Abstract
Rationale: Novel tuberculosis (TB) vaccines should be safe and effective in populations infected with Mycobacterium tuberculosis (M.tb) and/or HIV for effective TB control.
Objective: To determine the safety and immunogenicity of MVA85A, a novel TB vaccine, among M.tb- and/or HIV-infected persons in a setting where TB and HIV are endemic.
Methods: An open-label, phase IIa trial was conducted in 48 adults with M.tb and/or HIV infection. Safety and immunogenicity were analyzed up to 52 weeks after intradermal vaccination with 5 × 107 plaque-forming units of MVA85A. Specific T-cell responses were characterized by IFN-γ enzyme-linked immunospot and whole blood intracellular cytokine staining assays.
Measurements and Main Results: MVA85A was well tolerated and no vaccine-related serious adverse events were recorded. MVA85A induced robust and durable response of mostly polyfunctional CD4+ T cells, coexpressing IFN-γ, tumor necrosis factor-α, and IL-2. Magnitudes of pre- and postvaccination T-cell responses were lower in HIV-infected, compared with HIV-uninfected, vaccinees. No significant effect of antiretroviral therapy on immunogenicity of MVA85A was observed.
Conclusions: MVA85A was safe and immunogenic in persons with HIV and/or M.tb infection. These results support further evaluation of safety and efficacy of this vaccine for prevention of TB in these target populations.
Keywords: tuberculosis, HIV-1, vaccine, MVA85A, clinical trial
At a Glance Commentary
Scientific Knowledge on the Subject
New, more effective tuberculosis vaccines are urgently required for people infected with Mycobacterium tuberculosis (M.tb) and/or HIV. We conducted an open-label, phase IIa trial to determine the safety and immunogenicity of MVA85A vaccination in 48 adults with M.tb and/or HIV infection, off and on antiretroviral therapy.
What This Study Adds to the Field
MVA85A vaccination induced mild local reactions in all participants with M.tb and/or HIV infection, but was well tolerated. The vaccine induced long-lived, predominantly polyfunctional CD4 T-cell responses. Infection with HIV resulted in lower vaccination-induced immune responses than observed in HIV-uninfected subjects. Our findings support further evaluation and efficacy testing of MVA85A as a booster vaccine for BCG in these important target populations.
Infection with Mycobacterium tuberculosis (M.tb) carries an approximate 10% lifetime risk of developing tuberculosis (TB) disease in individuals without HIV infection. HIV infection may raise this risk more than 10-fold (1), even in those with relatively high CD4+ T-cell counts (2).
Epidemiological modeling suggests that an effective TB vaccine will be critical for TB elimination (3, 4). Although infant bacillus Calmette-Guérin (BCG) vaccination is routine in settings where TB is endemic, BCG confers reliable protection only against severe forms of TB in infancy (5, 6). Protection against pulmonary disease in children and adults is variable and mostly poor (7). A more efficacious TB vaccine for all ages is urgently needed.
Sub-Saharan Africa is affected by a disproportionate burden of the global HIV and TB epidemics. In South Africa, more than 10% of the population is HIV-infected, and almost 1% develop TB annually (8). In these high-burden settings, TB elimination is likely to require mass campaigns targeting adults with a new, better vaccine (9). Modeling suggests that a 92% reduction in TB incidence by 2050 may be achievable if preexposure vaccination of all M.tb-uninfected individuals is combined with mass postexposure vaccination of individuals with latent M.tb infection (LTBI) (4). Given the high prevalence of HIV infection in many TB-endemic countries, these targets are feasible only if new TB vaccines are also safe and effective in HIV-infected persons.
Fourteen new TB vaccines have entered human clinical trials (9, 10). Few trials of these products have targeted individuals with LTBI and/or HIV infection. MVA85A was the first novel TB vaccine to enter safety and immunogenicity studies in persons with LTBI (11). Three other novel TB vaccines, M72 and Aeras402 (both unpublished, ClinicalTrials.gov reference numbers NCT00707967 and NCT01017536, respectively) and heat-inactivated Mycobacterium vaccae (12, 13), are being or have been evaluated in HIV-infected subjects, the latter in a phase III efficacy trial.
Here we describe the safety profile and characterize the immune response induced by MVA85A vaccination of M.tb- and/or HIV-infected persons in a setting where TB and HIV are endemic.
Methods
Study Design and Enrollment
We conducted an open label, phase IIa trial in adults. The protocol and amendments were approved by the Medicines Control Council of South Africa and the Human Research Ethics Committees of the University of Cape Town (Cape Town, South Africa) and the University of Oxford (Oxford, UK). The trial was conducted according to International Conference on Harmonization-Good Clinical Practice guidelines, was externally monitored by an independent contract research organization, and registered at ClinicalTrials.gov (NCT00480558). Written, informed consent was obtained from all participants.
Adults aged 18–50 years were recruited from the general population of Worcester, 110 km from Cape Town, South Africa. The aim was to enroll 12 participants into each of 4 groups, as follows: group 1, healthy, HIV-uninfected volunteers with LTBI; group 2, HIV-infected volunteers with no evidence of LTBI, who were not receiving antiretroviral therapy (ART); group 3, HIV-infected volunteers with LTBI, not receiving ART; group 4, healthy, HIV-infected volunteers who had been stable while receiving ART for more than 1 year, irrespective of LTBI status. LTBI was defined as a positive tuberculin skin test (TST, induration ≥ 10 mm) and a positive response to early secretory antigenic target (ESAT)-6/culture filtrate protein (CFP)-10 and M.tb purified protein derivative (PPD) in an IFN-γ enzyme-linked immunospot (ELISPOT) assay (see below and the online supplement). For groups 2 and 3, we enrolled individuals with HIV infection diagnosed more than 3 months before screening. HIV infection was confirmed by an HIV antigen/antibody combination assay (Abbott, Johannesburg, South Africa). Individuals were included only if they had a CD4 count above 300 cells/mm3. “Stable on ART” was defined as at least 1 year of continuous therapy, with undetectable plasma viral load (pVL, below 40 RNA copies/ml) for at least 1 year, and two most recent CD4 counts greater than 300 cells/mm3, with a CD4 count nadir greater than 100 cells/mm3.
Vaccination
MVA85A, contract manufactured at Impfstoffwerk Dessau-Tornau (IDT Biologika GmbH, Dessau-Rosslau, Germany), was administered intradermally at 5 × 107 plaque-forming units (70 μl) over the deltoid region of the left arm.
Follow-up and Safety Evaluation
After vaccination, participants were evaluated on-site for at least 60 minutes. They also returned on Days 2, 7, 14, 28, 56, 84, 168, and 364 postvaccination for evaluation. Blood for safety evaluation, which included biochemistry and hematology tests, was collected on Days 7 and 84. CD4 counts and pVL were measured in HIV-positive participants at screening, on Day 0, and at all follow-up visits, except Day 2. Assessment of vaccine safety was done as previously described (14); details are in the online supplement.
Immunogenicity Evaluations and Data Analysis
Blood was collected for immunogenicity tests 7–14 days before vaccination and on Days 7, 14, 28, 56, 84, 168, and 364 postvaccination. The ex vivo IFN-γ ELISPOT assay was the primary immunological end point, and was performed as previously described (14). A whole blood intracellular cytokine staining assay was performed as described previously (14) prevaccination (Day 0) and on Days 7, 28, 84, and 364 postvaccination; details are in the online supplement.
