Abstract
Rationale
Studies in laboratory animals have demonstrated an influence of environmentally derived stress and enrichment on the reinforcing effects of stimulants.
Objective
To characterize the effects of acute exposure to ethologically valid environmental stimuli on the reinforcing strength of cocaine relative to food in socially housed monkeys.
Materials and Methods
Choice between cocaine and food was assessed in subsets of sixteen socially housed (4/pen) male cynomolgus monkeys immediately after the following manipulations: (1) treats placed in homecage; (2) a 10-minute exposure to a rubber snake or (3) three to seven days of living in a larger environment without cage mates.
Results
Placing treats in the homecage shifted the cocaine dose-response curve to the left in 5 monkeys tested and to the right in 4 of 12 animals. The rubber snake significantly shifted the cocaine choice curve to the left in dominant monkeys. Exposure to an enlarged environment decreased cocaine choice in 9 of 15 monkeys; this effect was transient and not related to social rank. Repeated testing did not affect cocaine choice.
Conclusions
Brief exposure to environmental events hypothesized to be stressors or enrichment altered cocaine choice, although not all individuals were affected and the effects were transient. Importantly, the data suggest that implementing positive changes in the environment produced effects that are clinically desirable. The behavioral and neurobiological mechanisms mediating sensitivity to environmental events in socially housed animals will lead to better treatment strategies for drug addiction.
Keywords: social rank, environmental enrichment, vulnerability, nonhuman primates, drug self-administration
Estimates from epidemiological studies suggest that fewer that 20% of individuals who try cocaine eventually become addicted (O’Brien and Anthony 2005). Almost nothing is known about the factors that increase or decrease the likelihood of an individual progressing to addiction. Whereas genetic factors are likely to play a role (Drgon et al. 2010; Yuferov et al. 2010), it has also become clear that the environment can influence vulnerability and resilience to the abuse-related effects of cocaine. Specifically, several studies have demonstrated that sensitivity to the reinforcing effects of cocaine and other drugs is enhanced by stress and decreased by exposure to environmental enrichment (e.g., Goeders 2002; Stairs and Bardo 2009). Social interactions represent one particularly relevant, ethologically valid source of stress and enrichment. For example, it has been demonstrated unequivocally that exposure to repeated social defeat increases cocaine self-administration in rodents (for review see Miczek et al. 2008), whereas housing rats in an enriched environment can decrease a number of measures of sensitivity to stimulant drugs, including acquisition and reinstatement of self-administration (e.g., Green et al. 2002; Thiel et al. 2009).
For over a decade, we have studied in monkeys the neurobiological and behavioral consequences of assuming a particular rank in the social dominance hierarchy. A great deal of evidence has documented the adverse health-related consequences of chronic social stress in subordinate monkeys (e.g., Kaplan et al. 1982). Moreover, social dominance is associated with brain and behavioral changes similar to those seen in rodents housed in enriched conditions (e.g. Bowling et al. 1993, Hall et al. 1997). Dominant animals are groomed more often than others in the pen, have access to the entire pen and are the recipients of the fewest aggressive acts, while subordinates receive the most aggression and are groomed the least (Kaplan et al. 1982; Morgan et al. 2000). Thus, we have conceptualized the dominance hierarchy as consisting of a continuum from environmental enrichment in dominant monkeys to chronic social stress in subordinates (Nader and Czoty 2005). Becoming dominant (but not subordinate) is associated with increases in availability of brain dopamine D2 receptors—a neurobiological substrate closely linked to cocaine’s abuse-related effects—and, subsequently, a decreased sensitivity to the reinforcing effects of cocaine compared to subordinate monkeys (Morgan et al. 2002). Although, with continued exposure to cocaine, the social rank-related differences in D2 receptor availability and self-administration dissipated, when cocaine access was discontinued while monkeys remained in social groups, the rank-related differences in D2 receptor availability re-emerged (Czoty et al. 2005, 2010). These studies demonstrated the enduring ability of social variables to influence brain substrates closely linked to the abuse-related effects of cocaine.
