Abstract
We previously reported that hypoxia attenuates nitric oxide–cyclic guanosine monophosphate (NO-cGMP)–mediated fetal pulmonary vessel relaxation by inhibiting cGMP-dependent protein kinase 1 (PKG1) activity, but not all the mechanisms by which acute hypoxia inhibits PKG1 activity have been delineated. Here we demonstrate for the first time, to the best of our knowledge, that acute hypoxia induces an accumulation of ubiquitinated PKG1 in ovine fetal and newborn pulmonary artery smooth muscle cells. Such a modification was not evident in ovine fetal systemic (cerebral) artery smooth muscle cells. The accumulation of polyubiquitinated PKG1 observed after 4 hours of hypoxia was affected neither by the activation of PKG1 kinase activity with the cell-permeable cGMP analogue 8-bromo-cGMP, nor by its inhibition with DT-3 in fetal pulmonary artery smooth muscle cells. Ubiquitinated PKG1α was unable to bind the cGMP analogue 8-(2-aminoethyl)thioguanosine-3′,5′ (AET)-cGMP, a ligand for the unmodified protein. Inhibition of the proteasomal complex with MG132 led to the accumulation of polyubiquitinated PKG1 in normoxia, indicating the involvement of the ubiquitin-26S proteasomal system in degradation and clearance of this protein under normoxic conditions. The ubiquitinated PKG1 under hypoxic conditions, however, was not predominantly targeted for proteasomal degradation. Importantly, reoxygenation reversed the acute hypoxia-induced accumulation of ubiquitinated PKG1. Our results suggest that the PKG1 ubiquitination induced by acute hypoxia plays a unique role in the regulation of the pulmonary vascular smooth muscle cell vasoreactivity and relaxation mediated by the NO-cGMP–PKG1 pathway.
Keywords: cGMP-dependent protein kinase, proteasome, ubiquitin conjugation, vascular smooth muscle, hypoxia
Clinical Relevance
Understanding the control of vasomotor tone in the fetal and neonatal circulations is critical to the treatment and prevention of pulmonary vascular disorders such as persistent pulmonary hypertension of the newborn. Our study implicates a new ubiquitin-mediated pathway in the regulation of nitric oxide–cyclic guanosine monophosphate–dependent protein kinase function in hypoxia, providing additional insights into the mechanisms controlling vasorelaxation, and potentially elucidating some mechanisms of hypoxic pulmonary vasoconstriction.
Cyclic guanosine monophosphate (cGMP)–dependent protein kinase (PKG1) is a serine–threonine kinase that plays an important role in nitric oxide–cyclic guanosine monophosphate (NO-cGMP)–mediated relaxation (1). In the canonical pathway, endothelium-derived NO diffuses into the vascular smooth muscle cell (VSMC) layers and activates soluble guanylate cyclase (sGC) to elevate concentrations of cyclic guanosine 3′,5′-monophosphate (cGMP). cGMP activates PKG1 to phosphorylate a wide range of downstream targets involved in the regulation of actomyosin cytoskeleton, calcium-mediated signaling mechanisms, and K+ channel function, resulting in vasodilatation (reviewed in detail elsewhere) (2, 3). The N-terminus of PKG1 (∼ 100 amino acids) is encoded by two alternative exons, resulting in the expression of two isoforms, PKG1α and PKG1β (apparent molecular weights of ∼ 77 and ∼ 80 kD, respectively) in an apparently spatiotemporal manner (4). Together, the PKGs regulate the smooth muscle cell “contractile state,” phenotype, and proliferative behavior (5–7).
Acute hypoxia is a known physiological stimulus for pulmonary vasoconstriction and increased pulmonary vascular resistance, unlike the systemic circulation. We showed previously that both fetal and neonatal ovine pulmonary vessels exhibit a vigorous dilator response to the nitric oxide–cGMP pathway, and that PKG1 plays a prominent role (8, 9). We also showed that acute hypoxia attenuates PKG1 activity in fetal and newborn pulmonary vessels and vascular smooth muscle cells, and that one mechanism by which this occurs involves reactive species–mediated PKG1 nitration and inactivation (10–12). Other mechanisms may be involved in the regulation of PKG1 activity in hypoxia. Sustained hypoxia-induced decreases in concentrations of PKG1 are associated with changes in smooth muscle contractile function and phenotypic modulation (7, 13, 14). However, the actual mechanisms involved in the regulation of PKG1 activity in hypoxia are unclear. The ubiquitin proteasome pathway is a major intracellular proteolytic system in eukaryotes, responsible for the protein homeostasis that also plays a critical role in the cellular hypoxic response (15–17). Here, we tested the hypothesis that acute hypoxia modulates PKG1 activity by posttranslational modification via an ubiquitin-dependent pathway in pulmonary vascular smooth muscle cells.
Materials and Methods
Fetal and Newborn Lamb Pulmonary Artery Smooth Muscle Cell Culture
All animal procedures and protocols described were reviewed and approved by the Animal Care and Use Committee at the University of Illinois. Fetal pulmonary artery smooth muscle cells (FPASMCs) and newborn pulmonary artery smooth muscle cells were isolated from intrapulmonary arteries (fourth to sixth generation vessels; see the online supplement) of near-term ovine fetuses and 1–2-day-old newborn lambs, using methods previously described (18), and cultured in Dulbecco's Modified Eagle's Medium containing 10% FBS. Cells were immunostained for α-smooth muscle actin (monoclonal antibody; Sigma-Aldrich, St. Louis, MO) to ascertain the smooth muscle phenotype (greater than 98%), and used between passages 3–6. Similarly, systemic vascular smooth muscle cells were isolated from fetal cerebral arteries (FCASMCs).
