Abstract
Experimental asthma increases eosinophil and collagen deposition in the lungs of sickle cell disease (SCD) mice to a greater extent than in control mice. However, the effects of asthma on inflammation and airway physiology remain unclear. To determine effects of asthma on pulmonary inflammation and airway mechanics in SCD mice, hematopoietic stem cell transplantation was used to generate chimeric SCD and hemoglobin A mice. Experimental asthma was induced by sensitizing mice with ovalbumin (OVA). Airway mechanics were assessed using forced oscillation techniques. Mouse lungs were examined histologically and physiologically. Cytokine, chemokine, and growth factors in bronchoalveolar lavage fluid were determined by multiplex. IgE was quantified by ELISA. LDH was quantified using a colorimetric enzymatic assay. At baseline (nonsensitized), chimeric SCD mice developed hemolytic anemia with sickled red blood cells, mild leukocytosis, and increased vascular endothelial growth factor and IL-13 compared with chimeric hemoglobin A mice. Experimental asthma increased perialveolar eosinophils, plasma IgE, and bronchoalveolar lavage fluid IL-1β, IL-4, IL-6, and monocyte chemotactic protein 1 in chimeric hemoglobin A and SCD mice. IFN-γ levels were reduced in both groups. IL-5 was preferentially increased in chimeric SCD mice but not in hemoglobin A mice. Positive end-expiratory pressures and methacholine studies revealed that chimeric SCD mice had greater resistance in large and small airways compared with hemoglobin A mice at baseline and after OVA sensitization. SCD alone induces a baseline lung pathology that increases large and small airway resistance and primes the lungs to increased inflammation and airway hyperresponsiveness after OVA sensitization.
Keywords: sickle cell disease, OVA sensitization, IgE, IL-5, airway hyperresponsiveness
Sickle cell disease (SCD) is the most common inherited blood disorder in the United States. This hemoglobinopathy manifests as a debilitating disease characterized by chronic and episodic pain, hemolytic anemia, severe infections, stroke, and pulmonary complications beginning in early childhood and lasting throughout life (1). Airway hyperresponsiveness, but not asthma, is more common in children with SCD than in control subjects (2). Accordingly, asthma is a common comorbid complication of SCD, affecting children with SCD at a similarly high rate (3–5).
As an inflammatory comorbid condition, asthma likely contributes to sickle hemoglobin–induced vascular and organ pathologies. In agreement, Boyd and colleagues reported that a diagnosis of asthma among children with SCD significantly increased the incidence of pain and acute chest syndrome when compared with children with SCD without asthma (3, 4) and that asthma is an independent risk factor for death in individuals with SCD (6). Although these clinical reports suggest that asthma contributes significantly to morbidity and premature death in children with SCD, the pathophysiological mechanisms driving airway disease in individuals with asthma with SCD remain unclear. The present studies were designed to determine how experimental asthma increases pulmonary inflammation and impairs airway physiology.
It is unclear why patients with SCD with asthma have an increased rate of vasoocclusive events. The most plausible explanation is the convergence of two independent pathophysiological processes—inflammation of the airways (asthma) and inflammation of the endothelium (SCD)—resulting in an overall greater airway inflammation than either disease alone and in a lower threshold for airway reactivity. In humans, asthma increases airway disease, which decreases lung function and oxygen exchange (7). Previous studies by our laboratories found increased oxidative stress and inflammation in the lungs of native SCD mice and that exposure to hypoxia to induce acute sickling further increased oxidative injury and vascular congestion in SCD mice compared with control C57BL/6J and heterozygote mice (8). These data are consistent with observations in humans showing that acute chest syndrome and death are increased more in individuals with SCD and asthma than in children with SCD alone (3, 4, 6).
Based on our previous work demonstrating that native SCD mice had a lower threshold for OVA sensitization and the clinical evidence that children with SCD have increased rates of airway hyperresponsiveness, we tested two hypotheses: (1) that the chimeric SCD (chi-SCD) mice would have greater airway inflammation and obstruction at baseline and (2) that OVA sensitization increases airway inflammation and hyperresponsiveness in chi-SCD mice.
Materials and Methods
Animals
All protocols and procedures were approved by the local Institutional Animal Care and Use Committee, conformed to the Guiding Principles for Research Involving Animals and Human Beings (9), and were in accordance with NIH Guide for the Care and Use of Laboratory Animals (10).
To minimize complications of mixed genetic background on the asthma phenotype, we generated chi-SCD mice by transplanting hematopoietic stem cells (HSCs) from Berkeley sickle mice that exclusively express human sickle hemoglobin (11, 12) into lethally irradiated (1,000 rads) C57BL/6J mice. In this way, the non–marrow-derived lung tissues would be on a genetically stable, inbred C57BL/6J background. We used HSCs (4 × 106 cells/mouse) derived from 14- to 16-day-old fetal livers to reduce the complications of graft versus host disease resulting from transplanting mixed genetic backgrounds (13–15). To control for effects of transplanting Berkeley strain HSCs into C57BL/6J mice, we generated control chi-HbA mice by transplanting fetal liver cells from healthy control HbA mice that exclusively express normal human hemoglobin A on the same Berkeley genetic background into irradiated C57BL/6J mice. Engraftment was confirmed 2 months after hematopoietic stem cell transplantation (HSCT) to allow sufficient time for the development of SCD hematologic, organ, and vascular pathologies (12).
Ova Sensitization
In the present study, OVA-sensitized mice (OVA-Sen) were exposed to low-dose OVA because we observed previously that the OVA-Sen native SCD mice were more susceptible to death when exposed to 30 minutes of aerosolized OVA (16). Upon completion of the study, blood, and bronchioalveolar lavage fluid (BALF) were collected or lungs were isolated and fixed for histopathologic study.