That the experience of socially housed monkeys appears to lie along a stress-enrichment continuum begs the question of whether dominant and subordinate monkeys would be similarly affected by acute exposure to environmental stimuli that could be hypothesized to function as stressors and enrichment. Determining whether an event is enriching or stressful is not straightforward, since biological markers are not always reliably increased or decreased following hypothesized stressful or enriching manipulations. For example, we found that during new social group formations, future subordinate monkeys had higher cortisol concentrations, but only during the first 3 days of exposure and once social hierarchies had stabilized, dominant animals had higher average cortisol concentrations (Czoty et al. 2009). For the present study, cocaine self-administration was examined using a food-cocaine choice procedure in which each day a range of doses of cocaine was made available in ascending order as the alternative to a food pellet (Negus, 2003). Of particular interest was whether the effects of acute exposure to events hypothesized to induce stress or enrichment would have similar effects on the reinforcing strength of cocaine relative to food in dominant and subordinate monkeys. Environmental events were operationally defined as enriching if they resulted in shifts to the right of the cocaine dose-response curve, while a stressor would shift the curve to the left.
Environmental stimuli used in the present studies were brief exposure to food treats, a rubber snake and an enlarged living space. Providing foraging opportunities for treats is a widely used method of providing environmental enrichment for monkeys, while exposure to a rubber snake in a laboratory setting has been shown to elicit acute fear responses in macaques (including fear grimaces, vocalizations, inhibited behavior and withdrawal) that can be comparable to those induced by a live snake in feral animals (Kalin et al. 2001; Prather et al. 2001; Nelson et al. 2003). In the final set of experiments, monkeys were allowed to live in their home pen in the absence of their three pen-mates for three days, or for multiple days, essentially quadrupling the size of their living space. Previous studies in monkeys have indicated that moving monkeys to larger cages results in decreases in stereotypic, abnormal and “tension-related” behaviors (e.g., threat, yawn, scratch, cage shake, and aggression; Draper and Bernstein 1963; Paulk et al. 1977; Kaufman et al. 2004). Moreover, in rodents, the converse—decreasing living space by increasing the density of individuals in a cage (or “crowding”)—functions as a stressor with several negative consequences including deleterious effects on hypothalamic-pituitary-adrenal axis function, decreased food and water consumption and increased anxiety-like behaviors (e.g., Bugajski et al., 1993; Brown and Grunberg, 1996; Gadek-Michalska and Bugajski, 2003; Botelho et al., 2007).
Materials and Methods
Subjects
Sixteen adult male cynomolgus monkeys (Macaca fascicularis) with a history of being housed in groups of three or four for over two years (Czoty et al. 2004, 2005) served as subjects. During these experiments, all monkeys were housed in groups of four. Eleven monkeys served as subjects for both experiments. One monkey participated in only Experiment 1 (snake exposure) and four monkeys participated in only Experiment 2 (enlarged living space). Each monkey was fitted with a nylon collar (Primate Products, Redwood City, CA) and trained to sit calmly in a standard primate chair (Primate Products). Monkeys were weighed weekly and fed enough food daily (Purina Monkey Chow and fresh fruit and vegetables) to maintain body weights at approximately 95% of free-feeding levels. Body weights did not change significantly during these studies and were not different between dominant and subordinate monkeys. Water was available ad libitum in the home cage.
Monkeys lived in stainless steel cages (0.71 × 1.73 × 1.83 m; Allentown Caging Equipment, Co., Allentown, NJ) with removable wire mesh partitions that separated monkeys into quadrants (0.71 × 0.84 × 0.84 m). Monkeys were separated daily for several hours during operant behavioral sessions and feeding. Social status had previously been determined for each monkey according to the outcomes of agonistic encounters using procedures similar to those described previously (see Kaplan et al. 1982; Czoty et al. 2005, 2009). Briefly, two observers separately conducted several 15-min observation sessions per pen. Aggressive, submissive and affiliative behaviors were recorded according to an ethogram described previously (see Table 1 in Morgan et al. 2000) utilizing Noldus Observer software (Noldus Information Technology; Wageningen, The Netherlands). In these focal group sessions, both initiators and recipients of behaviors were recorded. The monkey in each pen aggressing towards all others and submitting to none was ranked #1 (most dominant). The monkey designated most subordinate (#4) displayed a low frequency of aggressive behaviors and submitted to all other monkeys in the pen. Hierarchies were linear and transitive. For the present studies, #1- and #2-ranked monkeys were considered dominant and #3- and #4-ranked monkeys were considered to be subordinate. Animal housing and handling and all experimental procedures were performed in accordance with the 2003 National Research Council Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research and were approved by the Animal Care and Use Committee of Wake Forest University. Environmental enrichment was provided as outlined in the Animal Care and Use Committee of Wake Forest University Non-Human Primate Environmental Enrichment Plan.
