Abstract
Persistent inward currents (PICs) are present in many types of neurons and likely have diverse functions. In spinal motoneurons, PICs are especially strong, primarily located in dendritic regions, and subject to particularly strong neuromodulation by the monoamines serotonin and norepinephrine. Because motoneurons drive muscle fibers, it has been possible to study the functional role of their PICs in motor output and to identify PIC-mediated effects on motoneuron firing patterns in human subjects. The PIC markedly amplifies synaptic input, up to fivefold or more, depending on the level of monoaminergic input. PICs also tend to greatly prolong input time course, allowing brief inputs to initiate long-lasting self-sustained firing (i.e., bistable behavior). PIC deactivation usually requires inhibitory input and PIC amplitude can increase to repeated activation. All of these behaviors markedly increase motoneuron excitability. Thus, in the absence of monoaminergic input, motoneuron excitability is very low. Yet PICs have another effect: once active, they tend to sharply limit efficacy of additional synaptic input. All of these PIC effects have been detected in motoneuron firing patterns in human subjects and, hence, PICs are likely a fundamental component of normal motor output.
Keywords: Neuromodulation, Spinal neuron, Motor unit, Serotonin, Norepinephrine, Electrophysiology
Persistent inward currents (PICs), as considered in this review, are generated by voltage-sensitive Na and Ca currents that inactivate slowly. This voltage dependence distinguishes PICs from ion channels that simply remain open at all times, such as the K channels generating the resting membrane potential in neurons. PICs thus provide a sustained depolarization whenever the cell’s membrane potential exceeds the channel’s activation voltage. Here we focus on spinal motoneurons, where PICs are especially strong and where their functional role has been studied in detail.
Motoneurons are unique among CNS cells in two important respects. The first point to consider is that motoneurons are the primary output cells for the CNS, allowing us to act on our environment. Each motoneuron innervates a unique set of muscle fibers in one muscle, forming the motor unit. Hence, Sherrington thus referred to the motoneuron as the “final common pathway” for motor commands. Normally, muscle fiber action potentials of a motor unit are one-to-one with those of its motoneuron (Kernell 2006). This link provides the second unique aspect of the motoneuron: it is the only neuron in the CNS whose firing patterns can be readily measured in humans. It is relatively simple to insert fine wire electrodes in human muscles (as well as into muscles of intact animals) to record motor unit actions potentials. Recordings of motor unit firing patterns have long been a standard clinical evaluation tool for neurology as well as a basic tool for numerous scientific studies of human motor control. Thus, the motoneuron potentially provides a window on CNS function. A primary question addressed by this review is: to what degree can motor unit firing patterns reveal the cellular properties of motoneurons and organization of their synaptic inputs?
The key issue in addressing this question has proven to be neuromodulation of PICs. Like most neurons, the synaptic input to motoneurons includes both ionotropic inputs, to generate the fast excitatory postsynaptic potentials (EPSPs), and inhibitory postsynaptic potentials (IPSPs) that depolarize and hyperpolarize the cell, and neuromodulatory inputs, which act via G protein–coupled receptors to alter intrinsic electrical properties. The neuromodulatory control of PICs and other properties of motoneurons have proven to be unexpectedly strong, so that the motor output generated by a given pattern of ionotropic input can vary greatly, depending on the level and type of neuromodulatory input. To date, attempts to quantify the input-output relations of a motor pool (= the set of motoneurons innervating a single muscle) have assumed that, except for threshold behaviors, motoneuron firing patterns are largely determined by differences in the organization of ionotropic synaptic input (Heckman and Binder 1991; Fuglevand and others 1993; Heckman and Binder 1993a, 1993b). As this review will emphasize, this assumption can no longer be accepted. Instead, our understanding of motor output must be grounded in an understanding of the fundamental role played by neuromodulatory inputs to motoneurons.
Basic Electrical Properties of Motoneurons
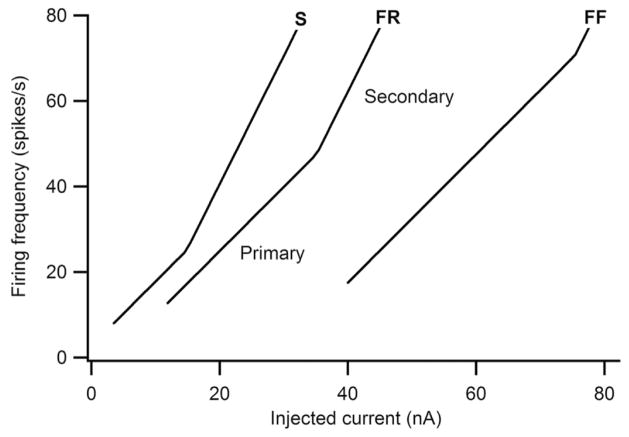
The properties of motoneurons have recently been reviewed in an outstanding monograph by Kernell (2006) (see also Binder and others 1996; Powers and Binder 2001; Alaburda and others 2002; Hornby and others 2002; Hultborn and others 2004) and are only briefly summarized here. All the initial studies of motoneuron properties were done in preparations where neuromodulatory input was suppressed by deep anesthesia. These studies thus revealed the basic properties of motoneurons without neuromodulation. Like many neurons, the motoneuron has a very distinct threshold for generating action potentials, typically referred to as its recruitment threshold. As injected current is increased to depolarize the motoneuron above its voltage threshold for spiking, firing rate increases more or less linearly. The resulting frequency-current function defines the basic behavior of the motoneuron. A fundamental point that lies at the very heart of how motoneurons function is that the amount of current required to reach their recruitment threshold has a very wide range, about 10-fold (Fig. 1). Note also that at high input levels, there is a transition from a primary range slope to a secondary range. Except for their thresholds, these frequency-current relations bear little relation to motor unit firing patterns in human subjects, where firing rates tend to be much lower (see section on PICs and motoneuron firing patterns in humans, below).
Fig. 1.
Motoneuron frequency-current functions in the absence of neuromodulatory input. Each function has a sharp threshold current (x axis) followed an approximately linear increase in firing rate. At high input-output levels there is a transition to a steeper, secondary range. Motoneuron-innervated slow (S) twitch muscle fibers have the lowest threshold currents, as shown by the example at the left. Those innervating fast, fatigue-resistant (FR) fibers have moderate thresholds and those innervating fast, fatiguable (FF) fibers the highest thresholds. Thus the motoneuron tends to be recruited in order of S, FR, and then FF. Data are based on realistic computer simulations of motoneuron properties (Heckman and Binder 1991).
Motoneurons with low current thresholds tend to innervate slow but highly fatigue-resistant muscle fibers, forming type (slow) S motor units. Motoneurons with progressively higher thresholds innervate progressively faster and less fatigue-resistant muscle fibers, forming type fast, fatigue-resistant (FR) and fast, fatiguable (FF) motor units (Burke 1981; Binder and others 1996; Kernell 2006). As a result, motoneurons are activated in a remarkably stereotyped sequence, proceeding from S to FR to FF. This sequence is often referred to as Henneman’s size principle, because Henneman and colleagues emphasized that motor unit force increases progressively from S to FR to FF motor units and that size of the motoneuron itself is a primary determinant of motoneuron input conductance, which in turn is the primary determinant of the current required to reach threshold for the action potential (Henneman and Mendell 1981; Binder and others 1996; Kernell 2006). Differences in membrane resistance are also important (Gustafsson and Pinter 1984). The recruitment pattern of motoneurons does have a significant degree of variability, but this variability is only about 10% to 15% (Cope and Clark 1991; Haftel and others 2001). The size principle of motor unit recruitment provides the fundamentally important advantages of minimizing fatigue and maximizing energy efficiency (Henneman and Mendell 1981). Moreover, this same recruitment pattern has been shown to apply across a very wide range of motor behaviors—indeed a clear exception has yet to be demonstrated (Binder and others 1996). This consistency of recruitment sequence is primarily due to the systematic differences in the threshold currents noted above, which exhibit an approximately 10-fold range that is larger than the variations in the organization of ionotropic synaptic inputs (Heckman and Binder 1993b; Haftel and others 2001). Henneman thus also emphasized the computational importance of the size principle (Henneman 1990): higher level motor systems do not have to compute which motor units to use for different motor behaviors, but instead can rely on the motoneurons themselves to determine their own activation order. The present review emphasizes the major changes in motoneuron properties due to neuromodulatory inputs, but it should be emphasized that not a single neuromodulatory effect alters the basic hierarchy of motoneuron thresholds (Lee and Heckman 1998a, 1998b). Thus, Henneman’s size principle of recruitment continues to apply as neuromodulatory input varies.
