Abstract
The effects of acute hyperglycemia on lung ischemia–reperfusion (IR) injury and the role of receptor for advanced glycation end-products (RAGE) signaling in this process are unknown. The objective of this study was twofold: (1) evaluate the impact of acute hyperglycemia on lung IR injury; and (2) determine if RAGE signaling is a mechanism of hyperglycemia-enhanced IR injury. We hypothesized that acute hyperglycemia worsens lung IR injury through a RAGE signaling mechanism. C57BL/6 wild-type (WT) and RAGE knockout (RAGE −/−) mice underwent sham thoracotomy or lung IR (1-h left hilar occlusion and 2-h reperfusion). Acute hyperglycemia was established by dextrose injection 30 minutes before ischemia. Lung injury was assessed by measuring lung function, cytokine expression in bronchoalveolar lavage fluid, leukocyte infiltration, and microvascular permeability via Evans blue dye. Mean blood glucose levels doubled in hyperglycemic mice 30 minutes after dextrose injection. Compared with IR in normoglycemic mice, IR in hyperglycemic mice significantly enhanced lung dysfunction, cytokine expression (TNF-α, keratinocyte chemoattractant, IL-6, monocyte chemotactic protein-1, regulated upon activation, normal T cell expressed and secreted), leukocyte infiltration, and microvascular permeability. Lung injury and dysfunction after IR were attenuated in normoglycemic RAGE −/− mice, and hyperglycemia failed to exacerbate IR injury in RAGE −/− mice. Thus, this study demonstrates that acute hyperglycemia exacerbates lung IR injury, whereas RAGE deficiency attenuates IR injury and also prevents exacerbation of IR injury in an acute hyperglycemic setting. These results suggest that hyperglycemia-enhanced lung IR injury is mediated, at least in part, by RAGE signaling, and identifies RAGE as a potential, novel therapeutic target to prevent post-transplant lung IR injury.
Keywords: lung transplant, receptor for advanced glycation end-products, hyperglycemia, inflammation
Clinical Relevance
This research demonstrates that acute hyperglycemia exacerbates experimental lung ischemia–reperfusion (IR) injury, and receptor for advanced glycation end products (RAGE) deficiency prevents the exacerbation of IR injury in the hyperglycemic setting. These results identify a therapeutic target for the future development of selective pharmacologic RAGE antagonists to ameliorate post-transplant lung IR injury.
Ischemia–reperfusion (IR) injury remains a leading cause of morbidity and mortality among patients undergoing lung transplantation. IR injury entails a rapid inflammatory response upon transplantation resulting in activation of the innate immune system, expression of proinflammatory cytokines, leukocyte infiltration, and edema, all of which contribute to subsequent acute graft failure. With a reported incidence of 25–30%, IR injury results in increased resource utilization, including prolonged mechanical ventilation, intensive care unit duration, and overall hospital lengths of stay (1, 2). Moreover, IR injury has also been identified as an important clinical risk factor for the development of chronic graft rejection in the form of bronchiolitis obliterans (3). As a result, studies aimed toward the development of therapeutic strategies to combat this complication hold tremendous clinical value.
The receptor for advanced glycation end-products (RAGE) is a ubiquitous, multiligand receptor implicated in a variety of pathological conditions, such as diabetes and its complications, cancer, cardiovascular disease, and inflammation. The RAGE gene is located on chromosome 6, and the membrane receptor is composed of three Ig-like regions with one “V” type and two “C” type domains (4, 5). Activation of RAGE occurs through the binding of a number of identified ligands, including amyloid-B peptide, amyloid A, a group of nonenzymatically glycated byproducts known as advanced glycated end-products (AGEs), the S100/calgranulin family of calcium-binding polypeptides, and the DNA-binding protein, high mobility group box 1 (HMGB1) (6–9). Once activated, RAGE mediates its downstream effects through several different signaling pathways, including generation of reactive oxygen species via activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, activation of NF-κB, and activation of extracellular signal–regulated kinase 1/2 mitogen-activated protein kinase, SAPK/JNK mitogen-activated protein kinase, or janus kinase–signal transducer and activator of transcription signaling pathways (10–14). RAGE is highly expressed in the lung, where it resides on the basolateral membrane of alveolar epithelial type I cells as well as other cell types, including smooth muscle cells, endothelial cells, and mononuclear phagocytes (15). Furthermore, RAGE can also exist as a soluble form (sRAGE), which is a truncated form of the receptor comprised of the extracellular domain that is cleaved from the cell surface by various matrix metalloproteinases (16, 17). sRAGE functions as a competitive, decoy receptor of membrane-bound RAGE to inhibit ligand binding and RAGE signaling. sRAGE has been reported as a primary marker of alveolar epithelial cell injury (18, 19). In fact, recent evidence has documented increased plasma levels of sRAGE in human transplant patients, which correlated with the development of primary graft dysfunction and prolonged intensive care unit duration (20, 21).
Acute hyperglycemia is known to be an established risk factor for many pathologic processes, and is also known to exacerbate several inflammatory mediators (22–24). Previous studies have documented the effects of acute, stress-induced hyperglycemia on many mechanistic processes, including oxidative stress and activation of select kinase pathways (22, 25–27). Most of these studies used cardiovascular models, and little is known regarding the effects of acute hyperglycemia on lung IR injury. Hagiwara and colleagues (28) investigated the impact of acute hyperglycemia on the expression and activity of HMGB1 using an endotoxin-induced rat model of acute lung injury. In the Hagiwara study, hyperglycemia was associated with higher HMGB1 levels and increased lung damage, which was significantly reduced after administration of insulin and normalization of blood glucose levels.