Results
Participants
We screened 340 adults and 48 were eligible for inclusion at 12 per group (CONSORT diagram; see Figure E1 in the online supplement). Demographic characteristics are shown in Table 1. More participants in group 1 were male and of mixed race, compared with mostly female black Africans in the other groups. No other demographic differences were observed between the groups.
TABLE 1.
DEMOGRAPHIC CHARACTERISTICS AT TIME OF ENROLLMENT
| Group 1: Infected with M.tb | Group 2: Infected with HIV | Group 3: Infected with M.tb and HIV | Group 4: Infected with HIV, Receiving ART | TB008: Not Infected with M.tb or HIV* | |
| (n = 12) | (n = 12) | (n = 12) | (n = 12) | (n = 24) | |
| Male, n (%) | 9 (75) | 1 (8.3)† | 1 (8.3)† | 0† | 8 (33) |
| Median age, yr (range) | 36 (27–49) | 34 (24–43) | 34 (18–41) | 35 (28–45) | 36 (21–49) |
| Ethnic origin, n (%) | |||||
| Black African | 2 (16.7) | 12 (100)† | 11 (91.7)† | 10 (83.3)† | 5 (21) |
| Mixed race | 10 (83.3) | 1 (8.3) | 2 (16.7) | 10 (42) | |
| White | 9 (38) | ||||
| Mean BMI, kg/m2 (range) | 30.5 (21.1–47.5) | 29.6 (21.5–42.8) | 29.3 (21.3–35.9) | 32.0 (22.3–43.2) | 28.4 (21.8–39.2) |
| Median TST induration, mm (IQR) | 22 (20–26) | 0 (0–5) | 20 (15–21) | 10 (0–16) | 5 (0–12) |
| Mean ESAT-6/CFP-10 response,‡ SFCs per million PBMCs (95% CI) | 354 (194–514) | 27 (8–45) | 452 (165–739) | 114 (25–202) | 13 (9–17) |
| Mean CD4 count, cells/mm3 (95% CI) | N/A | 519 (432–607) | 481 (357–605) | 578 (477–679) | N/A |
| Mean plasma VL, RNA copies/ml (95% CI) | N/A | 18,405 (2,811–34,000) | 17,289 (365–34,213) | <40§ (<40–<40) | N/A |
Definition of abbreviations: ART = antiretroviral therapy; BMI = body mass index; CI = confidence interval; IQR = interquartile range; M.tb = Mycobacterium tuberculosis; N/A = not applicable; PBMCs = peripheral blood mononuclear cells; SFCs = spot-forming cells; TST = tuberculin skin test; VL = viral load.
Previous trial of MVA85A vaccine (TB008), completed at the same clinical field site in the same population (16).
Compared with group 1: P < 0.001, Fisher exact test.
IFN-γ ELISPOT response.
Lower detection limit of plasma viral load test was 40 RNA copies/ml.
Safety of MVA85A Vaccination
All vaccinees reported adverse events (AEs) at the injection site. The most common local reactions were redness and swelling (100% of vaccinees), pruritus (81%), and desquamation (scaling, 79%); 48% also reported pain and 25% reported warmth. All local AEs were mild in nature; the majority had resolved by Day 14 and all had resolved by Day 28. A greater proportion of AEs in group 4 were classified as moderate, compared with groups 1 and 2 (Table 2). Frequencies of local or systemic AEs were not different between groups.
TABLE 2.
ADVERSE EVENTS SELF-REPORTED ON AT LEAST ONE DAY DURING THE FIRST 28 DAYS AFTER MVA85A VACCINATION
| Group 1 | Group 2 | Group 3 | Group 4 | |
| Mild | 81 | 67 | 78* | 52† |
| Moderate | 1 | 2 | 7 | |
| Severe | ||||
| Vaccine related | ||||
| Definitely | 70 | 61 | 64 | 42 |
| Probably | 6 | |||
| Possibly | 5 | 11 | ||
| Not | 5 | 7 | 11 | 6 |
| Local AEs, total | 58 | 52 | 55 | 43 |
| Redness | 12 | 12 | 12 | 12 |
| Swelling | 12 | 12 | 12 | 12 |
| Pruritus | 10 | 11 | 10 | 8 |
| Pain | 7 | 5 | 8 | 3 |
| Warmth | 5 | 3 | 3 | 1 |
| Desquamation | 11 | 9 | 10 | 7 |
| Limitation of arm movement | 1 | |||
| Systemic AEs, total | 23 | 16 | 25 | 16 |
| Feeling unwell | 5 | 1 | 2 | |
| Tiredness | 1 | 2 | 2 | 1 |
| Feeling feverish | 3 | 2 | 1 | |
| Documented fever > 37°C | 1 | |||
| Arthralgia | 1 | 1 | 1 | |
| Headache | 7 | 5 | 4 | 5 |
| Myalgia | 3 | 2 | 2 | 1 |
| Backache | 1 | 2 | ||
| Upper respiratory tract infection | 3 | 1 | 2 | |
| GIT symptoms | 3 | 1 | ||
| Cellulitis | 1 | |||
| Night sweat | 1 | |||
| Abnormal safety bloods | 5 | 1 | ||
| TST site reaction | 1 | |||
| Low CD4 count | 2‡ | |||
| Detectable pVL | 1 | |||
| Hypertension | 1 | |||
| Shingles | 1 | |||
Definition of abbreviations: AEs = adverse events; GIT = gastrointestinal; pVL = plasma viral load; TST = tuberculin skin test.
Compared with group 1: P = 0.017.
Compared with group 1: P < 0.001, Fisher exact tests.
These were in the same individual.
Systemic AEs peaked on Day 2, had resolved by Day 28, and included headache (27%), myalgia (15%), feeling unwell (15%), and tiredness (13%). Others included a subjective perception of fever, documented fever, arthralgia, and backache. Those considered definitely or probably related to vaccination occurred within 24–48 hours of vaccination and had resolved by Day 7. One participant, who had had a 24-mm TST reading at screening 12 days before enrollment, was noted to have a superficial infection at the TST site 7 days postvaccination. This was treated with an antiseptic cream and had resolved by the Day 14 visit. Because no reaction was documented at vaccination, this adverse event was classified as possibly related.
In addition, a case of shingles occurred 13 days postvaccination in a group 4 vaccinee.
