Abstract
Rationale: Sepsis, a leading cause of death worldwide, involves widespread activation of inflammation, massive activation of coagulation, and lymphocyte apoptosis. Calpains, calcium-activated cysteine proteases, have been shown to increase inflammatory reactions and lymphocyte apoptosis. Moreover, calpain plays an essential role in microparticle release.
Objectives: We investigated the contribution of calpain in eliciting tissue damage during sepsis.
Methods: To test our hypothesis, we induced polymicrobial sepsis by cecal ligation and puncture in wild-type (WT) mice and transgenic mice expressing high levels of calpastatin, a calpain-specific inhibitor.
Measurements and Main Results: In WT mice, calpain activity increased transiently peaking at 6 hours after cecal ligation and puncture surgery. Calpastatin overexpression improved survival, organ dysfunction (including lung, kidney, and liver damage), and lymphocyte apoptosis. It decreased the sepsis-induced systemic proinflammatory response and disseminated intravascular coagulation, by reducing the number of procoagulant circulating microparticles and therefore delaying thrombin generation. The deleterious effect of microparticles in this model was confirmed by transferring microparticles from septic WT to septic transgenic mice, worsening their survival and coagulopathy.
Conclusions: These results demonstrate an important role of the calpain/calpastatin system in coagulation/inflammation pathways during sepsis, because calpain inhibition is associated with less severe disseminated intravascular coagulation and better overall outcomes in sepsis.
Keywords: sepsis/multiple organ failure, calpain, calpastatin, microparticles, disseminated intravascular coagulation
At a Glance Commentary
Scientific Knowledge on the Subject
Activation of inflammatory and procoagulant pathways plays a crucial role in multiple organ failure during sepsis. Calpains are known to be activated during inflammatory processes. Calpains are also involved in microparticle release. However, their role in sepsis-induced multiple organ failure is still unclear.
What This Study Adds to the Field
This study demonstrates an important role for calpain in the development of disseminated intravascular coagulation in experimental sepsis via a mechanism involving procoagulant microparticle release.
Sepsis is the most common cause of death worldwide (1). Sepsis develops when the host response to an infection, initially appropriate, becomes amplified and then dysregulated (2, 3). This response leads to the activation of a number of host mediator systems, including leukocyte, cytokine, and hemostatic/thrombotic process, which contribute to the development of hypoperfusion, intravascular thrombosis, and subsequent multiple organ failure (4). This complex and multifocal intravascular activation induces cells to release circulating microparticles (MPs), which are membrane vesicles with both procoagulant and proinflammatory properties (5, 6). Patients with meningococcal sepsis display elevated numbers of MPs originating from platelets and granulocytes that are prothrombotic (7). However, the complex pathophysiology of sepsis is not limited to proinflammatory or procoagulant mechanisms. For example, when septic patients fail to control the bacterial infection, subsequent lymphocyte apoptosis and immunosuppression may predispose them to lethal nosocomial infections (8, 9).
Calpains are calcium-activated neutral cysteine proteases (10). Two ubiquitously expressed isoforms, calpain μ and m, are activated by micromolar and millimolar Ca2+ concentrations, respectively, whereas other isoforms have tissue-specific distributions. Calpastatin is a specific endogenous inhibitor of calpain activity through four equivalent inhibitory domains (11). First, calpains play an important role in inflammatory process. They are involved in the activation of nuclear factor (NF)-κB, and thereby in the NF-κB dependent expression of proinflammatory cytokines and adhesion molecules (12). Calpains are critical for inflammatory cell adhesion and chemotaxis, and inflammatory mediator processing (13). We have previously demonstrated that calpains participate in the development of inflammatory lesions in an acute model of antiglomerular basement membrane nephritis (14). Second, like caspases and other proteases, calpains are involved in programmed cell death or apoptosis, and some studies have suggested a broad role for calpain in various models of T-lymphocyte apoptosis (15, 16). Third, agonist-induced activation of calpains can cause the shedding of procoagulant microparticles from the membrane of aggregating platelets (17).
Although calpains can mediate several critical determinants of sepsis severity, their relative importance in sepsis has not been tested. Using a cecal ligation and puncture (CLP) experimental model of sepsis, we compared transgenic (TG) mice constitutively expressing high levels of calpastatin (14) with wild-type (WT) controls, with respect to survival, tissue lesions, inflammatory processes, lymphocyte apoptosis, and MP release/role in septic mice.
Some of the results of these studies have been previously reported in the form of an abstract (18).
Methods
Experimental details are provided in the online supplement.
Mice and Sepsis Model
The presence and the expression of the complementary DNA clone of rabbit calpastatin were identified in TG mice by polymerase chain reaction and reverse transcription-polymerase chain reaction analysis, respectively, as previously described (14). TG mice were backcrossed into the C57BL/6 background greater than nine generations, then bred to homozygosity. CLP was performed in aged (42–44 wk old) male TG or WT C57BL/6 mice as described previously (19).
Calpain Activity Assay
Calpain activity was measured spectrofluorometrically using the calpain substrate N-succinyl-Leu-Tyr-amidomethylcoumarin as previously described (14).
Isolation of Microparticles
Blood was collected from the heart and anticoagulated with 4% sodium citrate. Platelet-poor plasma (PPP) was obtained by centrifuging blood at 1,500 × g at 23°C for 25 minutes and then centrifuging for 2 minutes at 15,000 × g to remove contaminating cells from the plasma. PPP was stored at −80°C for further analysis (see Supplemental methods in online supplement).
Adoptive Transfer of Septic Microparticles into WT and TG Septic Mice
Three hundred microliters of PPP obtained from WT septic mice was centrifuged at 18,000 × g for 45 minutes to obtain an MP pellet and MP-free supernatant. In the first group of TG septic mice, MPs (diluted in 300 μl of phosphate-buffered saline [PBS]) were injected intravenously (retroorbitally) every 24 hours for 7 days. As a control, MP-free supernatants obtained from the high-speed centrifugation were diluted in PBS to reach a volume of 300 μl and then injected.
Thrombin Generation Assay
Thrombin generation was studied using the Calibrated Automated Thrombogram assay (Diagnostica Stago, Gennevilliers, France) according to the manufacturer's instructions and as standardized in our laboratory (20).
Statistics
For statistical analyses, we used Prism (GraphPad Software, San Diego, CA). All data are expressed as mean ± SEM. Differences between groups were analyzed for statistical significance by t test or one way analysis of variance for repeated measures and subsequent Bonferroni post hoc test. Survival studies were analyzed with the log-rank test. A P value less than 0.05 was accepted as statistically significant.