Neuromodulators and Motoneurons
Multiple neuromodulators influence the intrinsic excitability of motoneurons (reviewed in Rekling and others 2000; Powers and Binder 2001), including dopamine (Clemens and Hochman 2004) and acetylcholine (via muscarinic receptors; Miles and others 2007). Yet a wide range of studies show that the monoamines serotonin (5HT) and norepinephrine (NE) have such powerful effects that the descending monoaminergic input from the brainstem is probably the primary control system for motoneuron excitability (reviewed in Heckman and others 2003; Hultborn and others 2004). The effects of 5HT and NE on motoneuron excitability are diverse (reviewed in Rekling and others 2000; Powers and Binder 2001), ranging from hyperpolarization of spike voltage threshold (Krawitz and others 2001; Fedirchuk and Dai 2004) to reduction in the spike after hyperpolarization (AHP) amplitude to sub-threshold depolarization. The impact of monoamines on PICs in motoneurons is especially potent.
PICs in Motoneurons
PICs in motoneurons were first studied by Schwindt and Crill (1980a, 1980b), who used K channel blockers to reveal PICs in deeply anesthetized preparations. A major breakthrough was achieved by Hounsgaard and colleagues (1988) when they demonstrated that firing behaviors consistent with strong PICs were clearly present in the decerebrate cat preparation and that these behaviors were dependent on tonic activity in descending monoaminergic inputs to the cord. Initially, most attention was focused on the striking phenomenon of “bistability,” in which a short pulse of excitatory input could produce “self-sustained” firing, which then could be terminated by a short pulse of inhibition. When spikes were blocked, long-lasting plateau potentials were evident. Hounsgaard and colleagues (1988) emphasized the potential importance of self-sustained firing for maintenance of posture, allowing the steady motoneuron firing required for steady postural force to be generated without sustained descending input. A series of studies both in vitro (Hounsgaard and Kiehn 1993; Carlin and others 2000) and in vivo (Lee and Heckman 1996; Bennett and others 1998b) established that most of the PIC is of dendritic origin. As yet, studies of the distribution of voltage-sensitive ion channels that contribute to the PIC are just beginning, but these too are consistent with a dendritic origin (Carlin and others 2000; Simon and others 2003; Ballou and others 2006). The development of voltage clamp techniques in the decerebrate cat preparation (reviewed in Heckman and Lee 2001) focused attention on a different aspect of dendritic PICs: their potent amplification of synaptic input (Lee and Heckman 1996; Heckman and others 2000). These initial studies of amplification of synaptic input during voltage clamp also suggested that at least 70% of the PIC is generated in dendritic regions (Lee and Heckman 2000). Synaptic amplification by the PIC is discussed further below—its regulation by neuromodulation is likely a fundamental underpinning of motoneuron firing patterns.
Basic Properties of PICs
During a linearly rising voltage ramp, the PIC manifests as a strong downward deflection in the net current (Fig. 2A). If the PIC is large relative to the leak conductance, it induces a strong negative slope in the net current-voltage (I–V) relationship (Fig. 2B). Above the voltage range for PIC activation, activation of voltage-sensitive outward currents, presumably generated by K channels (Li and Bennett 2007), restore a positive slope to the I–V function. Note also that as the voltage is linearly returned to the original holding potential, the PIC remained on although the cell had been depolarized for more than 5 seconds (the sustained peak in Fig. 2A, B)—a clear indication of its persistence. Equally important, the deactivation of the PIC occurs at a more hyperpolarized voltage than does its activation (typically 10 to 20 mV; Lee and Heckman 1998a). This hysteresis in activation/deactivation is a hallmark of PIC behavior and produces a similar hysteresis in the relationship between injected current and firing frequency (Hounsgaard and others 1988; Lee and Heckman 1998b). Although initially assumed to result from the PICs largely dendritic location compared with the clamp applied at the soma of the cell, hysteresis is now known to be an intrinsic behavior of the Ca currents that generate a substantial fraction of the PIC (Moritz and others 2007).
Fig. 2.
Motoneuron current-voltage (I–V) functions in the presence of strong neuromodulatory input. (A) A slow linear increase and then decrease in voltage command (black triangular shaped trace) evokes a complex current response. The persistent inward current (PIC) is manifest as large downward deflections on both the rising (initial peak) and falling (sustained peak) phases. (B) I–V plot for the data in A. The amplitude of the PIC is calculated in comparison with the linear leak I–V function (dashed line). Data are from Lee and Heckman (1998a).
Two Components to the PIC in Motoneurons
The ionic basis of PICs in motoneurons was first studied by Hounsgaard and Kiehn (1985), using slices of the turtle spinal cord. This preparation was a major breakthrough, because motoneurons are exceptionally large (as shown in Fig. 4) and it is difficult to get them to survive in vitro. The turtle preparation continues to provide fundamentally important results on motoneuron properties and inputs (Alaburda and others 2002; Hornby and others 2002), but mammalian in vitro preparations have also been developed, for young adult motoneurons (Carlin and others 2000; Powers and Binder 2003) and fully adult motoneurons in the sacral portion of the cord (Bennett and others 2001; Jiang and Heckman 2006). Voltage clamp in vivo in the adult feline spinal cord has also contributed information on the cellular properties of PICs (Lee and Heckman 1999b). Overall, these studies have shown that the PIC in mammals is generated primarily by two types of channels: L-type Ca2+ channels (probably CaV 1.3) and persistent Na+ channels (molecular subtype unknown; Lee and Heckman 1999b; Li and others 2004a). Thus there is a CaPIC and an NaPIC. These two components have very different behaviors. The CaPIC activates slowly (time constant of around 50 ms) but is highly persistent (Svirskis and Hounsgaard 1997; Lee and Heckman 1998a; Li and Bennett 2003; Elbasiouny and others 2005, 2006; Li and others 2007). The CaPIC also exhibits warm-up, a tendency to increase in amplitude with repeated stimulation, and is likely the primary source of hysteresis in PIC onset/offset (Svirskis and Hounsgaard 1997; Bennett and others 1998a; Lee and Heckman 1998a; Moritz and others 2007). The NaPIC is fast activating but tends to show more rapid inactivation—although still generating a current that can last a few seconds (Lee and Heckman 1999b; Kuo and others 2003a; Li and Bennett 2003; Harvey and others 2005, 2006a). NaPIC also plays an essential role in initiating spikes during repetitive firing (Lee and Heckman 2001; Kuo and others 2003a; Harvey and others 2005; Theiss and others 2007).
Fig. 4.
Serotonin contacts on a spinal motoneuron. Each dot represents a synaptic bouton containing serotonin. Note the coverage includes distal dendrites. This figure was reproduced from Figure 4 of Alvarez and others (1998). Distribution of 5-hydroxytryptamine-immunoreactive boutons on alpha-motoneurons in the lumbar spinal cord of adult cats. J Comp Neurol 393:69–83. Copyright Wiley-Liss Inc. Reprinted with permission of Wiley-Liss, Inc, a subsidiary of John Wiley & Sons, Inc.