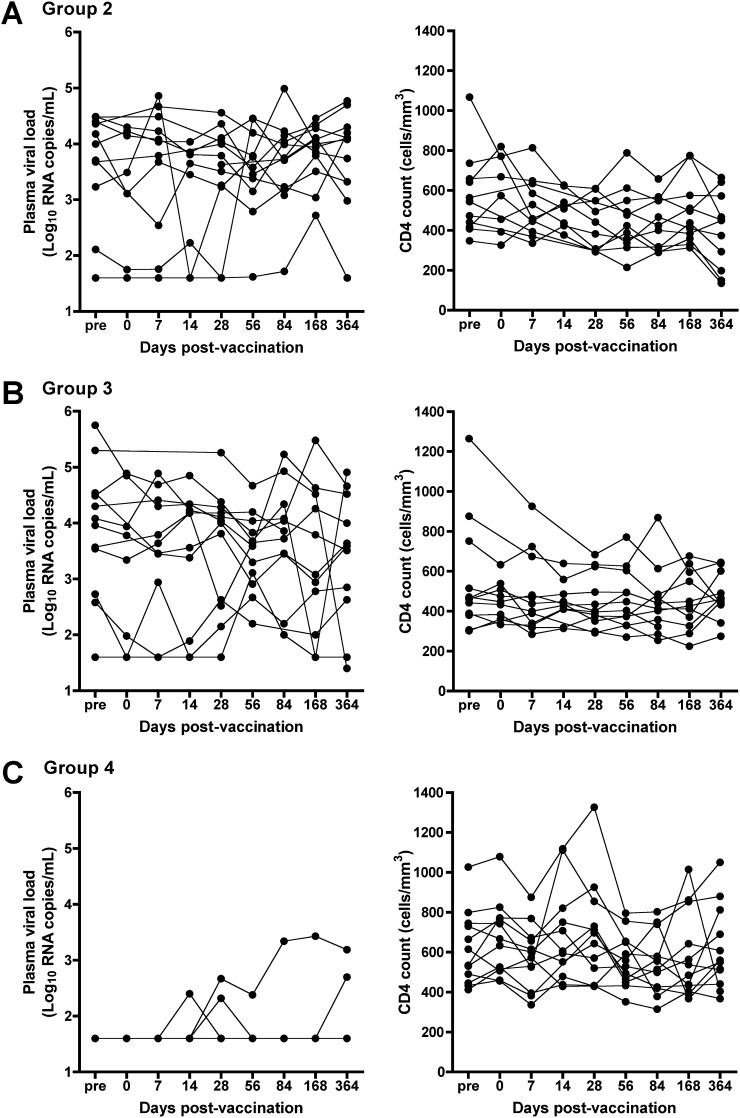
Although we observed some fluctuations in CD4 count and pVL in groups 2 and 3, and in CD4 count in group 4 (Figure 1), these were similar to fluctuations recorded before MVA85A vaccination, and typical of variation in untreated, chronic HIV infection (15). The participants remained clinically well and no changes were made to their ART regimens.
Figure 1.
Plasma HIV RNA load and CD4 counts before and after MVA85A vaccination in HIV-infected individuals from (A) group 2, (B) group 3, and (C) group 4. For individuals in group 4, plasma viral load (pVL) and CD4 counts recorded by their regular physicians before enrollment into this trial are shown as prevaccination (pre-) visits. Individual vaccinees are represented by each line.
A group 3 participant experienced a drop in CD4 count from 380 cells/mm3 prevaccination to 285 cells/mm3 7 days postvaccination. Although the CD4 count had returned to prevaccination levels by Day 14, it had decreased below 300 cells/mm3 by Day 28 and remained consistently at this level throughout the follow-up period. This change was classified as a mild AE, possibly vaccine related. The participant was clinically well, the CD4 percentage remained unchanged, and the pVL decreased.
Detectable pVLs, all less than 250 RNA copies/ml, were observed on single occasions in three group 4 participants between 14 and 56 days postvaccination. Detectable pVLs were also observed at 28 days in one and at 168 days postvaccination in another participant. In the former, detectable pVLs below 2,700 RNA copies/ml persisted throughout the follow-up period. In the latter participant, the pVL rose to 51,000 and decreased to 497 RNA copies/ml on Day 364. Whether this was due to nonadherence to ART was unknown. These were not considered clinically significant by their regular physicians and were classified as mild AEs. CD4 counts in group 4 participants did not increase or decrease significantly during the 1-year study follow-up.
Two serious AEs (SAEs) were recorded during follow-up. One was a right-sided cerebrovascular accident 10 months postvaccination in a group 2 participant, which was independently assessed as being “not related” to vaccination. The second was the admission of a group 3 participant to a state specialist TB hospital for TB of the spine, diagnosed 6 months postvaccination. On further investigation it was found that, contrary to the history given at screening, the participant had previously received treatment for pulmonary TB and had been receiving ART, but stopped treatment 3 months before study enrollment. The participant recommenced ART 1 month postvaccination. This SAE was also independently assessed as being “not related” to vaccination.
Effect of Underlying M.tb Infection on MVA85A-induced Response
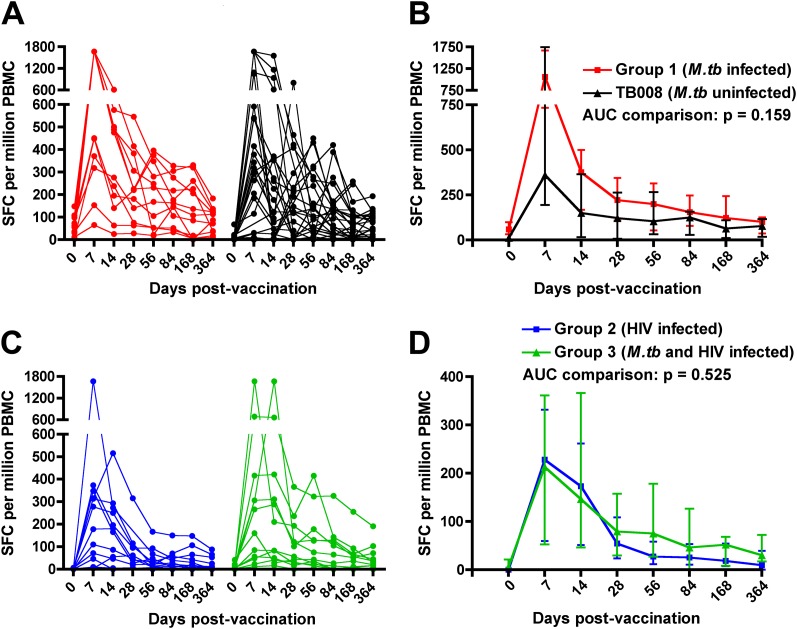
We previously showed that MVA85A vaccination induces robust and highly durable T-cell responses in M.tb-uninfected adults (Trial TB008 [16]). To investigate how underlying LTBI affects the MVA85A-induced T-cell response, we compared the magnitude and durability of Ag85A-specific T cells in M.tb-infected vaccinees (group 1) with M.tb-uninfected individuals from the same field site, who received 5 × 107 plaque-forming units of MVA85A in the previous TB008 study (Table 1) (16). The Ag85A-specific T-cell response measured before MVA85A vaccination by IFN-γ ELISPOT assay, was higher in M.tb-infected, compared with M.tb-uninfected, adults (P = 0.0002; Figures 2A and 2B). A similar result was observed in HIV-infected individuals; those coinfected with M.tb (group 3) had higher prevaccination Ag85A-specific T-cell responses than individuals infected with HIV only (group 2, P = 0.0072; Figures 2C and 2D). However, comparison of the kinetics of the longitudinal postvaccination T-cell responses by area-under-curve analysis revealed no significant differences between M.tb-infected and uninfected vaccinees (Figures 2B and 2D). Similarly, responses at any given postvaccination time point were not different (data not shown).
Figure 2.
Effect of underlying Mycobacterium tuberculosis (M.tb) infection on Ag85A-specific IFN-γ enzyme-linked immunospot (ELISPOT) responses in adults who received a single intradermal dose of 5 × 107 plaque-forming units of MVA85A. (A) Longitudinal responses in individual participants from group 1 (n = 12, M.tb infected) and M.tb-uninfected adults from trial TB008 (n = 24 [16]). All 12 group 1 participants had positive IFN-γ ELISPOT responses to Ag85A 7 days postvaccination (a stringent cutoff for positive response of 50 spot-forming cells [SFCs] per million peripheral blood mononuclear cells [PBMCs] was applied). (B) Medians (lines) and interquartile ranges (IQR, error bars) of Ag85A-specific IFN-γ ELISPOT responses. (C) Longitudinal responses in individual participants from group 2 (n = 12, HIV infected, M.tb uninfected) and group 3 (n = 12, M.tb and HIV infected). Nine participants from each group had positive IFN-γ ELISPOT responses to Ag85A 7 days postvaccination. (D) Medians (lines) and IQRs (error bars) of Ag85A-specific IFN-γ ELISPOT responses. Area under curve (AUC) comparisons of the groups were evaluated by Mann-Whitney U test. An adjusted P value of less than 0.025 was considered significant.