Histopathology, Blood, and BALF Studies
Eosinophil infiltration and basement membrane and vessel wall thickness were measured as we have previously described (16). Complete blood cell counts were performed on a Heska veterinary blood count analyzer per the manufacturer's guidelines for murine blood. Plasma lactate dehydrogenase (LDH) and IgE levels as well as BALF cytokine, chemokine, and vascular endothelial growth factor (VEGF) levels were measured as described in the online supplement.
Measurement of Pulmonary Airway and Tissue Mechanics
Airway and lung mechanics were assessed on anesthetized and intubated mice using the forced oscillation technique with Prime-2 perturbations (a 2-s pause in ventilation) with a computer-controlled small animal ventilator (flexiVent; SCIREQ, Tempe, AZ) as described in the online supplemental. At each level of increasing PEEP, the values of Newtonian resistance (RN), G, and H were averaged for each mouse. RN is a measure of flow resistance of the larger conducting pulmonary airways, G reflects viscous dissipation of energy in the respiratory tissues (tissue resistance), and H reflects elastic energy storage in the tissues (tissue stiffness). Pulmonary reactivity was assessed by administering increasing concentrations of methacholine (maximum 25 mg/ml) to induce bronchoconstriction (17, 18). These concentrations are lower than standard protocols (19) to minimize death in the fragile chi-SCD mice. Each mouse's maximal response (RN, G, and H) was plotted with respect to the dose of methacholine at a PEEP level of 3 cm H2O.
Details of the above methods and statistical analyses are provided in the online supplement.
Results
Effects of HSCT on Hematological Indices and LDH
Chi-HbA Berkeley strain transplant control mice had essentially normal blood cell counts, similar to the native HbA mice (Table 1). Chi-SCD mice developed hemolytic anemia, sickle red blood cells (not shown), mild leukocytosis, and elevated lactate dehydrogenase (LDH), similar to native SCD mice. After OVA sensitization, neither the chi-HbA nor the chi-SCD mice experienced significant changes in hemoglobin, hematocrit, reticulocytes, WBC counts, or LDH compared with the levels before OVA sensitization (Table 1).
TABLE 1.
BLOOD CELL COUNTS AND PLASMA LDH
| Chi-HbA Non-Sen | Chi-SCD Non-Sen | Chi-HbA OVA-Sen | Chi-SCD OVA-Sen | |
| Hb, g/dl | 11.8 ± 0.3 | 6.9 ± 0.3* | 12.8 ± 0.5 | 6.9 ± 0.3* |
| Hematocrit, % | 38.6 ± 1.2 | 22.6 ± 0.9* | 41.0 ± 1.7 | 21.2 ± 1.0* |
| Reticulocytes, % | 5.7 ± 0.5 | 53 ± 3* | 3.0 ± 0.6 | 45 ± 6* |
| WBC, K/μl | 7.4 ± 2.7 | 21.1 ± 2.2* | 3.9 ± 0.8 | 14.9 ± 1.1* |
| LDH, IU/l | 82 ± 7 | 140 ± 9* | 75 ± 9 | 147 ± 9* |
Definition of abbreviations: Hb = hemoglobin; LDH = lactate dehydrogenase; Non-Sen = nonsensitized; OVA-Sen = ovalbumin sensitized; WBC = white blood cells.
P < 0.001 compared with the chi-HbA Non-Sen or chi-HbA OVA-Sen groups. OVA sensitization had no effect on Hb, hematocrit, reticulocytes, WBC, or LDH values when comparing chi-SCD Non-Sen with OVA-Sen chi-SCD-Sen or chi-HbA Non-Sen with OVA-Sen chi-HbA-Sen (n = 7–12).
Histopathology
Chi-HbA mice had essentially normal organ histology. In contrast, chi-SCD mice had multiorgan pathology, including prominent sites of vascular congestion and tissue ischemia with infarcts in the kidneys, lungs, liver, and spleen, similar to the native Berkeley SCD mice (full pathology not shown).
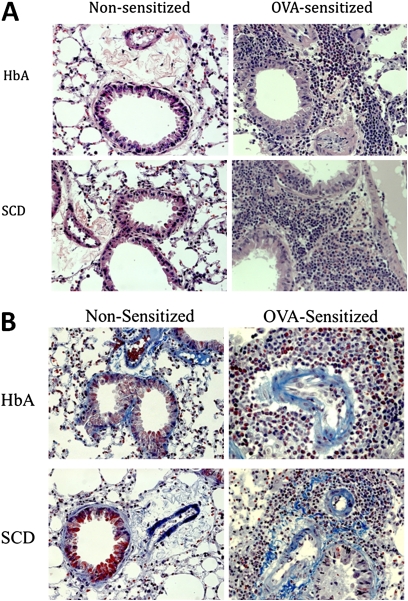
Representative H&E and trichrome stains of the lungs from chimeric mice can be seen in Figures 1A and 1B, respectively. H&E stains revealed essentially normal architecture and inflammatory cell counts in the control nonsensitized (Non-Sen) chi-HbA mice and increased inflammatory cell counts in the Non-Sen chi-SCD mice (Figure 1A, left). Trichrome stains revealed that collagen deposition in the Non-Sen chimeric mice was essentially equal (Figure 1B, left). After OVA sensitization, eosinophil infiltration (Figure 1A, right) and collagen deposition (Figure 1B, right, bright blue-green) were markedly increased in the chi-HbA and chi-SCD mice compared with the levels in unsensitized chimeric mice (Figure 1A, left; Figure 1B, left).
Figure 1.
Histology of lungs from nonsensitized (Non-Sen) and ovalbumin-sensitized (OVA-Sen) chimeric HbA and SCD mice. (A) Images showing H&E staining of eosinophil infiltration in the lungs of Non-Sen and OVA-Sen chimeric HbA and SCD mice. (B) Images showing McLetchie's trichrome staining for collagen in lungs of Non-Sen and OVA-Sen chi-HbA and chi-SCD mice.
