Abstract
It is widely held that exposure to pathogens such as fungi can be an agent of comorbidity, such as exacerbation of asthma or chronic obstructive pulmonary disease. Although many studies have examined allergic responses to fungi and their effects on pulmonary function, the possible pathologic implications of the early innate responses to fungal pathogens have not been explored. We examined early responses to the atypical fungus Pneumocystis in two common strains of mice in terms of overall immunological response and related pathology, such as cell damage and airway hyperresponsiveness (AHR). We found a strong strain-specific response in BALB/c mice that included recruitment of neutrophils, NK, NKT, and CD4 T cells. This response was accompanied by elevated indicators of lung damage (bronchoalveolar lavage fluid albumin and LDH) and profound AHR. This early response was absent in C57BL/6 mice, although both strains exhibited a later response associated with the clearance of Pneumocystis. We found that this AHR could not be attributed exclusively to the presence of recruited neutrophils, NKT, NK, or CD4 cells or to the actions of IFN-γ or IL-4. However, in the absence of STAT6 signaling, AHR and inflammatory cell recruitment were virtually absent. Gene expression analysis indicated that this early response included activation of several transcription factors that could be involved in pulmonary remodeling. These results show that exposure to a fungus such as Pneumocystis can elicit pulmonary responses that may contribute to morbidity, even without prior sensitization, in the context of certain genetic backgrounds.
Keywords: STAT6, airway hyperresponsiveness, Pneumocystis, pulmonary inflammation, strain-specific
Clinical Commentary
Fungal pathogens have long been believed to exacerbate airway and pulmonary pathology, although this has only been conclusively shown in the case of ongoing allergies to specific fungi. We show here that significant airway and pulmonary pathology can result from initial exposure to the fungus Pneumocystis due only to components of the innate immune response. We also show that there is a probable genetic predisposition of this response related to the regulatory molecule STAT6, which could facilitate identification of susceptible populations.
The immunological response to respiratory pathogens is normally calibrated to eliminate an infection without causing overt collateral damage to the respiratory tissue. In spite of this, an overexuberant or misdirected immune response can have pathological outcomes. This can range from phenomena that are transient and typically moderate, such as elevated airway hyperresponsiveness (AHR), to those that are permanent and progressive, such as pulmonary fibrosis. The health effects of these immunological responses depend on the context of the infection; those that are comorbid with other acute or chronic pulmonary conditions may incite serious respiratory distress in that context when otherwise they might have little noticeable effect.
One area in which the effects of comorbid respiratory illnesses are especially relevant is that of exacerbation of preexisting asthma or chronic obstructive pulmonary disease (COPD). Many potential pathogens have been implicated as causing exacerbation; the most well known are various viruses, including rhinovirus, metapneumovirus, and respiratory syncytial virus (1). Several respiratory bacteria, most notably the atypical bacteria Mycoplasma pneumoniae and Chlamydia pneumoniae, have also been implicated in the exacerbation of asthma symptoms (2). Common fungi have also been widely implicated in the perpetuation or exacerbation of asthma, including Aspergillus and Alternaria (reviewed in Reference 3). Although less widely studied, the atypical fungus Pneumocystis is strongly associated with the pathogenesis of COPD (4), and there are scattered reports of acute Pneumocystis pneumonia presenting as asthma (5, 6), although in the latter example no mechanistic associations have been implicated.
The majority of the studies that examine whether fungi or other pathogens can act as agents of exacerbation have focused on allergic asthma models, wherein an underlying asthmatic phenotype is perturbed by exposure to a fungus to which the host has been previously sensitized. In contrast, very little is known about the early innate response to pathogens that may be commonly encountered but for which no sensitization has occurred. There is some evidence that the early response to a viral infection can have these types of effects. For example, infection of respiratory epithelium by viral pathogens results in the rapid production of inflammatory cytokines that can induce AHR, such as IL-1 (7) and the IL-1–related cytokine IL-33 (8). In addition, viral-induced IL-8 production by epithelial cells results in the recruitment of neutrophils into the airways, and clinical correlations have been reported with asthma exacerbations and high sputum neutrophil percentages (9). Much less is known about possible innate immune responses to fungi that may be involved in exacerbation, in spite of the widely held belief that exposure to molds and other fungi can sometimes initiate symptoms of respiratory distress.
We report here that the early innate immune response to the fungal pathogen Pneumocystis can cause inflammation and AHR but that this is highly variable between two common strains of immunocompetent mice. In the C57BL/6 mouse strain, the immune response is gradual and leads to an acquired immune response that leads to the elimination of the fungus within 21 days. This same response occurs in BALB/c mice, but it is preceded by a strong but transient innate immune response that results in profound AHR and inflammation, which does not occur in C57BL/6 mice. We also show that this response is STAT6 dependent and results in broad and significant gene transcriptional changes that are suggestive of other long-term changes in the respiratory environment.
Materials and Methods
Animals
BALB/c mice and C57BL/6 mice were purchased from Charles River (Wilmington, MA). CD1−/− mice and STAT6−/− mice were purchased from Jackson Laboratories (Bar Harbor, ME). IL-4 receptor knockout mice, IFN-γ knockout mice, CXCR2 knockout mice, and C.B17 scid mice were bred at Montana State University from stock obtained from Jackson. Mice were judged to be free of prior exposure to Pneumocystis by periodic screening for anti-Pneumocystis antibodies and absence of infection in immunosuppressed sentinel mice (10).
Infection with Pneumocystis murina
Experimental mice were infected with 107 Pneumocystis via intratracheal inoculation as described (11). Additional details are provided in the online supplement.
Respiratory Measurements
AHR was assessed using unrestrained whole-body plethysmography (Buxco, Wilmington, NC) to measure the Penh respiratory parameter (12). Penh values do not always correlate with airway diameters; however, they are a useful noninvasive measurement of respiratory responses (13). Successive aerosol exposures to methacholine (Sigma Chemical, St. Louis, MO) at concentrations of 0, 2.5, 5, 10, and 20 mg/ml (or 30 mg/ml for C57BL/6 mice) were made, allowing for recovery to baseline Penh values between successive doses.
Cell and Tissue Collection
After respiratory measurements, mice were killed, serum was collected, bronchoalveolar lavage (BAL) was performed, and cells and BAL fluid (BALF) were collected as previously described (10). After lavage, right lung lobes were homogenized for enumeration of Pneumocystis, and the left lobe was instilled with PBS/formalin and embedded in paraffin for histological analysis (10). Tissue sections were processed for hematoxylin and eosin staining, and in some cases Gomori methenamine silver staining, using standard histological techniques.