Amplification and Prolongation of Excitatory Synaptic Input by Dendritic PICs
The possibility that motoneuron dendrites have active properties has long been considered, and evidence for active boosting was first obtained by Redman and colleagues using K channel blockers (Clements and others 1986). Because most of the PIC is generated in dendritic areas, it is perfectly placed to influence synaptic input. Figure 3 illustrates the effects of the PIC on a sustained excitatory synaptic input generated by muscle spindle Ia afferents. High-frequency activation of this pathway generates a steady excitatory synaptic current with a crisp onset and offset—but only when the cell is clamped at a hyperpolarized potential (Fig. 3A). When the cell is clamped at a depolarized potential, approximately equal to the level at which spiking would occur in the unclamped state, the very same input generates much larger net synaptic current due to activation of the dendritic PIC (baseline currents removed to allow traces to be superimposed). Equally important, the PIC continues to be active once the excitatory synaptic input is turned off. The difference between the depolarized and hyperpolarized currents provides an estimation of the effect of the dendritic PIC (Fig. 3B). This prolongation of current after excitation is removed is the very essence of motoneuron PICs and is the fundamental behavior that produces bistability and self-sustained firing, which are illustrated in Figure 3C. Therefore, a hyperpolarizing input is required to deactivate the PIC. This resistance to deactivation is probably due to both the largely dendritic generation of the PIC (Gutman 1991) and its intrinsic properties (hysteretic behavior, see above; Moritz and others 2007). It should be emphasized that the prolongation component of the PIC varies with the type of motoneuron. Low-threshold cells, which likely are part of slow twitch motor units (type S; Burke 1981), have very long lasting PICs and strong bistability (Lee and Heckman 1998a, 1998b). PIC amplitude and synaptic amplification remains just as strong in progressively higher threshold motoneurons (for type FR and FF motor units; Lee and Heckman 2000), but the input prolongation decreases in duration and bistability weakens. Nonetheless, even in high-threshold motoneurons, PICs can significantly outlast synaptic inputs.
Fig. 3.
Amplification and prolongation of synaptic input by the dendritic persistent inward current (PIC) in a low-threshold, type S motoneuron. (A) Steady synaptic input was generated by 1.5 seconds of high-frequency activation of a monosynaptic, ionotropic source, muscle spindle Ia afferents. At a hyperpolarized holding potential (−90 mV; green trace), this input produced a steady current with a sharp onset and offset. At depolarized holding potential (~−55 mV; red trace), the very same input is greatly amplified and prolonged by the PIC. Baseline holding currents are removed to allow the traces to be superimposed. (B) The difference between the currents in A reflects the net contribution of the dendritic PIC. (C) In unclamped conditions, the same input produces a steady excitatory postsynaptic potential (EPSP) at hyperpolarized levels (~−90 mV). At a more depolarized level (−70 mV), the same input evokes intense repetitive firing, following by continued, self-sustained firing at a lower level when the input is removed. Data are from Lee and Heckman (1996).
Neuromodulatory Control of Dendritic PICs
The source of nearly all of the 5HT found in the CNS arises from a handful of nuclei located in the pons and medulla of the brainstem. The rostral groups generally project to the forebrain; and the inferior groups supply 5HT terminals to the spinal cord (Jacobs and Azmitia 1992). Spinal motoneurons are densely covered with synapses containing 5HT, from the soma to very distal-most dendrites (Fig. 4; Alvarez and others 1998). The strong facilitation of PICs and other excitatory effects of 5HT are likely mediated via 5HT2 receptors, although it is not yet clear which of the 2a, b, or c subtypes are most important (Miller and others 1996; Perrier and Hounsgaard 2003; Harvey and others 2006a). The axons containing NE also arise from the brainstem, in the locus ceruleus and nearby subceruleus (SC), Kölliker-Fuse, and parabrachial nuclei (Björklund and Skagerberg 1982). Motoneurons are as densely covered by NE-containing synapses with 5HT synapses, again including the distal-most part of the dendritic tree (Rose PK, Queens University, personal communication). It is likely that the facilitatory effects of NE on motoneurons are mediated by NE α1-receptors (Wada and others 1997; Lee and Heckman 1998a, 1998b, 1999a; Harvey and others 2006b). The relative effects of NE and 5HT on the PIC have not been directly quantified but appear to be about equally strong (Lee and Heckman 1999a; Harvey and others 2006b).
PIC Mediated Amplification of Synaptic Input and its Neuromodulatory Control
PIC-mediated amplification of synaptic input is utterly dependent on descending monoaminergic drive. The cellular mechanisms of these neuromodulatory actions are not yet clear but may involve changes in intracellular Ca2+ levels (Perrier and others 2000). Figure 5 shows excitatory synaptic currents as a function of voltage clamp holding potential, in three cells from preparations with three different levels of monoaminergic drive. At low levels, synaptic current decreases as membrane potential is depolarized. This is the classic effect of decreasing drive force (note, however, the downward curvature—this probably indicates activation of voltage-sensitive outward [K+] currents in dendritic regions). At medium levels, synaptic current actually increases with depolarization due to activation of the dendritic PIC. Moreover, this increase occurs right at the membrane potential at which repetitive firing of action potentials would occur in the unclamped state. At high levels, the PIC amplification (green arrow) is really striking. Medium levels of monoaminergic drive result in two- to four-fold amplification of excitatory input; high levels produce five-to six-fold amplification (Lee and Heckman 1996; Lee and Heckman 2000). Note, however, once the PIC is activated, synaptic efficacy drops dramatically to less than at hyperpolarized levels. Thus, the PIC both amplifies and then limits synaptic input. This combination of effects is the basis for the paradoxical actions that PICs generate in motoneuron firing patterns.
Fig. 5.
Dependence of persistent inward current (PIC) amplification on level of neuromodulatory input. Each trace shows the synaptic current generated at the soma by a steady synaptic input while the voltage clamp holding potential is linearly depolarized. (Red trace) Low levels of drive (acutely spinalized preparation to eliminate descending monoaminergic input). (Blue trace) Medium level (intact cord decerebrate preparation, with tonic descending monoaminergic drive). (Green trace) High level (intact cord decerebrate with exongenous administration of the noradrenergic agonist α1-agonist methoxamine). The stronger the neuromodulatory drive, the larger the PIC-mediated amplification. But once the PIC is activated (above −40 mV), synaptic current decreases dramatically. Data are from Lee and Heckman (2000).
Neuromodulation of Motoneurons in Normal Motor Behavior
The output of brainstem monoaminergic nuclei is not constant but varies in different behaviors. The firing rate of neurons in the caudal raphe nuclei, which are the primary source of spinal 5HT, vary in proportion to intensity of motor outflow—for example, the faster the speed of locomotion, the higher the rate (Jacobs and others 2002). The noradrenergic system (locus ceruleus and nearby nuclei) primarily varies its activity with state of arousal (Aston-Jones and others 2001). Both systems are inactive in the sleeping state. The profound effect of monoaminergic input on motor output can be estimated with a reasonable degree of accuracy by computer simulations of motoneurons and muscle units. The initial simulations relied on data for motoneurons in the absence of neuromodulatory input (taken from studies in deeply anesthetized preparations; Heckman and Binder 1991; Fuglevand and others 1993). These simulations revealed that the pool-muscle input-output function is approximately sigmoidal, as illustrated in Figure 6 (right-most curve). The average gain of this “base” state input-output function has proven to be very low. The synaptic currents generated by a variety of ionotropic sensory and descending inputs have been quantified by Powers and Binder (2001). Comparison of the resulting set of current amplitudes to the simulated base-state input-output function showed that even maximal activation of all of these inputs would produce less than 30% to 40% of maximum motor output (Heckman 1994 and unpublished studies). A similar conclusion has been reached by Cushing and others (2005), who showed that 100% activation of all excitatory synapses on a morphological realistic motoneuron barely produced enough current at the soma to bring the cell to threshold, much less to generate sufficient firing for maximal force. The very low excitability of motoneurons in the absence of monoaminergic input is consistent with the effects of acute spinal transection, where motoneuron excitability is so low that reflexes are almost entirely absent (Miller and others 1996; Bennett and others 2001). In the simulations, adding the effects of monoamines on PICs, spike threshold, and so forth markedly increase gain, so that a medium to high level of monoaminergic input allows maximum force generation from a maximal input (Fig. 6, middle and left curves). Thus, at minimum, a moderate level of monoaminergic input appears to be required for normal motor behavior.
Fig. 6.
Effect of descending monoaminergic input on the net input-output gain of a motor pool and the muscle it innervates. Computer simulations closely based on the properties of the motor units of the cat medial gastrocnemius muscle and motor pool. As monoaminergic input increases, overall slope (gain) greatly increases. Dashed line and arrow: approximate maximum ionotropic input that descending and sensory systems can generate in motoneurons. Dotted line and arrow: force and input range required for posture in this muscle.