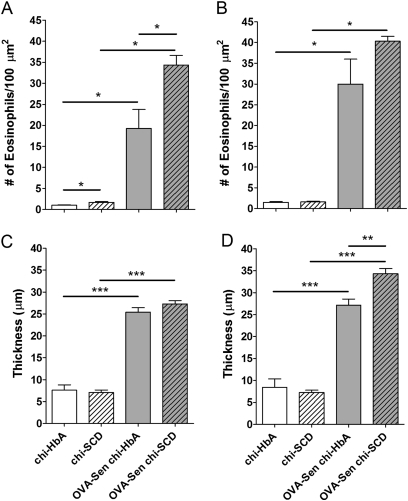
Morphometric analysis of the histology slides from the chimeric- and OVA-sensitized mice can be seen in Figure 2. The number of eosinophils was increased in the perialveolar regions in the chi-SCD mice compared with the number in Non-Sen chi-HbA mice in the absence of OVA sensitization. As expected, OVA sensitization increased eosinophils in the perialveolar and perivascular regions in sickle (OVA-Sen chi-SCD) mice and control (OVA-Sen chi-HbA) mice compared with the same strains of unsensitized mice (Figures 2A and 2B). Furthermore, after OVA sensitization, OVA-Sen chi-SCD mice had even more eosinophils in the perialveolar region than OVA-Sen chi-HbA mice (Figure 2A). Although SCD did not alter basement membrane thickness in the perialveolar and perivascular regions at baseline, OVA sensitization markedly increased collagen deposition in the OVA-Sen chi-HbA and OVA-Sen chi-SCD mice (Figures 2C and 2D). In addition, after OVA sensitization, perivascular collagen deposition was increased in OVA-Sen chi-SCD mice compared with OVA-Sen chi-HbA mice (Figure 2D).
Figure 2.
Eosinophil counts and basement membrane thickness. Morphometric quantification of eosinophils (A, B) and basement membrane thickness (C, D) in lungs from nonsensitized and OVA-Sen chimeric HbA and SCD mice in the perialveolar (A, C) and perivascular regions (B, D) (n = 6 for each). Asterisks: significantly different from the indicated group (*P < 0.05, **P < 0.01, ***P < 0.001). Open bars, chi-HbA mice; hatched bars, chi-SCD mice; shaded bars, OVA-Sen chi-HbA mice; shaded hatched bars, OVA-Sen chi-SCD mice.
Total IgE Levels
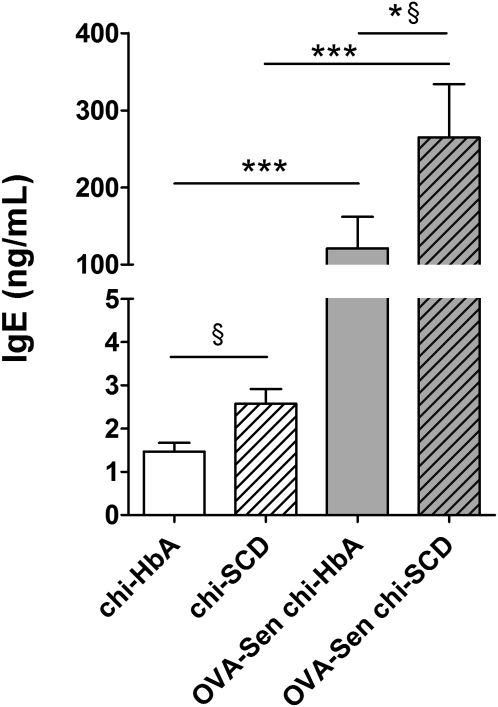
Total IgE in Non-Sen chi-SCD mice was increased compared with the levels in Non-Sen chi-HbA mice P < 0.05) (Figure 3). OVA sensitization markedly increased total IgE levels in the chimeric mice. In sensitized mice, total IgE in the OVA-Sen chi-SCD mice was further increased compared with OVA-Sen chi-HbA mice (Figure 3). These data suggest that SCD specifically contributes to total circulating IgE levels at baseline or after OVA sensitization, similar to humans with SCD (20, 21).
Figure 3.
Total IgE. Total IgE was quantified by ELISA and values compared with known IgE standards. §P < 0.05 directed comparison of Non-Sen chi-HbA mice with Non-Sen chi-SCD mice and OVA-Sen chi-SCD mice with OVA-Sen chi-HbA mice. *P < 0.05, comparing OVA-Sen chi-SCD mice versus OVA-Sen chi-HbA mice. ***P < 0.001, comparing OVA-Sen chi-HbA mice versus Non-Sen chi-HbA mice and OVA-Sen chi-SCD mice versus Non-Sen chi-SCD mice (n = 7–12). Open bars, chi-HbA mice. Hatched bars. chi-SCD mice. Shaded bars, OVA-Sen chi-HbA mice. Shaded hatched bars, OVA-Sen chi-SCD mice.
BALF Cytokines, Chemokine, and VEGF Levels
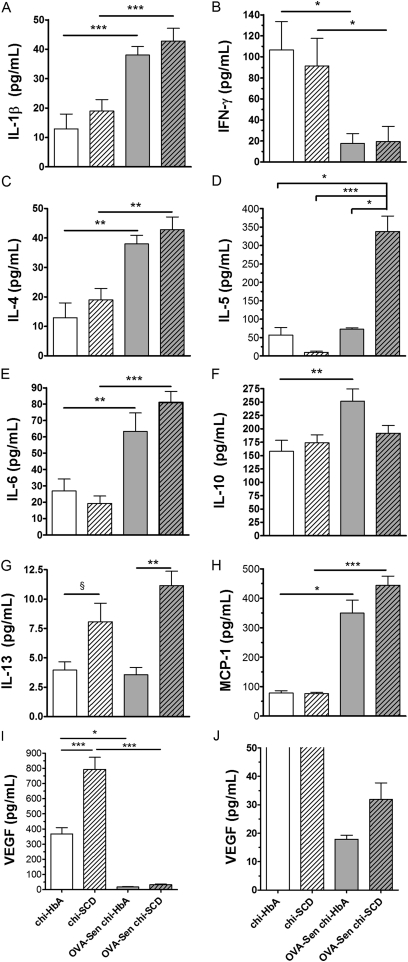
The major change in BALF cytokines, chemokines, and VEGF after SCD was induced by HSCT was a marked increase in IL-13 and VEGF production in the Non-Sen chi-SCD mice compared with chi-HbA mice (Figures 4G and 4I). After OVA sensitization, IL-1β, IL-4, IL-6, and monocyte chemotactic protein (MCP)-1 increased in the BALF of the OVA-Sen chi-HbA and OVA-Sen chi-SCD mice to essentially equal levels (Figures 4A, 4C, 4E, and 4H). In addition, BALF IL-13 was increased in OVA-Sen chi-SCD mice compared with OVA-Sen chi-HbA mice. A dramatic and preferential increase in BALF IL-5 was observed in the OVA-Sen chi-SCD mice (Figure 4D). In contrast, BALF IL-10 was increased in the OVA-Sen chi-HbA mice but was essentially unchanged in the OVA-Sen chi-SCD mice compared with the levels in Non-Sen chi-SCD mice (Figure 4F). OVA-sensitization also decreased BALF IFN-γ and VEGF in the OVA-Sen chi-HbA and OVA-Sen chi-SCD mice (Figures 4B and 4I).