Effect of Underlying HIV Infection on MVA85A-induced Response
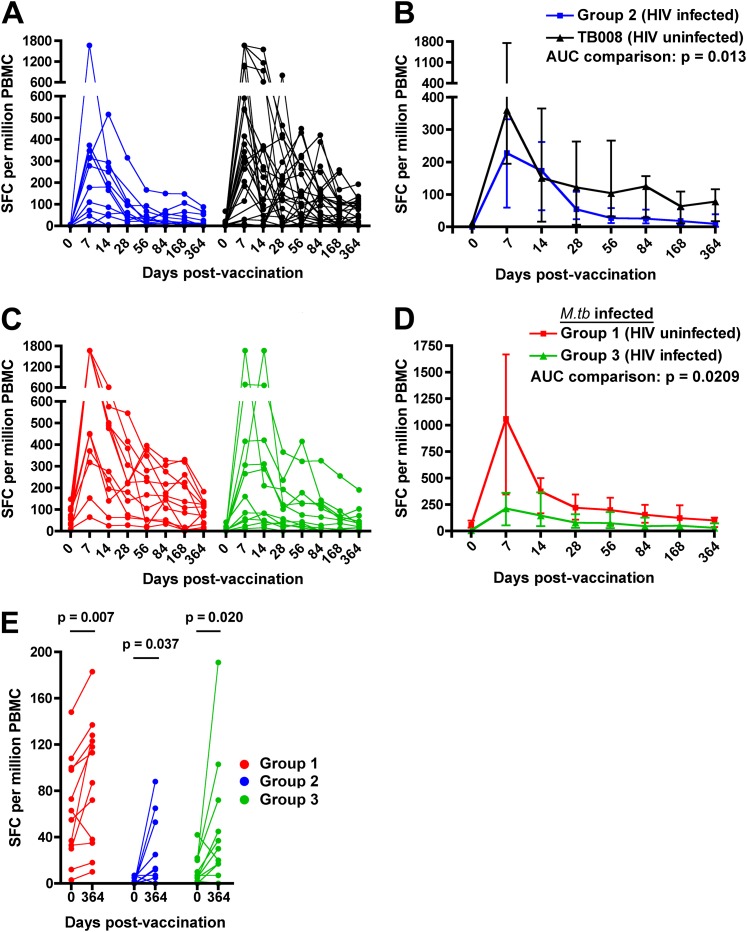
Next, we compared the magnitude and durability of the MVA85A-induced T-cell response in HIV-infected but M.tb-uninfected vaccinees (group 2) and HIV-uninfected, M.tb-uninfected vaccinees from the TB008 trial. Prevaccination Ag85A-specific T-cell frequencies were higher in uninfected, compared with HIV-infected, adults (P = 0.0018; Figure 3A). HIV infection also had an effect on the durability of the MVA85A-induced response; longitudinal postvaccination T-cell responses in HIV-infected vaccinees were lower compared with HIV-uninfected vaccinees (Figures 3A and 3B). In addition, concomitant HIV infection markedly affected the magnitude of T-cell responses in individuals with LTBI. Prevaccination Ag85A-specific T-cell frequencies were lower in HIV-infected, compared with HIV-uninfected, LTBI individuals (P = 0.0014) and longitudinal postvaccination responses were lower in HIV/M.tb-coinfected vaccinees compared with HIV-negative, M.tb-infected vaccinees (Figures 3C and 3D). No significant association was observed between Ag85A-specific T-cell frequencies and CD4 counts (data not shown).
Figure 3.
Effect of underlying HIV infection on MVA85A-induced Ag85A-specific IFN-γ enzyme-linked immunospot (ELISPOT) responses. (A) Longitudinal responses in individual participants from group 2 (n = 12, HIV infected) and HIV-uninfected adults from trial TB008 (n = 24 [16]). (B) Medians (lines) and interquartile ranges (IQR, error bars) of Ag85A-specific IFN-γ ELISPOT responses. (C) Longitudinal responses in individual participants from group 1 (n = 12, HIV uninfected, Mycobacterium tuberculosis [M.tb] infected) and group 3 (n = 12, M.tb and HIV infected). (D) Medians (lines) and IQRs (error bars) of Ag85A-specific IFN-γ ELISPOT responses. Area under curve (AUC) comparisons of the groups were evaluated by Mann-Whitney U test. An adjusted P value of less than 0.025 was considered significant for the AUC comparisons. (E) Comparisons between the Ag85A-specific T-cell response before (Day 0) and 1 year after (Day 364) MVA85A vaccination. Differences were evaluated by Wilcoxon matched pairs test and a P value less than 0.05 was considered significant. SFC = spot-forming cells.
Importantly, however, the MVA85A-induced T-cell response was long-lived in vaccinees from groups 1–3, including HIV-infected vaccinees. Frequencies of IFN-γ–expressing Ag85A-specific T cells observed up to 1 year postvaccination exceeded those observed prevaccination (Figure 3E).
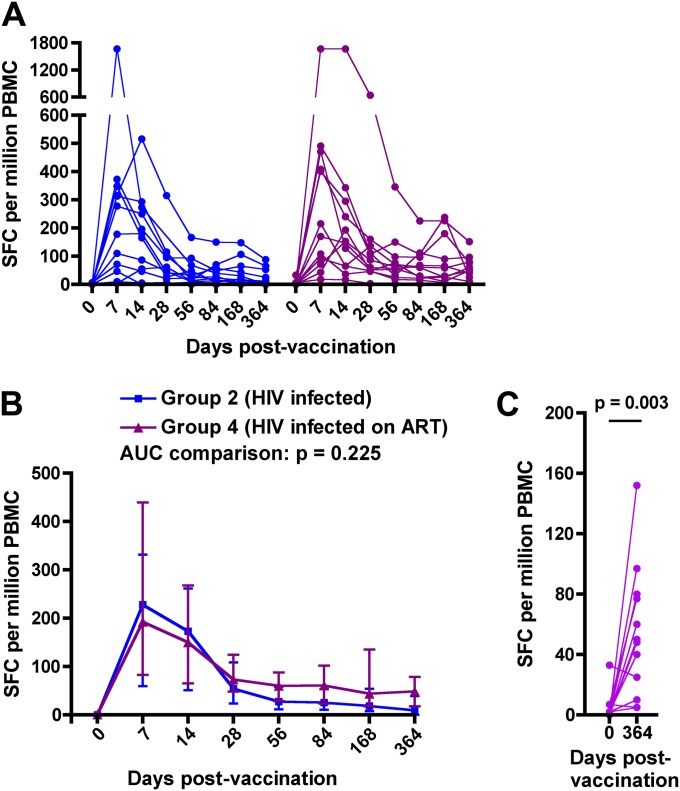
Effect of ART on MVA85A-induced Response
We also assessed the Ag85A-specific T-cell response in HIV-infected persons who were stable while receiving ART. Magnitudes of the pre- or postvaccination response to Ag85A were not different in ART-treated and untreated vaccinees (Figures 4A and 4B). The MVA85A-induced response was also durable in persons receiving ART; Ag85A-specific T-cell frequencies observed up to 1 year postvaccination exceeded those observed prevaccination (Figure 4C).
