Abstract
The mechanisms by which the exposure of mice to Cl2 decreases vectorial Na+ transport and fluid clearance across their distal lung spaces have not been elucidated. We examined the biophysical, biochemical, and physiological changes of rodent lung epithelial Na+ channels (ENaCs) after exposure to Cl2, and identified the mechanisms involved. We measured amiloride-sensitive short-circuit currents (Iamil) across isolated alveolar Type II (ATII) cell monolayers and ENaC single-channel properties by patching ATII and ATI cells in situ. α-ENaC, γ-ENaC, total and phosphorylated extracellular signal-related kinase (ERK)1/2, and advanced products of lipid peroxidation in ATII cells were measured by Western blot analysis. Concentrations of reactive intermediates were assessed by electron spin resonance (ESR). Amiloride-sensitive Na+ channels with conductances of 4.5 and 18 pS were evident in ATI and ATII cells in situ of air-breathing mice. At 1 hour and 24 hours after exposure to Cl2, the open probabilities of these two channels decreased. This effect was prevented by incubating lung slices with inhibitors of ERK1/2 or of proteasomes and lysosomes. The exposure of ATII cell monolayers to Cl2 increased concentrations of reactive intermediates, leading to ERK1/2 phosphorylation and decreased Iamil and α-ENaC concentrations at 1 hour and 24 hours after exposure. The administration of antioxidants to ATII cells before and after exposure to Cl2 decreased concentrations of reactive intermediates and ERK1/2 activation, which mitigated the decrease in Iamil and ENaC concentrations. The reactive intermediates formed during and after exposure to Cl2 activated ERK1/2 in ATII cells in vitro and in vivo, leading to decreased ENaC concentrations and activity.
Keywords: lung slices, patch clamp, radicals
Clinical Relevance
Chlorine (Cl2) is the ninth most abundantly produced chemical in the United States. Persons exposed to Cl2 released into the atmosphere during industrial accidents or acts or terrorism may develop respiratory failure and alveolar edema. Previously, we showed that exposure of animals to Cl2 damages alveolar epithelial cells by decreasing their ability to actively transport sodium (Na+) and clear excess alveolar fluid. However, the fundamental mechanisms by which Cl2 and its reactive intermediates damage distal lung epithelial sodium (Na+) channels, which are the main conduits for the entry of Na+ ions into epithelial cells, have not been identified. We used electrophysiological techniques to demonstrate direct injury to amiloride-sensitive Na+ channels (ENaC) in alveolar Type I and Type II cells in situ in mice at 1 hour and 24 hours after exposure to Cl2. We then exposed alveolar Type II cells in primary culture to Cl2 and demonstrated that Cl2-induced injury to Na+ channels was mediated by the phosphorylation and activation of ERK1/2. Treatment with antioxidants, administered before or after the exposure of alveolar Type II cells to Cl2, prevented and partly reversed these effects. The results of our experiments form the rational basis for the development of new treatments to restore ENaC function and decrease lung injury after exposure to Cl2.
After birth, lung liquid secretion and absorption are maintained by the activities of the cystic fibrosis transmembrane conductance regulator and epithelial Na+ channels (ENaCs), located at the apical membranes of epithelial cells, and by basolateral Na/K-ATPase (1, 2). In cases where this process is disturbed, the lungs become either dry because of excessive fluid absorption, as in cystic fibrosis (3), or flooded, as in acute lung injury, which hampers gas exchange (1, 4–7).
Components of the epithelial lining fluid (ELF) and epithelial cells are continuously subjected to assaults by the reactive intermediates in environmental pollutants and oxidant gases. The removal of inhaled particles and pathogens from ELF is controlled by macrophages and neutrophils, both of which produce a variety of reactive species such as hypochlorous acid (HOCl) (8–10), in close proximity to apical epithelial cell surfaces. Large quantities of HOCl can be generated in ELF during exposure to Cl2 (11, 12).
Cl2 is a yellowish-green gas of the halogen group, used in the production of bleach and other disinfectants. It is water-soluble and reacts rapidly with water to generate hydrochloric acid (HCl) and HOCl. Exposure of mice to Cl2 in concentrations likely to be encountered in the vicinity of industrial accidents (400 parts per million) impaired their ability to clear fluid across their distal lung spaces (13). Furthermore, HOCl and its byproducts such as chloramines formed by the reaction of HOCl with protein tyrosine and lysine residues, inhibited the activity of human ENaCs expressed in Xenopus oocytes by oxidatively modifying residues in γ-ENaC, thereby locking the ENaC in its closed state (13). However, the mechanisms by which Cl2, HOCl, and their reactive intermediates inhibit ENaCs, the rate-limiting step in Na+ transport, and fluid clearance across alveolar epithelial cells have not been elucidated.
Previous studies showed that the activation of extracellular signal-related kinase (ERK)1/2 inhibits ENaCs by phosphorylating residues in the C-termini of the β and γ subunits, and by enhancing the docking of the ubiquitin ligase Nedd4-2 (14, 15). Furthermore, ERK1/2 is known to be activated by reactive species (16, 17). We therefore hypothesized that the inhalation of Cl2 increased concentrations of reactive species, inducing ERK1/2 activation, and in turn decreasing ENaC concentrations and activity in alveolar Type II (ATII) cells in primary culture and in lung slices. To test this hypothesis, we exposed mice to 400 parts per million (ppm) Cl2 for 30 minutes, returned them to room air for 1 hour or 24 hours, prepared lung slices and patched ATII and alveolar Type I (ATI) cells in situ. We also exposed rat ATII cell monolayers cultured on permeable supports to 100 and 200 ppm Cl2, and measured amiloride-sensitive short-circuit currents (Iamil) α-ENaC and γ-ENaC, and total and phosphorylated ERK1/2 levels by Western blot analysis, and the concentrations of reactive intermediates by electron spin resonance spectroscopy and lipid peroxidation at 1 hour and 24 hours after exposure. Our results show that the activation of ERK1/2 by reactive intermediates contributed at least in part to decrease ENaC protein and Iamil in vitro and ENaC function in situ. These effects were partly prevented and reversed by the administration of ascorbate, desferal, and N-acetyl-cysteine in ATII cells.
Materials and Methods
Detailed descriptions of all methods are available in the online supplement.
Animals
Procedures involving animals (rats and mice) were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham (animal protocol numbers 091107999 and 090808541).
Preparation of Lung Slices
Eight-week-old C57BL/6 male mice (∼20–25 g body weight) were purchased from The Jackson Laboratory (Bar Harbor, ME). Lung slices were prepared as previously described (18). The right lower lobes were dissected (without being filled with agar solution) and stored in Krebs solution at 4°C. They were then attached to the jig of an OTS 5000 slicer (FHC, Inc., Bowdoinham, ME), using cyanoacrylate adhesive gel, and sectioned longitudinally with a zirconium blade into 200- to 250-μm-thick slices at 4°C. The slices were then transferred to a six-well plate containing approximately 2 ml Dulbecco's Modified Eagle's Medium without serum, supplemented with penicillin–streptomycin, and allowed to recover at 37°C in a humidified environment of 95% air/5% CO2 for 2 to 3 hours.
Recordings from Mouse Lungs of ENaC Single-Channel Activity
Lung slices were transferred to a chamber on the stage of an Olympus microscope EX51WI (Olympus, Pittsburgh, PA). Single-channel activity in both ATII and ATI cells was recorded using the cell-attached mode of the patch clamp technique (19, 20). ATII cells were identified by the presence of scattered green fluorescence after incubation with Lysotracker Green (catalogue number DND-26; Invitrogen, Eugene, OR). ATI cells were identified by their characteristic appearance.