Figure 4.
Bronchoalveolar lavage fluid (BALF) cytokine, chemokine, and vascular endothelial growth factor (VEGF) studies. Bio-Plex quantification of IL-1β, IFN-γ, IL-4, IL5, IL-6, IL-10, Il-13, monocyte chemotactic protein (MCP)-1, and VEGF in BALF from Non-Sen and OVA-Sen chi-HbA and chi-SCD mice (n = 8–10). **P < 0.01. ***P < 0.001. Open bars, chi-HbA mice. Hatched bars, chi-SCD mice. Shaded bars, OVA-Sen chi-HbA mice. Shaded hatched bars, OVA-Sen chi-SCD mice.
Pulmonary Airway and Tissue Mechanics
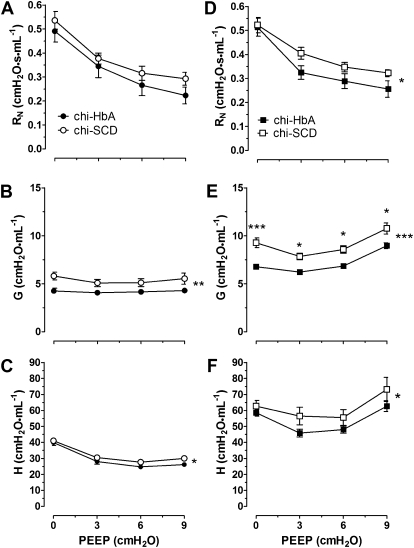
Airway resistance in response to increasing PEEP revealed that the curves for G and H, but not RN, were significantly increased in Non-Sen chi-SCD mice compared with the curves for Non-Sen chi-HbA mice. In contrast, after OVA sensitization, the curves for RN, G, and H were increased in the OVA-Sen chi-SCD mice compared with the curves for the OVA-Sen chi-HbA mice (P < 0.05, P < 0.001, and P < 0.05, respectively) (Figure 5). OVA-sensitization had little effect on RN in response to PEEP within the same strain but did significantly increase G and H compared with the baseline responses. These data suggest that the lungs of chi-SCD mice exhibit a baseline airway resistance that limits alveoli recruitment and amplifies pulmonary inflammation after OVA sensitization.
Figure 5.
Positive end-expiratory pressure (PEEP) studies. Indices of pulmonary mechanics using four levels of (0, 3, 6, and 9 cm H2O) in (A–C) Non-Sen and (D–F) OVA-Sen chi-HbA and chi-SCD mice. Closed circles, chi-HbA Non-Sen mice. Open circles, chi-SCD Non-Sen mice. Closed square, chi-HbA OVA-Sen mice. Open square, chi-SCD OVA-Sen mice. ‡Significantly different from OVA-Sen chi-HbA mice at same level of PEEP (P < 0.02). The number of asterisks to the right of the curves indicates the levels of significance between the curves (*P < 0.05, **P < 0.01, ***P < 0.001).
Hysteresis
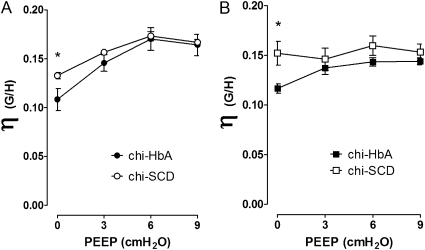
Forced oscillation techniques are often used to assess regional inhomogeneity of lungs with respect to viscoelastic properties and alveolar closing. The technique used here measures airway impedance (RN) and is connected to a constant phase viscoelastic component via measurements of G (tissue dampening) and H (tissue elastance). Dividing G by H yields η (hysteresisity), a sensitive measure of pulmonary resistance. Calculations of η show that when PEEP equals 0, hysteresis is greater in chi-SCD mice compared with chi-HbA mice (Figure 6A). As PEEP increases to 9, η increases in the chi-HbA mice to the levels observed in chi-SCD mice. After induction of experimental asthma, the PEEP η curve for OVA-Sen chi-SCD is essentially flat, whereas the curve for the OVA-Sen chi-HbA mice increases in response to increasing PEEP but is consistently lower than OVA-Sen chi-SCD mice (Figure 6B). These data are consistent with the idea that the lungs of OVA-Sen chi-SCD mice are less elastic and may experience greater alveolar closing than OVA-Sen chi-HbA mice.
Figure 6.
Changes in hysteresisity (η = G/H), a sensitive measure of pulmonary resistance, in response to increasing PEEP levels. An increase in η suggests that lung is stiffer, resulting from increased collagen deposition and/or a greater number of alveolae becoming filled with fluid. The η curves were generated by dividing G data by H data in Figure 5 and plotting the result against the four increasing levels of PEEP (0, 3, 6, and 9 cm H2O). Closed circles, chi-HbA Non-Sen mice. Open circles, chi-SCD Non-Sen mice. Closed squares, chi-HbA OVA-Sen mice. Open squares, chi-SCD OVA-Sen mice. *P < 0.05.