Figure 4.
Effect of antiretroviral treatment in HIV-infected adults on MVA85A-induced Ag85A-specific IFN-γ enzyme-linked immunospot (ELISPOT) responses. (A) Longitudinal responses in individual participants from group 2 (n = 12, HIV infected) and group 4 (n = 12, HIV infected, receiving antiretroviral therapy [ART]). Ten group 4 participants had positive IFN-γ ELISPOT responses to Ag85A 7 days postvaccination. (B) Medians (lines) and IQRs (error bars) of Ag85A-specific IFN-γ ELISPOT responses. Area under curve (AUC) comparisons of the groups were evaluated by Mann-Whitney U test. An adjusted P value of less than 0.025 was considered significant for the AUC comparisons. (C) Comparisons between the Ag85A-specific T-cell response before (Day 0) and 1 year after (Day 364) MVA85A vaccination in group 4 (n = 12, HIV infected, receiving ART). Differences were evaluated by Wilcoxon matched pairs test. SFC = spot-forming cells.
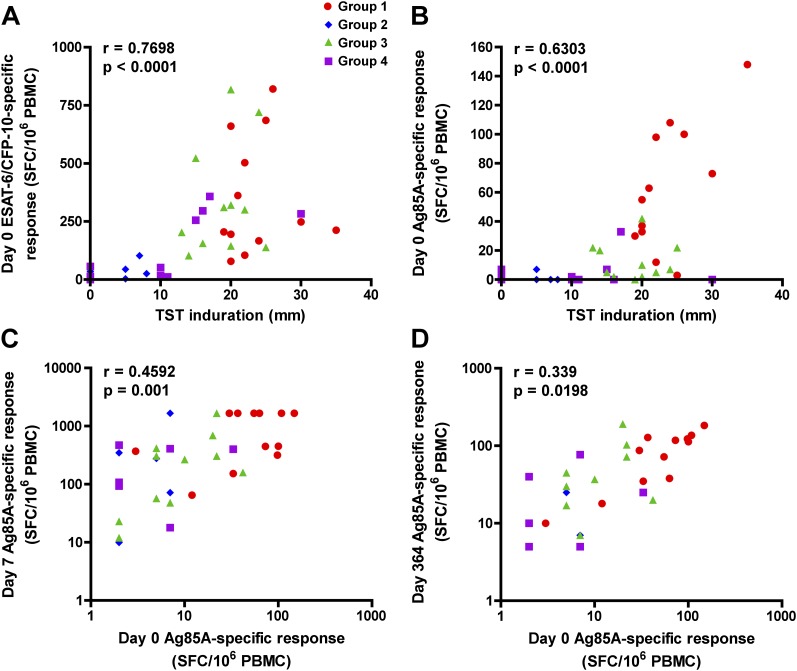
Relationship between M.tb Sensitization and T-cell Response
To explore whether the degree of M.tb sensitization may be associated with T-cell responses, we investigated associations between size of TST induration, measured during screening, and magnitudes of prevaccination T-cell responses. The size of TST induration correlated directly with prevaccination T-cell responses to ESAT-6 and CFP-10 (Figure 5A), as well as with Ag85A (Figure 5B). We also examined whether the prevaccination magnitude of Ag85A-specific T cells was a determinant of the subsequent MVA85A-induced response to Ag85A. Frequencies of Ag85A-specific T cells, detected 7 days (Figure 5C) as well as 364 days (Figure 5D) postvaccination, correlated directly with the prevaccination response to Ag85A.
Figure 5.
The degree of sensitization to Mycobacterium tuberculosis (M.tb) is an important determinant in the MVA85A-induced T-cell response. (A) Association between size of tuberculin skin test (TST) induration, measured at screening, and the summed early secretory antigenic target (ESAT)-6 and culture filtrate protein (CFP)-10–specific T-cell response, measured before MVA85A vaccination by IFN-γ enzyme-linked immunospot (ELISPOT) assay. (B) Association between size of TST induration and the Ag85A-specific T-cell response before MVA85A vaccination. (C) Association between the Ag85A-specific T-cell response before and 7 days after MVA85A vaccination. (D) Association between the Ag85A-specific T-cell response before and 1 year (364 d) after MVA85A vaccination. For (A–D), data from participants in all four groups were combined. Correlations were assessed by Spearman correlation test.
Characterization of CD4 T Cells
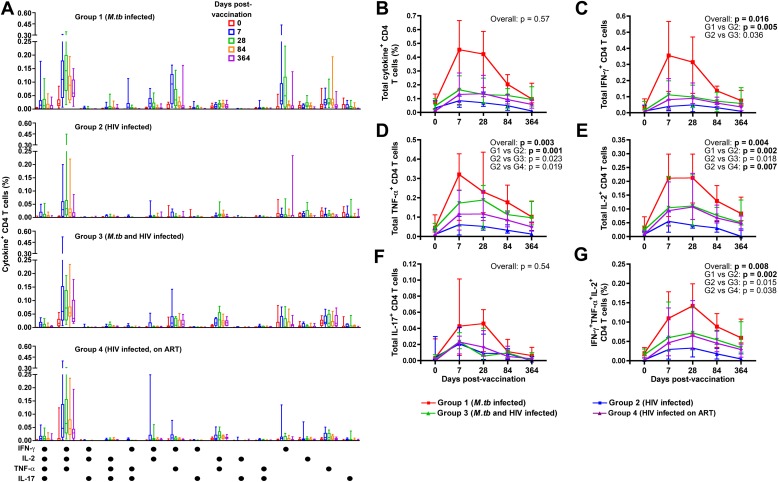
To assess the qualitative characteristics of MVA85A-induced T cells, we measured CD4 or CD8 T-cell coexpression of IFN-γ, TNF-α, IL-2, and IL-17 by flow cytometry (Figure E2). The Ag85A-specific CD4 T-cell response comprised multiple subsets. The predominant MVA85A-induced subset in all four groups coexpressed IFN-γ, TNF-α, and IL-2 (Figure 6A and Figure E2). In LTBI vaccinees (group 1), CD4 T cells coexpressing IFN-γ and TNF-α, IFN-γ and IL-2, or IFN-γ or TNF-α alone were also induced (Figure 6A). The latter CD4 T-cell subsets were not readily detectable in groups 2, 3, and 4, which had lower postvaccination frequencies of all these T-cell subsets. A small subset of CD4 T cells that coexpressed IL-17 with helper T-cell type 1 (Th1) cytokines was also detected in group 1 (Figure 6A).
Figure 6.