Exposure of Cells to Acute Hypoxia
Cells were starved for 3 hours in medium containing 0.2% FBS before hypoxia treatment (unless mentioned otherwise) in a humidified incubator saturated with 5% CO2, 3% oxygen, and balance nitrogen (Forma 3130; Thermo Scientific, Asheville, NC). The medium contained 2% FBS in experiments involving greater than 4 hours of exposure to hypoxia. The medium was rendered hypoxic by pre-equilibrating it in the hypoxia incubator for 3 hours before use. Normoxic control samples were placed in a standard CO2 incubator with 21% oxygen (Heracell240; Thermo Scientific). The Po2 in the medium exposed to hypoxia or normoxia measured between 30–40 mm Hg and approximately 100 mm Hg, respectively, and was monitored using a dissolved oxygen meter (Model 407510; Extech Instruments, Waltham, MA). Reoxygenation experiments involving hypoxia-exposed cells were performed by exposing FPASMCs to 2 hours of hypoxia (3% O2), after which the hypoxic medium was replaced with normoxic medium (21% O2), and the cells were returned to the normoxic incubator for 2 hours.
Transfection, Immunoprecipitation, and Western Blot Analysis of PKG1 and Ubiquitinated PKG1
Transient transfection with a plasmid encoding myc-tagged, full-length PKG1α was performed using Lipofectamine (Invitrogen, Carlsbad, CA). Unless otherwise mentioned, cell extracts were prepared in cold RIPA (radioimmunoprecipitation assay) cell lysis buffer (50 mM Tris-Cl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 0.1% SDS, and 1% Triton X-100) containing a cocktail of protease inhibitors. Protein content was determined using a Micro BCA Protein Assay Kit (Pierce, Rockford, IL). Cell extracts containing same amount of protein were mixed with 2 μg of PKG1 (rabbit polyclonal; Assay Designs, Ann Arbor, MI) or the PKG1α (goat polyclonal; Santa Cruz Biotechnology, Santa Cruz, CA) antibody for immunoprecipitation. For Western blot analysis, the proteins were transferred onto nitrocellulose membranes (Bio-Rad, Hercules, CA), blocked with 5% nonfat milk in TBS-T (Tris-buffered saline-Tween; 20 mM Tris-Cl, 137 mM NaCl, and 0.05% Tween-20, pH 8.0), and incubated with anti-ubiquitin antibody (monoclonal; Assay Designs). After incubation with horseradish peroxidase–conjugated secondary antibody, the blots were developed by the Supersignal West Pico Chemiluminescent Substrate (Pierce). Cell extracts were also probed with PKG1 (rabbit polyclonal; Assay Designs) or actin (rabbit polyclonal; Santa Cruz Biotechnology) antibody. When necessary, the blots were stripped (Restore PLUS; Pierce) for reprobing with the indicated antibodies. Blots were scanned and band intensities were quantified using the free software ImageJ (National Institutes of Health, Bethesda, MD).
Results
Acute Hypoxic Exposure Leads to Ubiquitin Modification and Accumulation of Ubiquitinated PKG1 in Ovine FPASMCs
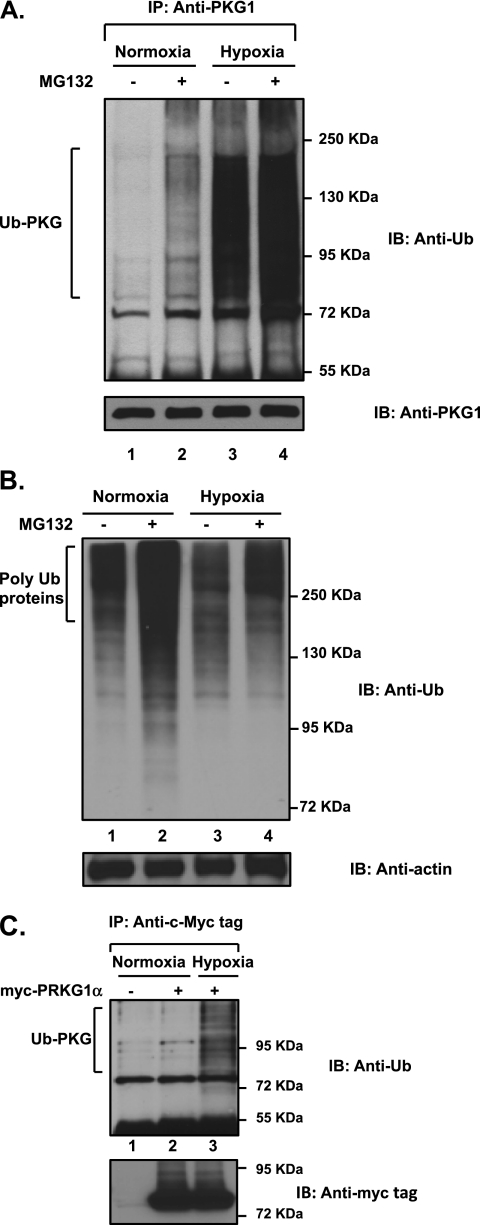
To determine whether hypoxia modifies PKG1 post-translationally by ubiquitin modification, resulting in decreased PKG1 activity, we analyzed ovine FPASMCs that had been exposed to acute hypoxia or normoxia in the presence or absence of MG132, a specific cell-permeable proteasome inhibitor. Ubiquitin conjugation was confirmed by immunoblot analysis with anti-ubiquitin (anti-Ub) antibody after the immunoprecipitation of PKG1 species, using a common anti-PKG1 antibody that recognizes both PKG1α and PKG1β. The inhibition of proteasomes with MG132 resulted in a noticeable accumulation of polyubiquitinated PKG1 (Ub-PKG) in normoxic cells (Figure 1A; compare lane 2 with lane 1), suggesting that PKG1 is degraded by the ubiquitin-26S proteasome system (UPS). Unexpectedly, the exposure of cells to hypoxia per se resulted in a drastic accumulation of Ub-PKG (Figure 1A; compare lane 3 with lane 1). Some additional accumulation of Ub-PKG species was evident after the inhibition of proteasomes with MG132 (Figure 1A; lane 4 versus lane 3), indicating that UPS degrades only a portion of Ub-PKG in hypoxia. Notably, hypoxia did not enhance the total concentration of ubiquitinated proteins in FPASMC lysates (Figure 1B; lane 3 versus lane 1), and the inhibition of proteasomes with MG132 resulted in an accumulation of polyubiquitinated proteins in both normoxic and hypoxic cells (Figure 1B; compare lane 2 with lane 1, and lane 4 with lane 3). In addition, exogenous PKG1α was also modified by ubiquitin during acute hypoxia in pulmonary artery smooth muscle cells (Figure 1C; compare lane 3 with lane 2). The modification by ubiquitin of PKG1 was not dependent on the serum concentration in the experimental culture medium, because the phenomenon was observed in low serum–containing (0.2% FBS) and high serum–containing (2–5% FBS) medium. Thus, acute hypoxia appears to activate PKG1-specific E2-ubiquitin–conjugating and/or E3-ubiquitin–conjugating enzymes in FPASMCs, and a significant proportion of Ub-PKG in hypoxia is not targeted for proteasomal degradation.