Methacholine Challenge
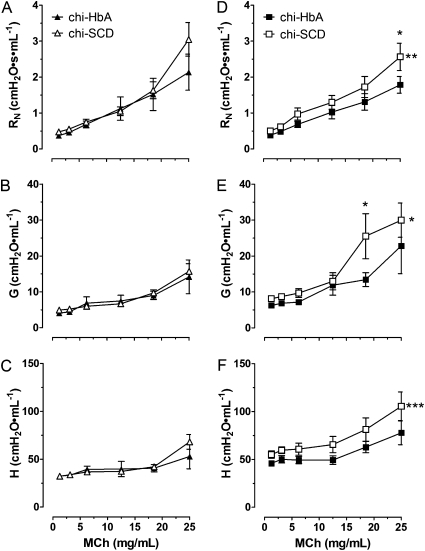
As a result of respiratory distress and death in chi-SCD mice (16), the studies were limited to low doses of methacholine. Although there was a tendency for airway resistance (RN and H, but not G) to increase in response to methacholine challenge to a greater extent in Non-Sen chi-SCD mice, two-way ANOVA revealed no significant differences in the response curves in Non-Sen chi-SCD mice compared with the curves for Non-Sen chi-HbA mice (Figures 7A, 7C, and 7B, respectively). However, sensitization increased the curves for RN, G, and H in chi-SCD mice compared with the curves for chi-HbA mice (Figures 7D, 7E, and 7F, respectively). The increase in RN was most notable at the highest methacholine dose (25 mg/ml). The greater increases in G and H in the OVA-Sen chi-SCD mice compared with OVA-Sen chi-HbA mice were also observed at the higher doses of methacholine (25 mg/ml) (Figures 7E and 7F). Sensitization did not induce significant increases in RN, G, or H in the OVA-Sen chi-HbA mice. In contrast, sensitization increased RN, G, and H in response to methacholine in OVA-Sen chi-SCD mice.
Figure 7.
Airway responses to aerosolized methacholine in nonsensitized and OVA-sensitized chimeric HbA and SCD mice. Closed triangles, chi-HbA nonsensitized mice. Open triangles, chi-SCD nonsensitized mice. Closed squares, chi-HbA OVA-sensitized mice. Open squares, chi-SCD OVA-sensitized mice. *Significantly different from nonsensitized mice at the same dosage of methacholine (P < 0.05). ‡Significantly different from chi-HbA mice at the same dosage of methacholine (P < 0.05). The number of asterisks to the right of the curves indicates the levels of significance between the curves. (*P < 0.05, **P < 0.01, ***P < 0.001).
Discussion
This study was designed to elucidate how the combination of asthma and SCD predisposes mice to exaggerated pulmonary inflammation and airway disease. OVA sensitization of chi-SCD mice causes a greater shift in the cytokine balance, increased inflammatory cell recruitment, increased collagen deposition, and increased airway hyperresponsiveness compared with Berkeley strain transplant control chi-HbA mice. For example, total IgE is increased in chi-SCD mice compared with chi-HbA mice whether or not the mice are sensitized to OVA. This is similar to reports in humans, where IgE is increased in individuals with SCD (with or without asthma) compared with the general population (20, 21). IgE production is stimulated by IL-13 (22). Accordingly, the increase in IL-13 levels in the Non-Sen chi-SCD mice at baseline may be one of the reasons that SCD mice have increased IgE and are more prone to allergic type inflammation. OVA sensitization preferentially increases IL-5 production in OVA-Sen chi-SCD mice. IL-5 plays an important role in the activation of eosinophils to increase pulmonary inflammation in response to allergens (23). Finally, the loss of antiinflammatory responses may also play a role in increasing pulmonary inflammation and airway hyperresponsiveness in SCD. For example, although IL-10, which decreases inflammation and prevents abnormal collagen deposition (24, 25), was similar in Non-Sen chi-HbA and chi-SCD mice, after OVA-sensitization, OVA-Sen chi-HbA mice were able to increase IL-10 production, but OVA-Sen chi-SCD mice were not. Failure to mount an antiinflammatory IL-10 response in the presence of robust increases in IL-5 and MCP-1 may account, at least in part, for the enhanced eosinophil recruitment and collagen deposition that occurred in the perialveolar and perivascular regions in the OVA-Sen chi-SCD mice. Similar to human SCD (15), VEGF was increased at baseline in chi-SCD compared with chi-HbA mice. However, it is not clear why VEGF, which usually increases in experimental and human asthma, decreased after OVA sensitization. That fact that VEGF decreased in chi-SCD and chi-HbA mice suggests that this may be a strain-specific response.
Airway studies provide additional support for the idea that SCD predisposes mice to greater pulmonary disease. PEEP is the pressure above ambient pressure in the airways upon expiration. PEEP is often used to improve oxygenation during mechanical ventilation (26, 27) and to assess the functional residual capacity of the lung. Thus, PEEP studies provide physiological information about the elastic properties of the lung and chest wall in health and disease (28). The PEEP studies here showed that SCD alone increased the resistance curves for G and H, but not RN, when compared with the curves for chi-HbA mice. After OVA-sensitization, the resistance curves for RN, G, and H were consistently increased in the OVA-Sen chi-SCD mice compared with the corresponding curves for OVA-Sen chi-HbA mice. In general, alveoli exist in three distinct states: (1) normal alveoli, which are always open and participating in oxygen exchange; (2) partially closed alveoli, which can be recruited with increasing PEEP; and (3) completely flooded alveoli, which can never be recovered. In this context, the increase in G and H at baseline suggests that SCD alone increases the number of alveoli that are resistant to recruitment by increasing PEEP. Therefore, experimental asthma likely induces an increase in the number of alveoli in chi-SCD mice that cannot be recruited by increasing PEEP. Accordingly, the functional residual capacity of the lung in chi-SCD mice will be diminished.