Isolation and Culture of ATII Cells
ATII cells were isolated from Sprague-Dawley rat lungs, seeded on semipermeable filters, and cultured for 4–5 days, until they formed confluent, high-resistance monolayers. In some cases, cells were immunostained with antibodies against surfactant protein C (SP-C), aquaporin-5 (APQ5), and α-ENaC.
Exposure of Rat ATII Cell Monolayers to Cl2
Confluent ATII monolayers were exposed to Cl2 (100–200 ppm) and incubated at 37°C in a humidified atmosphere of 95% air/5% CO2 for up to 24 hours. Just before exposures, all monolayers were covered with an “artificial ELF” consisting of 50 μl of normal Ringers solution (120 mM NaCl, 25 mM NaHCO3, 3.3 mM KH2PO4, 0.83 mM K2HPO4, 1.2 mM CaCl2, and 1.2 mM MgCl2) containing ascorbic acid (1 mM), reduced glutathione (0.12 mM), and urate (0.03 mM). The concentrations of antioxidants were based on their values in rat ELF (11, 21). In some cases, a mixture of antioxidants containing acetylcysteine, ascorbate, and deferoxamine (desferal; Hospira Inc., Lake Forest, IL) was added to the apical and basolateral compartments either before or after exposure to Cl2. However, just before exposure to Cl2, the apical fluid was removed and replaced with the “artificial ELF.”
Measurements of ATII Cell Short-Circuit Currents and Transepithelial Resistances
ATII cell monolayers were mounted in Ussing chambers at 1 hour or 24 hours after exposure to Cl2, as previously described (22, 23).
Detection of Reactive Intermediates in ATII Cells Exposed to Cl2
The presence of reactive intermediates in the apical fluid and cellular compartments of ATII cells was verified by (1) electron spin resonance (ESR), (2) the presence of advanced products of lipid peroxidation, and (3) the oxidation of MitoSOX Red (Invitrogen, Grand Island, NY).
Western Blotting
Total and phosphorylated ERK1/2, α-ENaC, and γ-ENaC in ATII cells before and after exposure to Cl2 were assessed by Western blot analysis, using commercially available antibodies.
Results
Single-Channel Recordings from Murine Alveolar Cells In Situ
Recordings of ENaC activity in ATI and ATII cells revealed the presence of two channels: a channel with a conductance (g) of 4.45 ± 0.061 pS that reversed at very positive voltage (+60 mV), indicative of its high selectivity for Na+ over K+, and a second channel with a conductance of 18 ± 0.45 pS that reversed at +10 mV, characteristic of a cation channel with near equal selectivity for Na+ and K+ ions (Figures 1A–1E). The addition of amiloride (5 μM) to the patch pipette solution decreased the activity of both channels (Figure 1F). Similar recordings were obtained from dozens of ATII cells and nine ATI cells, both in slices from five mice, with identical results. These findings are in agreement with our recently published results (18). Alveolar macrophages (identified by their size, shape, and morphology) did not show amiloride sensitive-channel activity (data not shown), in agreement with our previous report (24). We chose to study ATII cells, because they were easier to patch than ATI cells and the patches lasted longer. Furthermore, we were able to correlate biophysical measurements obtained from lung slices with biophysical and biochemical data obtained from isolated ATI cells exposed to Cl2.
Figure 1.
Alveolar Type I (ATI) and alveolar Type II (ATII) cells express two amiloride-sensitive Na+ conductances. In situ recordings of Na+ channel activity were obtained from an ATII (A) and an ATI (B) cell in a lung slice of an air-breathing mouse. The membrane potential across the patch was −100 mV. The corresponding amplitude histograms (C and D) show the presence of two distinct amplitudes at 0.45 pA and 1.8 pA, corresponding to conductances of 4.5 and 18 pS, respectively. Current–voltage (I-V) relationships of the 4.5 and 18 pS channels in ATII and ATI cells are shown in E. Values represent the means ± 1 SEM (n = 5 patches obtained from five different mice). (F) The addition of 5 μM amiloride in the patch pipette resulted in a gradual inhibition of the activities of both channels, as the amiloride diffused from the pipette solution onto the patch surface.
Acute Exposure to Cl2 Inhibits ENaC Activity
Both the 4 and 18 pS channels were present in ATII cells in lung slices from mice exposed to Cl2 (400 ppm for 30 minutes) and returned to 95% air/5% CO2 for 1 hour (Figures 2A and 2B). However, their open probabilities (Po) were significantly lower from their corresponding air values (Figures 2E and 2F, respectively). The addition of 5 μM forskolin (an adenylate cyclase activator) to the patch pipettes increased the number of active channels (Figures 2C and 2D) and the open probabilities in Cl2-exposed lungs, although they were still lower than their corresponding control values (Figures 2E and 2F).
Figure 2.
Epithelial Na+ channel (ENaC) single-channel activity in ATII cells of murine lungs at 1 hour after exposure to Cl2. (A and B) Characteristic single-channel recordings at a membrane potential of −100 mV and the corresponding amplitude histogram of ATII cells in lung slices of mice at 1 hour after exposure to Cl2. (C and D) Same conditions, but with 5 μM forskolin in the patch pipette. (E and F) Open probabilities (Po) of the 4 and 18 pS channels in cell-attached patches of ATII cells from lung slices of air and Cl2-exposed mice, patched in the absence or presence of forskolin (Forsk.) in pipette solutions. Values represent means ± 1 SEM. Number of patches (from at least five mice): in E, air = 19; Cl2 = 16; forskolin–air = 14; forskolin–Cl2 = 17; in F, air = 17; Cl2 = 15; forskolin–air = 16; forskolin–Cl2 = 16. Overall significance for one-way ANOVA, P < 0.0001. Each group was compared with the rest using the Tukey–Kramer multiple comparisons test. *P < 0.01, compared with the corresponding air values. #P < 0.01, compared with the corresponding vehicle (Veh.) values.
In the next series of experiments, we assessed the effects of trypsin on ENaC activity in ATII cells from air-breathing and Cl2-breathing animals. Trypsin activates silent channels by cleaving α-ENaC and γ-ENaC extracellular domains (19, 25). As shown in Figures 3A–3D, the inclusion of trypsin (2 μM) in the patch pipettes increased the number of active channels and their Po of air-breathing mice, without affecting their unitary conductance. Trypsin also increased the Po of both the 4 and 18 pS channels of ATII cells in Cl2-exposed mice approximately 3-fold (Figures 3C and 3D). These results indicate that Cl2 and its reactive intermediates mainly damage channels already cleaved by endogenous proteases.
Figure 3.
Trypsin increases ATII cell ENaC channel activity in ATII cells of murine lungs at 1 hour after Cl2 exposure. (A and B) Characteristic single-channel recordings at a membrane potential of −100 mV and the corresponding amplitude histogram of ATII cells in lung slices of mice at 1 hour after Cl2 exposure with trypsin (2 μM) in the recording pipette solution. (C and D) Open probabilities (Po) of the 4 and 18 pS channels in cell-attached patches of ATII cells from air-exposed and Cl2-exposed mice in the absence or presence of trypsin in the pipette solutions. Values refer to means ± 1 SEM. Numbers of patches (from three mice): in C, n = 6 for all groups; in D, n = 5 for all groups. Overall significance for one-way ANOVA, P < 0.0001. *P < 0.01, compared with the corresponding air values. #P < 0.01, compared with the corresponding vehicle values.