In light of these findings, the purpose of the present study was twofold: (1) to evaluate the impact of acute perioperative hyperglycemia on lung IR injury; and (2) to evaluate the role of RAGE signaling in hyperglycemia-enhanced IR injury. We hypothesized that acute hyperglycemia worsens lung IR injury through a RAGE signaling mechanism.
Materials and Methods
Further details are provided in the online supplement.
Animals
C57BL/6 wild-type (WT) mice (Jackson Laboratory, Bar Harbor, ME) and RAGE knockout (RAGE −/−) mice (29) 8–12 weeks of age were used. RAGE −/− mice have been backcrossed onto C57BL/6 for 10 generations, and are congenic with C57BL/6. Mice were randomly assigned to eight groups (n = 5–12/group) that underwent sham surgery (left thoracotomy + 2-h perfusion) or IR (1-h left lung ischemia + 2-h reperfusion) in the presence or absence of acute hyperglycemia. Historically, lung function in sham animals is very reproducible with little variation, thus an n of 5 for these groups was used for measurements of lung function and cytokine expression. The remaining groups, which all underwent lung IR, were comprised of 8–12 animals each, as indicated, for measurements of lung function and cytokine expression. As described subsequently here, separate groups of animals (n = 6/group) were used to measure microvascular permeability and leukocyte infiltration.
Acute hyperglycemia was established via intraperitoneal injection of 20% dextrose 30 minutes before ischemia. After 30 minutes of dextrose administration, mean glucose levels were 307.8 ± 55.2 mg/dL compared with 160.3 ± 23.2 mg/dL in untreated, normoglycemic mice (P < 0.001). This study conformed to National Institutes of Health guidelines, and was conducted under animal protocols approved by the University of Virginia's Institutional Animal Care and Use Committee.
Lung IR Model
Mice undergoing IR underwent 1 hour of lung ischemia (via left hilar occlusion), followed by 2 hours of reperfusion using an established model, as previously described (30). Sham animals underwent left thoracotomy, followed by 2 hours of perfusion.
Pulmonary Function
At the end of reperfusion, pulmonary function was evaluated by measuring pulmonary compliance, airway resistance, and pulmonary artery pressure using an isolated mouse lung system (Hugo Sachs Elektronik, March-Hugstetten, Germany), as previously described (31).
Bronchoalveolar Lavage
After measurement of pulmonary function, the left lung underwent bronchoalveolar lavage (BAL) using 0.4 ml PBS. The BAL fluid was centrifuged at 4°C (500 × g, 5 min), and the supernatant was collected and stored at –80°C until further analysis.
Cytokine Measurements
Proinflammatory cytokines (TNF-α, keratinocyte chemoattractant, IL-6, monocyte chemotactic protein-1, and RANTES [regulated upon activation, normal T cell expressed and secreted]) in BAL fluid were measured using a Bioplex Bead Array technique and multiplex cytokine panel assay (Bio-Rad Laboratories, Hercules, CA).
Pulmonary Vascular Permeability
Using separate groups of animals (n = 6/group), vascular permeability in lungs was estimated with the Evans blue dye extravasation technique, which is an index of change in protein permeability.
Immunohistochemistry and Leukocyte Infiltration
Using separate groups of animals (n = 6/group), lungs were fixed with 4% paraformaldehyde, and immunostaining of lung sections was performed with anti-mouse neutrophil (GR1.1; Santa Cruz Biotechnology, Santa Cruz, CA) or anti-mouse macrophage (Mac-2; Accurate Chem, Westbury, NY) antibodies. Neutrophils or macrophages were counted in five random fields and averaged.
Statistical Analysis
Independent, pairwise group comparisons of differences in measured results were performed using ANOVA or the Mann-Whitney U test, where appropriate. All probability estimates (P values) were adjusted for the potential influence of multiple group comparisons using a post hoc Bonferroni correction to provide a conservative estimate of these differences. Results are expressed as means (±SEM). All P values less than 0.05 were considered significant.
Results
Pulmonary Dysfunction after IR Is Attenuated in RAGE −/− Mice
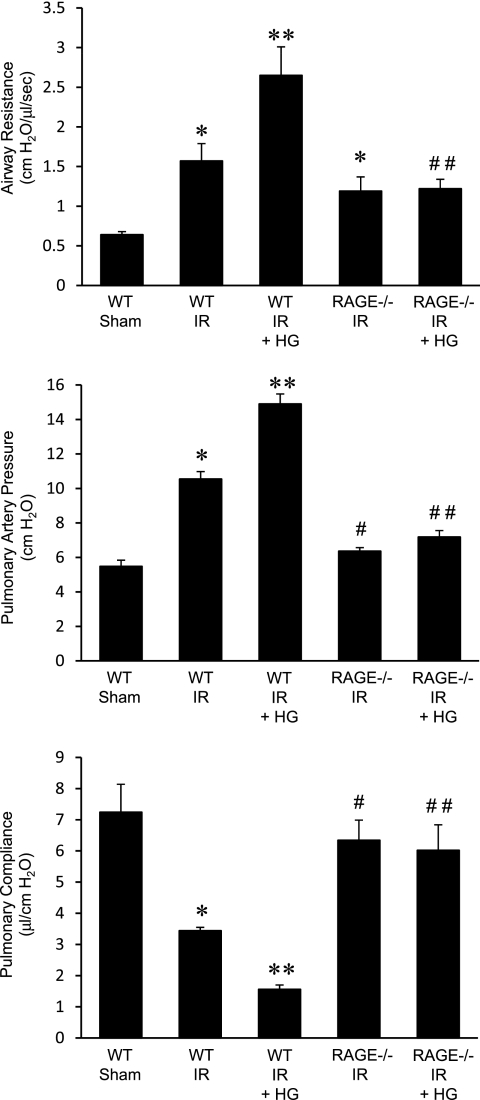
To determine the impact of acute perioperative hyperglycemia on lung function after IR, pulmonary function was assessed in hyperglycemic WT mice after sham surgery or IR. In normoglycemic WT mice, as expected, IR resulted in significant pulmonary dysfunction, as measured by increased airway resistance and pulmonary artery pressure, and decreased pulmonary compliance (Figure 1). Hyperglycemia in WT sham mice did not significantly alter lung function compared to normoglycemic sham mice (see Figure E1 in the online supplement). Hyperglycemia in WT mice after IR resulted in significantly worse lung function (i.e., enhanced airway resistance and pulmonary artery pressure and reduced pulmonary compliance) compared with IR in normoglycemic mice (Figure 1). These results suggest that acute perioperative hyperglycemia significantly exacerbates lung dysfunction after IR.