Ag85A-specific CD4 T-cell cytokine expression in whole blood measured before and after MVA85A vaccination by flow cytometry. (A) The graphs show patterns of single or combined expression of IFN-γ, IL-2, tumor necrosis factor (TNF)-α , and/or IL-17 by CD4 T cells from adults in group 1 (Mycobacterium tuberculosis [M.tb] infected, n = 12), group 2 (HIV infected, n = 12), group 3 (M.tb and HIV infected, n = 12), and group 4 (HIV infected, receiving antiretroviral therapy [ART], n = 12). The median frequency for each cytokine-expressing cell subset is represented by the horizontal line, the interquartile range (IQR) by the box, and the range by the whiskers. For each pattern, in each individual, background values (unstimulated) were subtracted. Each box plot represents a particular time point pre- or postvaccination, for the indicated cytokine-expressing cell subset. (B–G) Medians (lines) and IQRs (error bars) of Ag85A-specific CD4 T-cell frequencies measured before and after MVA85A vaccination in the four groups. (B) Total cytokine-expressing CD4 T cells. (C) Total IFN-γ–expressing CD4 T cells. (D) Total TNF-α–expressing CD4 T cells. (E) Total IL-2–expressing CD4 T cells. (F) Total IL-17–expressing CD4 T cells. (G) Polyfunctional CD4 T cells, coexpressing IFN-γ, TNF-α, and IL-2. Comparisons of area under curve (AUC) values for the groups were evaluated by Kruskal-Wallis test (overall effect), followed by the Mann-Whitney U test. An adjusted P value of less than 0.0083 (Bonferroni adjustment, 0.05 × 6) was considered significant. Significant differences are shown in boldface.
Although no intergroup differences were detected in frequencies of total cytokine-positive CD4 T cells (Figure 6B), total CD4 T cells expressing IFN-γ, TNF-α, or IL-2 were consistently less frequent in HIV-infected vaccinees (not TB infected and not receiving ART, group 2), compared with vaccinees in the other three groups (Figures 6C–6E). Notably, Ag85A-specific CD4 T cells in group 2 vaccinees waned to low levels by Day 364 postvaccination. Underlying LTBI in HIV-infected vaccinees appeared to result in greater postvaccination Ag85A-specific CD4 T-cell frequencies compared with HIV-infected vaccinees without LTBI, although significance was lost after correction for multiple comparisons. Ag85A-specific CD4 T-cell subsets expressing IFN-γ or TNF-α were not different in untreated or ART-treated HIV-infected vaccinees (Figures 6C and 6D). However, the frequencies of IL-2–expressing CD4 T cells were significantly higher in the ART group throughout the follow-up period, compared with untreated HIV-infected vaccinees (Figure 6E).
IL-17–expressing CD4 T cells were not different between the groups (Figure 6F). HIV-infected, group 2 vaccinees had lower frequencies of polyfunctional CD4 T cells compared with group 1 (Figure 6G).
Ag85A-specific CD8 T cells were not detected above background frequencies in any of the four groups (Figure E3).
Discussion
We studied MVA85A vaccination of HIV-infected adults with or without M.tb infection, and those receiving ART. Five major findings emerged from our study: (1) MVA85A was well tolerated in HIV and M.tb-infected persons; (2) MVA85A vaccination did not influence the effectiveness of ART; (3) MVA85A vaccination induced robust, long-lived, and predominantly polyfunctional CD4 T-cell responses in all groups, and these responses were lowest in HIV-infected vaccinees; (4) the prevaccination T-cell response, which was associated with M.tb sensitization, was a major determinant of the postvaccination response; and (5) successful ART of HIV-infected individuals did not significantly improve the MVA85A-induced response.
We observed no vaccine-related SAEs on MVA85A vaccination of HIV-immunocompromised individuals with or without LTBI. Fluctuations in pVL or CD4 count were within ranges previously reported in untreated, chronic HIV infection (15). The effectiveness of ART in group 4 participants was not markedly affected by MVA85A vaccination; pVL blips were similar to those observed after vaccination with other vaccines (17–19). These fluctuations were not considered clinically significant and the HIV physicians managing the care of these individuals made no adjustments to their treatment regimen. Although interpretation of these data is limited by the small sample size in our study, this is an important finding given the increasing number of persons receiving ART in developing countries. Because the risk of TB in HIV-infected persons receiving ART remains higher than for HIV-uninfected persons (2), effective TB vaccines are urgently required for this population.
The SAE involving diagnosis of TB of the spine was classified as not related to vaccination. This was based on the distal locality of disease and the 6-month timeframe between MVA85A vaccination and diagnosis. The individual had also previously received ART, but had stopped treatment 3 months before being screened for inclusion in the study. In addition, it emerged that the person had a previous episode of TB, a major risk factor for further episodes of TB in HIV-infected persons (20).
With respect to the case of shingles in a group 4 participant, no CD4 count decrease was observed after MVA85A vaccination. To date, a total of 2,012 subjects and 108 HIV-infected adults have received MVA85A, with no reports of herpes zoster reactivation (H. McShane, personal communication). Further, to our knowledge no associations between other vaccinations and zoster reactivation are described. On the basis of these factors, this AE was classified as not related to vaccination.
No vaccine-related SAEs were recorded in immunocompetent individuals with documented LTBI. Given the hypothetical concerns of immunopathogenic inflammation on administration of mycobacterial antigens to individuals with LTBI, as originally described by Robert Koch (21), this is an important finding. Nevertheless, we feel that reactions at the TST site should be carefully monitored in novel TB vaccine trials conducted in M.tb-infected persons. Our data add to the safety results of MVA85A in M.tb-infected adults from the United Kingdom, which also reported no vaccine-related immunopathology (11).
Our results complement the excellent safety profile of MVA85A in adults (11, 16, 22, 23), adolescents, children (14), and infants (24, 25) and pave the way for safety assessments of MVA85A in larger groups of M.tb- and/or HIV-infected persons.
MVA85A induced predominantly polyfunctional CD4 T-cell responses in all groups. The pattern of IFN-γ, TNF-α, IL-2, and IL-17 expression by CD4 T cells observed in M.tb-infected and/or HIV-infected vaccinees is similar to those reported previously (14). However, lower response frequencies were observed in HIV-infected individuals. It should be noted that participation was limited to HIV subjects with CD4 counts greater than 300 cells/μl. The specific Th1 response in HIV-infected vaccinees also waned to low levels, although Ag85A-specific IFN-γ–producing cells still exceeded prevaccination levels 1 year postvaccination. M.tb-specific CD4 T cells may be preferentially infected and depleted after HIV infection (26) (reviewed in Reference 27). It is unknown what the consequences of such lower responses on immunity against M.tb might be. In HIV-infected individuals, the elevated risk for developing TB may be associated with lower and functionally impaired mycobacteria-specific Th1 responses in the lungs (28). However, we showed that frequencies of polyfunctional Th1 cytokine-expressing T cells, measured 10 weeks after BCG vaccination in peripheral blood, did not correlate with risk of TB in infants (29). We also showed that T-cell functional capacity was associated with antigen load. Polyfunctionality of mycobacteria-specific T cells was highest in persons with LTBI, and progressively decreased in patients with smear-negative, and smear-positive pulmonary TB, respectively (30). Murine studies of vaccine-induced T-cell responses and their effect on control of M.tb burden have reported variable and contrasting outcomes (31–34). Until correlates of protection are identified in trials of effective vaccines, T-cell outcomes measured here may only be interpreted as vaccine take, even though these measures are known to be important in protection (35).