Biophysical and Biochemical Modifications of ENaC at 24 Hours after Exposure to Cl2
Patch clamp measurements performed on ATII cells from lungs of mice exposed to Cl2, returned to air/5% CO2, and killed 24 hours later revealed the presence of the highly selective 4 pS channel and the nonselective 18 pS channel, although their open probabilities were lower than their corresponding air values, namely, Po for 4 pS, air, 0.29 ± 0.012 (means ± SE; n = 6); 24 hours after Cl2, 0.133 ± 0.014 (n = 5) (P < 0.001; Student t test); and Po for 18 pS, air, 0.18 ± 0.011 (n = 5); 24 hours after Cl2, 0.04 ± 0.0004 (n = 5) (P < 0.001; Student t test). In addition to the 4 and 18 pS, a 25 pS nonselective cation channel (Figures 4A and 4B), not present either in air or 1 hour after exposure to Cl2, was evident (Figures 1A–1D). The complete inhibition of this channel required 100 μM amiloride (Figure 4C), instead of 5 μM in the pipette solution. The mean Po of this nonselective cation channel was almost doubled by the inclusion of forskolin in the pipette (Figure 4E).
Figure 4.
ENaC activity in ATII cells of murine lungs 24 hours after exposure to Cl2. (A and B) Characteristic single channel recordings at a membrane potential of −100 mV and the corresponding amplitude histogram of an ATII cells. 4.5 pS and 25 pS conductances can be seen. (C) Single-channel recordings with a pipette filled with a solution containing amiloride (100 μM). Note the gradual disappearance of channel activity as amiloride diffuses on the patch. (D) IV-relationship of the 25 pS channels. Values refer to means ± SEM, n = 5 patches from four different mice. (E) Open probabilities (Po) of the 25 pS in the absence and presence of forskolin (10 μM). Data are means ± SEM). Data are means ± SEM. Numbers of patches (from five mice): Veh., n = 21; Forsk., n = 15. *P < 0.001, using Student t test.
Immunocytochemical Detection of ENaCs in Lung Tissue
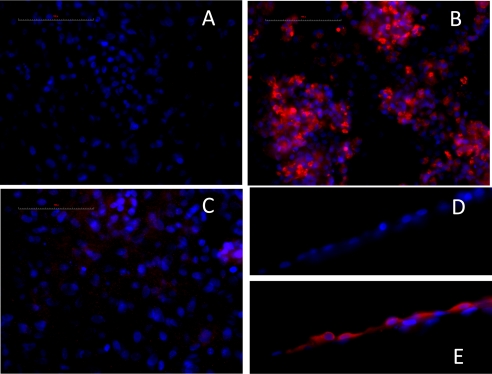
To obtain additional insights into levels of ENaC expression in epithelial (ATII and ATI) cells in vivo before and after exposure to Cl2, we immunostained lung tissues with specific antibodies against α-ENaC, β-ENaC, and γ-ENaC, followed by fluorescent secondary antibodies. As shown in Figure 5, a significant decrease in α-ENaC, β-ENaC, and γ-ENaC occurred at 1 hour after exposure to Cl2, compared with their corresponding air values. At 24 hours after exposure, levels of α-ENaC and γ-ENaC were not different from air values. However, β-ENaC remained significantly lower than the air control value. The decrease of β-ENaC may account for the appearance of the 25 pS nonselective conductance, because nonselective cation channels with larger conductance are thought to be formed mostly by α-ENaC subunits (26–28).
Figure 5.
Detection of ENaCs by indirect immunofluorescence. Mice were exposed to Cl2 (400 parts per million [ppm] for 30 minutes), returned to room air, and killed at 24 hours after exposure. Another group of mice was exposed to air. Fixed and permeabilized lung tissues were immunostained with antibodies against α-ENaC, β-ENaC, and γ-ENaC, followed by secondary antibodies with Alexa Fluor 594 goat anti-rabbit antibodies (1:400 dilution; Molecular Probes, Eugene, OR). Nuclei were stained with 4’,6-diamidino-2-phenylindole. Image acquisition was performed on a Leica DM6000 epifluorescence microscope with Simple PCI software (Compix, Inc., Cranberry Township, PA). Note the prominent increase in α-ENaC and decrease in β-ENaC at 24 hours after exposure, compared with air values. These experiments were repeated three times with similar results.
Immunocytochemical Characterization of Isolated Epithelial Cells
Approximately 92% of epithelial cells cultured on semipermeable supports for 3 days under a liquid–liquid interface, and then for 2 days under an air–liquid interface, stained positive for SP-C (Figure 6B). The remaining cells immunostained positive for AQP5 (Figure 6C), a marker of ATI cells. These results indicate that at the time of electrophysiological measurements, the majority of epithelial cells exhibit an ATII cell phenotype. The immunohistochemical staining of monolayers with an anti-αENaC antibody showed robust expression of this subunit at the apical or subapical membranes (Figure 6E). In contrast, no staining was observed when nonimmune IgG was used (Figures 6A–6D).
Figure 6.
Phenotypic characterization of alveolar epithelial cells seeded on semipermeable supports. Rat alveolar epithelial cells were immunostained with antibodies against nonimmune rabbit IgG (A), anti-rabbit surfactant protein C (a marker of ATII cells) (B), or rabbit aquaporin-5, a marker of ATI cells (C), and a secondary fluorescent antibody (goat anti-rabbit IgG conjugated to Alexa 594). In subsequent experiments, cells were immunostained with nonimmune IgG (D) or anti-rabbit α-ENaC (E), followed by goat anti-rabbit IgG coupled to Alexa 594 (D and E). Filters shown in D and E were folded to visualize the apical surfaces. In all cases, nuclei were counterstained with Hoechst 33258 dye (blue). The magnification was ×40 oil for all figures. Typical images from five different experiments with similar results are presented. In all cases, scale bar = 100 μm.
ENaC Function and Levels of ATII Cells Exposed to Cl2
Monolayers exposed to Cl2 were mounted in Ussing chambers at 1 hour and 24 hours after exposure for the measurements of short-circuit currents (Isc) before and after the addition of amiloride to the apical side of monolayers, as well as transepithelial resistances. No changes in total Isc at either 1 hour or 24 hours after exposure to 100 ppm Cl2 were detected (Figures 7A and 7B). However, Isc values decreased by more than 60% at both time points in monolayers exposed to 200 ppm Cl2 (Figures 7D and 7E). We note that exposure to either 100 or 200 ppm Cl2 decreased the amiloride-sensitive currents (Iamil) at both 1 hour and 24 hours after exposure (Figures 7A, 7B, 7D, and 7E). Transepithelial resistance decreased to approximately 200 Ω × cm2 (Figures 7C and 7F) after exposure to either 100 or 200 ppm Cl2, but remained higher than the resistance of an empty filter (∼ 50 Ω × cm2). The presence of significant amounts of Iamil currents across Cl2-exposed ATII cells indicates that the monolayers remained confluent.
Figure 7.
Cl2 decreases short-circuit currents (Isc) across monolayers of rat alveolar cells. Confluent monolayers of rat alveolar epithelial cells were exposed to either 100 ppm Cl2 (A and B) or 200 ppm Cl2 (C and D) with 5% CO2 at 37°C for 30 minutes, and placed in an incubator at 37°C in 95% air/5% CO2 for either 1 hour (A and D) or 24 hours (B and E). They were then mounted in Ussing chambers for measurements of Isc and transepithelial resistance (Rt), before and after the addition of amiloride (100 μM) into their apical compartments. Values for transepithelial resistances (R) are shown in C (100 ppm) and F (200 ppm). Values refer to means ± 1 SEM for numbers of monolayers: A, air, n = 6; Cl2, n = 8; B, air, n = 9; Cl2, n = 10; D, air, n = 16; Cl2, n = 19; E, n = 14 for all groups. *P < 0.05, compared with corresponding air control value (ANOVA followed by Tukey–Kramer multiple comparisons test).