Results
Tissue Calpain Activity and Expression in WT and TG Mice Undergoing CLP
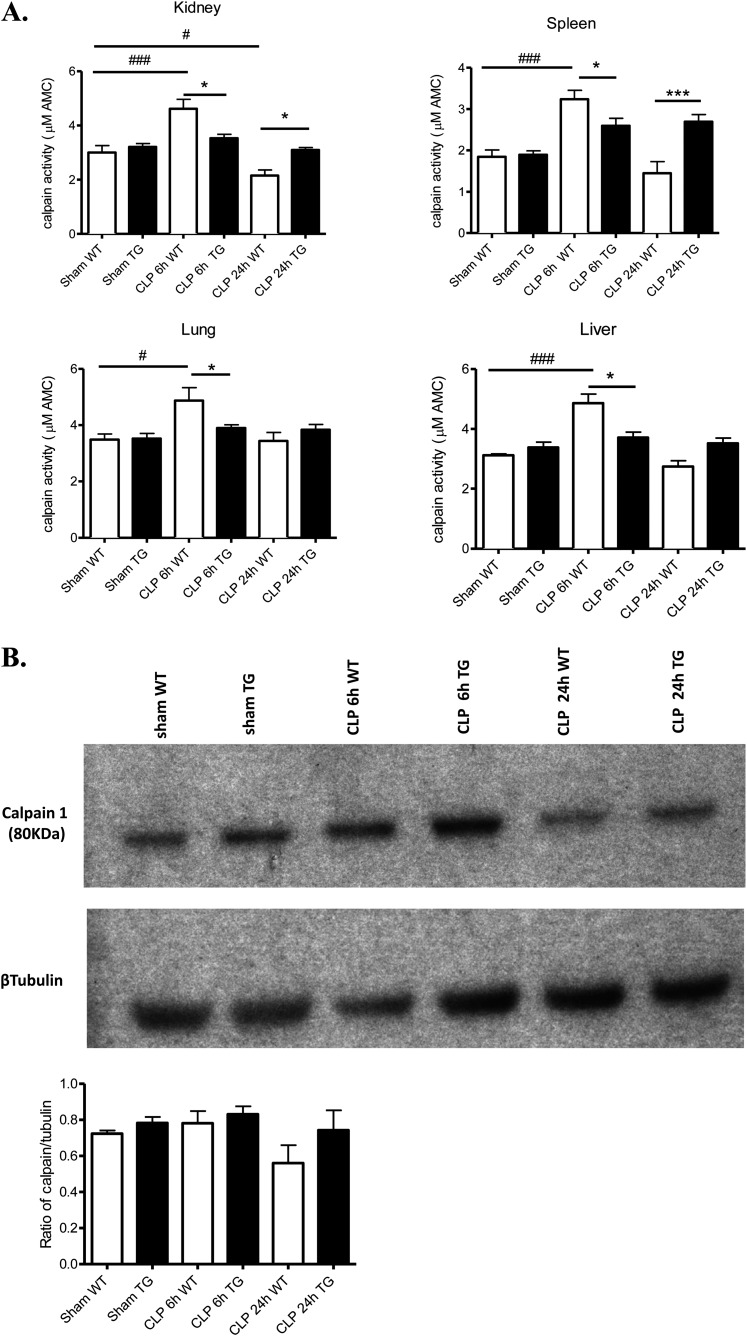
First, we determined whether CLP altered tissue calpain activity, by measuring the calpain-specific cleavage of fluorescent N-succinyl-Leu-Tyr-amidomethylcoumarin. Calpain activity in kidney, liver, spleen, and lung was significantly increased at 6 hours, and returned to basal levels at 24 hours in WT mice. Calpastatin excess did not alter tissue calpain activity in TG mice under sham basal conditions but completely prevented alterations in calpain activity after CLP induction (Figure 1A). Kidney calpain content was similar in sham, WT, and TG mice 6 hours after CLP (Figure 1B). Although there was a trend to less calpain content in WT mice 24 hours after CLP compared with TG mice, it did not reach significance (Figure 1B).
Figure 1.
Alterations of calpain activity and expression in tissues of wild-type (WT) and transgenic (TG) mice following cecal ligation and puncture (CLP). (A) Kidney, liver, spleen, and lung were isolated from WT (white bars) and TG (black bars) mice 6 and 24 hours after CLP (n = 5 mice per group). Calpain activities were determined by measuring the calpain-specific cleavage of fluorescent N-succinyl-Leu-Tyr-amidomethylcoumarin, a calpain-specific substrate. (B) Western blot analysis of calpain 1 in kidney from WT and TG before and 6 and 24 hours after CLP. Quantification was done by densitometry (n = 3). *P < 0.05 WT versus TG, #P < 0.05, ##P < 0.01, ###P < 0.001 CLP versus sham. Error bars represent means ± SEM.
Calpastatin Overexpression Improves Survival and Organ Function after CLP
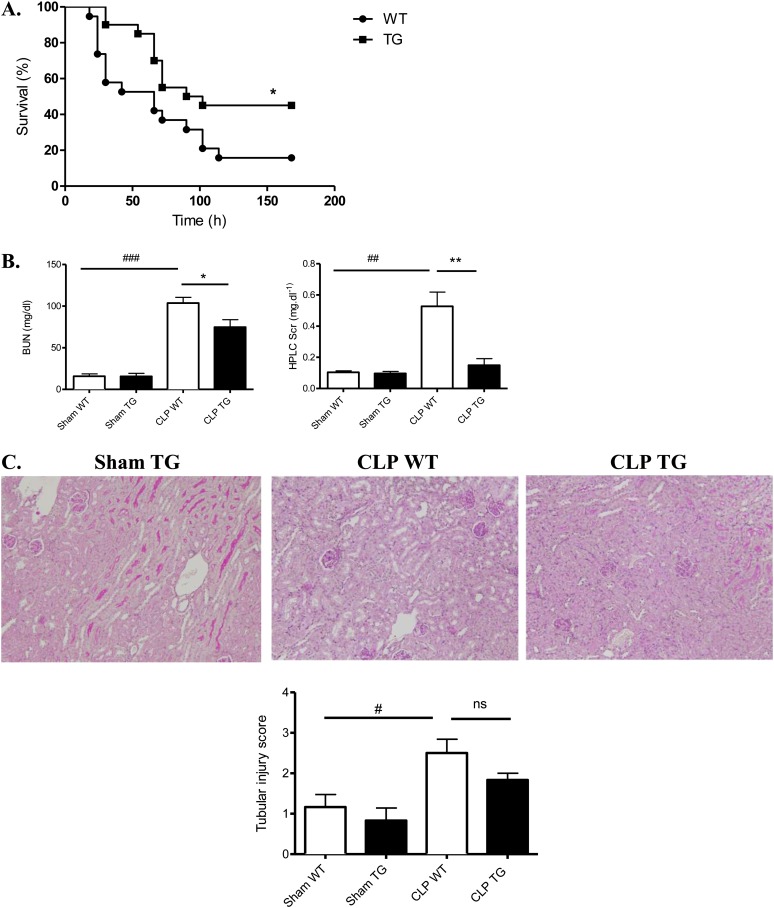
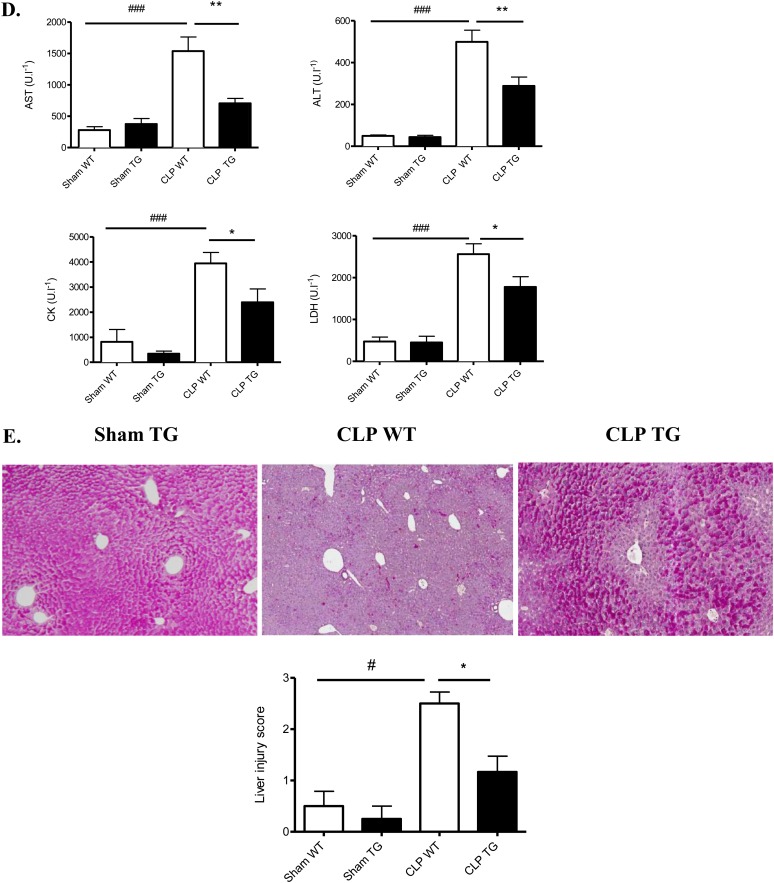
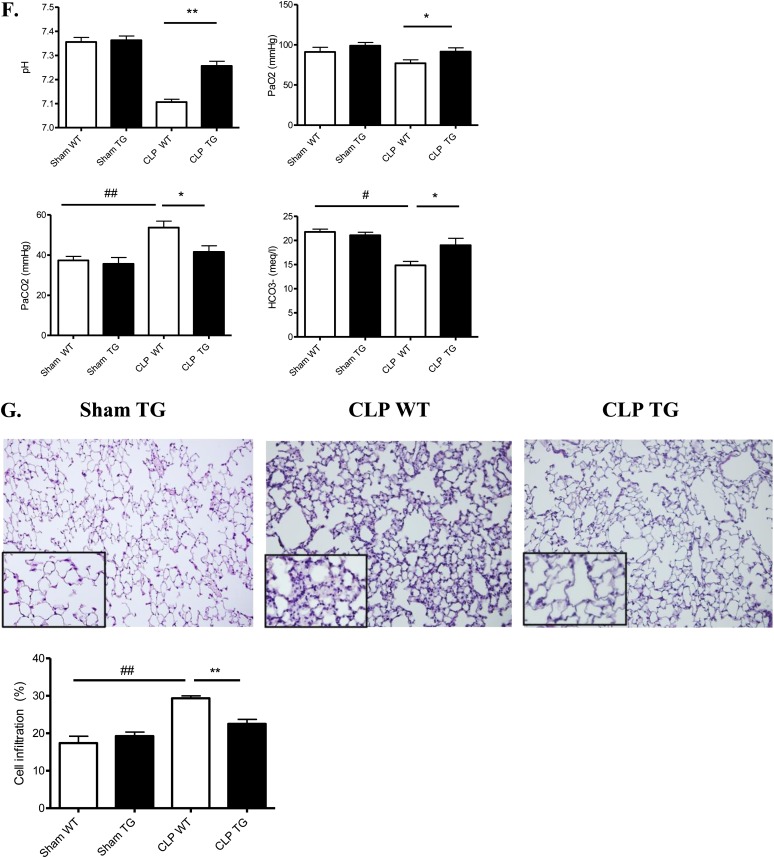
We measured the survival rates after CLP in TG and WT mice. There was a statistically significant improvement in the survival of TG mice: 50% of the TG mice survived until the end of day 7, and 16% of the WT mice survived until the end of day 7 (Figure 2A). Because the lethality in sepsis is associated with organ failure, we examined the pathology and function of major organs often injured in human subjects. Kidney function as measured by serum urea (P ≤ 0.02) and creatinine (P ≤ 0.001) was improved in TG mice (vs. WT) after CLP (Figure 2B). However, tubular injury scores were not statistically different (Figure 2C). In the liver, we observed an improvement in glycogen storage in TG mice versus WT mice. CLP-induced increases in alanine aminotransferase, aspartate aminotransferase, lactate dehydrogenase, and creatine kinase, surrogate markers for liver damage, muscle damage, and cell death, were also significantly decreased in the serum of TG mice (respectively 1.7-, 2.2-, 1.4-, and 1.6-fold decrease) (Figure 2D). Moreover, we observed more hypoxemia (P ≤ 0.04) and more mixed acidosis (P ≤ 0.002) (hypercapnia and metabolic acidosis) in WT septic mice compared with TG septic mice at 24 hours (Figure 2F). The lung pathology (alveolar septal thickening and cell infiltration) was less severe in TG septic than in WT septic mice (Figure 2G).