After OVA sensitization, the height and shape of the PEEP curves gives additional insight into the physiological mechanisms impairing airway mechanics. The PEEP curves for G and H do not decrease fully with increasing PEEP for the OVA-Sen chi-HbA and OVA-Sen chi-SCD mice. In contrast to the PEEP curves for Non-Sen chimeric mice, which simply decreased and/or plateaued, the PEEP curves for OVA-sensitized chimeric mice initially decreased and then increased. In some instances, G and H at higher PEEP levels increased to the same level or greater levels than at 0 PEEP. The shape of these curves suggests that experimental asthma increases the number of alveoli that are filled or resistant to recruitment with increasing PEEP. These findings seem to suggest that the lungs in chi-SCD mice are resistant to expansion during inspiration and limit recoil during expiration. In other words, chi-SCD lungs are stiffer, which is consistent with the observed increases in collagen deposition. These data support the idea that chi-SCD mice have baseline lung pathology resulting from sickle hemoglobin even in the absence of experimental asthma.
An increase in hysteresis (η) suggests that an increase in airway closure or stiffening of lung tissues has occurred. Such changes might occur in pulmonary fibrosis or when collagen deposition increases in the interstitium or plural surfaces. Because lung tissue resistance was increased in Non-Sen and OVA-Sen chi-SCD mice compared with the corresponding chi-HbA mice and, more importantly, the increase in resistance coincided with increased tissue collagen deposition in SCD, it is likely that the increase in η in the chi-SCD mice developed more because of tissue impedance than airway closure. Several studies have shown that one of the most common factors for increasing inhomogeneity of the lung is airway closure (29–33).
Airway lability can be measured in patients with asthma by methacholine challenge, which induces a mild bronchoconstriction of normal airways but marked bronchoconstriction of asthmatic airways. Methacholine response curves for RN, G, and H show little difference in the resistance between the two groups of unsensitized chimeric mice. Although there was a tendency for RN and H to increase in the Non-Sen chi-SCD mice at the highest dose of methacholine, statistical analysis using two-way ANOVA revealed no significant differences. In contrast, experimental asthma significantly increased the methacholine response curves for RN, G, and H for the OVA-Sen chi-SCD mice compared with the response curves for OVA-Sen chi-HbA mice. Thus, the airways of OVA-Sen chi-SCD mice are more prone to bronchoconstriction than the airways of OVA-Sen chi-HbA mice and provide insight into why more OVA-sensitized native SCD mice died during our previous studies (16). Indeed, SCD alone may confer a phenotype that is characterized by increased small and large airway hyperresponsiveness.
Previous studies in humans showed that LDH, a marker of hemolysis or tissue injury, was associated with airway hyperresponsiveness to methacholine challenge (34). Although LDH is increased in the chi-SCD mice compared with the chi-HbA mice, it was not increased further by OVA sensitization. In agreement with these data, no change in hemogobin or reticulocyte counts after OVA sensitization was observed in either group of mice. However, total IgE was increased in OVA-Sen chi-SCD mice compared with OVA-Sen chi-HbA mice, which correlates with the marked increase in airway resistance that develops in the chi-SCD mice after OVA sensitization. Thus, total IgE data in the chimeric mice are consistent with findings in human studies (34).
Although the findings presented here provide new insight into the mechanisms mediating pulmonary inflammation and airway lability in SCD, limitations in our study may restrict interpretation. For example, the studies here were performed on chimeric mice rather than native mice. Although this made it easier to obtain a sufficient number of mice for our studies and likely reduced variation because the pulmonary tissues were on an inbred genetic background, the chimeric SCD mice had SCD for approximately 3 months rather than 6 to 7 months, as would be the case with native SCD mice. Such shorter durations of SCD might induce a milder airway and vascular pathology.
Another difference between the present study and our previous study (16) is that in this study chimeric mice were exposed to 15 minutes of aerosolized OVA (“low dose”) rather than both a low and a standard dose of OVA. This change in protocol was based on our previous experience showing that 30 minutes of aerosolized OVA significantly increased death in the OVA-sensitized native sickle mice (16). The lung histology from the chimeric OVA-sensitized SCD and HbA mice were similar to that observed in our previous study in “low-dose” OVA-sensitized native SCD and HbA mice (16). These observations suggest that the generation of SCD and control HbA mice by lethal irradiation and HSCT did not adversely affect the phenotype of the chimeric mice. However, HSCT induced at least one difference that deserves mention. HSCT appears to increase the density of eosinophils in the lungs of OVA-sensitized chimeric mice by approximately 20 to 90% compared with the number of eosinophils in the lungs of OVA-sensitized native mice in our previous study (16). This accentuated inflammatory response was only observed in the OVA-sensitized chimeric mice but not in the nonsensitized chimeric mice. Accordingly, HSCT may predispose the lungs of the engrafted mice to exaggerated inflammatory responses to allergen-induced airway disease. We suspect that the notable increase in inflammatory cell recruitment may have contributed to the increase in frailty of the chimeric SCD mice, which limited the maximal methacholine dose that could be used and likely reduced our ability to detect differences in the response of the chi-SCD mice to methacholine. OVA-sensitized SCD mice demonstrated respiratory distress and tended to die when challenged with methacholine concentrations greater than 25 mg/ml (unpublished observations). In contrast, healthy control C57BL/6J mice can be subjected to methacholine doses as high as 100 mg/ml (19).
Methacholine challenge prompts contraction of hyperresponsive airways while having little effect on “healthy” airways. At a concentration of 25 mg/ml, values of RN and H for the OVA-sensitized chimeric SCD mice were significantly increased compared with chimeric HbA mice, yet the response curves for RN, G, and H are not significantly different. Thus, although there is a tendency for large airways, small airways, and lung parenchyma to be hyperresponsive to methacholine, this change in physiology appears to manifest itself only at the highest methacholine dose feasible for this study. The exact reasons for the minimal changes in response to methacholine are unclear. Higher doses of methacholine might have revealed greater differences in airway physiology between these two experimental groups. However, OVA-sensitized SCD mice exhibited respiratory distress and tended to die when challenged with methacholine concentrations greater than 25 mg/ml (unpublished observations), limiting the doses used for this study.