Western blot analysis of total proteins from ATII cells using an anti α-ENaC antibody (Figure 8A) showed a prominent band around 100 kD, indicative of uncleaved α-ENaC. Two additional bands (at 65 and 55 kD), characteristic of proteolytically cleaved α-ENaC, were also present (29, 30). A 150-kD band was also present, in agreement with previous observations in adult rabbit ATII cells (24) and fetal murine ATII cells (31). The bands were absent when proteins were blotted with the α-ENaC antibody in the presence of the immunizing peptide (Figure 8A). Significant decreases in all α-ENaC–specific bands (150, 100, and 65 kD) were evident at 1 hour and 24 hours after the exposure of cell monolayers to Cl2 (Figures 8A–8C). Exposure to Cl2 also decreased γ-ENaC in ATII cells at 1 hour after exposure, whereas at 24 hours after exposure, concentrations of γ-ENaC were not different from control values (see Figure E1 in the online supplement for more details). Despite numerous attempts with a variety of commercially available antibodies against β-ENaC, we were unable to obtain reproducible signals in Western blots (although we were able to detect β-ENaC in epithelial cells in vivo with indirect immunofluorescence) (Figure 5).
Figure 8.
Total α-ENaC concentrations in rat ATII cells are decreased at 1 hour and 24 hours after exposure to 200 ppm Cl2. (A) Representative Western blot from three independent isolations is shown. Left: A blot for α-ENaC. Right: The same membrane was stripped and reblotted, using the same antibody against α-ENaC plus a blocking peptide specific for this antibody, according to the manufacturer's recommendations (Thermo Scientific, Rockford, IL). (B and C) For each blot, we summed the optical densities for all specific ENaC bands (150, 100, 65, and 55) for the indicated groups (B, 1 hour after exposure; C, 24 hours after exposure). Mean values ± SE are presented from three independent isolations and four independent samples. *P < 0.02, compared with the corresponding air control.
Detection of Reactive Intermediates after Cl2 Exposure with ESR
ESR is widely used to detect paramagnetic species with an unpaired electron, and is considered a reliable and “orthogonal” method for the detection of reactive species (32). In practice, reagents called “spin traps” are used to scavenge reactive oxygen species, and the relatively stable adducts formed are detected by ESR. 5-5-dimethyl-1-pyrroline-N-oxide (DMPO), as used here, is one of the most common spin traps. As shown in Figure 9A, an ESR signal rises 1 hour after Cl2 exposure and 30 minutes of incubation with DMPO. This signal features the typical ESR spectra of a hydroxyl adduct, indicating the formation of reactive oxygen species. The addition of the low molecular weight oxidant scavengers ascorbate, desferal, and N-acetyl-cysteine (AO) before (but not during) exposure to Cl2 decreased the DMPO adducts to the air level (Figure 9A). Similarly, the posttreatment of ATII cells with AO returned the DMPO adduct signal at 24 hours after Cl2 exposure to the control level (Figure 9B).
Figure 9.
Detection of reactive intermediates in ATII cells exposed to Cl2 and returned to room air. (A) Rat ATII cells were isolated and cultured as described in the online supplement. A mixture of low molecular weight oxidant scavengers (ascorbate, desferal, and N-acetyl-cysteine; 0.849 mg/ml acetadote, 1.2 mg/ml ascorbate, and 0.0849 mg/ml deferoxamine in cell culture medium), or an equivalent amount of cell culture medium, was added to apical and basolateral solutions at 16 hours and 1 hour before exposure to Cl2. Five minutes before exposure, the apical mixture was aspirated and replaced with 50 μl of artificial epithelial lining fluid (ELF). Monolayers were exposed to Cl2 (200 ppm for 30 minutes) and returned to 95% air/5% CO2 for 1–2 hours. At those times, the ELF was removed, and an equal volume of PBS with 5-5-dimethyl-1-pyrroline-N-oxide (DMPO; 50 mM) was added at the apical surfaces of ATII cells for 30–60 minutes. The apical fluid was then aspirated and injected into the Aqua-X sample cell of a Bruker Elexsys E500 instrument (Bruker BioSpin, Billerica, MA) for the measurement of DMPO-adduct electron spin resonance (ESR) spectra, using the settings of 20 mW power, 1 g modulation amplitude, 160 ms time constant, and 320 ms conversion time. Four scans were performed. Records: I, air + DMPO for 30 minutes; II, air + AO + DMPO for 30 minutes; III, 1 hour after Cl2 + DMPO for 30 minutes; IV, 1 hour after Cl2 + DMPO for 30 minutes; and V, 1 hour after Cl2 + AO + DMPO for 40 minutes. Typical results were reproduced three different times, using cells isolated from three different rats; g = ΔE/μBB0, where μB is the Bohr magneton, B0 the strength of the magnetic field (Gaus), and ΔE the energy difference as electrons align parallel and antiparallel to the magnetic field. AO indicates pretreatment with antioxidants, as already described. (B) Rat ATII cells were isolated, cultured, exposed to 200 ppm Cl2 for 30 minutes, and returned to 95% air/5% CO2 for 24 hours. Antioxidants (already described) or vehicle were added at 1, 7, and 21 hours after exposure to Cl2 (200 ppm). ESR spectra were obtained at 24 hours after exposure, using exactly the same procedures as described in Figure 8A. Records: I, air + DMPO for 30 minutes; II, air + AO + DMPO for 30 minutes; III, 24 hours after Cl2 + DMPO for 30 minutes; and IV, 24 hours after Cl2 + AO + DMPO for 30 minutes. Typical results were reproduced three different times, using cells isolated from three different rats.
Evidence for the Existence of Advanced Products of Lipid Peroxidation after Exposure to Cl2
The data shown in Figure 10 indicate the presence of significant levels of malondialdehyde (MDA)–protein adduct in ATII cells exposed to Cl2 and returned to 95% air/5% CO2 for 24 hours. Post-treatment with AO totally eliminated the formation of MDA adducts. No MDA adducts were seen at 1 hour after exposure (data not shown), because the products of these reactions accumulate over time.
Figure 10.
Detection of malondialdehyde (MDA) adducts in ATII cells exposed to Cl2 and returned to room air for 24 hours, and reactive intermediates in ATII cells exposed to Cl2 and returned to room air for 24 hours. Rat ATII cells were isolated, cultured, exposed to 200 ppm Cl2 for 30 minutes, and returned to 95% air/5% CO2 for 24 hours. Antioxidants (described in legend of Figure 8A) or vehicle were added at 1, 7, and 21 hours after exposure to Cl2 (200 ppm). At that time, ATII cell monolayers were lysed, and equal amounts of the proteins (20 μg) were loaded onto a 12.5% SDS-PAGE gel. The proteins were transferred to a polyvinylidine difluoride membrane, and MDA protein adducts were detected, using the OxiSelect Malondialdehyde Immunoblot Kit (Cell Biolabs, Inc., San Diego, CA), according to the manufacturer's instructions, as previously described (52). Gels were then stripped and reprobed with an antibody against β-actin (below). Note the considerably higher concentrations of MDA adducts at 24 hours after Cl2 exposure, compared with either the air-exposed ATII cells or ATII cells exposed to Cl2 and treated with antioxidants. Typical blots were repeated three times with similar results. MW, molecular weight.