Catheter implantation
Each monkey had been prepared with an indwelling venous catheter and subcutaneous vascular access port (VAP; Access Technologies, Skokie, IL) under sterile surgical conditions. An antibiotic (30 mg/kg kefzol, i.m.; Cefazolin sodium, Marsam Pharmaceuticals, Inc., Cherry Hill, NJ) was administered 1 hour prior to surgery. Anesthesia was induced with ketamine (15 mg/kg, i.m.) and maintained with ketamine supplements. A catheter was inserted into a major vein (femoral or internal or external jugular) to the level of the posterior vena cava. The distal end of the catheter was passed subcutaneously to a point slightly off the midline of the back, where an incision was made. The end of the catheter was attached to a VAP, which was placed in a pocket formed by blunt dissection.
Apparatus and general behavioral procedures
Five days per week, monkeys were separated by partitioning the living space into quadrants. Next, each monkey was seated in a primate chair and placed into a ventilated, sound-attenuating chamber (1.5 × 0.74 × 0.76 m; Med Associates, St. Albans, VT). The back of the animal was cleaned with 95% ethanol and chlorhexidine, and the VAP was connected to an infusion pump (Cole-Parmer Instrument Co., Niles, IL) located outside the chamber via a 20-gauge Huber Point Needle (Access Technologies). The pump was operated for approximately 3 sec to fill the port and catheter with the concentration of (−)cocaine (National Institute on Drug Abuse, Bethesda, MD) available for the session. Two photo-optic switches (Model 117-1007; Stewart Ergonomics, Inc., Furlong, PA) were located on one side of the chamber with a horizontal row of three stimulus lights positioned 14 cm above each switch. The switches were positioned to be easily within reach of the monkey seated in the primate chair. A food receptacle, above which was a single white stimulus light, was located between the switches and connected with a Tygon tube to a pellet dispenser (Med Associates) located on the top of the chamber for delivery of 1-g banana-flavored food pellets (Bio-Serv, Frenchtown, NJ). At the conclusion of each behavioral session, monkeys were returned to their home cages. Partitions were left in place for 60–90 min during which monkeys were fed, except when Experiment 2 was being conducted (see below).
Cocaine self-administration
Monkeys were trained to self-administer cocaine under a concurrent fixed-ratio (FR) schedule of food and cocaine availability using a procedure similar to that described by Negus (2003). Monkeys were initially trained to respond using food reinforcement. To make a response, the monkey inserted his finger into a 2.5-cm opening in the device which broke a photobeam, recorded a response and activated a relay that provided auditory feedback to the monkey. It was necessary for the monkey to completely withdraw his finger before another response could be counted. Following initial exposure to FR contingencies on each switch, training under the choice procedure began under a concurrent FR 30 schedule. Responding on one switch (henceforth termed the “food switch”) always resulted in delivery of a single food pellet; the yellow light above this switch was illuminated during pellet availability. Responding on the other switch (henceforth termed the “drug switch”) resulted in activation of the infusion pump and an injection of cocaine (0.003–0.1 mg/kg per injection). Availability of each cocaine dose was associated with illumination of a different set of stimulus lights above the switch; different cocaine doses were studied by varying the duration of pump activation (Table 1). If a response was emitted on the alternate switch before an FR was completed, the response requirement on the first switch was reset. Assignment of food or drug to a switch was counterbalanced across monkeys. Delivery of either reinforcer was accompanied by illumination of the red light above the corresponding switch (for 5 sec after a pellet delivery or during an injection) and a subsequent period during which all lights remained off and responding had no scheduled consequences. The total timeout (TO) duration was 30 seconds. Initial training sessions consisted of one component in which monkeys chose between food and a single dose of cocaine in the presence of the appropriate discriminative stimuli. These sessions ended after 60 min elapsed or 30 total reinforcers were earned, whichever occurred first. Once monkeys had experienced approximately 10 such sessions per cocaine dose, terminal schedule conditions were enacted for subsequent sessions.
Table 1.