Flow Cytometry
BAL cells were stained with antibodies against typical surface discrimination markers (10). Complete details are provided in the online supplement.
Cytokine and Antibody Analysis
Concentrations of cytokines were measured in BALF using flow cytometric bead array kits from Becton Dickinson (Mountain View, CA) or commercial ELISAs (eBioscience, San Diego, CA) in the case of IL-13. Pneumocystis-specific IgG antibody titers were measured in serum samples by ELISA against a P. murina protein preparation (14).
RNA Collection and Analysis
Total RNA was collected from lungs using the Qiagen (Valencia, CA) Maxi-Kit procedure. RNA of sufficient quality was amplified, biotin labeled, and hybridized to Affymetrix GeneChip Mouse 430A 2.0 (Affymetrix, Santa Clara, CA) and used for gene expression analysis. Complete details are given in the online supplement.
Statistical Analysis
GraphPad Prism (San Diego, CA) was used for statistical analysis. One-way ANOVA analysis, followed by Tukey's pairwise comparisons, was performed when more than two groups were compared; otherwise, two-sided t tests were used.
Results
Time Course of AHR in BALB/c versus C57BL/6 Mice
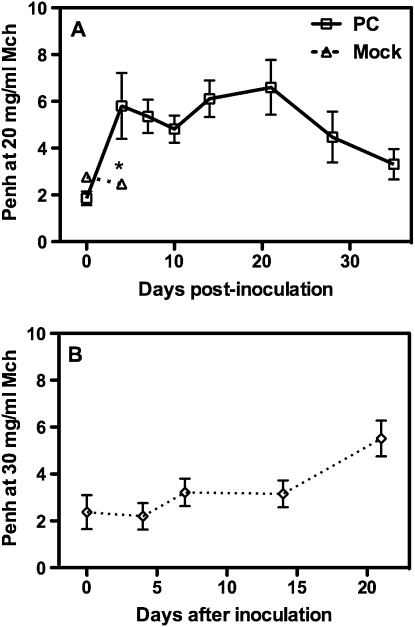
When BALB/c mice were inoculated with Pneumocystis, they exhibited a biphasic response of AHR such that Penh values were strongly elevated between 4 and 7 days after inoculation, declined slightly by 10 days, and reached a second peak near 21 days after infection, after which there was a gradual decline until Penh values returned to near baseline values after 30 days (Figure 1). C57BL/6 mice tend to have a reduced AHR response in comparison to BALB/c mice (15, 16), and this was the case in response to Pneumocystis as well. The C57BL/6 mice we tested required a higher dose of methacholine than did BALB/c mice (30 versus 20 mg/ml aerosolization) to reach significantly elevated Penh values. Furthermore, C57BL/6 mice lacked an early AHR response to Pneumocystis and only exhibited the later (∼ 20 d) elevation in Penh values (Figure 1). These kinetics are only statistically relevant with the applied dosage (107 organisms) of Pneumocystis (see Figure E1 in the online supplement). At lower doses, AHR is not significantly elevated at 7 days, although there is a trend toward a dose-response function. It is possible that when a smaller inoculum is given, the immune response is muted until a threshold level of organisms has grown, and the same response would occur at a later time, but this is not clear at this time.
Figure 1.
Airway hyperresponsiveness (Penh) values during a methacholine (Mch) challenge consisting of successive exposures to (A) 0, 2.5, 5, 10, and 20 mg/ml Mch for BALB/c mice or (B) 0, 3.75, 7.5, 15, and 30 mg/ml Mch for C57BL/6 mice at the indicated day after intratracheal Pneumocystis inoculation. Mock-infected mice were given intratracheal inoculation of lung homogenates from uninfected SCID mice, and had significantly lower Penh values at the highest dose (*P < 0.05). Values are means ± SEM, n = 4–5, representative of three or more independent experiments.
The General Immune Response to Pneumocystis Is Strain Specific
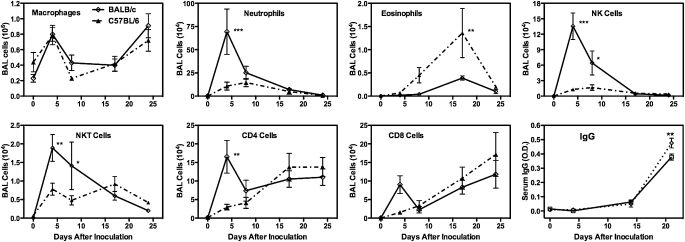
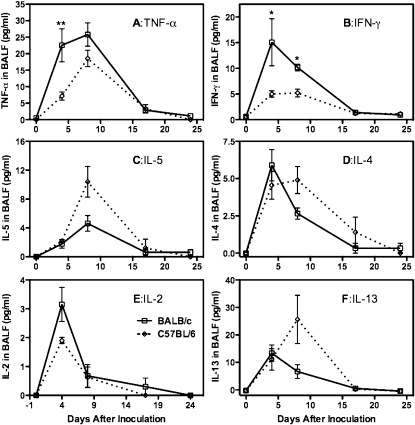
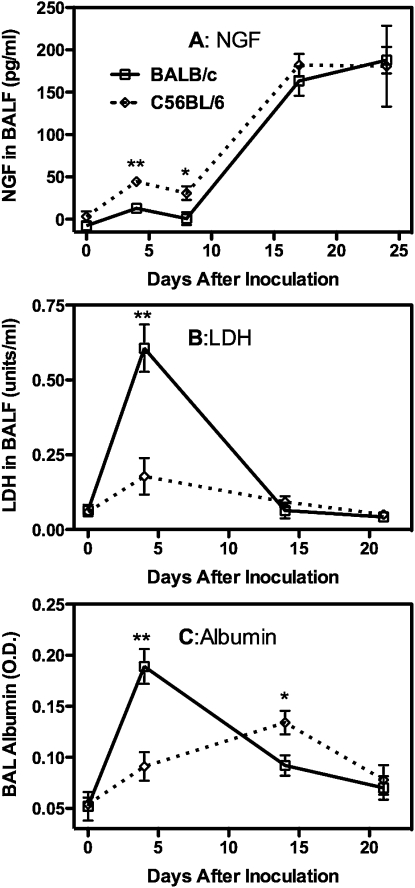
This strain difference in AHR responses to Pneumocystis inoculation is strongly reflected in the inflammatory responses that we observed. BALB/c and C57BL/6 mice developed a robust inflammatory response at 14 to 21 days after inoculation (Figure 2), and in both cases there were increased numbers of eosinophils in the BALF, which has been implicated in the induction of increased AHR in mouse experimental models (17, 18). This time period is also when an adaptive immune response is mounted to clear the Pneumocystis, and this is reflected by increased numbers of CD4 and CD8 T cells in the BALF and elevated titers of anti-Pneumocystis antibodies in circulating blood. In contrast to these similarities, the 4- to 7-day postinoculation inflammatory response is distinctly different between BALB/c and C57BL/6 mice. This early response in BALB/c mice is characterized by a very strong innate immune response, with large numbers of neutrophils and NK cells being recruited to the alveolar compartment as well as smaller numbers of NKT, CD8, and CD4 cells (Figure 2). This response is mostly absent in C57BL/6 mice, with the exception of a moderate increase in NKT cell numbers. Levels of inflammatory cytokines in the BALF also reflect a different response to Pneumocystis in the two types of mice. Although both strains reflect the secretion of cytokines that will set the stage for the response that clears the Pneumocystis, BALB/c mice also exhibit an early secretion of IFN-γ and TNF-α that is absent in C57BL/6 mice (Figure 3). The C57BL/6 mice, which are often reported to have more of a Th1 bias as compared with the Th2 bias of BALB/c mice (19, 20), have higher levels of IL-5 and IL-13 (Figure 3) in the BALF, which is followed by higher numbers of eosinophils later in the inflammatory response. Nerve growth factor, although not strictly a cytokine, has been proposed as a mediator of AHR in airway tissue (21, 22). We found that it was sharply elevated in the 14- to 24-day time period after inoculation in both strains of mice (Figure 4A), which may imply a role in the later onset of AHR. However, the fact that levels of this molecule were quite low in the 4- to 7-day period in BALB/c mice (although they were slightly elevated in C57BL/6 mice at this time) suggests it is not involved in the early AHR response. This early immune response in BALB/c mice does not bring about more rapid clearance of Pneumocystis and might slightly retard this process because C57BL/6 completely cleared the infection sooner than BALB/c mice (Figure E2).
