j.aem.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 39.Ince C. The microcirculation is the motor of sepsis. Crit Care. 2005;9(Suppl 4):S13–S19. doi: 10.1186/cc3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Dorze M, Legrand M, Payen D, et al. The role of the microcirculation in acute kidney injury. Curr Opin Crit Care. 2009;15:503–508. doi: 10.1097/MCC.0b013e328332f6cf. [DOI] [PubMed] [Google Scholar]
- 41.Walle T, Hsieh F, DeLegge MH, et al. High absorption but very low bioavailability of oral resveratrol in humans. Drug Met Dis. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 42.Vallon V, Traynor T, Barajas L, et al. Feedback control of glomerular vascular tone in neuronal nitric oxide synthase knockout mice. J Am Soc Nephrol. 2001;12:1599–1606. doi: 10.1681/ASN.V1281599. [DOI] [PubMed] [Google Scholar]
- 43.Langenberg C, Wan L, Egi M, et al. Renal blood flow in experimental septic acute renal failure. Kidney Int. 2006;69:1996–2002. doi: 10.1038/sj.ki.5000440. [DOI] [PubMed] [Google Scholar]
- 44.Brandt S, Regueira T, Bracht H, et al. Effect of fluid resuscitation on mortality and organ function in experimental sepsis models. Crit Care. 2009;13:R186. doi: 10.1186/cc8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gojkovic-Bukarica L, Novakovic A, Kanjuh V, et al. A role of ion channels in the endothelium-independent relaxation of rat mesenteric artery induced by resveratrol. J Pharmacol Sci. 2008;108:124–130. doi: 10.1254/jphs.08128fp. [DOI] [PubMed] [Google Scholar]
- 46.Novakovic A, Bukarica LG, Kanjuh V, et al. Potassium channels-mediated vasorelaxation of rat aorta induced by resveratrol. Basic Clin Pharmacol Toxicol. 2006;99:360–364. doi: 10.1111/j.1742-7843.2006.pto_531.x. [DOI] [PubMed] [Google Scholar]
- 47.Chilton L, Loutzenhiser K, Morales E, et al. Inward rectifier K(+) currents and Kir2. 1 expression in renal afferent and efferent arterioles. J Am Soc Nephrol. 2008;19:69–76. doi: 10.1681/ASN.2007010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zager RA, Johnson AC, Lund S, et al. Levosimendan protects against experimental endotoxemic acute renal failure. Am J Physiol Renal Physiol. 2006;290:F1453–F1462. doi: 10.1152/ajprenal.00485.2005. [DOI] [PubMed] [Google Scholar]
- 49.Salgado DR, Rocco JR, Silva E, et al. Modulation of the renin-angiotensin-aldosterone system in sepsis: a new therapeutic approach? Expert Opin Ther Targets. 2010;14:11–20. doi: 10.1517/14728220903460332. [DOI] [PubMed] [Google Scholar]
- 50.Marier JF, Vachon P, Gritsas A, et al. Metabolism and disposition of resveratrol in rats: extent of absorption, glucuronidation, and enterohepatic recirculation evidenced by a linked-rat model. J Pharmacol Exp Ther. 2002;302:369–373. doi: 10.1124/jpet.102.033340. [DOI] [PubMed] [Google Scholar]
- 51.Colom H, Alfaras I, Maijo M, et al. Population pharmacokinetic modeling of trans-resveratrol and its glucuronide and sulfate conjugates after oral and intravenous administration in rats. Pharm Res. 2011;28:1606–1621. doi: 10.1007/s11095-011-0395-8. [DOI] [PubMed] [Google Scholar]
- 52.Tanner GA, Sophasan S. Kidney pressures after temporary renal artery occlusion in the rat. Am J Physiol. 1976;230:1173–1181. doi: 10.1152/ajplegacy.1976.230.4.1173. [DOI] [PubMed] [Google Scholar]
- 53.Sutton TA. Alteration of microvascular permeability in acute kidney injury. Microvasc Res. 2009;77:4–7. doi: 10.1016/j.mvr.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leonard SS, Xia C, Jiang BH, et al. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem Biophys Res Comm. 2003;309:1017–1026. doi: 10.1016/j.bbrc.2003.08.105. [DOI] [PubMed] [Google Scholar]
- 55.Hoshino J, Park EJ, Kondratyuk TP, et al. Selective synthesis and biological evaluation of sulfate-conjugated resveratrol metabolites. J Med Chem. 2010;53:5033–5043. doi: 10.1021/jm100274c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morales AI, Buitrago JM, Santiago JM, et al. Protective effect of trans-resveratrol on gentamicin-induced nephrotoxicity. Antioxid Redox Signal. 2002;4:893–898. doi: 10.1089/152308602762197434. [DOI] [PubMed] [Google Scholar]
- 57.Chung S, Yao H, Caito S, et al. Regulation of SIRT1 in cellular functions: role of polyphenols. Arch Biochem Biophys. 2010;501:79–90. doi: 10.1016/j.abb.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weinberg JM. Mitochondrial biogenesis in kidney disease. J Am Soc Nephrol. 2011;22:431–436. doi: 10.1681/ASN.2010060643. [DOI] [PubMed] [Google Scholar]
- 59.Galley HF. Bench-to-bedside review: targeting antioxidants to mitochondria in sepsis. Crit Care. 2010;14:230. doi: 10.1186/cc9098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qi Z, Whitt I, Mehta A, et al. Serial determination of glomerular filtration rate in conscious mice using FITC-inulin clearance. Am J Physiol Renal Physiol. 2004;286:F590–F596. doi: 10.1152/ajprenal.00324.2003. [DOI] [PubMed] [Google Scholar]