Figure 1.
Accumulation of the polyubiquitinated cyclic guanosine monophosphate (cGMP)–dependent protein kinase (PKG1) in acute hypoxia is not attributable to the inactivation of the proteasomal degradation pathway. Ovine fetal pulmonary artery smooth muscle cells were exposed to hypoxia or normoxia in the presence of the cell-permeable proteasome inhibitor MG132 (5 μM) for 4 hours. (A) Cell lysates were immunoprecipitated with a common PKG1 antibody and analyzed, using Western blotting with an anti-ubiquitin–specific monoclonal antibody. Blots were reprobed for concentrations of PKG1. Experiments were repeated three times, and representative blots are shown. An increased accumulation of ubiquitinated PKG1 (Ub-PKG) was observed after exposure to hypoxia. Treatment with MG132 indicates that PKG1 protein is targeted for proteasomal degradation under normoxic conditions, and an enhanced accumulation of Ub-PKG is observed during hypoxia. (B) Cell lysates were analyzed using Western blotting, and probed with anti-ubiquitin monoclonal antibody. (C) Exogenously transfected PKG1 is ubiquitin-modified during acute hypoxia in pulmonary artery smooth muscle cells. Fetal pulmonary artery smooth muscle cells (FPASMCs) were transiently transfected with the plasmid vector containing myc-PKG1α, as described in Materials and Methods, and exposed to normoxia or hypoxia for 4 hours. Cell extracts were immunoprecipitated with a c-Myc tag antibody and analyzed by Western blotting, using anti-ubiquitin antibody. Cell lysates containing the same amount of protein were probed with the c-Myc tag antibody. Experiments were repeated three times, and a representative blot is shown. IP, immunoprecipitation; IB, immunoblot.
Ubiquitination of PKG1 in Hypoxia Is Specific to Pulmonary Arterial Smooth Muscle Cells
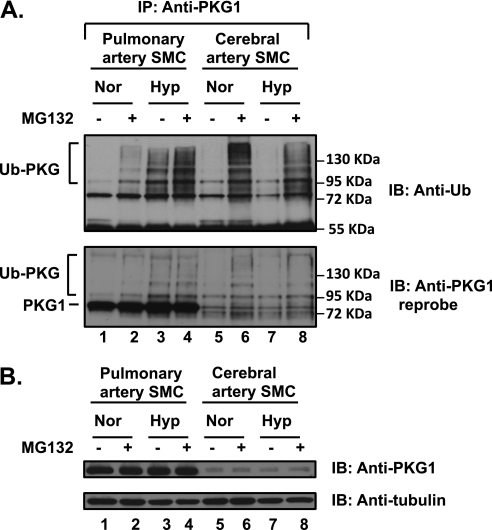
To determine the specificity of acute hypoxia-induced PKG1 ubiquitination in pulmonary VSMCs versus systemic VSMCs, isolated ovine FCASMCs were studied in the same manner as already described, in the presence or absence of the proteasomal inhibitor MG132 (Figure 2). No Ub-PKG was observed under normoxic conditions in any smooth muscle cells (Figure 2A, top; lanes 1 and 5). MG132 treatment under normoxic conditions resulted in an accumulation of Ub-PKG in both cell types (Figure 2A, top; compare lane 2 with lane 1, and lane 6 with lane 5), suggesting that UPS regulates concentrations of PKG1, and the ubiquitinated PKG1 is rapidly degraded. The amount of Ub-PKG in cerebral artery smooth muscle cells (SMCs) after the inhibition of proteasomes was significantly greater compared with that in pulmonary artery SMCs (Figure 2A, top; compare lane 6 with lane 2), suggesting a shorter half-life of PKG1 in these cells. We noticed that cerebral SMCs contain substantially lower concentrations of endogenous PKG1, compared with FPASMCs (Figure 2B), consistent with its increased degradation rate via the UPS. Remarkably, hypoxic exposure per se induced the accumulation of Ub-PKG species only in pulmonary SMCs (Figure 2A; compare lane 3 with lane 7). Notably, the stripped blots reprobed with PKG1 antibody also showed polyubiquitinated PKG1 species (Figure 2A, bottom).
Figure 2.
Hypoxia-induced ubiquitination of PKG1 is specific to pulmonary artery smooth muscle cells (SMCs). Isolated FPASMCs and fetal cerebral artery smooth muscle cells (FCASMCs) were both exposed to hypoxia (Hyo) or normoxia (Nor) in the presence or absence of the proteasomal inhibitor MG132 (5 μM) for 4 hours. (A) The cell lysates were immunoprecipitated with the common PKG1 antibody and assessed using Western blotting with anti-ubiquitin monoclonal antibody, as described in Materials and Methods. Blots were stripped and reprobed with anti-PKG1 antibody. (B) The cell lysates were probed with a common PKG1 antibody, and tubulin served as the loading control. Experiments were repeated 3–4 times, and a representative blot is shown. The ubiquitination of PKG (Ub-PKG accumulation) was evident in hypoxia only in FPASMCs, and not in FCASMCs.
Fetal and Newborn Pulmonary Arteries Do Not Demonstrate Increased Ubiquitinated PKG1
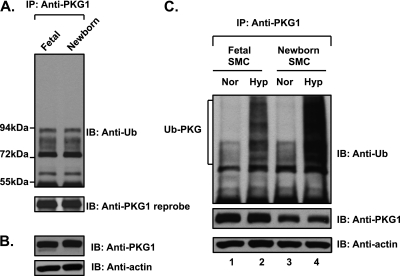
Fetal arteries, when dissected in a hypoxic chamber so that they remain chronically hypoxic as in utero, did not contain an increased amount of ubiquitinated PKG1. Neither did newborn pulmonary arteries that were normoxic (Figures 3A and 3B). However, the modification by ubiquitin of PKG1 was increased in both fetal and newborn pulmonary artery SMCs upon exposure to acute hypoxia (Figure 3C).