Figure 1.
Lung function is improved in receptor for advanced glycation end products (RAGE) −/− mice after ischemia–reperfusion (IR), and is not exacerbated by hyperglycemia. Pulmonary function (airway resistance, pulmonary artery pressure, and pulmonary compliance) was measured in wild-type (WT) and RAGE −/− mice that underwent sham surgery or IR in the absence or presence of acute perioperative hyperglycemia (HG). The numbers of animals per group were as follows: n = 5 for WT sham; n = 8 for WT IR, RAGE −/− IR, and RAGE −/− IR + HG; and n = 12 for WT IR + HG. *P < 0.05 versus WT sham; **P < 0.05 versus WT IR; #P < 0.05 versus WT IR; and ##P < 0.05 versus WT IR + HG.
To investigate the role of RAGE signaling in lung function after IR and hyperglycemia, the effects of acute hyperglycemia on pulmonary function was evaluated in RAGE −/− mice. Pulmonary function in normoglycemic and hyperglycemic sham RAGE −/− mice was not significantly different from sham WT mice (Figure E1). After IR, pulmonary dysfunction was significantly attenuated in RAGE −/− mice, which had reduced pulmonary artery pressure and increased pulmonary compliance compared with WT mice after IR (Figure 1). Airway resistance in RAGE −/− mice was not significantly reduced after IR compared with WT IR (Figure 1). Importantly, hyperglycemia failed to exacerbate lung dysfunction after IR in RAGE −/− mice, and no significant differences in lung function were observed after IR between normoglycemic and hyperglycemic RAGE −/− mice (Figure 1).
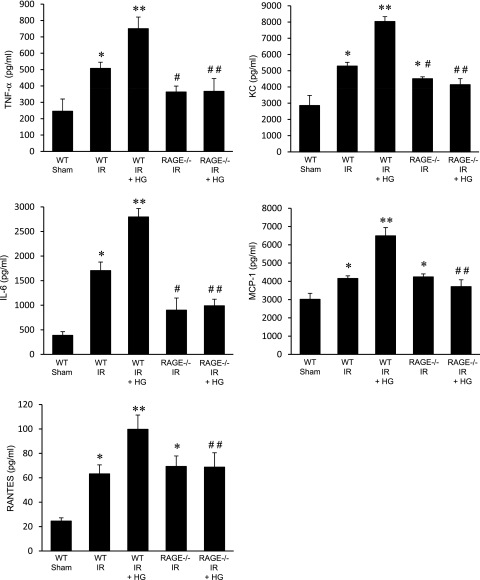
Proinflammatory Cytokines Are Enhanced by Hyperglycemia after IR, but Attenuated in RAGE −/− Mice
To determine the level of inflammation after IR and hyperglycemia, proinflammatory cytokine levels (TNF-α, KC, IL-6, MCP-1, RANTES) were measured in BAL fluid of WT and RAGE −/− mice after hyperglycemia. As expected, the expression of TNF-α, KC (CXCL1), IL-6, MCP-1, and RANTES were significantly increased in lungs of normoglycemic WT mice after IR compared with sham (Figure 2). Hyperglycemia resulted in a significant enhancement of TNF-α, KC, IL-6, MCP-1, and RANTES expression after IR in WT mice compared with normoglycemic mice. The expression of TNF-α, KC, and IL-6 were significantly attenuated in RAGE −/− mice after IR compared with WT mice (Figure 2). The expression of KC in RAGE −/− mice after IR, although significantly reduced compared with WT IR, remained significantly higher than in sham animals, and the expression of MCP-1 and RANTES was not significantly reduced compared with WT IR (Figure 2). Importantly, hyperglycemia did not enhance cytokine expression in RAGE −/− mice after IR, and no significant differences in cytokine expression after IR were observed between normoglycemic and hyperglycemic RAGE −/− mice (Figure 2). Cytokine levels were similar between sham RAGE −/− mice (normoglycemic or hyperglycemic) and sham WT mice (Figure E2).
Figure 2.
Hyperglycemia does not exacerbate cytokine expression in RAGE −/− mice after IR. Proinflammatory cytokines (TNF-α, keratinocyte chemoattractant, IL-6, monocyte chemotactic protein-1, and regulated upon activation, normal T cell expressed and secreted [RANTES]) in bronchoalveolar lavage (BAL) fluid were measured in WT and RAGE −/− mice that underwent sham surgery or IR in the absence or presence of acute perioperative hyperglycemia (HG). The numbers of animals per group were as follows: n = 5 for WT sham; n = 8 for WT IR, RAGE −/− IR, and RAGE −/− IR + HG; and n = 12 for WT IR + HG. *P < 0.05 versus WT sham; **P < 0.05 versus WT IR; #P < 0.05 versus WT IR; and ##P < 0.05 versus WT IR + HG.