Figure 2.
Early inflammatory response to Pneumocystis in BALB/c and C57BL/6 mice. Inflammatory cell numbers are those found in bronchoalveolar lavage fluid (BALF), while anti-Pneumocystis IgG values are those in serum. All values are means ± SEM, n = 4\x{2013}5. Strain difference significance is ***P ≤ 0.001, **P ≤ 0.01, *P ≤ 0.05. Representative of three independent experiments.
Figure 3.
Early inflammatory cytokine response to Pneumocystis inoculation in BALB/c and C57BL/6 mice. Cytokines were measured in BALF at the indicated day after Pneumocystis inoculation. Values are means ± SEM, n = 4–5, representative of three independent experiments. Significant difference as in Figure 2.
Figure 4.
Indicators of pulmonary damage in BALF in BALB/c and C57BL/6 mice at the indicated days after Pneumocystis inoculation. Values are means ± SEM, n = 4–5, representative of three independent experiments. Significant difference between the two strains as in Figure 2.
Effects of Inflammation on Cell and Barrier Integrity
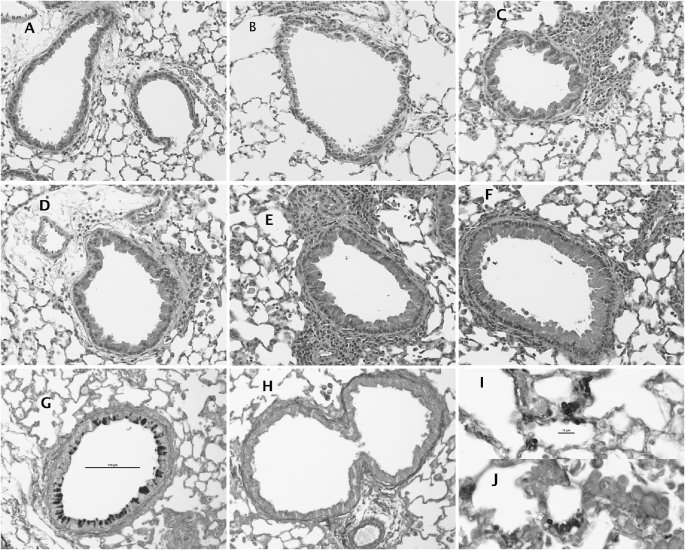
The strong early innate response to Pneumocystis in BALB/c mice appears to temporally affect the integrity of the lung epithelial barrier because BALF levels of serum albumin were strongly elevated at 4 days after inoculation (Figure 4B). Additional pulmonary pathology in BALB/c mice at this time was indicated by a sharp increase in BALF LDH (Figure 4C), suggesting that the strong immune response was causing destruction of some host cells. For the most part, histological appearance of the lung tissue was similar in both strains of mice. An early pulmonary response to Pneumocystis inoculation seems to involve the airway epithelium in both strains because marked hypertrophy of these cells is present at 4 and especially at 7 days after infection (Figures 5A–5F). However, it is apparent that the functional response of epithelial cells is also strain specific because silver-stained sections indicate significant activation of mucus-secreting cells in BALB/c mice but not in C57BL/6 mice at 4 days after inoculation (Figures 5G and 5H). Both strains exhibit some positive staining for mucus at later time points, but this is less than the early exuberant response in BALB/c mice (not shown). There was also some perivascular inflammation present, although generally more so in the BALB/c mice. Therefore, just as the early recruitment of inflammatory cells is significantly enhanced in BALB/c mice only, the functional response of the airway epithelium appears to be as well. There were no strain differences in the apparent distribution of Pneumocystis organisms of infected mice; positively stained cysts were widely scattered in the lungs of both strains and tended to occur in small clusters in smaller alveoli (Figures 5I and 5J).
Figure 5.
Histologic appearance of pulmonary tissue (A and B) before, and (C, D, G, and H) 4 and (E, F, I, and J) 7 days after Pneumocystis inoculation in (A, C, E, G, and I) BALB/c mice and (B, D, F, H, and J) C57BL/6 mice. Stain is standard hematoxylin and eosin in A–F, Gomori silver stain in G–J. Total magnification: ×200 (except for I and J: ×600).