Figure 3.
Fetal and newborn pulmonary arteries do not demonstrate increased ubiquitin modification of PKG1. The ubiquitin modification of PKG1 is increased in both fetal and newborn pulmonary artery SMCs upon exposure to acute hypoxia. (A) Fetal arteries were isolated under hypoxic conditions as in utero. Ovine fetal and newborn pulmonary artery extracts were immunoprecipitated with anti-PKG1 antibody and analyzed by Western blotting, using an anti-ubiquitin antibody. The blot was reprobed with anti-PKG1 antibody. (B) The arterial tissue extracts were analyzed by Western blotting, using anti-PKG1 and actin antibodies. (C) Fetal and newborn ovine pulmonary artery SMCs were exposed to hypoxia or normoxia for 3 hours. Cell lysates were immunoprecipitated with PKG1 antibody and assessed by Western blotting, using an anti-ubiquitin monoclonal antibody (top). Blots were reprobed with PKG1 antibody (middle). Actin in cell extracts served as loading control (bottom). A representative blot from three experiments is shown.
Acute Hypoxia Induces Transient Accumulation of Ubiquitinated PKG1 in Ovine FPASMCs
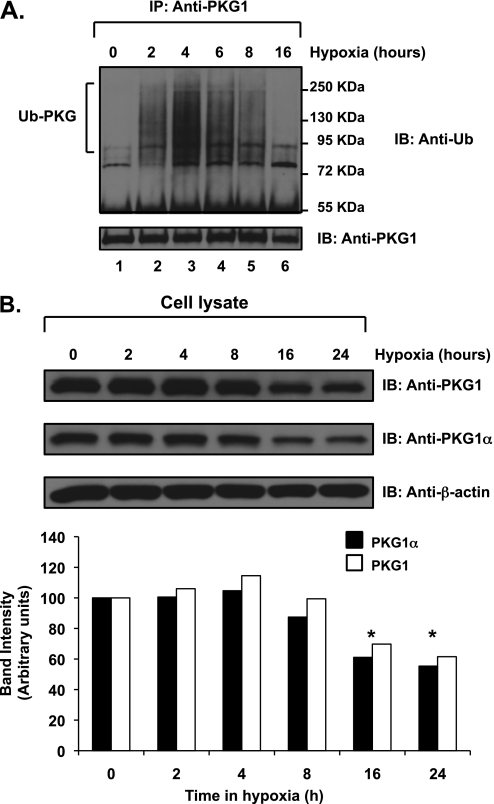
To characterize the observed phenomenon further, FPASMCs were exposed to hypoxia for different time intervals. We found that Ub-PKG rapidly accumulated until 4 hours of hypoxic exposure, and thereafter the amount of polyubiquitinated PKG1 species gradually decreased to an undetectable level by 16 hours of hypoxic treatment (prolonged) (Figure 4A, top). An immunoblot analysis of cellular lysates revealed that the concentration of PKG1 was markedly reduced after 16 hours of hypoxic exposure (Figure 4B; by ∼ 30% and ∼ 40% for total PKG1 and PKG1α, respectively). No accumulation of polyubiquitinated PKG1 species was evident in cells maintained in normoxia during this same time period (data not shown).
Figure 4.
PKG1 is subject to time-dependant ubiquitination in acute hypoxia. Ovine fetal pulmonary vascular smooth muscle cells were exposed to normoxia or hypoxia. (A) Cell extracts were immunoprecipitated with a common PKG1 antibody and analyzed by SDS-PAGE and Western blotting, using an anti-ubiquitin monoclonal antibody. The blots were stripped and reprobed with PKG1 antibody. (B) Cell lysates were analyzed by Western blotting, using a common PKG1 antibody or PKG1α isoform–specific antibody. Gel loading controls were assessed by probing with an actin antibody. Bottom: A plot of the relative PKG1 protein band intensities, normalized to actin levels, was quantified using ImageJ software (National Institutes of Health), and expressed as a percentage. Experiments were repeated 4–5 times, and representative blots are shown. *P < 0.05. The data shown are for hypoxia experiments. No PKG1 ubiquitination during normoxia was observed (data not shown).
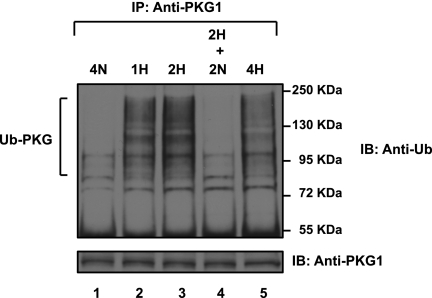
PKG1 Ubiquitination in Acute Hypoxia Is Reversible upon Reoxygenation
An analysis of cell extracts revealed that PKG1 was progressively ubiquitin-conjugated during the 2 hours of hypoxic exposure (Figure 5, top; lanes 2 and 3), but after 2 hours of reoxygenation of the cells, polyubiquitinated PKG1 was effectively cleared (Figure 5; lane 4). In these experiments, the protein concentrations of PKG1 were not decreased significantly after 4 hours of hypoxia. The disappearance of the polyubiquitinated PKG1 species after reoxygenation suggests that the ubiquitinated protein was either rapidly degraded or de-ubiquitinated upon reoxygenation. Thus, the hypoxically induced accumulation of Ub-PKG is temporary and reversible.
Figure 5.
Reoxygenation reverses acute hypoxia-induced PKG1 ubiquitin modification. FPASMCs were exposed to hypoxia for 2 hours and transferred to normoxia for an additional 2 hours. Cell lysates were immunoprecipitated with PKG1 antibody and analyzed according to Western blotting, using anti-ubiquitin monoclonal antibody, and were reprobed with PKG1 antibody. The experiment was repeated three times, and a representative blot is shown. Duration of treatment time in hours is indicated. H, hypoxia (3% O2); N, normoxia (21% O2). Hypoxic cells that were reoxygenated contained no ubiquitinated PKG1 (Ub-PKG), similar to normoxic cells that had never been exposed to hypoxia.