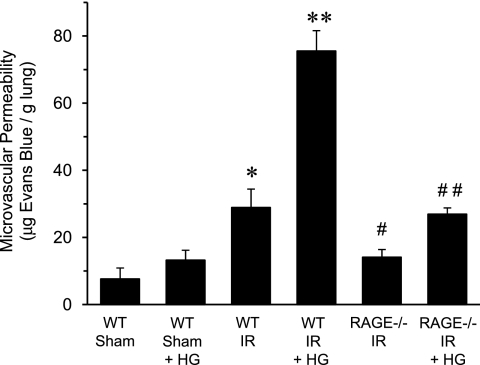
Pulmonary Vascular Permeability after IR Is Attenuated in RAGE −/− Mice
To determine the effect of hyperglycemia on the integrity of the pulmonary vasculature, vascular permeability was assessed via Evans blue dye extravasation. As expected, vascular permeability was significantly increased in WT mice after IR compared with sham (Figure 3). Hyperglycemia significantly enhanced vascular permeability in WT mice after IR by 2.6-fold. A comparison of WT and RAGE −/− mice revealed that vascular permeability was significantly decreased in RAGE −/− mice after IR, and perioperative hyperglycemia did not significantly increase vascular permeability in RAGE −/− mice (Figure 3).
Figure 3.
RAGE −/− mice have reduced vascular permeability after IR, which is not significantly exacerbated by hyperglycemia. Vascular permeability was quantified using Evans blue dye extravasation in WT and RAGE −/− mice that underwent either sham surgery or IR in the absence or presence of acute perioperative hyperglycemia (HG) (n = 6/group). *P < 0.05 versus WT sham; **P < 0.05 versus WT IR; #P < 0.05 versus WT IR; and ##P < 0.05 versus WT IR + HG.
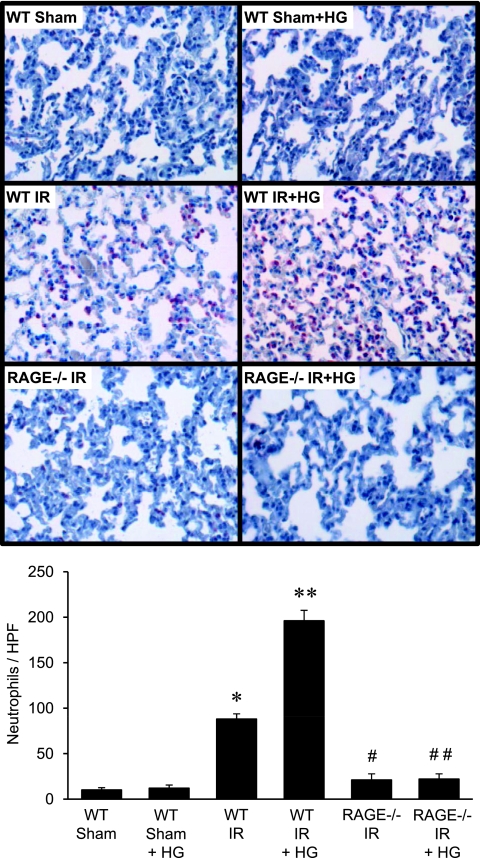
RAGE Deficiency Attenuates Leukocyte Infiltration after IR
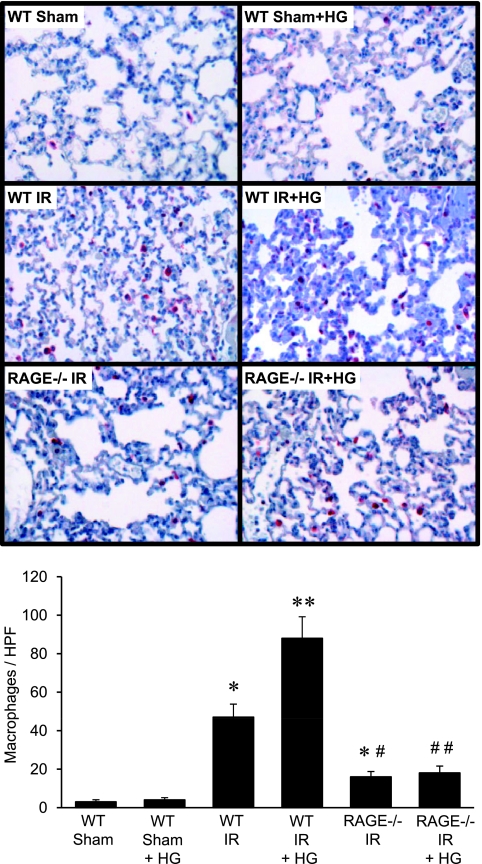
To determine the effect of hyperglycemia and RAGE signaling on leukocyte infiltration after IR, macrophages and neutrophils were counted in lung sections from WT and RAGE −/− mice after IR and hyperglycemia. The infiltration of neutrophils (Figure 4) and macrophages (Figure 5) were significantly increased in lungs of WT mice after IR compared with sham, but were significantly reduced in RAGE −/− mice after IR. The infiltration of macrophages in RAGE −/− mice after IR, although significantly reduced compared with WT IR, remained significantly higher than in sham animals (Figure 5). Hyperglycemia in WT mice after IR significantly enhanced neutrophil and macrophage infiltration compared with normoglycemia; however, hyperglycemia failed to increase neutrophil or macrophage infiltration in RAGE −/− mice after IR (Figures 4 and 5).