Figure 2.
Effect of calpastatin overexpression on survival and multiple organ failure during sepsis. (A) Survival curves of wild-type (WT) and transgenic (TG) mice after cecal ligation and puncture (CLP) (P ≤ 0.02). (B) Calpastatin overexpression effect on kidney function, as reflected by serum creatinine concentration (HPLC SCr) and serum urea (BUN) in sham and septic mice 24 hours after CLP (n = 8 in each sham group and 14 in each septic group). (C) Representative periodic acid Schiff staining in kidney of sham TG, septic WT, and TG mice (original magnification, ×20) and tubular injury scores 24 hours after CLP (n = 4 in sham group and 6 in septic group). (D) Alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatine kinase (CK), and lactate dehydrogenase (LDH) in sham WT, sham TG, septic WT, and septic TG mice 24 hours after CLP (n = 8 mice in each sham group and 14 mice in each septic group). (E) Representative liver histology in sham TG, septic WT, and septic TG mice (original magnification, ×10) and liver injury score 24 hours after CLP. The red staining by periodic acid Schiff in hepatocytes indicates glycogen storage capacity (n = 6 per group). (F) Arterial blood gas analysis was performed in sham and septic WT and TG mice at 24 hours (n = 6 per group). (G) Hematoxylin and eosin staining was performed on lung sections from sham TG, septic WT, and septic TG mice at 24 hours (×20 and ×40) and morphometric analysis of cell infiltration was performed using an automated analysis system (n = 12 per group). *P < 0.05 WT versus TG, **P < 0.01 WT versus TG, #P < 0.05 CLP versus sham, ##P < 0.01 CLP versus sham, ###P < 0.001 CLP versus sham. Error bars represent means ± SEM. ns = nonsignificant.
Calpastatin Overexpression Does Not Modify Bacterial Load and Neutrophil Recruitment after Sepsis
Blood and peritoneal bacterial content at 6 and 24 hours after CLP surgery were not statistically different in WT and TG septic mice (see Figure E1 in the online supplement). As calpains have been implicated in regulating inflammatory cell recruitment to injury sites (21), we analyzed cells in the peritoneal cavity at 6 or 24 hours after CLP surgery. In septic mice, the number of neutrophils was similar in WT and TG mice. Consistent with these results, we found no difference in leukocyte rolling and adhesion to the endothelium of mesenteric veins between WT and TG mice, as measured in intravital microscopy experiments (Figure E1).