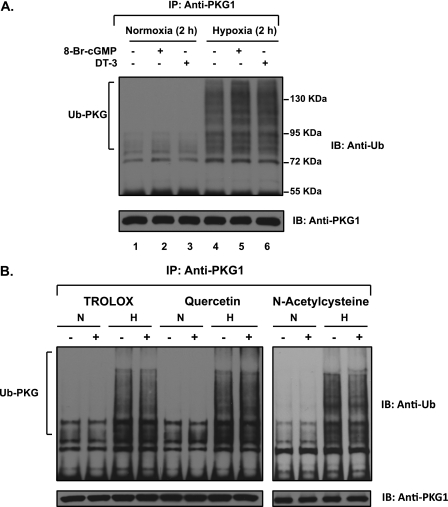
PKG1 Ubiquitination in Hypoxia Is Not Affected by PKG Kinase Activity, and Is Not Mediated by Reactive Oxygen Species
The relationship of endogenous intracellular PKG1 serine–threonine kinase activity to ubiquitination was evaluated in acute hypoxia. Neither a 2-hour treatment of FPASMCs with 0.5 mM 8-bromo-cGMP (the phosphodiesterase-resistant analogue of cGMP, used to stimulate PKG activity) nor an inhibition of constitutive PKG1 kinase activity with 5 μM of the cell-permeable peptide DT-3 changed the level of PKG1 ubiquitin modification during acute hypoxia (Figure 6 A, lanes 5 and 6 versus lane 4). Neither of the compounds induced the ubiquitination of PKG1 under normoxic conditions (Figure 6 A, lanes 2 and 3 versus lane 1). This indicates that the hypoxically induced accumulation of Ub-PKG is not dependent on constitutive cellular PKG1 kinase activity.
Figure 6.
Hypoxia-induced ubiquitin modification of PKG1 is unaffected by the endogenous activation or inhibition of its kinase activity or by common scavengers of reactive oxygen species or peroxynitrite. (A) FPASMCs were exposed to hypoxia for 2 hours, with or without the cell-permeable PKG1 inhibitor DT-3 (5 μM), or the phosphodiesterase-resistant activator 8-bromo (Br)-cGMP (0.5 mM). The cell lysates were immunoprecipitated with PKG1 and assessed by Western blotting, using an anti-ubiquitin monoclonal antibody. The blots were also reprobed with PKG1 antibody. The level of PKG activity exerted no effect on the accumulation of ubiquitinated PKG1 (Ub-PKG) during acute hypoxia. (B) FPASMCs were exposed to hypoxia or normoxia for 3 hours, with or without 0.2 mM trolox, 0.1 mM quercetin, or 10 mM N-acetylcysteine. Cells were preincubated for 30 minutes in normoxia (21% oxygen and 5% CO2) with the different scavengers before exposure to hypoxia (3% O2 and 5% CO2) in the presence of the same scavenger. The cell lysates were immunoprecipitated with PKG1 and assessed by Western blotting, using an anti-ubiquitin monoclonal antibody. The blots were also reprobed with PKG1 antibody. A representative blot from 3–4 experiments is shown.
To address whether the reactive oxygen species (ROS) and nitrogen species generated in hypoxia are involved in the ubiquitination of PKG1, FPASMCs were exposed to acute hypoxia in the absence or presence of various scavengers of reactive species, namely, trolox (a vitamin E–derivative and selective peroxynitrite scavenger), quercetin (an antioxidant), and N-acetylcysteine (a general ROS scavenger) (Figure 6B). In every case, treatment with the ROS inhibitor/scavenger did not affect PKG1 ubiquitination under hypoxic conditions. Thus, the reactive species generated in hypoxia may not be involved in acute hypoxia-induced PKG1 ubiquitination.
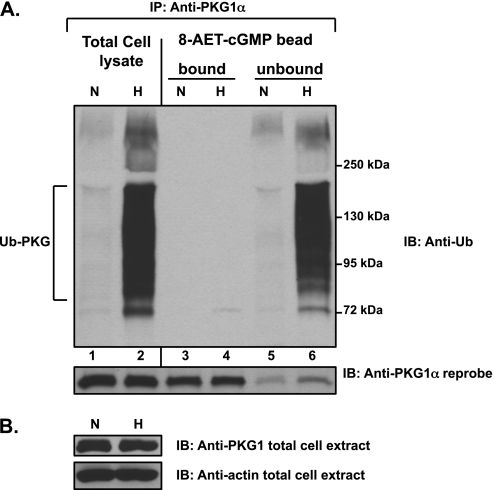
Ubiquitinated PKG Exhibits Altered Ligand Binding Affinity
To determine whether hypoxically induced PKG1 ubiquitination may alter some functional activity of the protein, we tested its ability to bind the cGMP analogue. We performed a large-scale purification of PKG1 species from hypoxic and normoxic FPASMC extracts, using 8-(2-aminoethyl)thioguanosine-3′,5′ (AET)-cGMP coupled to agarose-bead affinity chromatography. Polyubiquitinated PKG1α rapidly accumulated within the cells after 3 hours of hypoxic exposure (Figure 7A, top; lane 2 versus lane 1). Analysis of the 8-AET-cGMP bound and unbound protein fractions revealed that the hypoxia-induced polyubiquitinated PKG1α remained entirely in the unbound fraction (Figure 7A, top; lane 6 versus lane 4). PKG1α, unmodified by ubiquitin conjugation, was efficiently bound and eluted from the 8-AET-cGMP ligand beads by cGMP from either normoxic or hypoxic lysates (Figure 7A, bottom, lanes 3 and 4), with only a minor amount parting with the unbound fraction (Figure 7A, bottom, lanes 5 and 6). No significant difference was evident between the amount of bead-bound unmodified PKG1α in hypoxic and normoxic samples (Figure 7A, bottom; compare lane 4 with lane 3). Actin loading control and PKG1 protein levels were similar in the cell extracts. This result suggests that polyubiquitinated PKG1α is unable to bind the cGMP analogue 8-AET-cGMP.
Figure 7.