Figure 4.
Neutrophil infiltration in RAGE −/− mice is blocked after IR, and is not affected by hyperglycemia. Representative lung sections (top, 40× magnification) depict immunohistochemical staining of neutrophils (red-stained cells). Mean cell counts per high-powered field (HPF) are presented (bottom) for WT and RAGE −/− mice that underwent either sham surgery or IR in the absence or presence of acute perioperative hyperglycemia (HG) (n = 6/group). *P < 0.05 versus WT sham; **P < 0.05 versus WT IR; #P < 0.05 versus WT IR; and ##P < 0.05 versus WT IR + HG.
Figure 5.
Macrophage infiltration in RAGE −/− mice is attenuated after IR, and is not affected by hyperglycemia. Representative lung sections (top, 40× magnification) depict immunohistochemical staining of macrophages (red-stained cells). Mean cell counts per high-powered field (HPF) are presented (bottom) for WT and RAGE −/− mice that underwent either sham surgery or IR in the absence or presence of acute perioperative hyperglycemia (HG) (n = 6/group). *P < 0.05 versus WT sham; **P < 0.05 versus WT IR; #P < 0.05 versus WT IR; and ##P < 0.05 versus WT IR + HG.
Discussion
Herein, we present novel experimental evidence for the exacerbation of lung IR injury by acute perioperative hyperglycemia, and have implicated an important role for the RAGE signaling pathway in this process. Using an established in vivo hilar clamp model of lung IR-induced inflammation, the present study demonstrates that lung function, injury, and inflammation are significantly exacerbated after IR by acute hyperglycemia. Compared with normoglycemia, hyperglycemia in WT mice undergoing IR resulted in a significant enhancement of pulmonary dysfunction, proinflammatory cytokine expression, microvascular permeability, and infiltration of neutrophils and macrophages. Furthermore, to identify a potential mechanistic pathway underlying this process, we investigated the RAGE signaling pathway using RAGE −/− mice in the setting of acute hyperglycemia. As a result, lung dysfunction and injury after IR was significantly attenuated in RAGE −/− mice, and acute hyperglycemia did not significantly increase lung dysfunction, injury, or inflammation after IR in these mice. These findings suggest that the RAGE signaling pathway is an important mediator of lung IR injury, and also implicate a key role for RAGE signaling in hyperglycemic exacerbation of lung IR injury.
The inflammatory effects of acute hyperglycemia in this study correlate with published effects of stress-induced hyperglycemia in several models of experimental inflammation (27, 32–35). Stress-induced hyperglycemia is also a well documented risk factor for morbidity and mortality among patients undergoing cardiothoracic care and transplant (33, 36–38). Thus, to simulate stress-induced hyperglycemia in the present study, acute perioperative hyperglycemia was induced in mice by intraperitoneal injection of dextrose. The dosing strategy used was based upon our previous experience, wherein we demonstrated hyperglycemia after a single dose of dextrose administration, which peaks at 30 minutes after injection and remains elevated after 60 minutes (27). In our hyperglycemic animals, a twofold increase in blood glucose level was achieved 30 minutes after dextrose injection, which is similar to stress-induced changes in blood glucose among human transplant recipients (37). During hyperglycemia, ischemia of the left lung was performed as described in Materials and Methods. The reported results, therefore, represent the initiating effects of acute hyperglycemia during the ischemic period and the subsequent inflammatory cascade during reperfusion.
In the present study, we demonstrate that acute hyperglycemia in WT mice results in significant exacerbation of lung dysfunction, inflammation, and injury after IR. The similarities between normoglycemic and hyperglycemic WT sham mice indicate that the effects of elevated blood glucose levels are limited to the IR condition. Importantly, RAGE deficiency conferred a level of protection similar to that observed in WT sham mice in all parameters except macrophage infiltration and expression of KC, which were both slightly higher in RAGE −/− mice after IR compared with WT shams, but were significantly less than in WT mice after IR (Figure 2). Furthermore, we observed that the expression of MCP-1 and RANTES was not significantly reduced after IR in RAGE −/− versus WT mice. These results suggest that RAGE signaling may not play a significant role in the induction of MCP-1 and RANTES, but may play a more important role in the induction of other cytokines, such as TNF-α and IL-6, in the setting of lung IR. We have previously demonstrated a key role for TNF-α in murine lung IR injury (39), and thus it is possible that the attenuation of TNF-α production (in combination with reduced levels of KC and IL-6) in RAGE −/− mice after IR contributes importantly to the protection from IR injury in these mice.
Activation of RAGE represents one potential mechanistic pathway involved in lung IR injury under either normoglycemic or hyperglycemic conditions. Previous studies have provided evidence for a role of RAGE signaling in IR injury of other organs, such as heart and liver (40, 41). Considering the high expression of RAGE within the lung (15), and that several RAGE ligands (e.g., HMGB1 and other AGEs) have been shown to be increased by hyperglycemia (28, 42–44), we focused on the role of RAGE signaling in lung IR injury in the present study. By comparing the effects of IR in normoglycemic and hyperglycemic WT and RAGE −/− mice, the results of the present study corroborate results of other studies of RAGE in IR injury. For example, Sternberg and colleagues (45) used a similar left hilar clamp model to demonstrate reduced IR injury in RAGE −/− mice as well as in WT mice treated with sRAGE. This study differs from the present study in the timing of IR in their model and in the lack of pulmonary function data. In a more recent study, Reynolds and colleagues (46) examined the role of RAGE in a hyperoxic model of lung injury in which chronic hyperoxia exposure resulted in lung injury and increased RAGE expression on alveolar epithelial cells, whereas RAGE −/− mice were protected. This study demonstrated the impact of RAGE signaling in a more chronic model of lung injury. Consequently, the results of the present study serve to complement these reports, but, more importantly, extend our understanding of the role of RAGE signaling in lung IR injury in the setting of acute hyperglycemia.