PICs and Motoneuron Patterns in Humans
The PIC produces four effects on motoneuron firing that are unique and unlikely to be attributable to any other mechanisms. Induction of self-sustained firing by a relatively brief input is a particularly clear hallmark of PICs (Fig. 3; Schwindt and Crill 1980a; Hounsgaard and others 1988), especially in low-threshold motoneurons of type S motor units (Lee and Heckman 1998a, 1998b). As low-threshold motor unit firing patterns are the ones most easily measured in human subjects, the clear prediction is that self-sustained firing should be readily detectable in humans. This is indeed the case, with a short excitation via tendon vibration inducing long-lasting firing in motor units that were previously quiescent (Kiehn and Eken 1997; Gorassini and others 1998; Gorassini and others 2002a; Walton and others 2002). All of these studies used paired unit recordings, in which a steadily firing unit was used to monitor the background of synaptic input to avoid confusing self-sustained firing with an overall increase in background drive. An alternative, and striking, method of inducing PIC activation is high-frequency electrical stimulation, which Collins and colleagues have demonstrated consistently produces force enhancement that is probably due to PIC effects (Collins and others 2001; Nickolls and others 2004).
PICs also exhibit onset-offset hysteresis, in that they are deactivated at a more hyperpolarized level than they are activated (Fig. 2). In motor units in humans, hysteresis should be apparent by comparing recruitment versus derecruitment behaviors. Gorassini and colleagues (2002a) developed a paired motor unit method to demonstrate that low-threshold units manifest recruitment-derecruitment hysteresis. It should be noted here that, just as for demonstration of self-sustained firing, it is essential to use a paired, lower threshold unit as the estimate of synaptic input. A third manifestation of the PIC, not yet considered in this review, is the phenomenon of “warm-up.” This phenomenon is characteristic of L-type Ca channels (Lipscombe and others 2004) and has been shown to be strongly present in motoneurons with PICs (Svirskis and Hounsgaard 1997; Bennett and others 1998a). Basically, the PIC grows in amplitude with repeated, closely spaced activations. In human subjects (Gorassini and others 2002b), recruitment thresholds can be decreased with repeated stimuli, appropriately spaced to generate PIC warm-up.
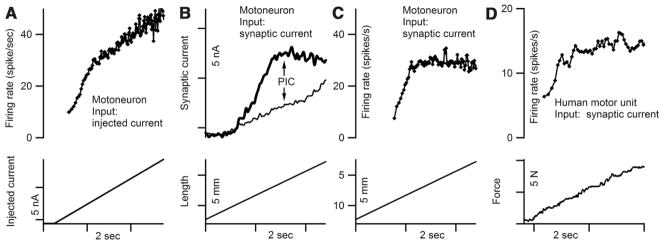
A fourth fundamental property of the PIC is the combination of amplification followed by saturation (Fig. 5). The prediction for firing patterns in human subjects is that firing rate should initially increase steeply but then switch to a much lower slope as the PIC becomes fully active. This dual slope pattern is, in fact, a very common feature of motor unit firing patterns in humans (Binder and others 1996; Hornby and others 2002; Heckman and others 2005; Kernell 2006), being evident even in studies where other signs of PICs may be absent (Fuglevand and others 2006). Although this pattern has been suggested to indicate an absence of PICs in humans, it is readily apparent in motoneurons with strong PICs in the decerebrate preparation as well (Lee and others 2003), as illustrated in Figure 7 (A, B, and C). When a linearly increasing current is injected into the cell, an inflection is evident although the change in slope is often moderate (Fig. 7A). If, however, synaptic input is applied (here evoked by linear muscle stretch) instead of artificial injected current, activation of the PIC produces a very strong saturation (as illustrated by the synaptic current in 7B). This strong saturation likely occurs because most of the synaptic input enters via the dendrites where most of the PIC is generated. In contrast, injected current enters at the soma and is relatively ineffective in activating the PIC (Lee and Heckman 1996; Bennett and others 1998b). Thus, synaptic current is more strongly amplified than injected current but also subject to stronger saturation. When the cell is allowed to fire (Fig. 7C) in response to this synaptic input, the same pattern occurs: initial steep rate modulation followed by saturation. This is the pattern often seen in human subjects, as illustrated by the example in Figure 7D. In fact, most of rate modulation in human subjects appears to take place once the PIC is already activated—this has been referred to as “rate limiting” (Heckman and Binder 1993a) or the “preferred firing range” (Kiehn and Eken 1997; Hornby and others 2002). This is really a surprising result: in human subjects, there is no trace of the primary range of motoneuron frequency-current functions seen in preparations without neuromodulatory input (Fig. 1). All of these PIC manifestations in human motoneuron firing patterns suggest that the PIC threshold is at or below recruitment threshold of type S motoneurons in humans (Heckman and others 2005). This possibility is entirely consistent with the PIC properties seen in cat and rat motoneurons (Lee and Heckman 1998a, 1998b; Li and Bennett 2003; Li and others 2004a). Overall, PICs have a paradoxical impact on firing patterns: a great increase in excitability due to amplification coupled to a restriction in rate modulation due to saturation.
Fig. 7.
Firing patterns in response to synaptic input in cat and man. (A) Firing response to linearly increasing injected current in cat motoneuron with a strong persistent inward current (PIC). (B) Synaptic currents in the same motoneuron generated by linear muscle stretch. The lower, thin trace was generated when the cell was held hyperpolarized to avoid activation of the dendritic PIC. The thick trace was the response to the same stretch, but with the clamp holding potential placed at the voltage at which spiking would occur in the unclamped state. The extra current and the saturation reflect PIC activation. (C) Firing pattern to the same stretch, same cell. (D) Example of motor unit firing in a human subject during a voluntary contraction in which joint torque increases linearly. Biceps muscle. Data are from Mottram C (unpublished studies).
Demonstrations of “PIC-like” firing patterns in humans are necessarily indirect. Studies with pharmacological agents that influence the PIC would be helpful in further identifying its role in motor behavior. Two initial studies are consistent with PIC predictions: caffeine, which increases the level of NE in the CNS, significantly increases the percent of motor units in humans that exhibit self-sustained firing (Walton and others 2002) and baclofen, which may suppress the PIC, appears to reduce the initial rate of increase in motoneuron firing that is likely to be dominated by PIC activation (Hornby and others 2004). Much further work along these lines is needed.
Diffuse Descending Neuromodulation, Specific Local Inhibition
Descending monoaminergic inputs to motoneurons are diffuse (Björklund and Skagerberg 1982), affecting many motor pools simultaneously, including agonists and antagonists. Once a PIC is activated, its persistent nature and its tendency for hysteresis means that simply withdrawing the activation does not turn it off. Thus, excitation could easily spread throughout the motor pools in a limb, both agonists and antagonists, strongly biasing the motor system to co-contraction. Although co-contraction is valuable in motor tasks requiring a high degree of stability, reciprocal activation is a hallmark of locomotion and many coordinated movement patterns.
Fortunately, the PIC is very sensitive to inhibitory synaptic input (Hultborn and others 2003; Kuo and others 2003b). The classic reciprocal inhibitory connections between antagonists, which are mediated by interneurons activated by muscle spindle Ia afferents (Jankowska 1992), may provide a solution to the problem of the diffuse nature of monoaminergic input. Ia reciprocal inhibition is a tightly focused pathway, shared only between strict mechanical antagonists (Eccles and Lundberg 1958; Nichols and others 1999). Ia afferent firing is generated by changes in muscle length and we have recently shown that moderate changes in joint position can modulate the PIC amplitude by as much as 50% (Hyngstrom and others 2007). This result supports a new concept: that the focused effects of Ia reciprocal inhibition in suppressing the PIC allow specific movement patterns to be “sculpted” from a diffuse neuromodulatory background. We thus argue that a specific local inhibitory circuit opposes the diffuse descending neuromodulation. At present, it is not entirely clear whether this local inhibitory control is purely ionotropic or perhaps has a neuromodulatory compontent via activation of GABAb receptors. Classically, Ia reciprocal inhibition is considered to be mediated via glycinergic receptors (Jankowska 1992), but a small GABAergic component has not been ruled out.
Different Motoneuron “Modes” for Different Motor Behaviors?