Hypoxia-induced PKG1 ubiquitination hinders its binding to 8-(2-aminoethyl)thioguanosine-3′,5′ (AET)-cGMP. Cell extracts were prepared from FPASMCs exposed to hypoxia (3% O2, 3 hours) in buffer A, as described in Materials and Methods. (A) The extracts were incubated with 8-AET-cGMP agarose beads. After washing the beads, the bound proteins were eluted with 25 mM cGMP. The total cell lysates, namely, the bead “bound” and “unbound” proteins, were immunoprecipitated with PKG1α antibody and assessed by Western blotting, using an anti-ubiquitin antibody. The blots were stripped and reprobed with PKG1α antibody. (B) The cell extracts were probed for PKG1 and actin. All experiments were repeated 3–4 times. A representative blot is shown. Ubiquitinated PKG1α (Ub-PKG) from hypoxically exposed cells exhibited defective binding to 8-AET-cGMP, compared with native, unmodified PKG1α.
Discussion
Here we describe a novel regulatory mechanism wherein acute hypoxia leads to the accumulation of Ub-PKG with altered ligand affinity, and protracted hypoxia likely inhibits the NO-cGMP–PKG vasodilatory pathway by engendering the degradation of polyubiquitinated PKG1. Acute hypoxia posttranslationally modifies PKG1 by ubiquitin conjugation in ovine fetal and newborn pulmonary artery SMCs (Figures 1, 2, and 4). Because the UPS is the major nonlysosomal protein-degradation pathway in eukaryotic cells, we surmised that PKG1 is also a target for this system during both normoxia and hypoxia. FPASMCs, after treatment with proteasomal inhibitors under normoxic conditions, accumulated polyubiquitinated PKG1 protein (Figures 1 and 2). The accumulation of polyubiquitinated PKG1 observed during acute hypoxia was further enhanced in the presence of the MG132 compound (Figures 1 and 2). This indicates that PKG1 is also a target for UPS-mediated degradation during acute hypoxia.
Hypoxically induced PKG1 ubiquitin conjugation was not evident when cells were reoxygenated after hypoxic exposure (Figure 5). This parallels our earlier observations that the nitration of PKG1 during hypoxia is not evident after reoxygenation, and that the recovery of PKG activity occurs upon reoxygenation. Our earlier experiments related to the nitration of PKG1 were performed from 30 minutes to 4 hours of exposure to hypoxia in pulmonary venous smooth muscle cells (11). The interrelationship between PKG1 nitration and PKG1 ubiquitination in hypoxia, if any, remains to be determined. The accumulation of polyubiquitinated PKG1 induced by hypoxia is not affected by the endogenous activation of PKG serine–threonine kinase activity by a cGMP analogue, or by the endogenous inhibition of PKG1 activity via a cell-permeable inhibitor (Figure 6A). This hypoxically induced acute effect on PKG1 in pulmonary SMCs is in direct contrast with, and kinetically different from, the observed down-regulation of PKG1 protein concentrations after the chronic (24-hour) activation of PKG1 in murine aortic SMCs by the cGMP analogue under normoxic conditions, as described elsewhere (20). The effect of chronic exposure to cGMP was dependent on PKG kinase activity, and has been attributed to autophosphorylation of the protein (20). Gao and colleagues reported a decrease in PKG1 protein amounts and activity when pulmonary veins were chronically (for up to 20 h) exposed to nitric oxide and elevated concentrations of cGMP (21). They found that the elevated concentrations of cGMP were exerting a negative feedback effect on concentrations of PKG1 (21). The decrease in PKG1 protein expression and activity was prevented by the administration of [1H-[1,2,4]oxadiazolo-[4, 3-a]quinoxalin-1-one] (ODQ), a sGC inhibitor or PKG inhibitor.
The specificity of ubiquitination of PKG1 during hypoxia in pulmonary vascular smooth muscle, compared with systemic vascular smooth muscle, is consistent with the physiologic response of pulmonary vessels to hypoxia, namely, vasodilatation in cerebral arteries versus vasoconstriction in pulmonary arteries (22, 23). Decreased PKG kinase activity during hypoxia augments the vasoconstrictor response of pulmonary vessels to hypoxia, especially in fetal pulmonary vessels (8, 24, 25). Cerebral artery SMCs express remarkably lower amounts of PKG1 (Figure 2) (26), and drastically reduced ubiquitinated PKG1 was noted during acute hypoxia, compared with pulmonary vascular SMCs. The regulation of the nitric oxide–cGMP pathway in cerebral vessels in hypoxia is most likely non–PKG1-mediated. Rather, a role for soluble guanylate cyclase was recently described (27). Thus the observed heterogeneity of the ubiquitin modification of PKG1 between cerebral and pulmonary vascular SMCs is consistent with their physiologic responses to acute hypoxia.
Previously, we reported that the attenuation of the NO-cGMP–PKG–dependent vasorelaxation pathway in hypoxia is related to the increased generation of reactive species. Reactive species led to the formation of nitrated PKG1 protein and decreased PKG1 activity in both intact pulmonary vessels and isolated cultured SMCs in acute hypoxia (11). Concentrations of PKG1 were markedly reduced (by 30%) after 16 hours of hypoxic treatment of pulmonary artery SMCs (Figure 4), a mechanism described earlier in freshly isolated ovine fetal pulmonary vessels and isolated ovine fetal pulmonary-vein SMCs (10, 11). We previously demonstrated that scavengers of reactive species and antioxidants restored most of the vascular dilation responses to cGMP in pulmonary veins during acute hypoxia (30 minutes), and we proposed that the ROS/reactive nitrogen species–induced nitration of PKG in hypoxia had resulted in the decreased dilation responses to cGMP during hypoxia (11). However, the treatment of FPASMCs with several known scavengers of ROS or peroxynitrite (N-acetylcysteine, trolox, or quercetin) did not block hypoxically induced PKG1 ubiquitination, suggesting that this modification occurs in a reactive species–independent manner (Figure 6B). Therefore, unlike the nitration of PKG, which was dependent on the generation of reactive oxygen and nitrogen species during hypoxia, the polyubiquitination of PKG1 was not mediated by reactive species. The interrelationship, if any, between PKG1 nitration and PKG1 ubiquitination during hypoxia remains to be determined.