Additional experiments indicated the presence of significant levels of MitoSOX fluorescence (an index of increased concentrations of reactive species in mitochondria) in ATII cells at 6 and 24 hours after exposure to 200 ppm Cl2 (Figures E2A–E2H in the online supplement) (33). Again, post-treatment with AO reduced most of the fluorescence. Taken as a whole, the ESR, lipid peroxidation, and MitoSOX provide convincing evidence for the existence of reactive intermediates in ATII cells, even at 24 hours after exposure.
The Cl2-Induced Down-Regulation of ATII Cell ENaC Function Is Attributable to the Activation of ERK1/2
The exposure of ATII cells to Cl2 resulted in the activation of ERK1/2, as evidenced by an increase of phospho-ERK1/2/total-ERK1/2 at 1 hour after exposure (Figure 11A). The pretreatment of ATII cells with either U0126, a specific inhibitor of mitogen-activated protein kinase kinase 1 and 2, or low-molecular-weight antioxidants (ascorbate, N-acetyl-cysteine, and deferoxamine) prevented the Cl2-induced phosphorylation of ERK1/2 (Figures 11A and 11B).
Figure 11.
Activation by Cl2 of ERK1/2 in ATII cells, 1 hour after exposure to 200 ppm Cl2 for 30-minute Western blots with phospo-ERK1/2 (upper lanes) or total ERK1/2 (lower lanes). Pretreatment with (A) U0126 (10 μM), a specific mitogen-activated protein kinase kinase 1 and 2 inhibitor, or (B) a mixture of low molecular weight oxidant scavengers (AO) prevented ERK1/2 phosphorylation. Positive control samples were treated with U0126, and negative control samples were treated with TPA (Bio-Rad, Hercules, CA). The quantification of digitized gels is depicted in the online supplement (Figure E3).
To investigate the relationship between reactive species formation, the activation of ERK1/2, and decreased ENaC function, we pretreated ATII cells with ascorbate, N-acetyl-cysteine, and deferoxamine (AO), and then exposed them to Cl2. Figures 12A and 12B show that pretreatment with AO prevented the Cl2-induced decrease in both basal and amiloride-sensitive currents and transepithelial resistance. In a second set of experiments, we added AO in the apical compartments of monolayers at 1, 7, and 21 hours after exposure to Cl2, and mounted the monolayers in Ussing chambers at 24 hours after exposure. Figures 12C and 12D show that posttreatment with antioxidants also prevented the decrease in amiloride-sensitive currents and transepithelial resistances. Finally, pretreatment with antioxidants restored concentrations of αENaC protein after Cl2 exposure to near control levels (Figure 12E).
Figure 12.
Antioxidants prevent and reverse the Cl2-induced decrease of epithelial sodium channel (ENaC) function. (A and B) A mixture of low molecular weight scavengers (0.849 mg/ml acetadote, 1.2 mg/ml ascorbate, and 0.0849 mg/ml deferoxamine in cell culture medium), or an equivalent amount of cell culture medium, were added into apical solutions (either 50 μl or 170 μl) and basolateral solutions (either 1 ml or 2 ml) at 16 hours and 1 hour before exposure to Cl2. Five minutes before exposure, the apical mixture was aspirated and replaced with 50 μl of normal Ringers solution containing 1 mM ascorbic acid (AA) and 0.12 mM reduced glutathione (GSH). Monolayers were exposed to Cl2 (200 ppm for 30 minutes), returned to room air, and mounted in Ussing chambers 1 hour after exposure for measurements of Isc (before and after the addition of amiloride) and Rt. Bars: black, air; white, Cl2; gray, air + antioxidants. diagonal lines, Cl2 + antioxidants. Values represent means ± 1 SEM. Numbers of measurements: air (Veh. or AO), n = 9; Cl2 + Veh., n = 11; Cl2 + AO, n = 13. *P < 0.001, compared with the air value in the group. +P < 0.05, compared with Cl2 + AO in the same group. (C and D) Antioxidants were added at 1, 7, and 21 hours after exposure to Cl2 (200 ppm). Cells were mounted in the Ussing chambers at 24 hours after exposure. Values represent means ± 1 SEM. Numbers of measurements: air (Veh. or AO), n = 25; Cl2 + Veh., n = 14; Cl2 + AO, n = 14. *P < 0.001, compared with the air value in the same group. +P < 0.05, compared with the Cl2 + AO in the same group. (E) Left: Western blot analysis shows the expression of α-ENaC in ATII cells pretreated with antioxidants at 1 hour after Cl2 exposure. Right: Same Western blot in the presence of the immunizing peptide. These blots were repeated twice. amil., amiloride.
Activation of ERK1/2 Contributes to Cl2-Induced Decrease of ENaC Activity In Vivo
To investigate the role of ERK1/2 in the acute down-regulation of ENaC activity in ATII cells by Cl2, we incubated lung slices from Cl2-exposed mice with 10 μM U0126 (an ERK1/2 inhibitor) for 3 hours and then patched ATII cells, as already described. As shown in Figures 13A and 13B, the inhibition of ERK1/2 partly restored the activities and Po of both 4 and 18 pS channels to near control values. Previous studies showed that the activation of ERK1/2 inhibited ENaC activity by phosphorylating residues in the C-termini of the β and γ subunits, enhancing the docking of the ubiquitin ligase (Nedd4-2) with these subunits (14, 15). Other studies showed that ubiquitinated ENaC is internalized and degraded by the proteasome and lysosome systems (34). We were unable to immunoprecipitate sufficient concentrations of ENaC from ATII cells to check for posttranslational modifications. However, the incubation of lung slices from Cl2-exposed mice with proteasome and lysosome inhibitors increased the open probabilities of both 4 and 18 pS channels (Figures 13C and 13D). These findings show that at least some ENaC inhibition is attributable to internalization and destruction by the proteasome and lysosome systems.
Figure 13.
Recovery of ENaC activity in Cl2-exposed lung by inhibition of ERK1/2 and of the proteasome/lysosome systems. (A and B) Open probabilities (P0) of 4 and 18 pS channels of lung slices from Cl2-exposed mice, incubated with either vehicle or 10 μM U0126 (an ERK inhibitor). Values represent the means ± SE, n = number of patches from five mice. Overall significance for one-way ANOVA, P < 0.0001. *P < 0.05, compared with the corresponding air vehicle value. #P < 0.05, compared with the corresponding air U0126 value. (C and D) Open probabilities (Po) of 4 and 18 pS channels of lung slices from Cl2-exposed mice, incubated with either vehicle or a combination of MG-132 (a proteasome inhibitor; 4 μM) and chloroquine (Chlorq.; an inhibitor of the lysosome system; 4 μM). Values represent the means ± SE; n ≥ 14 patches from five mice. Overall significance for one-way ANOVA, P < 0.0001. *P < 0.05, compared with the corresponding air vehicle value. #P < 0.05, compared with the corresponding air MG-132 + chloroquine value.