Perplexing aspects of the behavior of the PIC remain. Clearly the PIC tends to prolong input, especially in low-threshold type S motoneurons that are active during posture. Figure 3 gives the impression that it takes a rather substantial input to get the PIC going, but this is not the case. Figure 8 shows that an input pulse as short at 100 ms can produce a sustained PIC. Does this sustained behavior act as a temporal integrator? For posture, a tonic force level is needed as a base of support and having type S motoneurons generate this tonic force by self-sustained firing seems ideal—but does this mean that the motor commands in response to postural perturbation should be designed to assume that S motoneurons will integrate their synaptic input? Considerable further work is required to understand the dynamics of PIC processing of input. A related question occurs for very rapid inputs. The CaPIC is slow activating and is likely the main component that supports self-sustained firing. But the NaPIC is fast activating and thus the dendritic PIC does amplify fast synaptic inputs (Jones and Lee 2006). It is thus possible that the NaPIC forms a bridge to the sustained activation of the CaPIC. To what degree does this prolong or distort transient synaptic inputs? The way the PIC interacts with other voltage-sensitive currents is also not fully defined. For example, the PIC interaction with the H current in subthreshold regions could produce a resonant behavior in motoneurons (Manuel and others 2007).
Fig. 8.
A very short input can activate a persistent inward current (PIC) that continues after the input ends. Input generated by a short-lasting (100 ms) excitatory input mediated by high-frequency activation of muscle spindle Ia afferents. (Green trace) Current generated at a hyperpolarized holding potential, avoiding PIC activation. (Red trace) Depolarized holding potential, allowing PIC activation. Data from Heckman CJ (unpublished studies).
Given these questions, one possibility is that motoneurons have different “modes” of synaptic processing for different motor behaviors. For example, if inhibition is very strong, then the effects of the PIC can be minimized. Such a state has recently been demonstrated during the scratch reflex in turtle motoneurons: the inhibition increases in phase with the excitation, inducing a high conductance state similar to that likely to exist in cortical cells in vivo (Berg and others 2007). Figure 9 from Perreault (2002) provides an exceptionally clear demonstration of two modes in a single cell. The oscillations for the scratch reflex are generated against a baseline of strong hyperpolarization that substantially increases input conductance (Fig. 9B). Yet simply irritating the ear on the opposite side of the body generates sustained depolarization that is very likely due to a PIC (Fig. 9A). It is notable also that the scratch drive potentials increase dramatically with depolarization in mammalian motoneurons (Perreault 2002), unlike the case for turtle motoneurons (Berg and others 2007). Whether this “amplification” is due to a PIC that is not fully suppressed by the inhibitory background or is due instead to activation of N-methyl-D-aspartate receptors is an issue that requires further study. A diversity of neuromodulatory control is a hallmark of invertebrate neurons and our understanding of this issue for the motor output stage of vertebrate systems still lags that of invertebrate systems (Nusbaum and Beenhakker 2002; Marder and Bucher 2007). Overall, important unanswered questions remain about PICs and their interactions with other currents during the wide range of normal motor behaviors.
Fig. 9.
Two distinct modes of behavior in a single motoneuron. Spikes have been blocked (QX314 in the intracellular electrode). On the left, a scratch reflex has been evoked by irritating the ipsilateral ear. Note the onset of inhibition (arrow) before the scratch oscillations. On the right, irritation of the contralateral ear produced a completely different response in the same neuron, a weight support response mediated by a sustained plateau potential with no inhibition. Input conductance of the cell during the scratch was substantially larger than during the weight support responses. Data from Perreault (2002), used with permission of the author.
PICs, Neurotrauma, and Neurodegeneration
If, as argued here, the PIC is fundamental to motor output, then PICs would be expected to exhibit plasticity following spinal cord injury, which eliminates monoaminergic inputs along with all other descending inputs. Bennett and colleagues have shown that PICs do, in fact, exhibit remarkable plasticity following spinal injury (Li and Bennett 2003; Harvey and others 2006b). Initially following a complete spinal injury, motoneuron PICs are sharply reduced in amplitude, consistent with their requirement for facilitation by descending monoaminergic systems. As noted above, this loss of PICs and the other effects of monoamines on motoneuron properties result in a dramatic decrease in excitability. Yet, in rats, over the course of about 3 months, PIC undergo a remarkable up-regulation and recovery, to reattain an amplitude as large or larger than the normal state. Both the CaPIC and NaPIC are included in this recovery. Long-lasting spasms to a variety of stimuli recover with the same time course (Li and others 2004b).
Gorassini and colleagues (2004) have shown that these same events occur in human spinal cord–injured subjects and thus the PIC may play a central role in the spasms induced in spinal cord injury. For example, spinal cord–injured subjects at least 8 months after injury exhibit spontaneous and self-sustained firing of motor units that may continue for minutes at very low firing rates with low spike-to-spike variability. When voluntary muscle contractions were superimposed on the spontaneous firing (i.e., increasing synaptic drive or noise), mean firing rates and variability in firing rates increased. This suggests that the slow spontaneous firing observed in chronic spinal injury likely occurs without appreciable synaptic noise. Furthermore, Zijdewind and Thomas (2001) observed the start and termination of spontaneous activity in one motor unit in a hand muscle of chronic cervical spinal cord–injured subjects without a change in firing behavior of concurrently firing units, suggesting that the source of firing was intrinsic to the motoneuron. In fact, recent studies in rats provide evidence that this slow firing is caused by repetitive activation of the subthreshold portion of the NaPIC (Li and Bennett, 2003; Li and others 2004a). The slow and regular spontaneous firing profiles observed in spinal cord injury are in contrast to the higher firing rates and more variable firing profiles observed in the spontaneous firing of spastic stroke survivors while at rest (Mottram and others 2007). The wider range of variability in spontaneous firing of stroke survivors than spinal cord–injured patients suggests that the spontaneous firing in stroke survivors may be attributable to additional excitatory synaptic drive superimposed on NaPIC-mediated firing.
PIC plasticity might also be expected in amyotrophic lateral sclerosis (ALS), which is characterized by degeneration and death of motoneurons. As yet, only the NaPIC has been studied in the standard animal model of ALS, the mutant SOD1 mouse. In motoneurons cultured from these animals, only one electrical property was changed: the NaPIC was up-regulated, increasing mutant SOD1 motoneuron excitability (Kuo and others 2004, 2005). The changes in PICs in young and mature animals have not been systematically investigated as yet, but the increase in PICs in embryonic cell culture suggests that very early changes take place in motoneurons in this disease. Consistent with this, Durand and colleagues (Durand and others 2006; Bories and others 2007) have recently shown that neonatal motoneurons undergo an increase in input conductance, but whether this occurs in response to an elevated NaPIC or some other effect is presently unknown. Moreover, changes in the CaPIC have not yet been investigated, because cultured cells largely lack this fundamental component of motoneuron excitability. Nonetheless, it is evident that the PIC is subject to strong homeostatic regulation, so that PICs recover in response to spinal injury. It is possible that disruption of this regulation may be a major contributor to the progression of ALS.
Conclusion: Can Motoneuron Firing Patterns in Humans Reveal the Organization of Motor Commands?
Although many questions remain, progress in the past decade in understanding PICs in motoneuron dendrites and their impact on synaptic processing has been substantial. Serious efforts to create realistic models of motoneurons with PICs have begun (Elbasiouny and others 2005; Bui and others 2006; Elbasiouny and others 2006; Shapiro and Lee 2007). These successes suggest that models of entire motor pools that were initially attempted more than 10 years ago (Heckman and Binder 1991; Fuglevand and others 1993; Heckman and Binder 1993a, 1993b) should again be revisited and brought up to date with accurate PIC representations. It is also necessary to accurately represent the other potent effects of monoaminergic inputs, such as the hyperpolarization of spike thresholds (Krawitz and others 2001; Dai and others 2002). If such models can represent the dendritic PIC in a biologically realistic manner, then it may become possible to “reverse engineer” motor unit firing patterns in human subjects and intact animals. The goal of such a project would be to identify the organization of the synaptic input producing these patterns, in terms of the relative roles of ionotropic versus neuromodulatory input, the temporal dynamics of these two types of inputs, and their relative distribution of each among the S, FR, and FF motoneurons that make up the pool. A key problem for this endeavor will undoubtedly be that a given firing pattern can be reproduced by more than one input organization (compare Prinz and others 2004), but modeling methods to quantify this problem and identify potential “classes” of solutions are being developed by Lee and colleagues (Mitchell and others 2007). If this goal can be realized, then the unique window on CNS function provided by the motoneuron could be used to greatly deepen our insights into the structure of motor commands in both normal and abnormal states.