Our data also demonstrated that hypoxically induced PKG1 polyubiquitination alters the ability of PKG1 to bind 8-AET-cGMP, a cGMP analogue, which efficiently binds cGMP-dependent PKG1 and PKG1-associated proteins (19, 28). 8-AET-cGMP efficiently bound native, unmodified PKG1α in our experiments (Figure 7). We previously reported that the peroxynitrite-induced tyrosine nitration of PKG1 decreased the cGMP-dependent activation of this enzyme, but not its basal activity (11). In these experiments, the inability of 8-AET-cGMP to bind to ubiquitin-modified PKG1 means that the cGMP-mediated activation of this enzyme would be greatly impaired. This is consistent with the observed shift in concentration required to induce 50% relaxation for 8-bromo-cGMP, from 30 μM to 90 μM, when isolated pulmonary vessels were exposed to acute hypoxia (11). A systematic study with ubiquitin-modified PKG1 species is necessary to confirm its cGMP-dependent activation and conformational effects.
Interestingly, our data revealed that Ub-PKG is accumulated transiently during hypoxia (for up to 4 hours), suggesting that this modified PKG1 is degraded or de-ubiquitinated with time, perhaps as part of an adaptive process. To confirm further that the ubiquitination of PKG occurs only during acute hypoxia and not prolonged hypoxia, we measured the ubiquitination of PKG1 in pulmonary arteries isolated from fetal lungs that were isolated in a hypoxic chamber, so that they would remain hypoxic and not become oxygenated (as the fetal pulmonary artery SMCs cultured in room air were). No ubiquitinated PKG1 was evident in the fetal arteries. Similarly, normoxic newborn arteries contained little ubiquitinated PKG1. Newborn SMCs, similar to fetal SMCs, responded to acute hypoxia by accumulating ubiquitinated PKG1.
Specific Ub ligases that regulate the proteolysis of substrates in hypoxia maintain cellular adaptation and homeostasis, namely, seven in absentia homolog 2 (Drosophila), Siah2, which binds a kinase anchor protein 1 (AKAP1) 21-regulating protein kinase A function (29); the SMAD-specific E3 ubiquitin protein ligase 1 (Smurf1) regulation of bone morphogenic protein (BMP) receptors in hypoxia-induced pulmonary hypertension (30); and the S-phase kinase-associated protein 2 (Skp2) regulation of p27kip1 in cGMP-induced SMC proliferation (31). Thus the identification of the hypoxia-induced E3 Ub-ligase involved in the modification of PKG1 and the specific PKG1 lysine residues involved would facilitate the delineation of this mechanism. Much remains to be known in terms of the acute effects of hypoxia on the Ub conjugation of PKG1 and the subcellular targeting of PKG1 isoforms. Because PKG1 controls diverse cellular functions in vivo, understanding the regulation of this protein by changes in oxygen tension via ubiquitin modification will constitute an important step toward understanding the pathophysiology of pulmonary vascular diseases.
In conclusion, we have demonstrated a new phenomenon wherein acute hypoxia rapidly induces the conjugation of multiple ubiquitin molecules to PKG1 protein, leading to an enhanced accumulation of Ub-PKG, affecting its ligand affinity. Such a modification may be partly responsible for the inhibition of the NO-cGMP–PKG1 signaling pathway and decreased vasorelaxation seen in hypoxia. Our findings may have important implications in pulmonary vascular SMC contractility and the hypoxic pulmonary vascular hypertensive response.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge Drs. Lawrence Longo and Ravi Goyal (Loma Linda University, Loma Linda, CA) for providing the fetal lungs and cerebral artery SMCs, and Dr. Basil Ibe (Harbor–University of California at Los Angeles, Los Angeles, CA) for technical advice. The authors also thank Drs. Kurt Albertine and MarJanna Dahl of the School of Medicine at the University of Utah (Salt Lake City, UT) for the newborn lungs. The technical expertise of Drs. Harshavardhan Kumar and Prasanna Kumar Turaka is greatly appreciated.
Footnotes
This work was supported by National Institutes of Health grants R01 HL075187 and R01 HL059435 (to J.U.R.), RO1 HL066109 and ES011863 (to S.P.R), and R01 AI064489 (to E.P.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-0165OC on October 13, 2011
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hofmann F, Bernhard D, Lukowski R, Weinmeister P. cGMP regulated protein kinases (CGK). Handb Exp Pharmacol 2009;191:137–162 [DOI] [PubMed] [Google Scholar]
- 2.Schlossmann J, Desch M. CGK substrates. Handb Exp Pharmacol 2009;191:163–193 [DOI] [PubMed] [Google Scholar]
- 3.Francis SH, Busch JL, Corbin JD, Sibley D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev 2010;62:525–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hofmann F, Feil R, Kleppisch T, Schlossmann J. Function of cGMP-dependent protein kinases as revealed by gene deletion. Physiol Rev 2006;86:1–23 [DOI] [PubMed] [Google Scholar]
- 5.Surks HK, Mochizuki N, Kasai Y, Georgescu SP, Tang KM, Ito M, Lincoln TM, Mendelsohn ME. Regulation of myosin phosphatase by a specific interaction with cGMP-dependent protein kinase Ialpha. Science 1999;286:1583–1587 [DOI] [PubMed] [Google Scholar]
- 6.Feil R, Gappa N, Rutz M, Schlossmann J, Rose CR, Konnerth A, Brummer S, Kuhbandner S, Hofmann F. Functional reconstitution of vascular smooth muscle cells with cGMP-dependent protein kinase I isoforms. Circ Res 2002;90:1080–1086 [DOI] [PubMed] [Google Scholar]
- 7.Lincoln TM, Wu X, Sellak H, Dey N, Choi CS. Regulation of vascular smooth muscle cell phenotype by cyclic GMP and cyclic GMP– dependent protein kinase. Front Biosci 2006;11:356–367 [DOI] [PubMed] [Google Scholar]