We observed higher prevaccination frequencies of Ag85A-specific T cells in M.tb-infected adults, compared with uninfected adults studied previously at our field site (16). Furthermore, the magnitude of Ag85A-specific T cells correlated with the degree of M.tb sensitization and the prevaccination Ag85A-specific T-cell response correlated with the postvaccination response. These data highlight the importance of measuring prevaccination responses to mycobacterial antigens in TB vaccine trials, and imply that immunogenicity should be interpreted in the context of this preexisting response and levels of mycobacterial exposure. This is supported by our previous observation of greater magnitudes of Ag85A-specific T cells before MVA85A vaccination in adults and adolescents, compared with infants (24). We proposed that the frequency of Ag85A-specific cells before MVA85A vaccination may reflect BCG priming in infancy, while reflecting exposure to environmental mycobacteria and/or M.tb in adolescents and adults. Our results from M.tb-infected adults reinforce this finding. A limitation of these intergroup comparisons was the small number of individuals per group, which may have provided inadequate statistical power for detecting differences.
It has been proposed that the BCG-induced response may be blocked or masked in developing countries because of preexisting sensitization to mycobacterial antigens (36, 37). We observed that greater mycobacterial sensitization, in our case mostly M.tb, was associated with greater MVA85A-induced Th1 responses. Whether this also applies to BCG-induced responses is not known. However, boosting the BCG-induced response with BCG elicited lower Ag85A-specific T-cell responses than boosting with MVA85A (38), suggesting that vaccine formulation or nature of antigen may be important variables. Nevertheless, we propose that masking due to mycobacterial sensitization may be less important for vaccination with MVA85A, than has been suggested for BCG. Interestingly, sensitization of mice with nontuberculous mycobacteria interfered with immune responses induced by subsequent vaccination with BCG, but not a subunit TB vaccine (39). Further investigation is required to understand this better.
Compared with ART-naive HIV-infected vaccinees, MVA85A vaccination of HIV-infected, ART-treated individuals did not induce profoundly greater frequencies of Th1 responses, with the exception of IL-2–expressing CD4 T cells. Loss of IL-2 expression by HIV-specific T cells is a well-described consequence of immune activation associated with high HIV replication (40, 41). Furthermore, IL-2–expressing CD4 cells, which were predominant among M.tb-specific cells, were shown to be more susceptible to HIV infection than macrophage inflammatory protein-1β–expressing CD4 cells (26). ART-mediated suppression of HIV replication may thus preferentially restore IL-2–expressing mycobacteria-specific CD4 T cells, which may be important for immunity against M.tb (26).
Ag85A-specific CD8 T cells were not readily detectable after MVA85A vaccination. This contrasts with some previous MVA85A trials in different populations, in which low frequencies of specific CD8 T cells were reported (23, 24, 38). We propose that frequencies of CD8 T cells induced by MVA85A were too low to be detected with our ex vivo assay systems. This is supported by the observation that in vitro expansion of specific T cells enabled detection of MVA85A-induced CD8 T cells (38).
In conclusion, we show that MVA85A is safe and immunogenic in HIV- and/or M.tb-infected adults from a region where TB is endemic. These data support further studies to evaluate the safety and efficacy of MVA85A in HIV-infected and M.tb-infected populations.
Supplementary Material
Acknowledgments
The authors thank the participants in this trial.
Footnotes
Supported by a EuropeAID European Commission grant and by a TB-VAC grant, also of the European Commission (LSHP-CT-2003-503367). T.J.S. was a Wellcome Trust Research Training Fellow (080929/Z/06/Z), H.McS. is a Wellcome Trust Senior Clinical Fellow, and A.V.S.H. is a Wellcome Trust Principle Research Fellow. T.J.S. and W.A.H. are supported by the NIH (RO1AI087915). W.A.H. is also supported by the TB Research Unit of the NIH (NO1AI70022) and by the Wellcome Trust–supported Clinical Infectious Disease Research Initiative of the University of Cape Town.
Author Contributions: T.J.S., M.T., A.V.S.H., A.H., G.D.H., W.A.H., H.McS., and Ha.Ma. contributed to conception and design. T.J.S., M.T., E.S., L.v.d.M., E.J.H., B.K., K.M., S.M., N.B., A.L., Hu.Mu., M.d.K., L.M., E.J.v.R., S.G., A.V., M.H., H.G., W.A.H., H.McS., and Ha.Ma. acquired and/or analyzed the data. T.J.S., M.T., A.V., M.H., H.G., W.A.H., H.McS., and Ha.Ma. contributed to interpretation of data. T.J.S., M.T., H.McS., and Ha.Ma. drafted the manuscript, and all authors revised the manuscript and gave final approval of the version to be published.
This article has an online supplement, which is available from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201108-1548OC on January 26, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, Dye C. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med 2003;163:1009–1021 [DOI] [PubMed] [Google Scholar]
- 2.Lawn SD, Myer L, Edwards D, Bekker LG, Wood R. Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS 2009;23:1717–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abu-Raddad LJ, Sabatelli L, Achterberg JT, Sugimoto JD, Longini IM, Jr, Dye C, Halloran ME. Epidemiological benefits of more-effective tuberculosis vaccines, drugs, and diagnostics. Proc Natl Acad Sci USA 2009;106:13980–13985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dye C, Williams BG. Eliminating human tuberculosis in the twenty-first century. J R Soc Interface 2008;5:653–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodrigues LC, Diwan VK, Wheeler JG. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int J Epidemiol 1993;22:1154–1158 [DOI] [PubMed] [Google Scholar]
- 6.Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet 2006;367:1173–1180 [DOI] [PubMed] [Google Scholar]
- 7.Fine PEM, Carneiro IAM, Milstein JB, Clements CJ. Issues relating to the use of BCG in immunization programmes: a discussion document. Geneva, Switzerland: World Health Organization; 1999. Available from: http://www.who.int/vaccine_research/documents/en/bcg_vaccines.pdf
- 8.World Health Organization Global tuberculosis control. Geneva, Switzerland: World Health Organization; 2010. Available from: http://www.who.int/tb/publications/global_report/en/ [Google Scholar]
- 9.Hatherill M. Prospects for elimination of childhood tuberculosis: the role of new vaccines. Arch Dis Child 2011;96:851–856 [DOI] [PubMed] [Google Scholar]
- 10.Stop TB Partnership. Working group on new TB vaccines. Geneva, Switzerland: Stp TB Partnership; 2009. Available from: http://www.stoptb.org/wg/new_vaccines/