Other potential mechanisms related to exacerbation of IR injury by hyperglycemia have been previously reported, and the role of oxidative stress in this process has been shown to be of particular importance (24–26, 30, 47). Several nonenzymatic and enzymatic processes have been shown to result in an increase in reactive oxygen species during hyperglycemia including auto-oxidation of glucose and formation of AGEs, increased enzymatic activities of nitric oxide synthase, NADPH oxidase, or xanthine oxidase, as well as increased activity of the mitochondrial respiratory chain (25, 27, 48). In fact, accumulating data suggest an important role of NADPH oxidase in acute hyperglycemia-mediated oxidative stress (25, 27, 48). We have previously demonstrated that acute hyperglycemia increases oxidative stress and IR injury in a murine model of myocardial infarction through NADPH oxidase activation (27), and we have also implicated NADPH oxidase in experimental lung IR injury (30). In light of these findings, we believe that it is plausible that an interaction between the RAGE signaling pathway and the NADPH oxidase pathway could be involved in the mechanism underlying hyperglycemia-mediated lung IR injury. Such an interaction may occur by which increased NADPH oxidase activity during hyperglycemia results in elevated levels of AGEs, which subsequently activate RAGE signaling. Thus, this process may be further propagated in part by the downstream activation of NADPH oxidase after RAGE activation, ultimately serving to exacerbate this cyclical process.
Several aspects of the present study deserve further discussion. Although the blood glucose levels in hyperglycemic mice were slightly lower than that achieved previously (27), the level of hyperglycemia in the present study is similar to that of acute hyperglycemia among humans undergoing organ transplantation (37, 38). Second, this study documents the protection conferred by RAGE deficiency by using RAGE −/− mice, and does not evaluate the result of direct blockage of native RAGE in WT mice (e.g., with the use of decoy sRAGE). The results of the current study provide a foundation for several avenues of future investigation, including the identification of the specific RAGE ligands involved in hyperglycemia-mediated lung IR injury. In addition, further scrutiny of the downstream effects of RAGE activation will help to identify more precisely the exact mechanistic pathways involved in hyperglycemia exacerbation of lung IR injury.
In conclusion, acute hyperglycemia significantly exacerbates experimental lung IR injury, and RAGE deficiency prevents exacerbation of IR injury in the hyperglycemic setting. These results serve to not only corroborate the protective effects of RAGE deficiency in IR injury, but also suggest that hyperglycemia-enhanced lung IR injury is mediated, at least in part, by RAGE signaling. These results identify a novel therapeutic target for the future development of selective pharmacologic RAGE antagonists to ameliorate post-transplant lung IR injury.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health awards R01HL077301 (to V.E.L.), T32HL007849 (to I.L.K.), and by a University of Virginia Research & Development Award (to A.K.S.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-0247OC on October 6, 2011
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Ailawadi G, Lau CL, Smith PW, Swenson BR, Hennessy SA, Kuhn CJ, Fedoruk LM, Kozower BD, Kron IL, Jones DR. Does reperfusion injury still cause significant mortality after lung transplantation? J Thorac Cardiovasc Surg 2009;137:688–694 [DOI] [PubMed] [Google Scholar]
- 2.Trulock EP, Edwards LB, Taylor DO, Boucek MM, Keck BM, Hertz MI. Registry of the International Society for Heart and Lung Transplantation: twenty-third official adult lung and heart-lung transplantation report—2006. J Heart Lung Transplant 2006;25:880–892 [DOI] [PubMed] [Google Scholar]
- 3.Fiser SM, Tribble CG, Long SM, Kaza AK, Kern JA, Jones DR, Robbins MK, Kron IL. Ischemia–reperfusion injury after lung transplantation increases risk of late bronchiolitis obliterans syndrome. Ann Thorac Surg 2002;73:1041–1047 [DOI] [PubMed] [Google Scholar]
- 4.Malherbe P, Richards JG, Gaillard H, Thompson A, Diener C, Schuler A, Huber G. cDNA cloning of a novel secreted isoform of the human receptor for advanced glycation end products and characterization of cells co-expressing cell-surface scavenger receptors and Swedish mutant amyloid precursor protein. Brain Res Mol Brain Res 1999;71:159–170 [DOI] [PubMed] [Google Scholar]
- 5.Schmidt AM, Mora R, Cao R, Yan SD, Brett J, Ramakrishnan R, Tsang TC, Simionescu M, Stern D. The endothelial cell binding site for advanced glycation end products consists of a complex: an integral membrane protein and a lactoferrin-like polypeptide. J Biol Chem 1994;269:9882–9888 [PubMed] [Google Scholar]