Discussion
ENaC is a heterotrimeric ion channel formed by the assembly of three homologous subunits (α, β, and γ). The channel is usually expressed at the apical membranes of epithelial cells, where it plays a major role in Na+ absorption and homeostasis. Our results show that alveolar epithelial cells in situ express two amiloride-sensitive Na+ channels at their apical membranes. One is a highly selective channel with a conductance of 4–5 pS, and the other is less selective, with a conductance of approximately 18 pS. Both channels are activated by cyclic adenosine monophosphate and inhibited by amiloride. These findings are consistent with previous reports on the presence of various types of amiloride-sensitive Na+ channels in alveolar epithelial cells both ex vivo and in vitro (35–39). Highly selective channels are composed of α-ENaC, β-ENaC, and γ-ENaC subunits, whereas nonselective channels consist mainly of α-ENaC (37). The results presented here indicate that the exposure of rat ATII cells in vitro to Cl2 decreases the activity of ATI and ATII cell Na+ channels, and compromises vectorial Na+ transport across ATII cells by increasing steady-state levels of reactive intermediates, which in turn activates ERK1/2, an inhibitor of vectorial sodium transport, via a reduction of the number of channels at the membrane. A similar decrease of ENaC activity was also evident in both ATI and ATII cells in lung slices of mice exposed to Cl2 and returned to room air at 1 hour after exposure. In addition, at 24 hours after exposure, a nonselective 25 pS Na+ channel appeared, which was much less sensitive to amiloride than the 4 and 18 pS channels. This corresponded with the observed decrease of β-ENaC, which most likely altered the stoichiometry of ENaC channels, altering their biophysical properties (28). These findings are in agreement with a previous report showing a significant decrease of amiloride-sensitive alveolar fluid clearance (AFC) in the lungs of mice exposed to 400 ppm Cl2 and returned to room air for 1 hour (13). However, AFC returned to air control values at 24 hours. Apparently, the 25 pS nonselective channel compensated for the noted decrease in activity of the 4 and 18 pS channels, and helped restored normal vectorial Na+ transport and AFC values.
The exposure of animals and ATII cells to Cl2 will result in the formation of reactive intermediates via a number of different pathways. Both Cl2 and HOCl (its main hydration product, likely to be present in much larger amounts than Cl2 in the ELF) will react with functional groups of proteins and amino acids, predominantly sulfhydryl groups (40, 41), free amine groups of plasma amino acids (yielding chlorinated amines) (42), and aromatic amino acids (yielding chlorotyrosine) (43–45). Chloramines are a relatively longer-lasting species, and are likely to be present for some time after exposure, and capable of initiating radical–radical reactions. We previously detected various organic chloramines at 6 hours after the addition of HOCl in amine-containing media (13). The addition of N-acetyl-cysteine in the medium decreased concentrations of organic chloramine to background levels (13).
A variety of methods (including chemiluminescence, redox-sensitive dyes, antibodies against immunogenic stable adducts, colorimetry, fluorimetry, and chemiluminescence) were developed for the identification and detection of reactive intermediates, and each has distinct advantages and disadvantages (46). We therefore used three different methods to verify the presence of reactive intermediates in ATII cells after exposure to Cl2. Redox-sensitive dyes (which become fluorescent when oxidized) such as MitoSox Red allow for the detection of reactive intermediates at the single-cell level. In addition, MitoSox Red concentrates at the mitochondria. However, the specificity of redox-sensitive dyes and their ability to distinguish among various species (such as superoxide, hydroxyl radicals, and peroxynitrite) are limited. Lipid peroxides decompose to form MDA products, which bind to proteins forming stable adducts, the presence of which is widely accepted as an index of oxidative stress (46). However, MDA levels are measures of the accumulation of the products, and do not provide a real-time measurement. In addition, the concentration will also depend on the rate of its clearance. There is general agreement that ESR spectroscopy, using spin traps, is the most reliable and informative method to detect reactive intermediates, and has the potential to distinguish various species (32). By adding DMPO (a classic spin trap that is rapidly accessible to the cell interior and will trap intracellular species), we documented the presence of hydroxyl adducts at 1 hour and 24 hours after exposure to Cl2. However, the superoxide adduct can convert to the hydroxyl adduct relatively rapidly (t1/2 = 1 minute), and thus the specific reactive intermediates cannot be determined. More specific information about the exact nature of reactive intermediates can be obtained via redox-sensitive proteins such as HyPER, the oxidation of which can be attributed to hydrogen peroxide (46, 47). However, using these probes with cells in primary culture is difficult because of their low transfection efficiency. The incubation of ATII cells with antioxidants either before or after exposure decreased oxidant concentrations to air control values. Taken in aggregate, we believe we have provided convincing evidence for the existence of reactive intermediates in ATII cells after exposure to Cl2. We previously showed the presence of F2a-isoprostanes, a group of prostaglandin F2-like compounds derived from the nonenzymatic oxidation of arachidonic acid by HOCl or chloramines, in the lung tissue of rats exposed to 400 ppm Cl2 for 30 minutes and returned to room air for 6 hours (48).
It is important to stress that during exposure to Cl2, ATII cells were covered on the apical surface by a thin layer of normal Ringers solution containing the most prominent low molecular weight scavengers found in the epithelial lining fluid (ascorbate, reduced glutathione, and urate). We believe this system closely mimics the in vivo exposure conditions where Cl2 first reacts with the components of ELF (11, 12).
We then provided cause-and-effect relationships among increased concentrations of reactive species, the activation of ERK1/2, decreased levels of ENaC function (as assessed by measurements of amiloride-sensitive currents), and levels of α-ENaC and γ-ENaC. Antioxidants prevented the presence and also decreased concentrations of reactive species in ATII cells, prevented the activation of ERK1/2 by Cl2, and partly reversed the decrease of amiloride-sensitive currents and α-ENaC concentrations. Similar results were obtained when we prevented and reversed the activation of ERK1/2 both in vitro and ex vivo (in lung slices) with ERK1/2 inhibitors. Previous studies showed that ERK1/2 inhibits ENaC activity by phosphorylating residues in the C-termini of the β and γ subunits, and by enhancing the docking of the ubiquitin ligase Nedd4-2 (14, 15). Ubiquitinated ENaC is then internalized and degraded by the proteasome or lysosome systems (34). We were unable to immunoprecipitate sufficient levels of ENaC from ATII cells (mainly because of the low levels of ENaC in these cells) to assess the presence of posttranslational modifications after exposure to Cl2. However, we note that inhibitors of the proteasome and lysosome systems reversed to some extent the decrease of ENaC activity in lung slices.
In previous studies, we demonstrated that HOCl and chloramines interact and modify residues of a 51-amino acid segment of γ-ENaC, causing channels to remain locked in the closed state (13). Trypsin partly restored ENaC activity in Xenopus oocytes exposed to HOCl, most likely because of the activation of silent channels already present at the plasma membrane of oocytes. This observation led us to conclude that Cl2 and its byproducts mainly affected the cleaved and active ENaC channels, but not the noncleaved channels. The data presented here also show that trypsin increased the open probability of the 4 pS channels.
It is reasonable to ask whether the levels of exposure used in this study (up to 400 ppm Cl2 for 30 minutes) mimic the concentrations likely to be encountered in the vicinity of industrial accidents or acts of terrorism involving the use of Cl2. Weill and colleagues (49) reported concentrations of Cl2 at 400 ppm within 75 yards of an accident involving a spill of Cl2 from rail cars. Ten of the exposed individuals were hospitalized with pulmonary edema. Exposure to Cl2 released into the atmosphere during transportation and industrial accidents, as well as during acts of terrorism, has resulted in the development of acute lung injury requiring treatment with mechanical ventilation and supplemental oxygen (50, 51). Thus, although measurements of Cl2 concentrations in the vicinity of chemical accidents are not practical or feasible, we conclude that the levels of exposures chosen for our experiments mimic those likely to be encountered during industrial accidents and acts of terrorism.