These results suggest that calpain did not attenuate sepsis in increasing the clearance of bacterial pathogens by neutrophil recruitment or changing antibiotic effectiveness.
Effect of Calpastatin Overexpression on Cytokines and NF-κB Activity
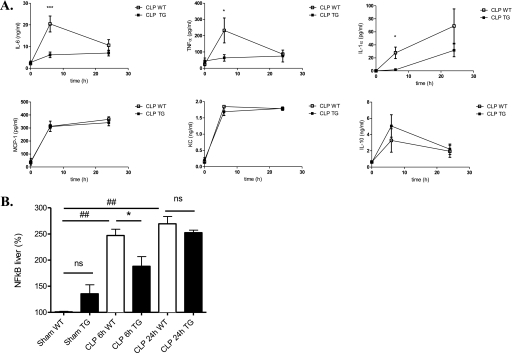
To further investigate the effect of calpastatin overexpression on systemic inflammatory response during sepsis, we evaluated plasma levels of the cytokines interleukin (IL)-6, tumor necrosis factor α(TNF-α), IL-1α, monocyte chemotactic protein-1, keratinocyte-derived chemokine, and IL-10. Calpastatin overexpression significantly reduced IL-6, TNF-α, and IL-1α levels at 6 hours after CLP surgery (vs. WT mice) (Figure 3A).
Figure 3.
Effect of calpastatin overexpression on cytokine concentrations and nuclear factor (NF)-κB activity. (A) Serum interleukin (IL)-6, tumor necrosis factor α(TNFα), IL-1α, keratinocyte-derived chemokine (KC), monocyte chemotactic protein (MCP)-1, and IL-10 concentrations 6 and 24 hours after cecal ligation and puncture (CLP) in wild-type (WT) and transgenic (TG) mice measured by ELISA (n = 4–6 mice per group). (B) NF-κB nuclear activity in liver of sham and septic mice, WT and TG, at 6 and 24 hours (n = 4 mice per group). Error bars represent means ± SEM; *P < 0.05 WT versus TG, ***P < 0.001 WT versus TG, ##P < 0.01 CLP versus sham. ns = nonsignificant.
To determine the inflammatory signaling pathways regulated by calpastatin overexpression, we also investigated the kinetics of NF-κB activation, because calpain activity can participate in the NF-κB activation process. We found that NF-κB was activated in WT and TG septic mice at 6 and 24 hours (Figure 3B). At 6 hours, NF-κB nuclear activity in liver was significantly decreased in TG mice (24% decrease) versus WT mice but not at 24 hours.
Calpastatin Transgenic Mice Have Decreased Lymphocyte Apoptosis
Sepsis is characterized by an initial hyperinflammatory response followed by a period of immunosuppression related to the development of lymphocyte apoptosis (8). Using two different techniques, we showed that lymphocyte apoptosis but not necrosis decreased significantly in TG mice versus WT mice during sepsis (Figure E2). However, transfer experiments of TG or WT lymphocytes into Rag2−/− mice did not show any difference after CLP in terms of survival (Figure E2C).
Sepsis-induced Release of Circulating MPs Is Prevented in Calpastatin Transgenic Mice, Independent of Their Cellular Origin
We next tested the potential role of procoagulant MPs. First, we assessed the number of circulating MPs in sham WT, sham TG, septic WT, and septic TG mice. Microparticles were isolated from plasma samples, labeled, and analyzed by flow cytometry (Figure E5).
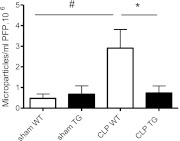
MPs were detected in plasma of sham WT and TG animals at similar levels. Twenty-four hours after CLP surgery, this level increased markedly in WT mice (P ≤ 0.02) while being unchanged in TG mice (Figure 4).
Figure 4.
Flow cytometry data on circulating microparticles (MPs) in sham and septic mice at 24 hours after cecal ligation and puncture (CLP) surgery. Data are given as absolute count of MPs per milliliter of platelet-free plasma (PFP) · 106 (n = 6 per group). Error bars represent means ± SEM; *P < 0.05 WT versus CLP TG, #P < 0.05 CLP versus sham.
We then assessed the cellular origin of circulating MP in sham and septic animals. Most of circulating MPs from septic mice were from platelets (85%); a minority originated from endothelial cells and monocytes (Table 1); a small number of MPs contained erythrocyte markers (data not shown). There was no difference in cellular origin between WT and TG mice (Table 1). Furthermore, there was no correlation between MP level and platelet levels (Table E1).
TABLE 1.
DISTRIBUTION OF ANNEXIN V+ CIRCULATING MPS DIFFERENTIATED BY CELL OF ORIGIN
| Platelets | Endothelial cells | Monocytes | |
| CD41+ MPs (%) | CD144+ MPs (%) | CD14+ MPs (%) | |
| Sham WT | 66.7 (±18) | 3.6 (±1) | 1.4 (±0.4) |
| Sham TG | 59.8 (±5) | 6.3 (±2.5) | 6.6 (±0.6) |
| CLP WT | 84.5 (±17) | 15 (±2.3) | 6.4 (±4.7) |
| CLP TG | 73.8 (±21) | 11 (±1.5) | 4.6 (±1.2) |
Definition of abbreviations: CLP = cecal ligation and puncture; MPs = microparticles; TG = transgenic; WT = wild-type.
No difference was observed between WT and TG mice. Results are expressed in mean (±SEM).
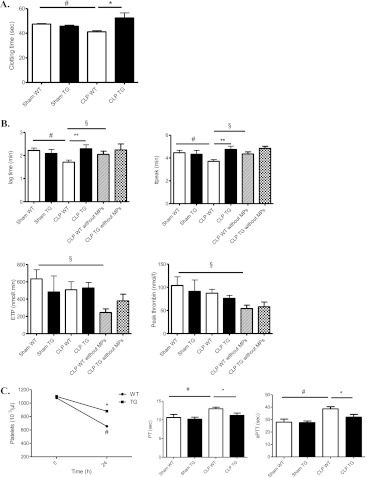
To assess the role of these MPs, we measured the procoagulant activity of their procoagulant phospholipids. MPs (normalized by volume) isolated from WT septic mice were significantly more procoagulant than MPs isolated from sham mice (P ≤ 0.01); this was reversed in TG septic mice (P ≤ 0.04) (Figure 5A).
Figure 5.
Procoagulant activity of microparticles (MPs). Effect of calpastatin overexpression on thrombin generation and global test of hemostasis. (A) Procoagulant activity of MPs was assessed by functional assay (STA-Procoag-PPL, Diagnostica Stago) in sham wild-type (WT), sham transgenic (TG), septic WT, and septic TG mice 24 hours after cecal ligation and puncture (CLP) surgery (n = 8 per group) (P ≤ 0.04). Error bars represent means ± SEM; *P < 0.05 WT versus TG, #P < 0.05 CLP versus sham. (B) Calibrated thrombography in PPP samples from sham WT, sham TG, septic WT, and septic TG mice and PPP depleted in MPs from CLP WT and CLP TG mice, 24 hours after CLP surgery. The prolongation of the lag time and time to peak thrombin generation suggest a delay in the activation of hemostasis in TG mice. **P < 0.01 versus CLP WT; #P < 0.05 versus sham WT; §P < 0.05 CLP WT versus PPP depleted in MPs. Results are expressed as mean (±SEM). (C) Platelets and hemostatic parameters. Measurement of platelets are shown before and 24 hours after CLP surgery (n = 5 per group). Prothrombin time (PT) and activated partial thromboplastin time (aPTT) are shown in plasma from sham WT, sham TG, septic WT, and septic TG mice, 24 hours after CLP surgery. PT and aPTT are significantly increased in septic WT mice (n = 5 per group). *P < 0.05, versus CLP WT; #P < 0.05 versus sham WT. Results are expressed as mean (±SEM).