Parameters used across components of the choice procedure
| Component | Switch 1
|
Switch 2
|
|||
|---|---|---|---|---|---|
| stimulus | light | stimulus1 | light (s) | pump (sec) | |
| 1 | food | yellow | -- | none | 0 |
| 2 | food | yellow | 0.003 | yellow | 1 |
| 3 | food | yellow | 0.01 | white & yellow | 3 |
| 4 | food | yellow | 0.03 | green | 10 |
| 5 | food | yellow | 0.1 | white & green | 30 |
cocaine dose (mg/kg per injection)
Each daily session consisted of 5 components in which monkeys chose between food pellets and ascending doses of cocaine (i.e., no injection, 0.003, 0.01, 0.03 and 0.1 mg/kg per injection cocaine in components 1–5, respectively). Each component ended when 10 total reinforcers had been earned or 20 minutes had elapsed, whichever came first; a 120-sec TO followed each component. Ratio requirements for food and cocaine were adjusted for each monkey such that allocation of responding to the drug switch increased over the session as the available dose of cocaine increased (Table 2). There was not a significant difference in ratio values between social ranks according to an unpaired t-test. Responding was considered stable when ≤20% of reinforcers were earned on the drug switch when the alternative to food was no injection (component 1) or 0.003 mg/kg per injection cocaine (component 2) and ≥80% of reinforcers were earned on the drug switch when the alternative to food was 0.1 mg/kg per injection cocaine (component 5). An additional criterion was observation of a dose-related increase in drug choice. A complete dose-effect curve was determined in each monkey each day, typically 5 days per week.
Table 2.
Social ranks, parameters and outcomes for individual subjects used in the food-cocaine choice procedurea
| Monkey | Rank | Food FR | Cocaine FR | Fd:Coc | Exp1A | Exp1B | Exp2 |
|---|---|---|---|---|---|---|---|
| C-6526 | 1 | 100 | 60 | 1.67 | L | L | L |
| C-6628 | 1 | 50 | 100 | 0.50 | R | R | Rc |
| C-7079 | 1 | 100 | 50 | 2.00 | - | - | - |
| C-7426b | 1/2 | 125 | 75 | 1.67 | R | L | -d |
| C-7428 | 2 | 75 | 50 | 1.50 | L | L | R |
| C-6529 | 2 | 50 | 175 | 0.29 | R d | ||
| C-6625 | 2 | 50 | 50 | 1.00 | L | L | R |
| C-6629 | 2 | 75 | 125 | 0.60 | L | - | Rc |
| C-7081 | 2 | 125 | 60 | 2.08 | -d | ||
|
| |||||||
| C-6216 | 3 | 50 | 75 | 0.67 | L | L | -c |
| C-6527 | 3 | 50 | 75 | 0.67 | R | - | - |
| C-6214 | 4 | 50 | 200 | 0.25 | - | - | |
| C-6530 | 4 | 100 | 60 | 1.67 | R | - | Rc |
| C-7083 | 4 | 125 | 75 | 1.67 | R | ||
| C-7424 | 4 | 150 | 100 | 1.50 | R | ||
| C-7425 | 4 | 100 | 75 | 1.33 | - | - | R |
R = right; L=left; - = no shift
#1 rank for Exp. 1 and #2 rank for Exp. 2
tested after 1 consecutive week in the enlarged pen
tested after spending consecutive weekends in the enlarged pen
Experiment 1A: Exposure to preferred treats and Experiment 1B: Exposure to a rubber snake
These experiments occurred shortly after the monkeys were separated into quadrants at the start of an experimental session and when responding was deemed stable (three consecutive days using the criteria described above). First, the monkey in the quadrant horizontally adjacent to the test subject was removed, the test subject was moved into the vacant quadrant and the partition between the quadrants was replaced. Preferred treats (approximately 10 total marshmallows and candy) were placed into a red bucket (18 cm deep × 23 in diameter) and covered with sawdust to a depth of approximately 10 cm. The bucket was placed into a monkey’s home cage and after 10 min, during which the monkey approached the bucket and retrieved the treats, the monkey was placed into a primate chair and put into the behavioral chamber for a self-administration session. Monkeys were exposed to the bucket with treats for three consecutive days. On the fourth day, rather than treats, a rubber snake (length, 43.2 cm; diameter, 2.5 cm; yellow body with black rings) was placed in the bucket on top of the sawdust, and the bucket was put into the empty quadrant. Next, the partition was again removed granting the monkey access to the bucket. After 10 minutes of exposure to the bucket, the monkey was placed into a primate chair and transported into the behavioral chamber for a self-administration session. Twelve monkeys (7 dominant, 5 subordinate) were used in this experiment. Sessions were videotaped and latency to touch the bucket and time spent interacting with the bucket were determined from these videos. For these studies, baseline was defined as the mean performance during the three sessions preceding the placement of the red bucket in the homecage.