Gene Expression Differences in BALB/c versus C57BL/6 Mice
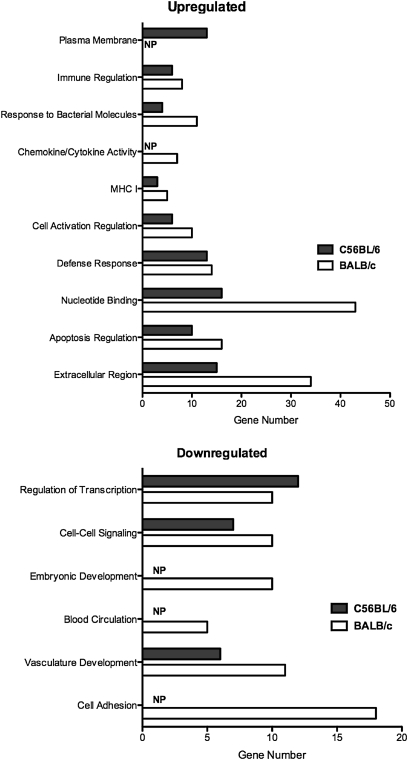
The profound difference in the early innate immune response between these two strains of mice was also evident at the transcriptional level. We analyzed gene expression data to compare genes that exhibited a change in expression that was greater than 2-fold, of which there were 574, and then grouped genes into simplified gene ontology clusters to simplify comparisons. The breadth of the early response to Pneumocystis exhibited by BALB/c mice is evident in the observation that, in most of these gene ontology clusters, there is a greater number of regulated genes in BALB/c mice than in C57BL/6 mice (Figure 6). When we applied more specific analysis, we found a large number of genes that were differentially regulated in BALB/c mice 7 days after Pneumocystis inoculation that were not comparably regulated in C57BL/6 mice. Using a minimum cutoff point of at least 2-fold up- or down-regulation, we found 116 genes that were uniquely up-regulated and 91 genes that were uniquely down-regulated. Investigation into the potential functions of these regulated genes indicated that, in addition to immune response genes, there were several genes that could be categorized as important in growth regulation, tissue remodeling, extracellular matrix, and transcription regulation (Table 1).
Figure 6.
Differential gene transcription in BALB/c (open bars) versus C57BL/6 (solid bars) mice at 7 days after Pneumocystis inoculation. Bars represent gene numbers in each simplified gene ontology (GO) group that were regulated up or down as compared to control mice at a level of ≤ 2-fold. NP indicates no genes present in the indicated group that met the level of up- or down-regulation.
TABLE 1.
DIFFERENTIALLY EXPRESSED GENES IN PNEUMOCYSTIS-INFECTED BALB/c MICE
| Symbol | Subset/Gene Name | vs. CON | vs. C57BL/6 |
| Transcription Factors | |||
| Egr1 | Early growth response 1 | 2.63 ↑ | 2.62 ↑ |
| Sox11 | SRY-box containing gene 11 | 3.39 ↑ | 4.13 ↑ |
| Runx1 | Runt related transcription factor 1 | 10.48 ↑ | 6.42 ↑ |
| Growth Factors | |||
| Mga | MAX gene associated | 12.01 ↑ | 12.82 ↑ |
| Nov | Nephroblastoma overexpressed gene | 2.35 ↑ | 2.19 ↑ |
| Fos | FBJ osteosarcoma oncogene | 2.51 ↑ | 2.28 ↑ |
| Areg | Amphiregulin | 2.31 ↑ | 2.75 ↑ |
| Extracellular Matrix | |||
| Spon2 | Spondin 2; extracellular matrix protein | 4.37 ↑ | 2.21 ↑ |
| Spon1 | Spondin 1, (f-spondin) extracellular matrix protein | 19.57 ↓ | 18.21 ↓ |
| Fn1 | Fibronectin 1 | 2.36 ↓ | 2.14 ↓ |
| Chemokines | |||
| Cxcl14 | Chemokine (C-X-C motif) ligand 14 | 2.40 ↑ | 2.30 ↑ |
| Ccl27 | Chemokine (C-C motif) ligand 27 | 2.76 ↑ | 4.24 ↑ |
| Proteases and Inhibitors | |||
| Adamts5 | A disintegrin and metalloproteinase with thrombospondin motifs 5 | 2.33 ↑ | 3.12 ↑ |
| Mmp3 | Matrix metallopeptidase 3 | 9.26 ↑ | 9.24 ↑ |
| Serpina1a | Serine (or cysteine) peptidase inhibitor, clade A, member 1a | 95.80 ↓ | 92.70 ↓ |
| Serpina1b | Serine (or cysteine) preptidase inhibitor; clade A; member 1b | 89.64 ↓ | 85.65 ↓ |
| Serpinb3b | Serine (or cysteine) peptidase inhibitor, clade B, member 3B | 8.48 ↓ | 2.44 ↓ |
| Receptor Type Molecules | |||
| Clec11a | C-type lectin domain family 11, member a | 3.37 ↑ | 3.69 ↑ |
| Sdc4 | Syndecan 4 | 3.53 ↑ | 2.10 ↑ |
| Klra7 | Killer cell lectin-like receptor, subfamily A, member 7 | 4.57 ↑ | 4.26 ↑ |
| Marco | Macrophage receptor with collagenous structure | 4.32 ↑ | 4.83 ↑ |
| Klra14 | Killer cell lectin-like receptor subfamily A, member 14 | 9.91 ↑ | 9.52 ↑ |
| Tnfrsf14 | Tumor necrosis factor receptor superfamily, member 14 | 2.18 ↓ | 2.95 ↓ |
| Other | |||
| Cd38 | CD38 antigen | 5.39 ↑ | 3.34 ↑ |
| Ang | Angiogenin, ribonuclease, RNase A family, 5 | 3.57 ↑ | 4.66 ↑ |
| Prdx2 | Peroxiredoxin 2 | 13.46 ↑ | 14.70 ↑ |
Selected genes are shown categorized by simplified gene ontology (GO) groups, as relative expression of the gene in Pneumocystis-infected BALB/c mice at 6 days compared to control noninfected mice (CON) or infected C57BL/6 mice at the same stage of infection. ↑ Indicates relative up-regulation, ↓ indicates relative down-regulation.
Investigation of Essential Factors in the Early Inflammatory Response
The broadness of this differential response in BALB/c mice suggested many potential cellular and cytokine candidates as being mechanistically responsible for the strain-specific AHR that we observed. We performed a number of experiments with mouse knockout models and antibody-mediated depletions to find specific factors implicated with the observed increase in AHR. Most of these experiments produced negative or ambiguous results. Although the presence of large numbers of neutrophils suggested a possible role in AHR, CXCR2 KO mice, in which recruitment of neutrophils to the alveolar compartment during Pneumocystis infection is blocked (23), still had elevated Penh values 4 to 7 days after Pneumocystis inoculation, as did BALB/c mice, in which circulating neutrophils were depleted with an anti-Gr1 antibody before and during the Pneumocystis infection (Table 2). Furthermore, although NKT cells have been implicated as a causal agent in elevated AHR (24), when we infected CD1 KO mice, which lack functional NKT cells, they still demonstrated AHR as high or higher than wild-type BALB/c mice (Table 2).