Restriction of MP Levels in TG Septic Mice Delayed Thrombin Generation and Decreased Disseminated Intravascular Coagulation Compared with WT Mice during Sepsis
MPs originating from platelets have the capacity to generate thrombin. We therefore looked at four parameters of thrombin generation (the endogenous thrombin potential, lag time, time to peak, and peak height) in plasma samples from control and septic mice, before and after removing MPs. There was a significant decrease in lag time and time to peak in WT septic mice compared with control WT and control and septic TG mice (Figure 5B). The presence of MPs in plasma did influence thrombin generation, since depletion of MPs from PPP delayed and reduced thrombin generation (Figure 5B). Taken together, this suggests that septic MPs are responsible for this procoagulant phenotype.
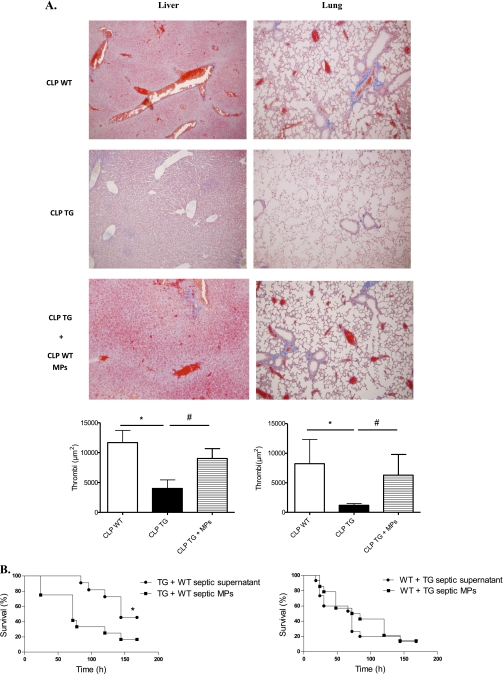
Interestingly, calpastatin overexpression lessened the decline in platelet counts during sepsis (P ≤ 0.01) (Figure 5C), lessened the consumption of coagulation factors FVII, FII, FVIII, and FIX (Table 2), and shortened the prothrombin time and the activated partial thromboplastin time compared with WT mice (14 and 17% decrease, respectively) (Figure 5C), which is consistent with milder coagulopathy in TG mice. We therefore assessed the presence of thrombi on tissue sections (lung, liver, and kidney) in WT and TG mice, and TG mice supplemented with WT septic MPs at 6 and 24 hours and in “pre-mortem” mice (mice that exhibit signs of distress/extreme morbidity as assessed by a clinical score described in Supplemental methods and Table E2). Livers and lungs at 6 hours did not show any thrombi in vessels (data not shown). At a later stage (“pre-mortem mice”), thrombi were dramatically increased in WT septic mice compared with TG mice (Figure 6A). To ascertain the presence of fibrin in these vessels, we used a fluorescein isothiocyanate–conjugated anti-fibrin for immunofluorescence analysis (Figure E3). When TG mice were supplemented with MPs originating from WT septic mice, thrombi in lung and liver increased (2.2- and 5.2-fold increase, respectively). Thrombi in kidneys were very scarce in WT and TG mice, even in pre-mortem mice (data not shown).
TABLE 2.
COAGULATION FACTORS IN SHAM WT AND TG MICE AND 24 HOURS AFTER CLP SURGERY IN WT AND TG MICE
| Sham WT | Sham TG | CLP WT | CLP TG | P | |
| F VIIII, % | 96.9 (±2.3) | 97.2 (±6.8) | 29.5 (±7) | 62.3 (±7.1) | <0.05 |
| F IX, % | 95.7 (±5) | 98 (±6.8) | 55.1 (±1.6) | 89.6 (±2.2) | <0.05 |
| F XI, % | 96.6 (±4.9) | 90.7 (±4.3) | 76.7 (±7.9) | 82.7 (±8.6) | ns |
| F XII, % | 88.6 (±4.7) | 86.1 (±4.3) | 69.7 (±2.4) | 77.5 (±2.5) | ns |
| F V, % | 98.1 (±6.6) | 88.6 (±7.4) | 63.1 (±6.4) | 82.9 (±8.2) | ns |
| F VII, % | 94.7 (±6.4) | 83.3 (±3.3) | 40.6 (±3.7) | 76.3 (±8) | <0.05 |
| F II, % | 90.4 (±5.6) | 102.2 (±5.7) | 56 (±5.6) | 71.7 (±1.8) | <0.05 |
| F X, % | 101 (±5.4) | 94.6 (±3.2) | 56.9 (±12.4) | 96.7 (±11.6) | ns |
Definition of abbreviation: CLP = cecal ligation and puncture; MPs = microparticles; ns = nonsignificant; TG = transgenic; WT = wild-type.
Results are expressed in mean (±SEM) (n = 5 per group).
*P < 0.05 CLP TG versus CLP WT.
Figure 6.
Effect of microparticles (MPs) on fibrin deposit and survival. (A) Representative photographs of sections stained with Marcius Scarlet Blue for fibrin. Results shown correspond to livers (original magnification, ×20) and lungs (original magnification, ×10) in “pre-mortem” mice. Thrombi (red blood cells mixed with fibrin in red) were seen in wild-type (WT) septic mice and transgenic (TG) mice supplemented with WT septic MPs. Quantification of red area was performed using an automated analysis system (n = 6 per group). Results are means ± SEM. *P < 0.05 versus WT, #P < 0.05 CLP TG + MPs versus CLP TG. (B) Effects of MPs on survival. (Left) Survival curves of septic TG mice supplemented with MPs from septic WT mice (n = 16) or corresponding supernatant (n = 14) (P ≤ 0.04); (right) survival curves of septic WT mice supplemented with MPs from septic TG mice (n = 12) or corresponding supernatant (n = 12) (P = nonsignificant). CLP = cecal ligation and puncture.
Sepsis Survival Benefit in Calpastatin Transgenic Mice Can Be Alleviated by Transfer of Wild-Type Septic Microparticles
To evaluate the impact of MPs on the survival advantage observed in TG mice during sepsis, we injected TG mice with either circulating MPs from WT septic mice or the MP-depleted supernatant of the high-speed centrifugation, every 24 hours after CLP. Injection of MPs from WT septic mice worsened the prognosis of TG mice during sepsis, as assessed by survival curves (P ≤ 0.03) (Figure 6B) and markers of organ dysfunction (Figure E4). Conversely, supplementation of WT septic mice with MPs from TG mice did not change the survival outcome (Figure 6B). In a control experiment, MP fractions and supernatant (originating from frozen PPP) were plated onto tryptic soy agar and colonies were counted after 24-hour incubation at 37°C. We did not find any bacteria at 24 hours, excluding the fact that circulating bacteria in MP fractions could be responsible for the worse outcome in TG mice supplemented with MP fractions.