- 8.Gao Y, Dhanakoti S, Tolsa JF, Raj JU. Role of protein kinase G in nitric oxide and cGMP-induced relaxation of newborn ovine pulmonary veins. J Appl Physiol 1999;87:993–998 [DOI] [PubMed] [Google Scholar]
- 9.Gao Y, Zhou H, Raj JU. Endothelium-derived nitric oxide plays a larger role in pulmonary veins than in arteries of newborn lambs. Circ Res 1995;76:559–565 [DOI] [PubMed] [Google Scholar]
- 10.Gao Y, Dhanakoti S, Trevino EM, Sander FC, Portugal AM, Raj JU. Effect of oxygen on cyclic GMP-dependent protein kinase– mediated relaxation in ovine fetal pulmonary arteries and veins. Am J Physiol Lung Cell Mol Physiol 2003;285:L611–L618 [DOI] [PubMed] [Google Scholar]
- 11.Negash S, Gao Y, Zhou W, Liu J, Chinta S, Raj JU. Regulation of cGMP-dependent protein kinase–mediated vasodilation by hypoxia-induced reactive species in ovine fetal pulmonary veins. Am J Physiol Lung Cell Mol Physiol 2007;293:L1012–L1020 [DOI] [PubMed] [Google Scholar]
- 12.Negash S, Narasimhan SR, Zhou W, Liu J, Wei FL, Tian J, Raj JU. Role of cGMP-dependent protein kinase in regulation of pulmonary vascular smooth muscle cell adhesion and migration: effect of hypoxia. Am J Physiol Heart Circ Physiol 2009;297:H304–H312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou W, Dasgupta C, Negash S, Raj JU. Modulation of pulmonary vascular smooth muscle cell phenotype in hypoxia: role of cGMP-dependent protein kinase. Am J Physiol Lung Cell Mol Physiol 2007;292:L1459–L1466 [DOI] [PubMed] [Google Scholar]
- 14.Zhou W, Negash S, Liu J, Raj JU. Modulation of pulmonary vascular smooth muscle cell phenotype in hypoxia: role of cGMP-dependent protein kinase and myocardin. Am J Physiol Lung Cell Mol Physiol 2009;296:L780–L789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz AL, Ciechanover A. Targeting proteins for destruction by the ubiquitin system: implications for human pathobiology. Annu Rev Pharmacol Toxicol 2009;49:73–96 [DOI] [PubMed] [Google Scholar]
- 16.Semenza GL. Oxygen-regulated transcription factors and their role in pulmonary disease. Respir Res 2000;1:159–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakayama K. Cellular signal transduction of the hypoxia response. J Biochem 2009;146:757–765 [DOI] [PubMed] [Google Scholar]
- 18.Golovina VA, Blaustein MP. Preparation of primary cultured mesenteric artery smooth muscle cells for fluorescent imaging and physiological studies. Nat Protoc 2006;1:2681–2687 [DOI] [PubMed] [Google Scholar]
- 19.Schlossmann J, Ammendola A, Ashman K, Zong X, Huber A, Neubauer G, Wang GX, Allescher HD, Korth M, Wilm M, et al. Regulation of intracellular calcium by a signalling complex of IRAG, IP3 receptor and cGMP kinase Ibeta. Nature 2000;404:197–201 [DOI] [PubMed] [Google Scholar]
- 20.Dey NB, Busch JL, Francis SH, Corbin JD, Lincoln TM. Cyclic GMP specifically suppresses type-Ialpha cGMP-dependent protein kinase expression by ubiquitination. Cell Signal 2009;21:859–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao Y, Dhanakoti S, Trevino EM, Wang X, Sander FC, Portugal AD, Raj JU. Role of cGMP-dependent protein kinase in development of tolerance to nitric oxide in pulmonary veins of newborn lambs. Am J Physiol Lung Cell Mol Physiol 2004;286:L786–L792 [DOI] [PubMed] [Google Scholar]
- 22.Hunter CJ, Blood AB, White CR, Pearce WJ, Power GG. Role of nitric oxide in hypoxic cerebral vasodilatation in the ovine fetus. J Physiol 2003;549:625–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raj U, Shimoda L. Oxygen-dependent signaling in pulmonary vascular smooth muscle. Am J Physiol Lung Cell Mol Physiol 2002;283:L671–L677 [DOI] [PubMed] [Google Scholar]
- 24.Bloch KD, Filippov G, Sanchez LS, Nakane M, de la Monte SM. Pulmonary soluble guanylate cyclase, a nitric oxide receptor, is increased during the perinatal period. Am J Physiol 1997;272:L400–L406 [DOI] [PubMed] [Google Scholar]
- 25.Gao Y, Raj JU. Regulation of the pulmonary circulation in the fetus and newborn. Physiol Rev 2010;90:1291–1335 [DOI] [PubMed] [Google Scholar]
- 26.Robertson BE, Schubert R, Hescheler J, Nelson MT. cGMP-dependent protein kinase activates Ca-activated K channels in cerebral artery smooth muscle cells. Am J Physiol 1993;265:C299–C303 [DOI] [PubMed] [Google Scholar]
- 27.Pearce WJ, Williams JM, White CR, Lincoln TM. Effects of chronic hypoxia on soluble guanylate cyclase activity in fetal and adult ovine cerebral arteries. J Appl Physiol 2009;107:192–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim E, Park JM. Identification of novel target proteins of cyclic GMP signaling pathways using chemical proteomics. J Biochem Mol Biol 2003;36:299–304 [DOI] [PubMed] [Google Scholar]
- 29.Carlucci A, Adornetto A, Scorziello A, Viggiano D, Foca M, Cuomo O, Annunziato L, Gottesman M, Feliciello A. Proteolysis of AKAP121 regulates mitochondrial activity during cellular hypoxia and brain ischaemia. EMBO J 2008;27:1073–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murakami K, Mathew R, Huang J, Farahani R, Peng H, Olson SC, Etlinger JD. Smurf1 ubiquitin ligase causes downregulation of BMP receptors and is induced in monocrotaline and hypoxia models of pulmonary arterial hypertension. Exp Biol Med (Maywood) 2010;235:805–813 [DOI] [PubMed] [Google Scholar]
- 31.Wu YJ, Bond M, Sala-Newby GB, Newby AC. Altered S-phase kinase– associated protein– 2 levels are a major mediator of cyclic nucleotide– induced inhibition of vascular smooth muscle cell proliferation. Circ Res 2006;98:1141–1150 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.