In conclusion, we present evidence that the reactive species formed during and after exposure to Cl2 activate ERK1/2 in ATII cells in vitro and in vivo, leading to decreased ENaC concentrations and activity. The postexposure administration of low molecular weight scavengers of reactive species and the inhibitors of ERK1/2 prevent these changes. These findings offer significant insights into the mechanisms of lung injury and fluid accumulation after exposure to Cl2, and form the rational basis for the development of new strategies for mitigations of such injury. Indeed, we recently reported that the postexposure administration of low molecular weight oxidant scavengers (ascorbate and desferal) in mice exposed to lethal concentrations of Cl2 increased their survival and decreased concentrations of lung MDA adducts (48).
Supplementary Material
Acknowledgments
The authors acknowledge numerous informative discussions on Cl2-induced lung injury with Drs. Rakesh P. Patel, Edward M. Postlethwait, and Giuseppe L. Squadrito. The authors also thank Dr. James Collawn, Dr. Judy Creighton, Dr. Karen E. Iles, and Dr. Weifeng Song for reading the manuscript and providing many helpful comments and suggestions. Finally, the authors acknowledge the editorial assistance of Ms. Gloria Y. Son.
Footnotes
This work was supported by National Heart, Lung, and Blood Institute grants 5R01HL031197–25 (S.M.), HL105473 (G.L.), and HL097218 (G.L.), National Institute of Environmental Health Sciences grants 5U01ES015676–05 and 5U54ES017218–03 (S.M.), and National Cancer Institute grant 5R01CA131653 (JR.L.).
Author Contributions: A.L. was responsible for the study design, data acquisition and analysis, and interpretation of information, and contributed to the writing of the manuscript. L.C. was responsible for data acquisition and analysis. A.J. was responsible for data acquisition and analysis and the interpretation of information, and contributed to the writing of the manuscript. S.F.D. was responsible for data acquisition and analysis. G.L. was responsible for the study design. Q.L. was responsible for data acquisition and analysis. J.R.L. was responsible for the study design and data interpretation. S.M. was responsible for the study design, data acquisition and analysis, interpretation of the information, the writing of the manuscript, and quality control.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-0309OC on October 13, 2011
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev 2002;82:569–600 [DOI] [PubMed] [Google Scholar]
- 2.Eaton DC, Helms MN, Koval M, Bao HF, Jain L. The contribution of epithelial sodium channels to alveolar function in health and disease. Annu Rev Physiol 2009;71:403–423 [DOI] [PubMed] [Google Scholar]
- 3.Burch LH, Talbot CR, Knowles MR, Canessa CM, Rossier BC, Boucher RC. Relative expression of the human epithelial Na+ channel subunits in normal and cystic fibrosis airways. Am J Physiol 1995;269:C511–C518 [DOI] [PubMed] [Google Scholar]
- 4.Matalon S, O'Brodovich H. Sodium channels in alveolar epithelial cells: molecular characterization, biophysical properties, and physiological significance. Annu Rev Physiol 1999;61:627–661 [DOI] [PubMed] [Google Scholar]
- 5.Hickman-Davis JM, Nicholas-Bevensee C, Davis IC, Ma HP, Davis GC, Bosworth CA, Matalon S. Reactive species mediate inhibition of alveolar Type II sodium transport during mycoplasma infection. Am J Respir Crit Care Med 2006;173:334–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dada LA, Chandel NS, Ridge KM, Pedemonte C, Bertorello AM, Sznajder JI. Hypoxia-induced endocytosis of Na, K-ATPase in alveolar epithelial cells is mediated by mitochondrial reactive oxygen species and PKC-zeta. J Clin Invest 2003;111:1057–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vadasz I, Dada LA, Briva A, Trejo HE, Welch LC, Chen J, Toth PT, Lecuona E, Witters LA, Schumacker PT, et al. AMP-activated protein kinase regulates CO2-induced alveolar epithelial dysfunction in rats and human cells by promoting Na, K-ATPase endocytosis. J Clin Invest 2008;118:752–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crouch EC, Hirche TO, Shao B, Boxio R, Wartelle J, Benabid R, McDonald B, Heinecke J, Matalon S, Belaaouaj A. Myeloperoxidase-dependent inactivation of surfactant protein D in vitro and in vivo. J Biol Chem 2010;285:16757–16770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med 2010;17:293–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ward PA. Phagocytes and the lung. Ann NY Acad Sci 1997;832:304–310 [DOI] [PubMed] [Google Scholar]
- 11.Yadav AK, Bracher A, Doran SF, Leustik M, Squadrito GL, Postlethwait EM, Matalon S. Mechanisms and modification of chlorine-induced lung injury in animals. Proc Am Thorac Soc 2010;7:278–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Squadrito GL, Postlethwait EM, Matalon S. Elucidating mechanisms of chlorine toxicity: reaction kinetics, thermodynamics, and physiological implications. Am J Physiol Lung Cell Mol Physiol 2010;299:L289–L300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song W, Wei S, Zhou Y, Lazrak A, Liu G, Londino JD, Squadrito GL, Matalon S. Inhibition of lung fluid clearance and epithelial Na+ channels by chlorine, hypochlorous acid, and chloramines. J Biol Chem 2010;285:9716–9728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimkets RA, Lifton R, Canessa CM. In vivo phosphorylation of the epithelial sodium channel. Proc Natl Acad Sci USA 1998;95:3301–3305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Staub O, Abriel H, Plant P, Ishikawa T, Kanelis V, Saleki R, Horisberger JD, Schild L, Rotin D. Regulation of the epithelial Na+ channel by Nedd4 and ubiquitination. Kidney Int 2000;57:809–815 [DOI] [PubMed] [Google Scholar]
- 16.Khan NM, Sandur SK, Checker R, Sharma D, Poduval TB, Sainis KB. Pro-oxidants ameliorate radiation-induced apoptosis through activation of the calcium–ERK1/2-Nrf2 pathway. Free Radic Biol Med 2011;51:115–128 [DOI] [PubMed] [Google Scholar]
- 17.Carreras MC, Poderoso JJ. Mitochondrial nitric oxide in the signaling of cell integrated responses. Am J Physiol Cell Physiol 2007;292:C1569–C1580 [DOI] [PubMed] [Google Scholar]
- 18.Lazrak A, Jurkuvenaite A, Chen L, Keeling KM, Collawn JF, Bedwell DM, Matalon S. Enhancement of alveolar epithelial sodium channel activity with decreased cystic fibrosis transmembrane conductance regulator expression in mouse lung. Am J Physiol Lung Cell Mol Physiol 2011; 301:L557–L567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lazrak A, Nita I, Subramaniyam D, Wei S, Song W, Ji HL, Janciauskiene S, Matalon S. Alpha(1)-antitrypsin inhibits epithelial Na+ transport in vitro and in vivo. Am J Respir Cell Mol Biol 2009;41:261–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lazrak A, Iles KE, Liu G, Noah DL, Noah JW, Matalon S. Influenza virus M2 protein inhibits epithelial sodium channels by increasing reactive oxygen species. FASEB J 2009;23:3829–3842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leustik M, Doran S, Bracher A, Williams S, Squadrito GL, Schoeb TR, Postlethwait E, Matalon S. Mitigation of chlorine-induced lung injury by low-molecular-weight antioxidants. Am J Physiol Lung Cell Mol Physiol 2008;295:L733–L743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen L, Song W, Davis IC, Shrestha K, Schwiebert E, Sullender WM, Matalon S. Inhibition of Na+ transport in lung epithelial cells by respiratory syncytial virus infection. Am J Respir Cell Mol Biol 2009;40:588–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen L, Bosworth CA, Pico T, Collawn JF, Varga K, Gao Z, Clancy JP, Fortenberry JA, Lancaster JR, Jr, Matalon S. DETANO and nitrated lipids increase chloride secretion across lung airway cells. Am J Respir Cell Mol Biol 2008;39:150–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matalon S, Kirk KL, Bubien JK, Oh Y, Hu P, Yue G, Shoemaker R, Cragoe EJ, Jr, Benos DJ. Immunocytochemical and functional characterization of Na+ conductance in adult alveolar pneumocytes. Am J Physiol 1992;262:C1228–C1238 [DOI] [PubMed] [Google Scholar]