Discussion
This study demonstrates for the first time that calpain inhibition attenuates inflammatory and disseminated coagulopathy processes and improves splenic apoptosis, organ dysfunction, and survival during sepsis in mice, in part, by decreasing procoagulant microparticles.
Previous studies showed that treatment with pharmacological calpain inhibitors attenuated myocardial dysfunction or diaphragmatic weakness in models of endotoxemia (22, 23) and reduced organ injury in hemorrhagic shock (24). However, such inhibitors usually lack specificity among cysteine proteases and other proteolytic enzymes (25). Moreover, most of these studies examined a model of endotoxemia, in which the inflammatory response is entirely deleterious, as there is no infectious focus, and eradication of pathogenic organisms by the host's immune system is not essential for recovery. As a model of sepsis, we chose CLP, a model closer than endotoxemia to the human disease. Indeed, in patients with sepsis, while the inflammatory response is critical for the pathogenesis of organ system dysfunction, it is also a critical component of the host's defense against the invading microorganisms.
This study demonstrates for the first time that calpastatin overexpression, a more specific mode of calpain inhibition, is beneficial in terms of acute lung injury, hepatic dysfunction, and renal failure, three major organ dysfunctions that are prominent features during sepsis (26, 27). Moreover, Li et al. found a protective effect of calpastatin overexpression on myocardial dysfunction in endotoxemia (28). All these results suggest a key role of calpains during sepsis.
We first studied the time course of calpain activation during sepsis. Calpain activity significantly increased 6 hours after CLP surgery and paradoxically decreased at 24 hours in WT mice, whereas calpain activity remained at a steady state during the course of sepsis in TG mice. Calpains have been shown to be activated in multiple inflammatory models (14, 21, 29) and after endotoxemia (28), consistent with our results at 6 hours. When looking at the 24-hour results, three non–mutually exclusive mechanisms can explain the decrease of calpain activity in WT septic mice. First, excess of calpain activation during the early phase of sepsis could induce a consumption of this protein and its decrease at a later phase. Second, cell death could be responsible for calpain release into the extracellular environment (30). As WT mice have more severe organ dysfunction than TG mice 24 hours after CLP, calpain may have been released by necrotic and apoptotic cells. However, we found a trend toward less calpain content in WT mice 24 hours after CLP compared with TG mice (Figure 1B), but it did not reach significance, strengthening the view that these two mechanisms are not sufficient to explain our results. A third mechanism may have been involved: at 24 hours of sepsis, WT mice were more acidotic than TG mice as assessed by arterial blood gas measurements. Since the optimum pH for calpain activity is between 7.2 and 8.2 (10), acidosis may have inhibited calpain activity in WT septic mice.
However, if calpain activation is only an early and transient event, it still plays a crucial role in the course of sepsis and multiple organ failure as assessed by differences in terms of outcome in WT and TG mice, even at a later stage of sepsis (7 d). Transient, early mediators would not be unprecedented, as there are numerous observations that only early interventions during sepsis provide better outcomes. For example, in clinical studies, delays in the recognition of the sepsis and therapeutic management of critically ill patients have been associated with higher mortality rates and increased utilization of hospital resources (31–33).
We therefore focused on what underlying mechanisms could explain the impact of calpastatin overexpression on multiorgan dysfunction and survival during sepsis.
A growing body of evidence supports the contribution of lymphocyte apoptosis in worsening the prognosis during sepsis both in experimental and clinical settings (34–37). As calpains have been involved in lymphocyte apoptosis (15), we first explored this mechanism. We tested whether lymphocyte apoptosis could contribute to the benefit we observed, by using RAG2−/− mice, then reconstituting them with TG or WT lymphocytes. Within this context, calpastatin overexpression in lymphocytes did not change the survival rate of septic mice. There are two main interpretations of this result: first, the effect of calpastatin overexpression on lymphocyte apoptosis is not sufficient to modify the survival after CLP. Alternatively, there may be a reason why lymphocyte reconstitution does not adequately represent lymphocyte-specific overexpression of calpastatin, such as developmental differences between RAG2−/− mice and C57BL6 mice. Therefore, our study does not eliminate the possibility that calpastatin overexpression in lymphocytes participates in the protection observed on our TG mice during sepsis.
We then focused our study on MPs, which increased dramatically after CLP in WT mice, but was significantly blunted in TG mice after CLP. Calpain have a central role in MP release from cells, especially platelets, as Ca2+-activated calpains degrade cytoskeletal proteins such as talin or vinculin, directly allowing MP release into the extracellular fluid (17, 38). Here, we found that CD41+ platelet MPs represented the most abundant type (85%) of MPs in septic plasma, consistent with previous studies in humans (39). The importance of the role of platelet MPs in procoagulant activity was illustrated by Sinauridze and colleagues (40), who demonstrated that the platelet MP surface was 50- to 100-fold more procoagulant than activated platelets. Their procoagulant properties are based on the combined presence of phosphatidylserine, a procoagulant aminophospholipid exposed after stimulation that supports the assembly of the blood-clotting enzyme complexes, and tissue factor, a major initiator and promoter of the coagulation cascade and inflammation. Despite a dramatic difference in MP concentration in WT CLP and TG CLP mice, we did not find any differences in the cellular origin of MP in WT and TG septic mice. The lack of shift in cellular origin is consistent with the ubiquity of calpastatin overexpression.
The high amount of platelet-derived MPs can participate in the coagulation activation in our model: since the earliest demonstration of MP generation by platelets, it has been known that PS expression on MPs contributes to thrombin generation by facilitating assembly of the prothrombinase complex (41). Indeed, TG septic mice had a longer lag time and time to peak in the thrombin generation test compared with WT septic mice, consistent with previous studies in human sepsis (42). Moreover, when the WT septic PPP was depleted in MPs by high-speed centrifugation, lag time and time to peak became closer to the values observed in TG septic mice. This strongly suggests that the lower abundance of MPs in TG mice is responsible for the delay in thrombin generation.
The coagulation system is pathologically activated in severe sepsis and is a major factor of subsequent organ dysfunction. Converging animal and clinical data highlight a role for procoagulant MPs in the initiation of disseminated intravascular coagulation (DIC) (7, 43, 44). Here, the beneficial effect of calpastatin transgene expression on survival and DIC tissue injury disappeared when TG mice were supplemented with septic WT MPs, providing evidence for the deleterious effect of procoagulant MPs in this model. As the supernatant from the high-speed centrifugation had no effect on outcome, this effect was not caused by injection of bacterial products or cytokines.
The role of MPs during sepsis is complex; previous studies describe both protective (45) and deleterious (46) effects. In this study, we did not differentiate the precise role of endothelial-/monocyte-/erythrocyte-/platelet-derived MPs, and we cannot exclude that some might be beneficial. However, the predominant effect in our model is procoagulant and deleterious.