TABLE 2.
EFFECT OF CHANGES OF MOUSE PHENOTYPE ON PNEUMOCYSTIS-ASSOCIATED INCREASE IN AHR
| Mouse Phenotype | Functional Effect | Penh @ 20 mg/ml Mch |
| Wild Type | — | 5.61 ± 1.96 (4) |
| RB6 treated | Neutrophil depletion | 5.05 ± 2.74 (4) |
| CXCR2-KO | Absence of neutrophil recruitment | 5.14 ± 2.39 (4) |
| GK1.5 treated | CD4 cell depletion | 8.61 ± 2.82 (5) |
| IFN-γ KO | Lack of IFN-γ and potentially Th1 responses | 3.97 ± 0.97 (4) |
| IL-4r KO | Lack of IL-4 signaling and potentially Th2 responses | 3.52 ± 2.65 (4) |
| CD1 KO | Absence of the CD1 molecule and functional NKT cells | 6.65 ± 1.58 (4) |
| Control uninfected | — | 1.34 ± 0.29 (4) |
Phenotypes represent the use of specific KO mouse strains on a BALB/c background, or experimental manipulation of wild-type BALB/c mice with antibody-mediated depletion to remove a specific cell type. Values are Penh at a dose of 20 mg/ml methacholine at 4–7 days after Pneumocystis inoculation [means ± SD (n)].
Because different experimental models have implicated Th1 (25, 26) and Th2 (27, 28) factors as being important in the development of AHR, we also tested whether IFN-γ KO mice (deficient in Th1 responses) and IL-4 receptor KO mice (deficient in Th2 responses) would develop AHR after Pneumocystis inoculation. In both mouse knockouts, however, AHR was only slightly, but not significantly, reduced from that seen in wild-type BALB/c mice. Therefore, although each of these factors may have affected the early AHR response to Pneumocystis, neither was exclusively responsible. In addition, IFN-γ KO mice exhibited only approximately 10% of the large increase in NK cell recruitment after Pneumocystis inoculation compared with wild-type mice, which suggests that these cell types were not a major factor in the development of early AHR.
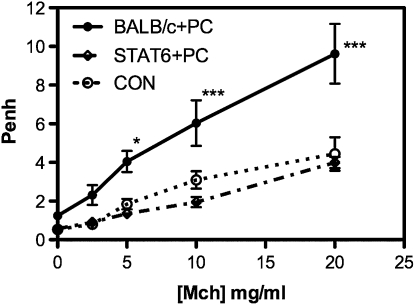
In contrast to all of these more obvious candidates, when we inoculated mice that were of a BALB/c background but deficient in the transcription factor STAT6, Penh values in the 4- to 7-day period were not significantly elevated above control, uninfected mice (Figure 7). In fact, most of the factors that we observed as part of the strong early innate immune response to Pneumocystis inoculation were sharply reduced, if not eliminated, in STAT6 KO mice, including neutrophils, NK cells, and CD4 and CD8 T cells (Table 3), indicating that most of the inflammatory and pathological responses we observed in BALB/c mice were dependent on the STAT6-associated pathways.
Figure 7.
Absence of elevated Penh values in STAT6 KO mice at 7 days after Pneumocystis inoculation. Values are means ± SEM, n = 4–5, * indicates that BALB/c+PC is significantly greater than STAT6+PC at P ≤ 0.005, while ** indicates significance at P ≤ 0.001; representative of three independent experiments.
TABLE 3.
COMPARISON OF THE INFLAMMATORY RESPONSE TO PNEUMOCYSTIS INOCULATION IN BALB/c AND STAT6 KO MICE
| Cell Type | BALB/c | STAT6-KO | Control |
| Total cells (106) | 1.388 ± 0.307 | 0.244 ± 0.085* | 0.182 ± 0.002 |
| Macrophages (106) | 0.386 ± 0.102 | 0.136 ± 0.023† | 0.178 ± 0.002 |
| Neutrophils (104) | 54.96 ± 15.26 | 3.50 ± 2.76* | 0.04 ± 0.04 |
| Eosinophils (104) | 0.20 ± 0.20 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Total lymphocytes (106) | 0.440 ± 0.118 | 0.070 ± 0.037* | 0.004 ± 0.002 |
| CD4 cells (104) | 7.342 ± 1.829 | 1.328 ± 0.795* | 0.006 ± 0.004 |
| CD8 cells (104) | 4.888 ± 1.205 | 0.770 ± 0.307* | 0.126 ± 0.055 |
| NK cells (104) | 4.438 ± 0.955 | 1.484 ± 0.748* | 0.156 ± 0.067 |
| NKT cells (104) | 0.706 ± 0.944 | 0.322 ± 0.188 | 0.016 ± 0.006 |
| γδ cells (104) | 1.510 ± 0.412 | 0.522 ± 0.236 | 0.116 ± 0.059 |
| B cells (104) | 0.112 ± 0.028 | 0.034 ± 0.013† | 0.00 ± 0.00 |
Cell types in the bronochoalveolar lavage fluid at 7 days after Pneumocystis inoculation in BALB/c and STAT6 knockout mice, as well as Control uninfected BALB/c mice. Values are means ± SEM, n = 4–5. Representative of three independent experiments.
* Values in STAT6-KO mice are significantly lower than those in BALB/c infected mice; P ≤ 0.01.
† Values in STAT6-KO mice are significantly lower than those in BALB/c infected mice; P ≤ 0.05.
Discussion
Inoculation of Pneumocystis into the lungs of BALB/c mice initiates a profoundly different early immune response in BALB/c mice than it does in C57BL/6 mice. This difference is broadly based and is evident at the transcriptional, protein expression, and cellular recruitment levels of the immune response. Differential strain-specific responses to fungal pathogens are not uncommon and have been reported for Cryptococcus neoformans (29), Stachybotrys chartarum (30), and Aspergillus fumigatus (31). Unlike many of these examples, however, the differential response to Pneumocystis that we describe does not affect clearance of the fungus, as any immunocompetent host strain will easily clear a Pneumocystis infection once an adaptive immune response has been mounted. In that respect, the immune response to Pneumocystis that occurs at 10 to 21 days after inoculation is similar in quality and effectiveness in BALB/c and C57BL/6 mice, although it is somewhat more robust in the latter strain and consists of the recruitment of CD4, CD8, and B-lymphocytes and the initiation of the production of anti-Pneumocystis antibody. In contrast, the early innate response unique to BALB/c mice is characterized by the recruitment of neutrophils and NK cells and secretion of cytokines such as TNF-α and IFN-γ and may not be necessary for the clearance of Pneumocystis. In fact, this early response seems excessive and aberrant in that it generates pathology in the host in the form of leakage of serum components into the alveolar space, limited host cell death, and elevated AHR.