Experiment 2: Exposure to an enlarged living space
Three separate manipulations were performed: one weekend, consecutive weekends and one consecutive week in the enlarged cage. After a Friday self-administration session when responding was deemed stable (i.e. criteria for stability were met across the Wednesday-Friday sessions), one monkey was returned to the home pen in which one horizontal and both vertical partitions were removed. That is, the monkey had access to all four quadrants of the pen in the absence of the other three monkeys from his social group, who were temporarily housed in a different room. These conditions were in place until Monday morning (the monkey did not leave the cage over the weekend) when the next scheduled self-administration session occurred. Only the monkey living in the enlarged enclosure was studied in the operant chamber on Monday. After the Monday experiment, all monkeys were returned to quadrants of the home cage and fed. After 60–90 min, partitions were removed and monkeys were group-housed. Fifteen monkeys (10 dominant, 5 subordinate) were used in this experiment. For these studies, baseline was defined as the mean performance on Wednesday-Friday sessions. This experiment was repeated to determine whether a second consecutive weekend would result in enhanced effects on cocaine choice. Finally, a subset of monkeys lived in the enlarged enclosure for 7 consecutive days prior to being studied in a self-administration experiment.
Data analysis
The primary dependent measure in the present studies was cocaine choice, defined as the percent of reinforcers received as injections and calculated for each component as the number of fixed ratios completed on the injection-associated switch divided by the total number of fixed ratios completed on both switches. Percent reinforcers was used rather than percent responses because, as described above, FR response requirements differed across monkeys. Daily ED50 values were also determined using the linear portion of the curve which crossed 50% choice. ED50 values were compared using independent or paired t-tests as appropriate. In all cases, differences were considered statistically significant when p<0.05. For experiments in which only one manipulation was made (for example, the effects of snake presentation on cocaine choice), shifts of > 0.25 log units in the ED50 were considered significant.
Results
Experiment 1A: Exposure to preferred treats
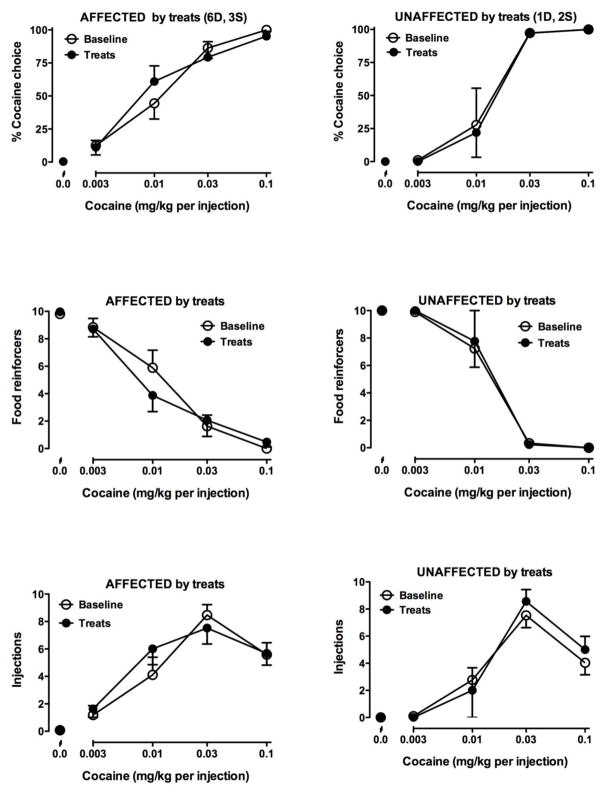
Under baseline conditions (Fig. 1, open symbols), when choice was between food and no injection, responding occurred exclusively on the food switch. When the choice was between a food pellet and a cocaine injection, a dose-related increase in responding on the cocaine switch was observed along with a dose-related decrease in the number of food pellets earned. The relationship between number of injections earned and available dose was characterized as an inverted U-shaped curve. Thus, when the highest dose of cocaine was available as an alternative to food, monkeys tended to take fewer than the maximum available cocaine injections. Videos of baseline sessions in which treats were placed in the bucket in the absence of the snake showed that, on the first day, most of the monkeys immediately approached and reached into the bucket after the partition was pulled. By the third day of treats being placed in the pen, all monkeys immediately approached the bucket, resulting in latencies of less than 3 seconds for all subjects. On average, monkeys spent 463.2 ± 47.0 seconds (out of 600) sitting near and interacting with the bucket during these baseline sessions. Some of this variability appeared to be due to monkeys finding and finishing all treats prior to the end of the 600-second observation session, which tended to decrease time near the bucket. In the 12 monkeys receiving treats before the session, 4 showed shifts to the right and 5 showed shifts to the left in the cocaine choice dose-response curves (Table 2) resulting in no overall effect when data from these monkeys were averaged (Fig. 1, left panels, closed symbols); the curves of three monkeys were unaffected (Fig. 1, right panels; Table 2). There was no relationship between social rank and effects of the environmental manipulation.