Another important aspect of calpains is their role in the inflammatory process. Data on NF-κB activity and cytokine concentration (IL-1, IL-6, and TNF-α) suggest that MPs are not the only explanation for the improved survival in TG mice during sepsis. Calpains have been shown to degrade the NF-κB inhibitor I-κB (12) and thereby to induce the nuclear translocation of NF-κB. In this regard, we found a substantial reduction of NF-κB activity at 6 hours in TG septic mice compared with WT septic mice. As NF-κB is an important mediator for IL-6, IL-1, and TNF-α gene expression (47), it can explain the differences observed in IL-6, IL-1, and TNF-α levels in WT and TG septic mice. Interestingly, IL-6, an inflammatory cytokine, is one of the major mediators involved in coagulation activation. A positive correlation between plasma IL-6 and platelet-derived MPs has been demonstrated in humans (48), and IL-6 is known to increase platelet reactivity and microparticle release. Six hours after CLP, we found a significant increase of IL-6 level in plasma of WT septic mice compared with TG septic mice. IL-6 may therefore have enhanced the coagulation activation in WT mice, suggesting a strong link between inflammation and coagulation in our model.
Thus, targeting the calpain/calpastatin system may allow selective reduction in inflammation, DIC, lymphocyte apoptosis, and later immunodepression, without impairing critically important innate immune functions (e.g., neutrophil infiltration). Furthermore, although calpastatin overexpression inhibits sepsis-induced calpain activity, it does not alter basal calpain activity. We did not find any changes in calpain activity, number of MPs, or thrombin generation in nonseptic WT versus TG mice. This has also been shown in other models (14, 21, 28). Structural and biochemical data indicate that calpastatin might bind preferentially to calcium-activated calpains (11), suggesting that calpastatin controls calpain activity only under stimulated conditions. Thus, therapeutic strategies focusing on increasing calpastatin may allow inhibiting maladaptive, overexuberant inflammatory and coagulation responses without interfering with basal calpain activity, which is required for normal cellular homeostasis.
In conclusion, the present study provides evidence for the first time that a specific inhibition of calpain increases survival and reduces multiorgan dysfunction during sepsis. The main mechanism involved is the decrease of procoagulant MPs release involved in the pathogenesis of DIC. This study shed new light on the pathophysiology of sepsis, and calpain may therefore constitute a novel target for therapeutic intervention in sepsis when coagulation and inflammation are abnormally enhanced.
Supplementary Material
Acknowledgments
The authors thank Marie-Paule Roman (Laboratory of Hematology, Tenon Hospital, France) for her participation in global tests of hemostasis, Pr. Gerard Eberl (Institut Pasteur, Laboratory of Lymphoid Tissue Development, CNRS URA 1961, Paris, France) and Pr. Michel Chignard (Unité de Défense Innée et Inflammation, Institut Pasteur, Paris, France) for providing RAG2−/− mice, and Patrick Vandreden (Diagnostica Stago, Asnières sur Seine, France) for his participation in the procoagulant activity of MPs experiment.
Footnotes
Supported by the Institut National de la Santé et de la Recherche Médicale and by the Intramural Research Program at National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
Author Contributions: L.Z. conceived, performed the experimental laboratory work and wrote the manuscript; G.G. and I.E. conceived the hemostasis part of the experiment; J.P, S.P., and C.L. helped to perform the laboratory work. C.B. and J.L.M. conceived and performed the flow cytometry experiment on MPs; X.H. helped to perform the histological analysis; E.L. and J.-p.H. helped to conceive the experiment. R.A.S., P.S.T.Y., and L.B. conceived the experiment and wrote the manuscript. All authors read and approved the final manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201109-1686OC on January 20, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009;302:2323–2329 [DOI] [PubMed] [Google Scholar]
- 2.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003;31:1250–1256 [DOI] [PubMed] [Google Scholar]
- 3.Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet 2005;365:63–78 [DOI] [PubMed] [Google Scholar]
- 4.Esmon CT. Sepsis: a myriad of responses. Lancet 2001;358:S61. [DOI] [PubMed] [Google Scholar]
- 5.Morel O, Toti F, Hugel B, Freyssinet JM. Cellular microparticles: a disseminated storage pool of bioactive vascular effectors. Curr Opin Hematol 2004;11:156–164 [DOI] [PubMed] [Google Scholar]
- 6.Mortaza S, Martinez MC, Baron-Menguy C, Burban M, de la Bourdonnaye M, Fizanne L, Pierrot M, Calès P, Henrion D, Andriantsitohaina R, et al. Detrimental hemodynamic and inflammatory effects of microparticles originating from septic rats. Crit Care Med 2009;37:2045–2050 [DOI] [PubMed] [Google Scholar]
- 7.Nieuwland R, Berckmans RJ, McGregor S, Böing AN, Romijn FP, Westendorp RG, Hack CE, Sturk A. Cellular origin and procoagulant properties of microparticles in meningococcal sepsis. Blood 2000;95:930–935 [PubMed] [Google Scholar]
- 8.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 2003;348:138–150 [DOI] [PubMed] [Google Scholar]
- 9.Munford RS, Pugin J. Normal responses to injury prevent systemic inflammation and can be immunosuppressive. Am J Respir Crit Care Med 2001;163:316–321 [DOI] [PubMed] [Google Scholar]
- 10.Sorimachi H, Suzuki K. The structure of calpain. J Biochem 2001;129:653–664 [DOI] [PubMed] [Google Scholar]
- 11.Hanna RA, Campbell RL, Davies PL. Calcium-bound structure of calpain and its mechanism of inhibition by calpastatin. Nature 2008;456:409–412 [DOI] [PubMed] [Google Scholar]
- 12.Shumway SD, Maki M, Miyamoto S. The PEST domain of IkappaBalpha is necessary and sufficient for in vitro degradation by mu-calpain. J Biol Chem 1999;274:30874–30881 [DOI] [PubMed] [Google Scholar]
- 13.Lokuta MA, Nuzzi PA, Huttenlocher A. Calpain regulates neutrophil chemotaxis. Proc Natl Acad Sci USA 2003;100:4006–4011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peltier J, Bellocq A, Perez J, Doublier S, Dubois YC, Haymann JP, Camussi G, Baud L. Calpain activation and secretion promote glomerular injury in experimental glomerulonephritis: evidence from calpastatin-transgenic mice. J Am Soc Nephrol 2006;17:3415–3423 [DOI] [PubMed] [Google Scholar]
- 15.Squier MK, Cohen JJ. Calpain, an upstream regulator of thymocyte apoptosis. J Immunol 1997;158:3690–3697 [PubMed] [Google Scholar]
- 16.Sarin A, Nakajima H, Henkart PA. A protease-dependent TCR-induced death pathway in mature lymphocytes. J Immunol 1995;154:5806–5812 [PubMed] [Google Scholar]
- 17.Pasquet JM, Toti F, Nurden AT, Dachary-Prigent J. Procoagulant activity and active calpain in platelet-derived microparticles. Thromb Res 1996;82:509–522 [DOI] [PubMed] [Google Scholar]