The dependence of most of the components of this response, including AHR, on STAT6 signaling is not surprising, considering the strong connection between STAT6 and AHR and the Th2-associated airway inflammation that has been found in multiple studies of allergic asthma (32–34). The association of the inflammatory response we describe here in BALB/c mice with STAT6 is more complex than a simple Th2 differentiation decision. For example, the common description in the literature is that BALB/c mice tend to have a Th2-biased response, whereas C57BL/6 mice have a Th1 bias (35). In the early response to Pneumocystis infection, this Th1-Th2 discrepancy would seem to be not true because the C57BL/6 mice exhibit higher expression of IL-5 and IL-13, equal secretion of IL-4, and reduced secretion of IFN-γ, as compared with BALB/c mice, followed by greater numbers of eosinophils in the BALF. Besides the well known Th2 involvement, STAT6 has been shown to be involved in very early responses to pathogens. The most relevant of these is the observation that elevated AHR by direct stimulation (such as with IL-13) in the absence of a polarized Th2 phenotype is STAT6 dependent (36). What is notable here is that not only AHR but also almost all of the increases in inflammatory cell responses that were present in the BALB/c mice were absent in the STAT6 KO mice. Furthermore, in some cases the effect of the STAT6 KO was opposite that of the same KO in a Th2 asthma model. For example, although the early response to Pneumocystis in STAT6 KO mice was characterized by sharply reduced BALF neutrophils, in ova-induced allergic inflammation, BALF neutrophils are elevated in the absence of STAT6 signaling (37). This demonstrates that, besides implementing many aspects of Th2 differentiation, the STAT6 pathway is instrumental in many of the early innate responses to pathogens, such as fungi (e.g., Pneumocystis), at least in certain host strains. This strain specificity and STAT6 dependence is intriguing in light of the reported incidence of the association of human genetic variability in STAT6 with some asthma-associated pulmonary phenotypes, such as elevated IgE levels (38, 39), AHR (40), and eosinophilia (41). However, these studies primarily examined Th2-related phenotypes associated with established allergic asthma, and little is known of any possible associations of STAT6 variability with early innate response to pathogenic stimuli and pulmonary symptomatology and exacerbation of other lung conditions.
The possible presence and effects of potentially pathological early responses to Pneumocystis in humans are only speculations at this time. Although we demonstrate a clear and distinct murine strain–specific pathological pulmonary response to Pneumocystis, more work is needed to determine the detailed mechanism and the potential human implications of these findings. Although it is clear, for example, that STAT6 is essential in this response, we do not know if the strain difference is due to polymorphisms in that molecule itself, to upstream differences in the numbers or types of receptor molecules that initiate the responses to Pneumocystis, or to differences in downstream elements, such as transcription factors. Our results indicate a surprising number of strain-specific regulated genes in the early response to Pneumocystis; this helps to explain the intensity of the innate response and raises questions about possible consequences of this response. Several transcription factors are up-regulated in these mice, which may in turn initiate multiple downstream responses. Examples of these are EGR-1 (42), Runx1 (43, 44), and possibly factors like Mga and Sox11. Genes with more specific functions that may be involved in this early response and pathology are also up-regulated, such as chemokines, metalloproteases, and immune cell receptors, or down-regulated, such as peptidase inhibitors. The gene for one of the antioxidant proteins, peroxiredoxin, was one of the most strongly up-regulated genes in Pneumocystis-infected BALB/c mice. Although this might act as a protective agent in the presence of oxidants, peroxiredoxins also are implicated in the modification of immune cells function (45) as well as the onset of AHR in mice (46). Another interesting gene on this list is CD38. CD38 has potent effects on cellular Ca2+ signaling in the cell (47); CD38 is also present on airway smooth muscle cells and has been shown to be required for the maximal development of AHR in response to a variety of stimuli, including TNF-α (48), IL-13 (49), and allergen exposure (50). Therefore, the possibility exists that a number of downstream effectors may be the proximal mechanisms of the AHR and other events that we observe.
Although STAT6 signaling is necessary for the development of early inflammation and AHR after Pneumocystis inoculation, it is likely that the regulation of a number of these other molecules is required for the complete development of the phenotype that we observed. We do not know whether there may be long-term consequences to the host because of this response. Many of the modulated genes have potential effects on the remodeling of pulmonary tissue, and the possibility could exist that continued stimulation of a receptive host with a fungus like Pneumocystis may promote more long-term pathology than observed here. However, an innate response to an otherwise innocuous fungus, if a sufficient exposure occurs, can initiate a significant pulmonary inflammatory event in a susceptible host even in the absence of allergic response to the fungus.