When comparing OVA-sensitized with non-sensitized chi-SCD and chi-HbA mice, methacholine challenge causes greater change in G and H than in RN after OVA sensitization. This indicates that the greatest pathophysiological change in the SCD lungs likely resides in the peripheral airways and pulmonary tissues. This demonstrates that chronic exposure to allergens increases resistance at three distinct levels of pulmonary physiology (large airways, small airways, and pulmonary tissues) in the SCD mice but not in the HbA mice, where allergen sensitization induces mild increases in G and H with little to no change in RN. Our observations in mice are similar to those by DeBaun and colleagues, who recently showed that methacholine hyperresponsiveness in humans with SCD is strongly associated with the magnitude of hemolysis rather than more traditional measures of classical asthma (35, 36). Thus, when inflammation and oxidative stress induced by asthma team up with the chronic states of inflammation and oxidative stress in SCD, this combination takes an even greater toll on lung physiology, making it harder for patients with SCD to inhale and exhale.
In conclusion, the combination of SCD and experimental asthma induces profound increases in pulmonary inflammation, shifts in TH1 and TH2 cytokine production, and airway resistance. Differences in the patterns of cytokine and chemokine production between OVA-sensitized OVA-Sen chi-SCD and OVA-Sen chi-HbA mice provide new insight into the mechanisms regulating inflammation in SCD. Furthermore, the unique pattern of inflammation that is induced when chi-SCD mice are sensitized to OVA suggests that the mechanisms mediating experimental asthma synergize with those mediating SCD, with the final result being that it is more difficult for chi-SCD mice with “asthma” to inhale and exhale. Such changes may play important roles in the mechanisms by which asthma increases morbidity and mortality in SCD.
Supplementary Material
Acknowledgments
The authors thank Thomas Foster for assistance with animal husbandry, the HSC transplant procedure, and posttransplant care; Deron W. Jones for technical support; Dorothee Weihrauch, DVM, Ph.D., for consultation concerning histology; and Meghann Sytsma for assistance with the preparation of the manuscript.
Footnotes
Supported by NIH grants HL079937 (M.R.D. and K.A.P.), HL081139 and HL102836 (K.A.P. and C.A.H.), HL090503 (C.A.H.), HL44612 (C.A.H.), and HL079937 (M.R.D.) and by grants from the Burroughs Wellcome Foundation (M.R.D.) and the Midwest Athletes Against Childhood Cancer (MACC) Fund (C.A.H.).
Author Contributions: Research design and authoring the paper: T.R.F., C.A.H., and K.A.P.; performing the experiments and assisting with experimental design, S.D.N., S.L.H., and W.H.; interpreting results and editing the paper, M.L.S., R.C.S., and M.R.D.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-0097OC on October 27, 2011
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Steinberg MH. Sickle cell anemia, the first molecular disease: overview of molecular etiology, pathophysiology, and therapeutic approaches. ScientificWorldJournal 2008;8:1295–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knight-Madden JM, Forrester TS, Lewis NA, Greenough A. Asthma in children with sickle cell disease and its association with acute chest syndrome. Thorax 2005;60:206–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyd JH, Macklin EA, Strunk RC, DeBaun MR. Asthma is associated with acute chest syndrome and pain in children with sickle cell anemia. Blood 2006;108:2923–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyd JH, Moinuddin A, Strunk RC, DeBaun MR. Asthma and acute chest in sickle-cell disease. Pediatr Pulmonol 2004;38:229–232 [DOI] [PubMed] [Google Scholar]
- 5.Sylvester KP, Patey RA, Broughton S, Rafferty GF, Rees D, Thein SL, Greenough A. Temporal relationship of asthma to acute chest syndrome in sickle cell disease. Pediatr Pulmonol 2007;42:103–106 [DOI] [PubMed] [Google Scholar]
- 6.Boyd JH, Macklin EA, Strunk RC, DeBaun MR. Asthma is associated with increased mortality in individuals with sickle cell anemia. Haematologica 2007;92:1115–1118 [DOI] [PubMed] [Google Scholar]
- 7.Dal Negro R, Allegra L. Blood gas changes during and after nonspecific airway challenge in asthmatic and normal subjects. J Appl Physiol 1989;67:2627–2630 [DOI] [PubMed] [Google Scholar]
- 8.Pritchard KA, Jr, Ou J, Ou Z, Shi Y, Franciosi JP, Signorino P, Kaul S, Ackland-Berglund C, Witte K, Holzhauer S, et al. Hypoxia-induced acute lung injury in murine models of sickle cell disease. Am J Physiol Lung Cell Mol Physiol 2004;286:L705–L714 [DOI] [PubMed] [Google Scholar]
- 9.World Medical Association, American Phisiological Society Guiding principles for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol 2002;283:R281–R283 [DOI] [PubMed] [Google Scholar]
- 10.Anonymous Guide for the care and use of laboratory animals. Washington, DC: National Academy Press; 1996 [Google Scholar]
- 11.Paszty C, Brion CM, Manci E, Witkowska HE, Stevens ME, Mohandas N, Rubin EM. Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease. Science 1997;278:876–878 [DOI] [PubMed] [Google Scholar]
- 12.Manci EA, Hillery CA, Bodian CA, Zhang ZG, Lutty GA, Coller BS. Pathology of berkeley sickle cell mice: similarities and differences with human sickle cell disease. Blood 2006;107:1651–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plotnicky H, Touraine JL. Promotion of fetal liver cell engraftment in irradiated mice by activated t lymphocytes. Bone Marrow Transplant 1993;12:307–314 [PubMed] [Google Scholar]