Acknowledgments
Studies of PICs in motoneuron in the Heckman laboratory are supported by grants from the National Institutes of Health (NINDS NS03482 and NS051462) and the Les Turner ALS Foundation.
References
- Alaburda A, Perrier JF, Hounsgaard J. Mechanisms causing plateau potentials in spinal motoneurones. Adv Exp Med Biol. 2002;508:219–26. doi: 10.1007/978-1-4615-0713-0_27. [DOI] [PubMed] [Google Scholar]
- Alvarez FJ, Pearson JC, Harrington D, Dewey D, Torbeck L, Fyffe RE. Distribution of 5-hydroxytryptamine-immunoreactive boutons on alpha-motoneurons in the lumbar spinal cord of adult cats. J Comp Neurol. 1998;393:69–83. [PubMed] [Google Scholar]
- Aston-Jones G, Chen S, Zhu Y, Oshinsky ML. A neural circuit for circadian regulation of arousal. Nat Neurosci. 2001;4:732–8. doi: 10.1038/89522. [DOI] [PubMed] [Google Scholar]
- Ballou EW, Smith WB, Anelli R, Heckman CJ. Measuring dendritic distribution of membrane proteins. J Neurosci Methods. 2006;156:257–66. doi: 10.1016/j.jneumeth.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Short-term plasticity in hindlimb motoneurons of decerebrate cats. J Neurophysiol. 1998a;80:2038–45. doi: 10.1152/jn.1998.80.4.2038. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J Neurophysiol. 1998b;80:2023–37. doi: 10.1152/jn.1998.80.4.2023. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Li Y, Siu M. Plateau potentials in sacrocaudal motoneurons of chronic spinal rats, recorded in vitro. J Neurophysiol. 2001;86:1955–71. doi: 10.1152/jn.2001.86.4.1955. [DOI] [PubMed] [Google Scholar]
- Berg RW, Alaburda A, Hounsgaard J. Balanced inhibition and excitation drive spike activity in spinal half-centers. Science. 2007;315:390–3. doi: 10.1126/science.1134960. [DOI] [PubMed] [Google Scholar]
- Binder MD, Heckman CJ, Powers RK. The physiological control of motoneuron activity. In: Rowell LB, Shepherd JT, editors. Handbook of physiology exercise: regulation and integration of multiple systems. New York: Oxford University Press; 1996. pp. 1–53. [Google Scholar]
- Björklund A, Skagerberg G. Descending monoaminergic projections to the spinal cord. In: Sjolund B, Bjärklund A, editors. Brain stem control of spinal mechanisms. Amsterdam: Elsevier Biomedical Press; 1982. pp. 55–88. [Google Scholar]
- Bories C, Amendola J, Lamotte d’Incamps B, Durand J. Early electrophysiological abnormalities in lumbar motoneurons in a transgenic mouse model of amyotrophic lateral sclerosis. Eur J Neurosci. 2007;25:451–9. doi: 10.1111/j.1460-9568.2007.05306.x. [DOI] [PubMed] [Google Scholar]
- Bui TV, Ter-Mikaelian M, Bedrossian D, Rose PK. Computational estimation of the distribution of L-type Ca(2+) channels in motoneurons based on variable threshold of activation of persistent inward currents. J Neurophysiol. 2006;95:225–41. doi: 10.1152/jn.00646.2005. [DOI] [PubMed] [Google Scholar]
- Burke RE. Motor units: anatomy, physiology, and functional organization. In: Brooks VB, editor. Handbook of physiology, the nervous system, motor control. Bethesda (MD): American Physiological Society; 1981. pp. 345–422. [Google Scholar]
- Carlin KP, Jones KE, Jiang Z, Jordan LM, Brownstone RM. Dendritic L-type calcium currents in mouse spinal motoneurons: implications for bistability. Eur J Neurosci. 2000;12:1635–46. doi: 10.1046/j.1460-9568.2000.00055.x. [DOI] [PubMed] [Google Scholar]
- Clemens S, Hochman S. Conversion of the modulatory actions of dopamine on spinal reflexes from depression to facilitation in D3 receptor knock-out mice. J Neurosci. 2004;24:11337–45. doi: 10.1523/JNEUROSCI.3698-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JD, Nelson PG, Redman SJ. Intracellular tetraethy-lammonium ions enhance group Ia excitatory post-synaptic potentials evoked in cat motoneurones. J Physiol. 1986;377:267–82. doi: 10.1113/jphysiol.1986.sp016186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DF, Burke D, Gandevia SC. Large involuntary forces consistent with plateau-like behavior of human motoneurons. J Neurosci. 2001;21:4059–65. doi: 10.1523/JNEUROSCI.21-11-04059.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope TC, Clark BD. Motor-unit recruitment in the decerebrate cat: several unit properties are equally good predictors of order. J Neurophysiol. 1991;66:1127–38. doi: 10.1152/jn.1991.66.4.1127. [DOI] [PubMed] [Google Scholar]
- Cushing S, Bui T, Rose PK. Effect of nonlinear summation of synaptic currents on the input-output properties of spinal motoneurons. J Neurophysiol. 2005;94:3465–78. doi: 10.1152/jn.00439.2005. [DOI] [PubMed] [Google Scholar]
- Dai Y, Jones KE, Fedirchuk B, McCrea DA, Jordan LM. A modelling study of locomotion-induced hyperpolarization of voltage threshold in cat lumbar motoneurones. J Physiol. 2002;544:521–36. doi: 10.1113/jphysiol.2002.026005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand J, Amendola J, Bories C, Lamotte d’Incamps B. Early abnormalities in transgenic mouse models of amyotrophic lateral sclerosis. J Physiol Paris. 2006;99:211–20. doi: 10.1016/j.jphysparis.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Eccles RM, Lundberg A. Integrative pattern of Ia synaptic actions on motoneurones of hip and knee muscles. J Physiol. 1958;144:271–98. doi: 10.1113/jphysiol.1958.sp006101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbasiouny SM, Bennett DJ, Mushahwar VK. Simulation of dendritic CaV1.3 channels in cat lumbar motoneurons: spatial distribution. J Neurophysiol. 2005;94:3961–74. doi: 10.1152/jn.00391.2005. [DOI] [PubMed] [Google Scholar]
- Elbasiouny SM, Bennett DJ, Mushahwar VK. Simulation of Ca2+ persistent inward currents in spinal motoneurones: mode of activation and integration of synaptic inputs. J Physiol. 2006;570:355–74. doi: 10.1113/jphysiol.2005.099119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedirchuk B, Dai Y. Monoamines increase the excitability of spinal neurones in the neonatal rat by hyperpolarizing the threshold for action potential production. J Physiol. 2004;557:355–61. doi: 10.1113/jphysiol.2004.064022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuglevand AJ, Dutoit AP, Johns RK, Keen DA. Evaluation of plateau-potential-mediated ‘warm up’ in human motor units. J Physiol. 2006;571:683–93. doi: 10.1113/jphysiol.2005.099705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuglevand AJ, Winter DA, Patla AE. Models of recruitment and rate coding organization in motor-unit pools. J Neurophysiol. 1993;70:2470–88. doi: 10.1152/jn.1993.70.6.2470. [DOI] [PubMed] [Google Scholar]
- Gorassini M, Yang JF, Siu M, Bennett DJ. Intrinsic activation of human motoneurons: possible contribution to motor unit excitation. J Neurophysiol. 2002a;87:1850–8. doi: 10.1152/jn.00024.2001. [DOI] [PubMed] [Google Scholar]
- Gorassini M, Yang JF, Siu M, Bennett DJ. Intrinsic activation of human motoneurons: reduction of motor unit recruitment thresholds by repeated contractions. J Neurophysiol. 2002b;87:1859–66. doi: 10.1152/jn.00025.2001. [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Bennett DJ, Yang JF. Self-sustained firing of human motor units. Neurosci Lett. 1998;247:13–6. doi: 10.1016/s0304-3940(98)00277-8. [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Knash ME, Harvey PJ, Bennett DJ, Yang JF. Role of motoneurons in the generation of muscle spasms after spinal cord injury. Brain. 2004;127:2247–58. doi: 10.1093/brain/awh243. [DOI] [PubMed] [Google Scholar]
- Gustafsson B, Pinter MJ. An investigation of threshold properties among cat spinal alpha-motoneurones. J Physiol. 1984;357:453–83. doi: 10.1113/jphysiol.1984.sp015511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman AM. Bistability of dendrites. International Journal of Neural Systems. 1991;1:291–304. doi: 10.1142/s0129065794000104. [DOI] [PubMed] [Google Scholar]