Supplementary Material
Acknowledgments
The authors thank Tamera Marcotte, Gayle Callis, Soo Han, Joanna Gress, Katie Rowse, Larissa Jackiw, Trenton Bushmaker, Rebecca Pullen, Abigail Leary, Ann Harmsen, Mark McAlpine, and Albert Dorsett for technical assistance
Footnotes
This study was supported by National Institutes of Health grants RO1 RO1HL55002, PO1HL71659 (A.G.H), RO1 HL096464 (S.D.S.), NCRR INBRE P20 RR016455 (K.M.), and COBRE 5P20RR020185 (Dept. I.I. and D., MSU).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-0154OC on September 29, 2011
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Contoli M, Marku B, Conti V, Saturni S, Caramori G, Papi A. Viral infections in exacerbations of asthma and chronic obstructive pulmonary disease. Minerva Med 2009;100:467–478 [PubMed] [Google Scholar]
- 2.Esposito S, Principi N. Asthma in children: are chlamydia or mycoplasma involved? Paediatr Drugs 2001;3:159–168 [DOI] [PubMed] [Google Scholar]
- 3.Sevin CM, Peebles RS. Infections and asthma: new insights into old ideas. Clin Exp Allergy 2010;40:1142–1154 [DOI] [PubMed] [Google Scholar]
- 4.Norris KA, Morris A. Pneumocystis infection and the pathogenesis of chronic obstructive pulmonary disease. Immunol Res 2011;50:175–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ong EL, Hanley SP, Mandal BK. Bronchial responsiveness in aids patients with Pneumocystis carinii pneumonia. AIDS 1992;6:1331–1333 [DOI] [PubMed] [Google Scholar]
- 6.Schnipper S, Small CB, Lehach J, Kaufman A, Grizzanti JN, Rothstein M, Minkowitz S, Rosenstreich DL. Pneumocystis carinii pneumonia presenting as asthma: Increased bronchial hyperresponsiveness in Pneumocystis carinii pneumonia. Ann Allergy 1993;70:141–146 [PubMed] [Google Scholar]
- 7.Park J-W, Taube C, Swasey C, Kodama T, Joetham A, Balhorn A, Takeda K, Miyahara N, Allen CB, Dakhama A, et al. Interleukin-1 receptor antagonist attenuates airway hyperresponsiveness following exposure to ozone. Am J Respir Cell Mol Biol 2004;30:830–836 [DOI] [PubMed] [Google Scholar]
- 8.Kondo Y, Yoshimoto T, Yasuda K, Futatsugi-Yumikura S, Morimoto M, Hayashi N, Hoshino T, Fujimoto J, Nakanishi K. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int Immunol 2008;20:791–800 [DOI] [PubMed] [Google Scholar]
- 9.Ordoñez CL, Shaughnessy TE, Matthay MA, Fahy JV. Increased neutrophil numbers and IL-8 levels in airway secretions in acute severe asthma: clinical and biologic significance. Am J Respir Crit Care Med 2000;161:1185–1190 [DOI] [PubMed] [Google Scholar]
- 10.Swain SD, Meissner N, Han S, Harmsen A. Pneumocystis infection in an immunocompetent host can promote collateral sensitization to respiratory antigens. Infect Immun 2011;79:1905–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swain SD, Lee SJ, Nussenzweig MC, Harmsen AG. Absence of the macrophage mannose receptor in mice does not increase susceptibility to Pneumocystis carinii infection in vivo. Infect Immun 2003;71:6213–6221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwarze J, Hamelmann E, Bradley KL, Takeda K, Gelfand EW. Respiratory syncytial virus infection results in airway hyperresponsiveness and enhanced airway sensitization to allergen. J Clin Invest 1997;100:226–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwarze J, Hamelmann E, Gelfand EW. Barometric whole body plethysmography in mice. J Appl Physiol 2005;98:1955–1957; author reply 1956–1957 [DOI] [PubMed] [Google Scholar]
- 14.Harmsen AG, Chen W, Gigliotti F. Active immunity to Pneumocystis carinii reinfection in T-cell-depleted mice. Infect Immun 1995;63:2391–2395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gueders MM, Paulissen G, Crahay C, Quesada-Calvo F, Hacha J, Van Hove C, Tournoy K, Louis R, Foidart J-M, Noël A, et al. Mouse models of asthma: a comparison between C57BL/6 and BALB/c strains regarding bronchial responsiveness, inflammation, and cytokine production. Inflamm Res 2009;58:845–854 [DOI] [PubMed] [Google Scholar]
- 16.Leme AS, Berndt A, Williams LK, Tsaih S-W, Szatkiewicz JP, Verdugo R, Paigen B, Shapiro SD. A survey of airway responsiveness in 36 inbred mouse strains facilitates gene mapping studies and identification of quantitative trait loci. Mol Genet Genomics 2010;283:317–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomkinson A, Cieslewicz G, Duez C, Larson KA, Lee JJ, Gelfand EW. Temporal association between airway hyperresponsiveness and airway eosinophilia in ovalbumin-sensitized mice. Am J Respir Crit Care Med 2001;163:721–730 [DOI] [PubMed] [Google Scholar]
- 18.Shen HH, Ochkur SI, McGarry MP, Crosby JR, Hines EM, Borchers MT, Wang H, Biechelle TL, O'Neill KR, Ansay TL, et al. A causative relationship exists between eosinophils and the development of allergic pulmonary pathologies in the mouse. J Immunol 2003;170:3296–3305 [DOI] [PubMed] [Google Scholar]
- 19.Charles PC, Weber KS, Cipriani B, Brosnan CF. Cytokine, chemokine and chemokine receptor mRNA expression in different strains of normal mice: implications for establishment of a Th1/Th2 bias. J Neuroimmunol 1999;100:64–73 [DOI] [PubMed] [Google Scholar]
- 20.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol 2000;164:6166–6173 [DOI] [PubMed] [Google Scholar]
- 21.Braun A, Appel E, Baruch R, Herz U, Botchkarev V, Paus R, Brodie C, Renz H. Role of nerve growth factor in a mouse model of allergic airway inflammation and asthma. Eur J Immunol 1998;28:3240–3251 [DOI] [PubMed] [Google Scholar]
- 22.Frossard N, Naline E, Olgart Hoglund C, Georges O, Advenier C. Nerve growth factor is released by IL-1beta and induces hyperresponsiveness of the human isolated bronchus. Eur Respir J 2005;26:15–20 [DOI] [PubMed] [Google Scholar]
- 23.Swain SD, Wright TW, Degel PM, Gigliotti F, Harmsen AG. Neither neutrophils nor reactive oxygen species contribute to tissue damage during Pneumocystis pneumonia in mice. Infect Immun 2004;72:5722–5732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyer EH, Goya S, Akbari O, Berry GJ, Savage PB, Kronenberg M, Nakayama T, DeKruyff RH, Umetsu DT. Glycolipid activation of invariant t cell receptor+ nk t cells is sufficient to induce airway hyperreactivity independent of conventional CD4+ T cells. Proc Natl Acad Sci USA 2006;103:2782–2787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hessel EM, Van Oosterhout AJ, Van Ark I, Van Esch B, Hofman G, Van Loveren H, Savelkoul HF, Nijkamp FP. Development of airway hyperresponsiveness is dependent on interferon-gamma and independent of eosinophil infiltration. Am J Respir Cell Mol Biol 1997;16:325–334 [DOI] [PubMed] [Google Scholar]