- 14.Gale RP. Fetal liver transplants. Bone Marrow Transplant 1992;9:118–120 [PubMed] [Google Scholar]
- 15.Solovey A, Gui L, Ramakrishnan S, Steinberg MH, Hebbel RP. Sickle cell anemia as a possible state of enhanced anti-apoptotic tone: survival effect of vascular endothelial growth factor on circulating and unanchored endothelial cells. Blood 1999;93:3824–3830 [PubMed] [Google Scholar]
- 16.Nandedkar SD, Feroah TR, Hutchins W, Weihrauch D, Konduri KS, Wang J, Strunk RC, DeBaun MR, Hillery CA, Pritchard KA. Histopathology of experimentally induced asthma in a murine model of sickle cell disease. Blood 2008;112:2529–2538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomioka S, Bates JH, Irvin CG. Airway and tissue mechanics in a murine model of asthma: alveolar capsule vs. forced oscillations. J Appl Physiol 2002;93:263–270 [DOI] [PubMed] [Google Scholar]
- 18.Finotto S, Neurath MF, Glickman JN, Qin S, Lehr HA, Green FH, Ackerman K, Haley K, Galle PR, Szabo SJ, et al. Development of spontaneous airway changes consistent with human asthma in mice lacking t-bet. Science 2002;295:336–338 [DOI] [PubMed] [Google Scholar]
- 19.Wang W, Xu H, Shi Y, Nandedkar S, Zhang H, Gao H, Feroah T, Weihrauch D, Schulte ML, Jones DW, et al. Genetic deletion of apolipoprotein a-i increases airway hyperresponsiveness, inflammation, and collagen deposition in the lung. J Lipid Res 2010;51:2560–2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.An P, Barron-Casella EA, Strunk RC, Hamilton RG, Casella JF, DeBaun MR. Elevation of IgE in children with sickle cell disease is associated with doctor diagnosis of asthma and increased morbidity. J Allergy Clin Immunol 2011;127:1440–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ross JGC, Bernaudin F, Strunk RC, Kamdem A, Arnaud C, Herve M, Delacourt C, DeBaun MR. Asthma is a distinct co-morbid condition in children with sickle cell anemia with elevated total and allergen-specific IgE levels. J Pediatr Hematol Oncol 2011;33:e205–e208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 1998;282:2261–2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ochkur SI, Jacobsen EA, Protheroe CA, Biechele TL, Pero RS, McGarry MP, Wang H, O'Neill KR, Colbert DC, Colby TV, et al. Coexpression of il-5 and eotaxin-2 in mice creates an eosinophil-dependent model of respiratory inflammation with characteristics of severe asthma. J Immunol 2007;178:7879–7889 [DOI] [PubMed] [Google Scholar]
- 24.Burchfield JS, Iwasaki M, Koyanagi M, Urbich C, Rosenthal N, Zeiher AM, Dimmeler S. Interleukin-10 from transplanted bone marrow mononuclear cells contributes to cardiac protection after myocardial infarction. Circ Res 2008;103:203–211 [DOI] [PubMed] [Google Scholar]
- 25.Peranteau WH, Zhang L, Muvarak N, Badillo AT, Radu A, Zoltick PW, Liechty KW. Il-10 overexpression decreases inflammatory mediators and promotes regenerative healing in an adult model of scar formation. J Invest Dermatol 2008;128:1852–1860 [DOI] [PubMed] [Google Scholar]
- 26.Afzal M, Tharratt RS. Mechanical ventilation in severe asthma. Clin Rev Allergy Immunol 2001;20:385–397 [DOI] [PubMed] [Google Scholar]
- 27.Schuessler TF, Gottfried SB, Bates JH. A model of the spontaneously breathing patient: applications to intrinsic peep and work of breathing. J Appl Physiol 1997;82:1694–1703 [DOI] [PubMed] [Google Scholar]
- 28.Gomes RF, Shen X, Ramchandani R, Tepper RS, Bates JH. Comparative respiratory system mechanics in rodents. J Appl Physiol 2000;89:908–916 [DOI] [PubMed] [Google Scholar]
- 29.Brown RH, Mitzner W. Understanding airway pathophysiology with computed tomograpy. J Appl Physiol 2003;95:854–862 [DOI] [PubMed] [Google Scholar]
- 30.Rincon M, Anguita J, Nakamura T, Fikrig E, Flavell RA. Interleukin (il)-6 directs the differentiation of il-4-producing cd4+ t cells. J Exp Med 1997;185:461–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown RH, Scichilone N, Mudge B, Diemer FB, Permutt S, Togias A. High-resolution computed tomographic evaluation of airway distensibility and the effects of lung inflation on airway caliber in healthy subjects and individuals with asthma. Am J Respir Crit Care Med 2001;163:994–1001 [DOI] [PubMed] [Google Scholar]
- 32.Lutchen KR, Hantos Z, Petak F, Adamicza A, Suki B. Airway inhomogeneities contribute to apparent lung tissue mechanics during constriction. J Appl Physiol 1996;80:1841–1849 [DOI] [PubMed] [Google Scholar]
- 33.Hantos Z, Daroczy B, Suki B, Nagy S, Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol 1992;72:168–178 [DOI] [PubMed] [Google Scholar]
- 34.Field JJ, Stocks J, Kirkham FJ, Rosen CL, Dietzen DJ, Semon T, Kirkby J, Bates P, Seicean S, Debaun MR, et al. Airway hyperresponsiveness in children with sickle cell anemia. Chest 2011;139:563–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strunk RC, Brown MS, Boyd JH, Bates P, Field JJ, DeBaun MR. Methacholine challenge in children with sickle cell disease: a case series. Pediatr Pulmonol 2008;43:924–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Field JJ, Strunk RC, Knight-Perry JE, Blinder MA, Townsend RR, DeBaun MR. Urinary cysteinyl leukotriene e4 significantly increases during pain in children and adults with sickle cell disease. Am J Hematol 2009;84:231–233 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.