- 6.Du Yan S, Zhu H, Fu J, Yan SF, Roher A, Tourtellotte WW, Rajavashisth T, Chen X, Godman GC, Stern D, et al. Amyloid-beta peptide-receptor for advanced glycation endproduct interaction elicits neuronal expression of macrophage-colony stimulating factor: a proinflammatory pathway in Alzheimer disease. Proc Natl Acad Sci USA 1997;94:5296–5301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell 1999;97:889–901 [DOI] [PubMed] [Google Scholar]
- 8.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 1999;285:248–251 [DOI] [PubMed] [Google Scholar]
- 9.Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature 1996;382:685–691 [DOI] [PubMed] [Google Scholar]
- 10.Bierhaus A, Schiekofer S, Schwaninger M, Andrassy M, Humpert PM, Chen J, Hong M, Luther T, Henle T, Kloting I, et al. Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes 2001;50:2792–2808 [DOI] [PubMed] [Google Scholar]
- 11.Lander HM, Tauras JM, Ogiste JS, Hori O, Moss RA, Schmidt AM. Activation of the receptor for advanced glycation end products triggers a p21(ras)-dependent mitogen-activated protein kinase pathway regulated by oxidant stress. J Biol Chem 1997;272:17810–17814 [DOI] [PubMed] [Google Scholar]
- 12.Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, Tanji N, Lu Y, Lalla E, Fu C, et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature 2000;405:354–360 [DOI] [PubMed] [Google Scholar]
- 13.Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier JL. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab 2001;280:E685–E694 [DOI] [PubMed] [Google Scholar]
- 14.Yeh CH, Sturgis L, Haidacher J, Zhang XN, Sherwood SJ, Bjercke RJ, Juhasz O, Crow MT, Tilton RG, Denner L. Requirement for p38 and p44/p42 mitogen-activated protein kinases in RAGE-mediated nuclear factor-kappaB transcriptional activation and cytokine secretion. Diabetes 2001;50:1495–1504 [DOI] [PubMed] [Google Scholar]
- 15.Fehrenbach H, Kasper M, Tschernig T, Shearman MS, Schuh D, Muller M. Receptor for advanced glycation endproducts (RAGE) exhibits highly differential cellular and subcellular localisation in rat and human lung. Cell Mol Biol (Noisy-le-grand) 1998;44:1147–1157 [PubMed] [Google Scholar]
- 16.Hanford LE, Enghild JJ, Valnickova Z, Petersen SV, Schaefer LM, Schaefer TM, Reinhart TA, Oury TD. Purification and characterization of mouse soluble receptor for advanced glycation end products (sRAGE). J Biol Chem 2004;279:50019–50024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakurai S, Yonekura H, Yamamoto Y, Watanabe T, Tanaka N, Li H, Rahman AK, Myint KM, Kim CH, Yamamoto H. The AGE-RAGE system and diabetic nephropathy. J Am Soc Nephrol 2003;14:S259–S263 [DOI] [PubMed] [Google Scholar]
- 18.Calfee CS, Ware LB, Eisner MD, Parsons PE, Thompson BT, Wickersham N, Matthay MA. Plasma receptor for advanced glycation end products and clinical outcomes in acute lung injury. Thorax 2008;63:1083–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uchida T, Shirasawa M, Ware LB, Kojima K, Hata Y, Makita K, Mednick G, Matthay ZA, Matthay MA. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med 2006;173:1008–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calfee CS, Budev MM, Matthay MA, Church G, Brady S, Uchida T, Ishizaka A, Lara A, Ranes JL, deCamp MM, et al. Plasma receptor for advanced glycation end-products predicts duration of ICU stay and mechanical ventilation in patients after lung transplantation. J Heart Lung Transplant 2007;26:675–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christie JD, Shah CV, Kawut SM, Mangalmurti N, Lederer DJ, Sonett JR, Ahya VN, Palmer SM, Wille K, Lama V, et al. Plasma levels of receptor for advanced glycation end products, blood transfusion, and risk of primary graft dysfunction. Am J Respir Crit Care Med 2009;180:1010–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aljada A, Friedman J, Ghanim H, Mohanty P, Hofmeyer D, Chaudhuri A, Dandona P. Glucose ingestion induces an increase in intranuclear nuclear factor kappaB, a fall in cellular inhibitor kappaB, and an increase in tumor necrosis factor alpha messenger RNA by mononuclear cells in healthy human subjects. Metabolism 2006;55:1177–1185 [DOI] [PubMed] [Google Scholar]
- 23.Ling PR, Smith RJ, Bistrian BR. Hyperglycemia enhances the cytokine production and oxidative responses to a low but not high dose of endotoxin in rats. Crit Care Med 2005;33:1084–1089 [DOI] [PubMed] [Google Scholar]
- 24.Sudic D, Razmara M, Forslund M, Ji Q, Hjemdahl P, Li N. High glucose levels enhance platelet activation: involvement of multiple mechanisms. Br J Haematol 2006;133:315–322 [DOI] [PubMed] [Google Scholar]
- 25.Mohanty P, Hamouda W, Garg R, Aljada A, Ghanim H, Dandona P. Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J Clin Endocrinol Metab 2000;85:2970–2973 [DOI] [PubMed] [Google Scholar]
- 26.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006;295:1681–1687 [DOI] [PubMed] [Google Scholar]
- 27.Yang Z, Laubach VE, French BA, Kron IL. Acute hyperglycemia enhances oxidative stress and exacerbates myocardial infarction by activating nicotinamide adenine dinucleotide phosphate oxidase during reperfusion. J Thorac Cardiovasc Surg 2009;137:723–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hagiwara S, Iwasaka H, Hasegawa A, Koga H, Noguchi T. Effects of hyperglycemia and insulin therapy on high mobility group box 1 in endotoxin-induced acute lung injury in a rat model. Crit Care Med 2008;36:2407–2413 [DOI] [PubMed] [Google Scholar]