- 26.Cui J, Pazdziorko S, Miyashiro JS, Thakker P, Pelker JW, Declercq C, Jiao A, Gunn J, Mason L, Leonard JP, et al. Th1-mediated airway hyperresponsiveness independent of neutrophilic inflammation. J Allergy Clin Immunol 2005;115:309–315 [DOI] [PubMed] [Google Scholar]
- 27.Venkayya R, Lam M, Willkom M, Grunig G, Corry DB, Erle DJ. The th2 lymphocyte products IL-4 and IL-13 rapidly induce airway hyperresponsiveness through direct effects on resident airway cells. Am J Respir Cell Mol Biol 2002;26:202–208 [DOI] [PubMed] [Google Scholar]
- 28.Tournoy KG, Kips JC, Pauwels RA. The allergen-induced airway hyperresponsiveness in a human-mouse chimera model of asthma is T cell and IL-4 and IL-5 dependent. J Immunol 2001;166:6982–6991 [DOI] [PubMed] [Google Scholar]
- 29.Guillot L, Carroll SF, Homer R, Qureshi ST. Enhanced innate immune responsiveness to pulmonary Cryptococcus neoformans infection is associated with resistance to progressive infection. Infect Immun 2008;76:4745–4756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenblum Lichtenstein JH, Molina RM, Donaghey TC, Brain JD. Strain differences influence murine pulmonary responses to Stachybotrys chartarum. Am J Respir Cell Mol Biol 2006;35:415–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cenci E, Perito S, Enssle KH, Mosci P, Latgé JP, Romani L, Bistoni F. Th1 and Th2 cytokines in mice with invasive aspergillosis. Infect Immun 1997;65:564–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mullings RE, Wilson SJ, Puddicombe SM, Lordan JL, Bucchieri F, Djukanovic R, Howarth PH, Harper S, Holgate ST, Davies DE. Signal transducer and activator of transcription 6 (STAT-6) expression and function in asthmatic bronchial epithelium. J Allergy Clin Immunol 2001;108:832–838 [DOI] [PubMed] [Google Scholar]
- 33.Zosky GR, Larcombe AN, White OJ, Burchell JT, Janosi TZ, Hantos Z, Holt PG, Sly PD, Turner DJ. Ovalbumin-sensitized mice are good models for airway hyperresponsiveness but not acute physiological responses to allergen inhalation. Clin Exp Allergy 2008;38:829–838 [DOI] [PubMed] [Google Scholar]
- 34.Chiba Y, Todoroki M, Nishida Y, Tanabe M, Misawa M. A novel STAT6 inhibitor AS1517499 ameliorates antigen-induced bronchial hypercontractility in mice. Am J Respir Cell Mol Biol 2009;41:516–524 [DOI] [PubMed] [Google Scholar]
- 35.Watanabe H, Numata K, Ito T, Takagi K, Matsukawa A. Innate immune response in Th1- and Th2-dominant mouse strains. Shock 2004;22:460–466 [DOI] [PubMed] [Google Scholar]
- 36.Yang M, Hogan SP, Henry PJ, Matthaei KI, McKenzie AN, Young IG, Rothenberg ME, Foster PS. Interleukin-13 mediates airways hyperreactivity through the IL-4 receptor-alpha chain and stat-6 independently of IL-5 and eotaxin. Am J Respir Cell Mol Biol 2001;25:522–530 [DOI] [PubMed] [Google Scholar]
- 37.Fulkerson PC, Zimmermann N, Hassman LM, Finkelman FD, Rothenberg ME. Pulmonary chemokine expression is coordinately regulated by STAT1, STAT6, and IFN-gamma. J Immunol 2004;173:7565–7574 [DOI] [PubMed] [Google Scholar]
- 38.Nagarkatti R, B-Rao C, Vijayan V, Sharma SK, Ghosh B. Signal transducer and activator of transcription 6 haplotypes and asthma in the Indian population. Am J Respir Cell Mol Biol 2004;31:317–321 [DOI] [PubMed] [Google Scholar]
- 39.Weidinger S, Klopp N, Wagenpfeil S, Rümmler L, Schedel M, Kabesch M, Schäfer T, Darsow U, Jakob T, Behrendt H, et al. Association of a stat 6 haplotype with elevated serum IgE levels in a population based cohort of white adults. J Med Genet 2004;41:658–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daley D, Lemire M, Akhabir L, Chan-Yeung M, He JQ, McDonald T, Sandford A, Stefanowicz D, Tripp B, Zamar D, et al. Analyses of associations with asthma in four asthma population samples from canada and australia. Hum Genet 2009;125:445–459 [DOI] [PubMed] [Google Scholar]
- 41.Duetsch G, Illig T, Loesgen S, Rohde K, Klopp N, Herbon N, Gohlke H, Altmueller J, Wjst M. Stat6 as an asthma candidate gene: polymorphism-screening, association and haplotype analysis in a caucasian sib-pair study. Hum Mol Genet 2002;11:613–621 [DOI] [PubMed] [Google Scholar]
- 42.Ngiam N, Post M, Kavanagh BP. Early growth response factor-1 in acute lung injury. Am J Physiol Lung Cell Mol Physiol 2007;293:L1089–L1091 [DOI] [PubMed] [Google Scholar]
- 43.Zhang F, Meng G, Strober W. Interactions among the transcription factors Runx1, RORgammat and Foxp3 regulate the differentiation of Interleukin 17-producing T cells. Nat Immunol 2008;9:1297–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Himes SR, Cronau S, Mulford C, Hume DA. The Runx1 transcription factor controls CSF-1-dependent and -independent growth and survival of macrophages. Oncogene 2005;24:5278–5286 [DOI] [PubMed] [Google Scholar]
- 45.Robinson MW, Hutchinson AT, Dalton JP, Donnelly S. Peroxiredoxin: a central player in immune modulation. Parasite Immunol 2010;32:305–313 [DOI] [PubMed] [Google Scholar]
- 46.Walker JKL, Ahumada A, Frank B, Gaspard R, Berman K, Quackenbush J, Schwartz DA. Multistrain genetic comparisons reveal CCR5 as a receptor involved in airway hyperresponsiveness. Am J Respir Cell Mol Biol 2006;34:711–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Partida-Sanchez S, Cockayne DA, Monard S, Jacobson EL, Oppenheimer N, Garvy B, Kusser K, Goodrich S, Howard M, Harmsen A, et al. Cyclic ADP-ribose production by CD38 regulates intracellular calcium release, extracellular calcium influx and chemotaxis in neutrophils and is required for bacterial clearance in vivo. Nat Med 2001;7:1209–1216 [DOI] [PubMed] [Google Scholar]
- 48.Guedes AGP, Jude JA, Paulin J, Kita H, Lund FE, Kannan MS. Role of CD38 in TNF-alpha-induced airway hyperresponsiveness. Am J Physiol Lung Cell Mol Physiol 2008;294:L290–L299 [DOI] [PubMed] [Google Scholar]
- 49.Guedes AGP, Paulin J, Rivero-Nava L, Kita H, Lund FE, Kannan MS. CD38-deficient mice have reduced airway hyperresponsiveness following IL-13 challenge. Am J Physiol Lung Cell Mol Physiol 2006;291:L1286–L1293 [DOI] [PubMed] [Google Scholar]
- 50.Gally F, Hartney JM, Janssen WJ, Perraud A-L. CD38 plays a dual role in allergen-induced airway hyperresponsiveness. Am J Respir Cell Mol Biol 2009;40:433–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.