- 11.Sander CR, Pathan AA, Beveridge NE, Poulton I, Minassian A, Alder N, Van Wijgerden J, Hill AV, Gleeson FV, Davies RJ, et al. Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in Mycobacterium tuberculosis–infected individuals. Am J Respir Crit Care Med 2009;179:724–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Reyn CF, Mtei L, Arbeit RD, Waddell R, Cole B, Mackenzie T, Matee M, Bakari M, Tvaroha S, Adams LV, et al. Prevention of tuberculosis in bacille Calmette-Guérin–primed, HIV-infected adults boosted with an inactivated whole-cell mycobacterial vaccine. AIDS 2010;24:675–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lahey T, Arbeit RD, Bakari M, Horsburgh CR, Matee M, Waddell R, Mtei L, Vuola JM, Pallangyo K, von Reyn CF. Immunogenicity of a protective whole cell mycobacterial vaccine in HIV-infected adults: a phase III study in Tanzania. Vaccine 2010;28:7652–7658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scriba TJ, Tameris M, Mansoor N, Smit E, van der Merwe L, Isaacs F, Keyser A, Moyo S, Brittain N, Lawrie A, et al. Modified vaccinia Ankara-expressing Ag85A, a novel tuberculosis vaccine, is safe in adolescents and children, and induces polyfunctional CD4+ T cells. Eur J Immunol 2010;40:279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korenromp EL, Williams BG, Schmid GP, Dye C. Clinical prognostic value of RNA viral load and CD4 cell counts during untreated HIV-1 infection—a quantitative review. PLoS ONE 2009;4:e5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawkridge T, Scriba TJ, Gelderbloem S, Smit E, Tameris M, Moyo S, Lang T, Veldsman A, Hatherill M, Merwe L, et al. Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in healthy adults in South Africa. J Infect Dis 2008;198:544–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee PK, Kieffer TL, Siliciano RF, Nettles RE. HIV-1 viral load blips are of limited clinical significance. J Antimicrob Chemother 2006;57:803–805 [DOI] [PubMed] [Google Scholar]
- 18.Nettles RE, Kieffer TL. Update on HIV-1 viral load blips. Curr Opin HIV AIDS 2006;1:157–161 [DOI] [PubMed] [Google Scholar]
- 19.Moss WJ, Clements CJ, Halsey NA. Immunization of children at risk of infection with human immunodeficiency virus. Bull World Health Organ 2003;81:61–70 [PMC free article] [PubMed] [Google Scholar]
- 20.El-Sadr WM, Perlman DC, Denning E, Matts JP, Cohn DL. A review of efficacy studies of 6-month short-course therapy for tuberculosis among patients infected with human immunodeficiency virus: differences in study outcomes. Clin Infect Dis 2001;32:623–632 [DOI] [PubMed] [Google Scholar]
- 21.Koch R. A further communication on a remedy for tuberculosis. BMJ 1891;1:125–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McShane H, Pathan AA, Sander CR, Keating SM, Gilbert SC, Huygen K, Fletcher HA, Hill AV. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat Med 2004;10:1240–1244 [DOI] [PubMed] [Google Scholar]
- 23.Brookes RH, Hill PC, Owiafe PK, Ibanga HB, Jeffries DJ, Donkor SA, Fletcher HA, Hammond AS, Lienhardt C, Adegbola RA, et al. Safety and immunogenicity of the candidate tuberculosis vaccine MVA85A in West Africa. PLoS ONE 2008;3:e2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scriba TJ, Tameris M, Mansoor N, Smit E, van der Merwe L, Mauff K, Hughes EJ, Moyo S, Brittain N, Lawrie A, et al. Dose-finding study of the novel tuberculosis vaccine, MVA85A, in healthy BCG-vaccinated infants. J Infect Dis 2011;203:1832–1843 [DOI] [PubMed] [Google Scholar]
- 25.Ota MO, Odutola AA, Owiafe PK, Donkor S, Owolabi OA, Brittain NJ, Williams N, Rowland-Jones S, Hill AV, Adegbola RA, et al. Immunogenicity of the tuberculosis vaccine MVA85A is reduced by coadministration with EPI vaccines in a randomized controlled trial in Gambian infants. Sci Transl Med 2011;3:88ra56 [DOI] [PubMed] [Google Scholar]
- 26.Geldmacher C, Ngwenyama N, Schuetz A, Petrovas C, Reither K, Heeregrave EJ, Casazza JP, Ambrozak DR, Louder M, Ampofo W, et al. Preferential infection and depletion of Mycobacterium tuberculosis–specific CD4 T cells after HIV-1 infection. J Exp Med 2010;207:2869–2881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diedrich CR, Flynn JL. HIV-1/Mycobacterium tuberculosis coinfection immunology: how does HIV-1 exacerbate tuberculosis? Infect Immun 2011;79:1407–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalsdorf B, Scriba TJ, Wood K, Day CL, Dheda K, Dawson R, Hanekom WA, Lange C, Wilkinson RJ. HIV-1 infection impairs the bronchoalveolar T-cell response to mycobacteria. Am J Respir Crit Care Med 2009;180:1262–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kagina BM, Abel B, Scriba TJ, Hughes EJ, Keyser A, Soares A, Gamieldien H, Sidibana M, Hatherill M, Gelderbloem S, et al. Specific T cell frequency and cytokine expression profile do not correlate with protection against tuberculosis, following BCG vaccination of newborns. Am J Respir Crit Care Med 2010;182:1073–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Day CL, Abrahams DA, Lerumo L, Janse van Rensburg E, Stone L, O'Rie T, Pienaar B, de Kock M, Kaplan G, Mahomed H, et al. Functional capacity of Mycobacterium tuberculosis–specific T cell responses in humans is associated with mycobacterial load. J Immunol 2011;187:2222–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mittrucker HW, Steinhoff U, Kohler A, Krause M, Lazar D, Mex P, Miekley D, Kaufmann SH. Poor correlation between BCG vaccination–induced T cell responses and protection against tuberculosis. Proc Natl Acad Sci USA 2007;104:12434–12439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aagaard C, Hoang T, Dietrich J, Cardona PJ, Izzo A, Dolganov G, Schoolnik GK, Cassidy JP, Billeskov R, Andersen P. A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat Med 2011;17:189–194 [DOI] [PubMed] [Google Scholar]
- 33.Bennekov T, Dietrich J, Rosenkrands I, Stryhn A, Doherty TM, Andersen P. Alteration of epitope recognition pattern in Ag85B and ESAT-6 has a profound influence on vaccine-induced protection against Mycobacterium tuberculosis. Eur J Immunol 2006;36:3346–3355 [DOI] [PubMed] [Google Scholar]
- 34.Forbes EK, Sander C, Ronan EO, McShane H, Hill AVS, Beverley PCL, Tchilian EZ. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J Immunol 2008;181:4955–4964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanekom WA, Dockrell HM, Ottenhoff TH, Doherty TM, Fletcher H, McShane H, Weichold FF, Hoft DF, Parida SK, Fruth UJ. Immunological outcomes of new tuberculosis vaccine trials: WHO panel recommendations. PLoS Med 2008;5:e145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Black GF, Dockrell HM, Crampin AC, Floyd S, Weir RE, Bliss L, Sichali L, Mwaungulu L, Kanyongoloka H, Ngwira B, et al. Patterns and implications of naturally acquired immune responses to environmental and tuberculous mycobacterial antigens in northern Malawi. J Infect Dis 2001;184:322–329 [DOI] [PubMed] [Google Scholar]
- 37.Black GF, Weir RE, Floyd S, Bliss L, Warndorff DK, Crampin AC, Ngwira B, Sichali L, Nazareth B, Blackwell JM, et al. BCG-induced increase in interferon-γ response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: two randomised controlled studies. Lancet 2002;359:1393–1401 [DOI] [PubMed] [Google Scholar]
- 38.Whelan KT, Pathan AA, Sander CR, Fletcher HA, Poulton I, Alder NC, Hill AV, McShane H. Safety and immunogenicity of boosting BCG vaccinated subjects with BCG: comparison with boosting with a new TB vaccine, MVA85A. PLoS ONE 2009;4:e5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brandt L, Feino Cunha J, Weinreich Olsen A, Chilima B, Hirsch P, Appelberg R, Andersen P. Failure of the Mycobacterium bovis BCG vaccine: some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis. Infect Immun 2002;70:672–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palmer BE, Boritz E, Wilson CC. Effects of sustained HIV-1 plasma viremia on HIV-1 Gag-specific CD4+ T cell maturation and function. J Immunol 2004;172:3337–3347 [DOI] [PubMed] [Google Scholar]
- 41.Younes SA, Yassine-Diab B, Dumont AR, Boulassel MR, Grossman Z, Routy JP, Sekaly RP. HIV-1 viremia prevents the establishment of interleukin 2–producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J Exp Med 2003;198:1909–1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.