- 18.Zafrani L, Hu X, Dezman Z, Zhou H, Leelahavanichkul A, Perez J, Letavernier E, Tsuji T, Star RA, Baud L, et al. Calpastatin overexpression decreases sepsis-induced organ damage. J Am Soc Nephrol 2010;21:785A [Google Scholar]
- 19.Doi K, Leelahavanichkul A, Hu X, Sidransky KL, Zhou H, Qin Y, Eisner C, Schnermann J, Yuen PS, Star RA. Pre-existing renal disease promotes sepsis-induced acute kidney injury and worsens outcome. Kidney Int 2008;74:1017–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerotziafas GT, Depasse F, Busson J, Leflem L, Elalamy I, Samama MM. Towards a standardization of thrombin generation assessment: the influence of tissue factor, platelets and phospholipids concentration on the normal values of Thrombogram-Thrombinoscope assay. Thromb J 2005;3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Letavernier E, Perez J, Bellocq A, Mesnard L, de Castro Keller A, Haymann JP, Baud L. Targeting the calpain/calpastatin system as a new strategy to prevent cardiovascular remodeling in angiotensin II-induced hypertension. Circ Res 2008;102:720–728 [DOI] [PubMed] [Google Scholar]
- 22.Tissier S, Lancel S, Marechal X, Mordon S, Depontieu F, Scherpereel A, Chopin C, Neviere R. Calpain inhibitors improve myocardial dysfunction and inflammation induced by endotoxin in rats. Shock 2004;21:352–357 [DOI] [PubMed] [Google Scholar]
- 23.Supinski GS, Callahan LA. Calpain activation contributes to endotoxin-induced diaphragmatic dysfunction. Am J Respir Cell Mol Biol 2010;42:80–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDonald MC, Mota-Filipe H, Paul A, Cuzzocrea S, Abdelrahman M, Harwood S, Plevin R, Chatterjee PK, Yaqoob MM, Thiemermann C. Calpain inhibitor I reduces the activation of nuclear factor-kappaB and organ injury/dysfunction in hemorrhagic shock. FASEB J 2001;15:171–186 [DOI] [PubMed] [Google Scholar]
- 25.Carragher NO. Calpain inhibition: a therapeutic strategy targeting multiple disease states. Curr Pharm Des 2006;12:615–638 [DOI] [PubMed] [Google Scholar]
- 26.Matuschak GM, Lechner AJ. Acute lung injury and the acute respiratory distress syndrome: pathophysiology and treatment. Mo Med 2010;107:252–258 [PMC free article] [PubMed] [Google Scholar]
- 27.Ricci Z, Polito A, Ronco C. The implications and management of septic acute kidney injury. Nat Rev Nephrol 2011;7:218–225 [DOI] [PubMed] [Google Scholar]
- 28.Li X, Li Y, Shan L, Shen E, Chen R, Peng T. Over-expression of calpastatin inhibits calpain activation and attenuates myocardial dysfunction during endotoxaemia. Cardiovasc Res 2009;83:72–79 [DOI] [PubMed] [Google Scholar]
- 29.Hassen GW, Feliberti J, Kesner L, Stracher A, Mokhtarian F. A novel calpain inhibitor for the treatment of acute experimental autoimmune encephalomyelitis. J Neuroimmunol 2006;180:135–146 [DOI] [PubMed] [Google Scholar]
- 30.Mehendale HM, Limaye PB. Calpain: a death protein that mediates progression of liver injury. Trends Pharmacol Sci 2005;26:232–236 [DOI] [PubMed] [Google Scholar]
- 31.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M; Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001;345:1368–1377 [DOI] [PubMed] [Google Scholar]
- 32.Estenssoro E, González F, Laffaire E, Canales H, Sáenz G, Reina R, Dubin A. Shock on admission day is the best predictor of prolonged mechanical ventilation in the ICU. Chest 2005;127:598–603 [DOI] [PubMed] [Google Scholar]
- 33.Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, Gea-Banacloche J, Keh D, Marshall JC, Parker MM, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med 2004;32:858–873 [DOI] [PubMed] [Google Scholar]
- 34.Hotchkiss RS, Chang KC, Swanson PE, Tinsley KW, Hui JJ, Klender P, Xanthoudakis S, Roy S, Black C, Grimm E, et al. Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat Immunol 2000;1:496–501 [DOI] [PubMed] [Google Scholar]
- 35.Hotchkiss RS, Opal S. Immunotherapy for sepsis–a new approach against an ancient foe. N Engl J Med 2010;363:87–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leelahavanichkul A, Yasuda H, Doi K, Hu X, Zhou H, Yuen PS, Star RA. Methyl-2-acetamidoacrylate, an ethyl pyruvate analog, decreases sepsis-induced acute kidney injury in mice. Am J Physiol Renal Physiol 2008;295:F1825–F1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doi K, Hu X, Yuen PS, Leelahavanichkul A, Yasuda H, Kim SM, Schnermann J, Jonassen TE, Frøkiaer J, Nielsen S, et al. AP214, an analogue of alpha-melanocyte-stimulating hormone, ameliorates sepsis-induced acute kidney injury and mortality. Kidney Int 2008;73:1266–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fox JE, Austin CD, Reynolds CC, Steffen PK. Evidence that agonist-induced activation of calpain causes the shedding of procoagulant-containing microvesicles from the membrane of aggregating platelets. J Biol Chem 1991;266:13289–13295 [PubMed] [Google Scholar]
- 39.Flaumenhaft R. Formation and fate of platelet microparticles. Blood Cells Mol Dis 2006;36:182–187 [DOI] [PubMed] [Google Scholar]
- 40.Sinauridze EI, Kireev DA, Popenko NY, Pichugin AV, Panteleev MA, Krymskaya OV, Ataullakhanov FI. Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thromb Haemost 2007;97:425–434 [PubMed] [Google Scholar]
- 41.Freyssinet JM, Toti F. Formation of procoagulant microparticles and properties. Thromb Res 2010;125:S46–S48 [DOI] [PubMed] [Google Scholar]
- 42.Collins PW, Macchiavello LI, Lewis SJ, Macartney NJ, Saayman AG, Luddington R, Baglin T, Findlay GP. Global tests of haemostasis in critically ill patients with severe sepsis syndrome compared to controls. Br J Haematol 2006;135:220–227 [DOI] [PubMed] [Google Scholar]
- 43.Morel O, Morel N, Hugel B, Jesel L, Vinzio S, Goichot B, Bakouboula B, Grunebaum L, Freyssinet JM, Toti F. The significance of circulating microparticles in physiology, inflammatory and thrombotic diseases [in French]. Rev Med Interne 2005;26:791–801 [DOI] [PubMed] [Google Scholar]
- 44.Wang JG, Manly D, Kirchhofer D, Pawlinski R, Mackman N. Levels of microparticle tissue factor activity correlate with coagulation activation in endotoxemic mice. J Thromb Haemost 2009;7:1092–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mostefai HA, Meziani F, Mastronardi ML, Agouni A, Heymes C, Sargentini C, Asfar P, Martinez MC, Andriantsitohaina R. Circulating microparticles from patients with septic shock exert protective role in vascular function. Am J Respir Crit Care Med 2008;178:1148–1155 [DOI] [PubMed] [Google Scholar]
- 46.Mastronardi ML, Mostefai HA, Meziani F, Martinez MC, Asfar P, Andriantsitohaina R. Circulating microparticles from septic shock patients exert differential tissue expression of enzymes related to inflammation and oxidative stress. Crit Care Med 2011;39:1739–1748 [DOI] [PubMed] [Google Scholar]
- 47.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell 2002;109:S81–S96 [DOI] [PubMed] [Google Scholar]
- 48.Ueba T, Nomura S, Inami N, Nishikawa T, Kajiwara M, Iwata R, Yamashita K. Correlation and association of plasma interleukin-6 and plasma platelet-derived microparticles, markers of activated platelets, in healthy individuals. Thromb Res 2010;125:e329–e334 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.