- 29.Constien R, Forde A, Liliensiek B, Grone HJ, Nawroth P, Hammerling G, Arnold B. Characterization of a novel EGFP reporter mouse to monitor Cre recombination as demonstrated by a Tie2 Cre mouse line. Genesis 2001;30:36–44 [DOI] [PubMed] [Google Scholar]
- 30.Yang Z, Sharma AK, Marshall M, Kron IL, Laubach VE. NADPH oxidase in bone marrow–derived cells mediates pulmonary ischemia–reperfusion injury. Am J Respir Cell Mol Biol 2009;40:375–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma AK, Lapar DJ, Zhao Y, Li L, Lau CL, Kron IL, Iwakura Y, Okusa MD, Laubach VE. Natural killer T cell–derived IL-17 mediates lung ischemia–reperfusion injury. Am J Respir Crit Care Med 2011;183:1539–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aeba R, Griffith BP, Kormos RL, Armitage JM, Gasior TA, Fuhrman CR, Yousem SA, Hardesty RL. Effect of cardiopulmonary bypass on early graft dysfunction in clinical lung transplantation. Ann Thorac Surg 1994;57:715–722 [DOI] [PubMed] [Google Scholar]
- 33.Doenst T, Wijeysundera D, Karkouti K, Zechner C, Maganti M, Rao V, Borger MA. Hyperglycemia during cardiopulmonary bypass is an independent risk factor for mortality in patients undergoing cardiac surgery. J Thorac Cardiovasc Surg 2005;130:1144. [DOI] [PubMed] [Google Scholar]
- 34.Ishihara M, Kojima S, Sakamoto T, Asada Y, Tei C, Kimura K, Miyazaki S, Sonoda M, Tsuchihashi K, Yamagishi M, et al. Acute hyperglycemia is associated with adverse outcome after acute myocardial infarction in the coronary intervention era. Am Heart J 2005;150:814–820 [DOI] [PubMed] [Google Scholar]
- 35.Webster KA. Stress hyperglycemia and enhanced sensitivity to myocardial infarction. Curr Hypertens Rep 2008;10:78–84 [DOI] [PubMed] [Google Scholar]
- 36.Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet 2000;355:773–778 [DOI] [PubMed] [Google Scholar]
- 37.Thomas MC, Moran J, Mathew TH, Russ GR, Rao MM. Early peri-operative hyperglycaemia and renal allograft rejection in patients without diabetes. BMC Nephrol 2000;1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van den Berg TJ, Bogers H, Vriesendorp TM, Surachno JS, DeVries JH, ten Berge IJ, Hoekstra JB. No apparent impact of increased post-operative blood glucose levels on clinical outcome in kidney transplant recipients. Clin Transplant 2009;23:256–263 [DOI] [PubMed] [Google Scholar]
- 39.Maxey TS, Enelow RI, Gaston B, Kron IL, Laubach VE, Doctor A. Tumor necrosis factor-alpha from resident lung cells is a key initiating factor in pulmonary ischemia–reperfusion injury. J Thorac Cardiovasc Surg 2004;127:541–547 [DOI] [PubMed] [Google Scholar]
- 40.Andrassy M, Volz HC, Igwe JC, Funke B, Eichberger SN, Kaya Z, Buss S, Autschbach F, Pleger ST, Lukic IK, et al. High-mobility group box-1 in ischemia reperfusion injury of the heart. Circulation 2008;117:3216–3226 [DOI] [PubMed] [Google Scholar]
- 41.Zeng S, Feirt N, Goldstein M, Guarrera J, Ippagunta N, Ekong U, Dun H, Lu Y, Qu W, Schmidt AM, et al. Blockade of receptor for advanced glycation end product (RAGE) attenuates ischemia and reperfusion injury to the liver in mice. Hepatology 2004;39:422–432 [DOI] [PubMed] [Google Scholar]
- 42.Brownlee M. Negative consequences of glycation. Metabolism 2000;49:9–13 [DOI] [PubMed] [Google Scholar]
- 43.Schiekofer S, Andrassy M, Chen J, Rudofsky G, Schneider J, Wendt T, Stefan N, Humpert P, Fritsche A, Stumvoll M, et al. Acute hyperglycemia causes intracellular formation of CML and activation of ras, p42/44 MAPK, and nuclear factor kappaB in PBMCs. Diabetes 2003;52:621–633 [DOI] [PubMed] [Google Scholar]
- 44.Schmidt AM, Yan SD, Stern DM. The dark side of glucose. Nat Med 1995;1:1002–1004 [DOI] [PubMed] [Google Scholar]
- 45.Sternberg DI, Gowda R, Mehra D, Qu W, Weinberg A, Twaddell W, Sarkar J, Wallace A, Hudson B, D'Ovidio F, et al. Blockade of receptor for advanced glycation end product attenuates pulmonary reperfusion injury in mice. J Thorac Cardiovasc Surg 2008;136:1576–1585 [DOI] [PubMed] [Google Scholar]
- 46.Reynolds PR, Schmitt RE, Kasteler SD, Sturrock A, Sanders K, Bierhaus A, Nawroth PP, Paine R, III, Hoidal JR. Receptors for advanced glycation end-products targeting protect against hyperoxia-induced lung injury in mice. Am J Respir Cell Mol Biol 2010;42:545–551 [DOI] [PubMed] [Google Scholar]
- 47.Hu Y, Block G, Norkus EP, Morrow JD, Dietrich M, Hudes M. Relations of glycemic index and glycemic load with plasma oxidative stress markers. Am J Clin Nutr 2006;84:70–76, quiz 266–267 [DOI] [PubMed] [Google Scholar]
- 48.Dhindsa S, Tripathy D, Mohanty P, Ghanim H, Syed T, Aljada A, Dandona P. Differential effects of glucose and alcohol on reactive oxygen species generation and intranuclear nuclear factor-kappaB in mononuclear cells. Metabolism 2004;53:330–334 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.