- Haftel VK, Prather JF, Heckman CJ, Cope TC. Recruitment of cat motoneurons in the absence of homonymous afferent feedback. J Neurophysiol. 2001;86:616–28. doi: 10.1152/jn.2001.86.2.616. [DOI] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. 5-HT2 receptor activation facilitates a persistent sodium current and repetitive firing in spinal motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol. 2006a;96:1158–70. doi: 10.1152/jn.01088.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. Endogenous monoamine receptor activation is essential for enabling persistent sodium currents and repetitive firing in rat spinal motoneurons. J Neurophysiol. 2006b;96:1171–86. doi: 10.1152/jn.00341.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li Y, Li X, Bennett DJ. Persistent sodium currents and repetitive firing in motoneurons of the sacrocaudal spinal cord of adult rats. J Neurophysiol. 2005;96:1141–57. doi: 10.1152/jn.00335.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ. Computer simulations of the effects of different synaptic input systems on the steady-state input-output structure of the motoneuron pool. J Neurophysiol. 1994;71:1727–39. doi: 10.1152/jn.1994.71.5.1727. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Binder MD. Computer simulation of the steady-state input-output function of the cat medial gastrocnemius motoneuron pool. J Neurophysiol. 1991;65:952–67. doi: 10.1152/jn.1991.65.4.952. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Binder MD. Computer simulations of motoneuron firing rate modulation. J Neurophysiol. 1993a;69:1005–8. doi: 10.1152/jn.1993.69.4.1005. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Binder MD. Computer simulations of the effects of different synaptic input systems on motor unit recruitment. J Neurophysiol. 1993b;70:1827–40. doi: 10.1152/jn.1993.70.5.1827. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Gorassini MA, Bennett DJ. Persistent inward currents in motoneuron dendrites: Implications for motor output. Muscle Nerve. 2005;31:135–56. doi: 10.1002/mus.20261. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Lee RH. Advances in measuring active dendritic currents in spinal motoneuronsin vivo. In: Cope TC, editor. Motor neurobiology of the spinal cord. London: CRC Press; 2001. pp. 89–106. [Google Scholar]
- Heckman CJ, Lee RH, Brownstone RM. Hyperexcitable dendrites in motoneurons and their neuromodulatory control during motor behavior. Trends Neurosci. 2003;26:688–95. doi: 10.1016/j.tins.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Theiss RD, Johnson MD, Lee RH. Active dendrites markedly enhance input-output gain in motoneurons. Soc Neurosci. 2000:Abstr 257.212. [Google Scholar]
- Henneman E. Comments on the logical basis of muscle control. In: Binder MD, Mendell LM, editors. The segmental motor system. New York: Oxford University Press; 1990. pp. vi–x. [Google Scholar]
- Henneman E, Mendell LM. Functional organization of motoneuron pool and its inputs. In: Brooks VB, editor. Handbook of physiology, the nervous system, motor control. Bethesda (MD): American Physiological Society; 1981. pp. 423–507. [Google Scholar]
- Hornby TG, Heckman CJ, Harvey RL, Rymer WZ. Changes in voluntary torque and electromyographic activity following oral baclofen. Muscle Nerve. 2004;30:784–95. doi: 10.1002/mus.20176. [DOI] [PubMed] [Google Scholar]
- Hornby TG, McDonagh JC, Reinking RM, Stuart DG. Motoneurons: a preferred firing range across vertebrate species? Muscle Nerve. 2002;25:632–48. doi: 10.1002/mus.10105. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of alpha-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol. 1988;405:345–67. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Calcium spikes and calcium plateaux evoked by differential polarization in dendrites of turtle motoneurones in vitro. J Physiol Lond. 1993;468:245–59. doi: 10.1113/jphysiol.1993.sp019769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Ca++ dependent bistability induced by serotonin in spinal motoneurons. Exp Brain Res. 1985;57:422–5. doi: 10.1007/BF00236551. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Brownstone RB, Toth TI, Gossard JP. Key mechanisms for setting the input-output gain across the motoneuron pool. Prog Brain Res. 2004;143:77–95. doi: 10.1016/s0079-6123(03)43008-2. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Denton ME, Wienecke J, Nielsen JB. Variable amplification of synaptic input to cat spinal motoneurones by dendritic persistent inward current. J Physiol. 2003;552:945–52. doi: 10.1113/jphysiol.2003.050971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyngstrom AS, Johnson MD, Miller JF, Heckman CJ. Intrinsic electrical properties of spinal motoneurons vary with joint angle. Nat Neurosci. 2007;10:363–9. doi: 10.1038/nn1852. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Martin-Cora FJ, Fornal CA. Activity of medullary serotonergic neurons in freely moving animals. Brain Res Rev. 2002;40:45–52. doi: 10.1016/s0165-0173(02)00187-x. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol. 1992;38:335–78. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- Jiang MC, Heckman CJ. In vitro sacral cord preparation and motoneuron recording from adult mice. J Neurosci Methods. 2006;156:31–6. doi: 10.1016/j.jneumeth.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Jones SM, Lee RH. Fast amplification of dynamic synaptic inputs in spinal motoneurons in vivo. J Neurophysiol. 2006;96:2200–6. doi: 10.1152/jn.00537.2006. [DOI] [PubMed] [Google Scholar]
- Kernell D. The motoneurone and its muscle fibres. Oxford: Oxford University Press; 2006. [Google Scholar]
- Kiehn O, Eken T. Prolonged firing in motor units: evidence of plateau potentials in human motoneurons? J Neurophysiol. 1997;78:3061–8. doi: 10.1152/jn.1997.78.6.3061. [DOI] [PubMed] [Google Scholar]
- Krawitz S, Fedirchuk B, Dai Y, Jordan LM, McCrea DA. State-dependent hyperpolarization of voltage threshold enhances motoneurone excitability during fictive locomotion in the cat. J Physiol. 2001;532:271–81. doi: 10.1111/j.1469-7793.2001.0271g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo JJ, Jiang MC, Lee RH, Heckman CJ. Essential role of persistent sodium current in spike initiation and excitability. Soc Neurosci. 2003a:Abstr 496.16. [Google Scholar]
- Kuo JJ, Lee RH, Johnson MD, Heckman HM, Heckman CJ. Active dendritic integration of inhibitory synaptic inputs in vivo. J Neurophysiol. 2003b;90:3617–24. doi: 10.1152/jn.00521.2003. [DOI] [PubMed] [Google Scholar]
- Kuo JJ, Schonewille M, Siddique T, Schults AN, Fu R, Bar PR, et al. Hyperexcitability of cultured spinal motoneurons from presymptomatic ALS mice. J Neurophysiol. 2004;91:571–5. doi: 10.1152/jn.00665.2003. [DOI] [PubMed] [Google Scholar]
- Kuo JJ, Siddique T, Fu R, Heckman CJ. Increased persistent Na(+) current and its effect on excitability in motoneurones cultured from mutant SOD1 mice. J Physiol. 2005;563:843–54. doi: 10.1113/jphysiol.2004.074138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Influence of voltage-sensitive dendritic conductances on bistable firing and effective synaptic current in cat spinal motoneurons in vivo. J Neurophysiol. 1996;76:2107–10. doi: 10.1152/jn.1996.76.3.2107. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in persistent inward currents. J Neurophysiol. 1998a;80:583–93. doi: 10.1152/jn.1998.80.2.583. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in rhythmic firing patterns. J Neurophysiol. 1998b;80:572–82. doi: 10.1152/jn.1998.80.2.572. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Enhancement of bistability in spinal motoneurons in vivo by the noradrenergic alpha1 agonist methoxamine. J Neurophysiol. 1999a;81:2164–74. doi: 10.1152/jn.1999.81.5.2164. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Paradoxical effect of QX-314 on persistent inward currents and bistable behavior in spinal motoneurons in vivo. J Neurophysiol. 1999b;82:2518–27. doi: 10.1152/jn.1999.82.5.2518. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Adjustable amplification of synaptic input in the dendrites of spinal motoneurons in vivo. J Neurosci. 2000;20:6734–40. doi: 10.1523/JNEUROSCI.20-17-06734.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Essential role of a fast persistent inward current in action potential initiation and control of rhythmic firing. J Neurophysiol. 2001;85:472–5. doi: 10.1152/jn.2001.85.1.472. [DOI] [PubMed] [Google Scholar]