- 25.Kleyman TR, Carattino MD, Hughey RP. ENaC at the cutting edge: regulation of epithelial sodium channels by proteases. J Biol Chem 2009;284:20447–20451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kizer N, Guo XL, Hruska K. Reconstitution of stretch-activated cation channels by expression of the alpha-subunit of the epithelial sodium channel cloned from osteoblasts. Proc Natl Acad Sci USA 1997;94:1013–1018 [Published erratum appears in Proc Natl Acad Sci USA 1997;94:4233] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen L, Fuller CM, Kleyman TR, Matalon S. Mutations in the extracellular loop of alpha-rENaC alter sensitivity to amiloride and reactive species. Am J Physiol Renal Physiol 2004;286:F1202–F1208 [DOI] [PubMed] [Google Scholar]
- 28.Lazrak A, Samanta A, Venetsanou K, Barbry P, Matalon S. Modification of biophysical properties of lung epithelial Na(+) channels by dexamethasone. Am J Physiol Cell Physiol 2000;279:C762–C770 [DOI] [PubMed] [Google Scholar]
- 29.Hughey RP, Bruns JB, Kinlough CL, Kleyman TR. Distinct pools of epithelial sodium channels are expressed at the plasma membrane. J Biol Chem 2004;279:48491–48494 [DOI] [PubMed] [Google Scholar]
- 30.Hughey RP, Bruns JB, Kinlough CL, Harkleroad KL, Tong Q, Carattino MD, Johnson JP, Stockand JD, Kleyman TR. Epithelial sodium channels are activated by furin-dependent proteolysis. J Biol Chem 2004;279:18111–18114 [DOI] [PubMed] [Google Scholar]
- 31.Thome UH, Davis IC, Nguyen SV, Shelton BJ, Matalon S. Modulation of sodium transport in fetal alveolar epithelial cells by oxygen and corticosterone. Am J Physiol Lung Cell Mol Physiol 2003;284:L376–L385 [DOI] [PubMed] [Google Scholar]
- 32.Villamena FA, Zweier JL. Detection of reactive oxygen and nitrogen species by EPR spin trapping. Antioxid Redox Signal 2004;6:619–629 [DOI] [PubMed] [Google Scholar]
- 33.Robinson KM, Janes MS, Pehar M, Monette JS, Ross MF, Hagen TM, Murphy MP, Beckman JS. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc Natl Acad Sci USA 2006;103:15038–15043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malik B, Price SR, Mitch WE, Yue Q, Eaton DC. Regulation of epithelial sodium channels by the ubiquitin–proteasome proteolytic pathway. Am J Physiol Renal Physiol 2006;290:F1285–F1294 [DOI] [PubMed] [Google Scholar]
- 35.Helms MN, Jain L, Self JL, Eaton DC. Redox regulation of epithelial sodium channels examined in alveolar Type 1 and 2 cells patch-clamped in lung slice tissue. J Biol Chem 2008;283:22875–22883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson MD, Bao HF, Helms MN, Chen XJ, Tigue Z, Jain L, Dobbs LG, Eaton DC. Functional ion channels in pulmonary alveolar Type I cells support a role for Type I cells in lung ion transport. Proc Natl Acad Sci USA 2006;103:4964–4969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jain L, Chen XJ, Ramosevac S, Brown LA, Eaton DC. Expression of highly selective sodium channels in alveolar Type II cells is determined by culture conditions. Am J Physiol Lung Cell Mol Physiol 2001;280:L646–L658 [DOI] [PubMed] [Google Scholar]
- 38.Yue G, Russell WJ, Benos DJ, Jackson RM, Olman MA, Matalon S. Increased expression and activity of sodium channels in alveolar Type II cells of hyperoxic rats. Proc Natl Acad Sci USA 1995;92:8418–8422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shlyonsky V, Goolaerts A, Mies F, Naeije R. Electrophysiological characterization of rat Type II pneumocytes in situ. Am J Respir Cell Mol Biol 2008;39:36–44 [DOI] [PubMed] [Google Scholar]
- 40.den Hartog GJ, Haenen GR, Vegt E, van der Vijgh WJ, Bast A. Efficacy of HOCl scavenging by sulfur-containing compounds: antioxidant activity of glutathione disulfide? Biol Chem 2002;383:709–713 [DOI] [PubMed] [Google Scholar]
- 41.Hawkins CL, Pattison DI, Davies MJ. Hypochlorite-induced oxidation of amino acids, peptides and proteins. Amino Acids 2003;25:259–274 [DOI] [PubMed] [Google Scholar]
- 42.Weiss SJ, Klein R, Slivka A, Wei M. Chlorination of taurine by human neutrophils: evidence for hypochlorous acid generation. J Clin Invest 1982;70:598–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crow JP. Measurement and significance of free and protein-bound 3-nitrotyrosine, 3-chlorotyrosine, and free 3-nitro-4-hydroxyphenylacetic acid in biologic samples: a high-performance liquid chromatography method using electrochemical detection. Methods Enzymol 1999;301:151–160 [DOI] [PubMed] [Google Scholar]
- 44.Hazen SL, Hsu FF, Mueller DM, Crowley JR, Heinecke JW. Human neutrophils employ chlorine gas as an oxidant during phagocytosis. J Clin Invest 1996;98:1283–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hazen SL, Heinecke JW. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J Clin Invest 1997;99:2075–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murphy MP, Holmgren A, Larsson NG, Halliwell B, Chang CJ, Kalyanaraman B, Rhee SG, Thornalley PJ, Partridge L, Gems D, et al. Unraveling the biological roles of reactive oxygen species. Cell Metab 2011;13:361–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Belousov VV, Fradkov AF, Lukyanov KA, Staroverov DB, Shakhbazov KS, Terskikh AV, Lukyanov S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods 2006;3:281–286 [DOI] [PubMed] [Google Scholar]
- 48.Yadav AK, Doran SF, Samal AA, Sharma R, Vedagiri K, Postlethwait EM, Squadrito GL, Fanucchi MV, Roberts LJ, Patel RP, et al. Mitigation of chlorine gas lung injury in rats by postexposure administration of sodium nitrite. Am J Physiol Lung Cell Mol Physiol 2011;300:L362–L369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weill H, George R, Schwarz M, Ziskind M. Late evaluation of pulmonary function after acute exposure to chlorine gas. Am Rev Respir Dis 1969;99:374–379 [PubMed] [Google Scholar]
- 50.Cave D, Fadam A. Iraq insurgents employ chlorine in bomb attacks. New York Times 2/22/2007 [Google Scholar]
- 51.Van SD, Wenck MA, Belflower A, Drociuk D, Ferdinands J, Holguin F, Svendsen E, Bretous L, Jankelevich S, Gibson JJ, et al. Acute health effects after exposure to chlorine gas released after a train derailment. Am J Emerg Med 2009;27:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zarogiannis SG, Jurkuvenaite A, Fernandez S, Doran SF, Yadav AK, Squadrito GL, Postlethwait EM, Bowen L, Matalon S. Ascorbate and deferoxamine administration post chlorine exposure decrease mortality and lung injury in mice. Am J Respir Cell Mol Biol 2011;45:386–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.