- Lee RH, Kuo JJ, Jiang MC, Heckman CJ. Influence of active dendritic currents on input-output processing in spinal motoneurons in vivo. J Neurophysiol. 2003;89:27–39. doi: 10.1152/jn.00137.2002. [DOI] [PubMed] [Google Scholar]
- Li X, Bennett DJ. Apamin-sensitive calcium-activated potassium currents (SK) are activated by persistent calcium currents in rat motoneurons. J Neurophysiol. 2007;97:3314–30. doi: 10.1152/jn.01068.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Murray K, Harvey PJ, Ballou EW, Bennett DJ. Serotonin facilitates a persistent calcium current in motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol. 2007;97:1236–46. doi: 10.1152/jn.00995.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Bennett DJ. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J Neurophysiol. 2003;90:857–69. doi: 10.1152/jn.00236.2003. [DOI] [PubMed] [Google Scholar]
- Li Y, Gorassini MA, Bennett DJ. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J Neurophysiol. 2004a;91:767–83. doi: 10.1152/jn.00788.2003. [DOI] [PubMed] [Google Scholar]
- Li Y, Harvey PJ, Li X, Bennett DJ. Spastic long-lasting reflexes of the chronic spinal rat studied in vitro. J Neurophysiol. 2004b;91:2236–46. doi: 10.1152/jn.01010.2003. [DOI] [PubMed] [Google Scholar]
- Lipscombe D, Helton TD, Xu W. L-type calcium channels: the low down. J Neurophysiol. 2004;92:2633–41. doi: 10.1152/jn.00486.2004. [DOI] [PubMed] [Google Scholar]
- Manuel M, Meunier C, Donnet M, Zytnicki D. Resonant or not, two amplification modes of proprioceptive inputs by persistent inward currents in spinal motoneurons. J Neurosci. 2007;27:12977–88. doi: 10.1523/JNEUROSCI.3299-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol. 2007;69:291–316. doi: 10.1146/annurev.physiol.69.031905.161516. [DOI] [PubMed] [Google Scholar]
- Miles GB, Hartley R, Todd AJ, Brownstone RM. Spinal cholinergic interneurons regulate the excitability of motoneurons during locomotion. Proc Natl Acad Sci U S A. 2007;104:2448–53. doi: 10.1073/pnas.0611134104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JF, Paul KD, Lee RH, Rymer WZ, Heckman CJ. Restoration of extensor excitability in the acute spinal cat by the 5-HT2 agonist DOI. J Neurophysiol. 1996;75:620–8. doi: 10.1152/jn.1996.75.2.620. [DOI] [PubMed] [Google Scholar]
- Mitchell CS, Feng SS, Lee RH. An analysis of glutamate spillover on the N-methyl-D-aspartate receptors at the cerebellar glomerulus. J Neural Eng. 2007;4:276–82. doi: 10.1088/1741-2560/4/3/013. [DOI] [PubMed] [Google Scholar]
- Moritz AT, Newkirk G, Powers RK, Binder MD. Facilitation of somatic calcium channels can evoke prolonged tail currents in rat hypoglossal motoneurons. J Neurophysiol. 2007;98:1042–7. doi: 10.1152/jn.01294.2006. [DOI] [PubMed] [Google Scholar]
- Mottram CJ, Wallace CL, Chikando CN, Rymer WZ. Mechanisms contributing to spontaneous firing of motor units in the spastic biceps brachii of stroke survivors. Soc Neurosci. 2007:Abstr 726.10. doi: 10.1152/jn.00463.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TR, Cope TC, Abelew TA. Rapid spinal mechanisms of motor coordination. Exerc Sport Sci Rev. 1999;27:255–84. [PubMed] [Google Scholar]
- Nickolls P, Collins DF, Gorman RB, Burke D, Gandevia SC. Forces consistent with plateau-like behaviour of spinal neurons evoked in patients with spinal cord injuries. Brain. 2004;127:660–70. doi: 10.1093/brain/awh073. [DOI] [PubMed] [Google Scholar]
- Nusbaum MP, Beenhakker MP. A small-systems approach to motor pattern generation. Nature. 2002;417:343–50. doi: 10.1038/417343a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault MC. Motoneurons have different membrane resistance during fictive scratching and weight support. J Neurosci. 2002;22:8259–65. doi: 10.1523/JNEUROSCI.22-18-08259.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier JF, Hounsgaard J. 5-HT(2) Receptors promote plateau potentials in turtle spinal motoneurons by facilitating an L-type calcium current. J Neurophysiol. 2003;89:954–9. doi: 10.1152/jn.00753.2002. [DOI] [PubMed] [Google Scholar]
- Perrier JF, Mejia-Gervacio S, Hounsgaard J. Facilitation of plateau potentials in turtle motoneurones by a pathway dependent on calcium and calmodulin. J Physiol. 2000;528(Pt 1):107–13. doi: 10.1111/j.1469-7793.2000.t01-1-00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Input-output functions of mammalian motoneurons. Rev Physiol Biochem Pharmacol. 2001;143:137–263. doi: 10.1007/BFb0115594. [DOI] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Persistent sodium and calcium currents in rat hypoglossal motoneurons. J Neurophysiol. 2003;89:615–24. doi: 10.1152/jn.00241.2002. [DOI] [PubMed] [Google Scholar]
- Prinz AA, Bucher D, Marder E. Similar network activity from disparate circuit parameters. Nat Neurosci. 2004;7:1345–52. doi: 10.1038/nn1352. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev. 2000;80:767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. Properties of a persistent inward current in normal and TEA-injected motoneurons. J Neurophysiol. 1980a;43:1700–24. doi: 10.1152/jn.1980.43.6.1700. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. Role of a persistent inward current in motoneuron bursting during spinal seizures. J Neurophysiol. 1980b;43:1296–318. doi: 10.1152/jn.1980.43.5.1296. [DOI] [PubMed] [Google Scholar]
- Shapiro NP, Lee RH. Synaptic amplification versus bistability in motoneuron dendritic processing: a top-down modeling approach. J Neurophysiol. 2007;97:3948–60. doi: 10.1152/jn.00084.2007. [DOI] [PubMed] [Google Scholar]
- Simon M, Perrier JF, Hounsgaard J. Subcellular distribution of L-type Ca2+ channels responsible for plateau potentials in motoneurons from the lumbar spinal cord of the turtle. Eur J Neurosci. 2003;18:258–66. doi: 10.1046/j.1460-9568.2003.02783.x. [DOI] [PubMed] [Google Scholar]
- Svirskis G, Hounsgaard J. Depolarization-induced facilitation of a plateau-generating current in ventral horn neurons in the turtle spinal cord. J Neurophysiol. 1997;78:1740–2. doi: 10.1152/jn.1997.78.3.1740. [DOI] [PubMed] [Google Scholar]
- Theiss RD, Kuo JJ, Heckman CJ. Persistent inward currents in rat ventral horn neurones. J Physiol. 2007;580:507–22. doi: 10.1113/jphysiol.2006.124123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada T, Hasegawa Y, Ono H. Characterization of alpha1-adrenoceptor subtypes in facilitation of rat spinal motoneuron activity. Eur J Pharmacol. 1997;340:45–52. doi: 10.1016/s0014-2999(97)01406-4. [DOI] [PubMed] [Google Scholar]
- Walton C, Kalmar JM, Cafarelli E. Effect of caffeine on self-sustained firing in human motor units. J Physiol. 2002;545:671–9. doi: 10.1113/jphysiol.2002.025064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijdewind I, Thomas CK. Spontaneous motor unit behavior in human thenar muscles after spinal cord injury. Muscle Nerve. 2001;24:952–62. doi: 10.1002/mus.1094. [DOI] [PubMed] [Google Scholar]