Figure 1.
Cocaine choice (top panels), numbers of reinforcers earned as food pellets (center panels) and numbers of injections earned (bottom panels) in monkeys that were affected (left column) or unaffected (right column) by exposure to treats before the self-administration session. The heading of the top graph in each column indicates the number of dominant (D) and subordinate (S) monkeys in each column of graphs. Ordinates: percent of reinforcers earned as injections (top row) and absolute number of food reinforcers earned (center row) or injections earned (bottom row). Abscissae: cocaine dose available as an alternative to one food pellet. Data points are means ± SEM.
Experiment 1B: Exposure to a rubber snake
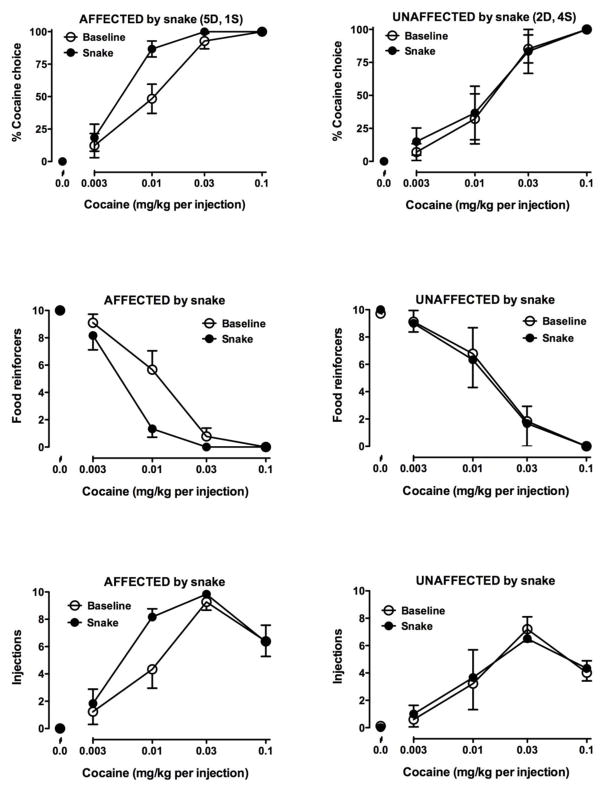
On the day of snake exposure, all latencies to approach the bucket were again less than 3 seconds. Most monkeys spent 5–10 seconds looking into the bucket, then retreated to the opposite cage, typically returning several times to further inspect the bucket. In all but one monkey (C-7079), the time spent sitting near and interacting with the bucket was reduced by more than 90% compared to baseline days. Exposure to a rubber snake for 10 min prior to the cocaine self-administration session resulted in a leftward shift in the cocaine choice dose-effect curve in 5 of the 12 monkeys (Fig. 2, left panels, closed symbols; Table 2). In these monkeys, there was a significant difference in ED50 values when average baseline and post-snake exposure choice curves were compared (0.022 ± 0.007 mg/kg vs. 0.004 ± 0.001 mg/kg, respectively; t(4)=3.034, p<0.05). In one monkey, the cocaine dose-effect curve was shifted to the right. Of the six monkeys affected by the rubber snake, five were dominant animals (Table 2). Affected monkeys decreased the number of food pellets earned as well as increased the number of cocaine injections earned when 0.01 mg/kg was the alternative to food pellets. In other words, the increase in choice was not simply due to a decrease in food-maintained responding. Average ED50 values for baseline curves (before the snake presentation) were not significantly different between affected (0.022 ± 0.007 mg/kg) and unaffected monkeys (0.016 ± 0.007 mg/kg).
Figure 2.
Cocaine self-administration in monkeys exposed to the toy snake. See Fig. 1 legend for details.
Experiment 2: Exposure to an enlarged living space
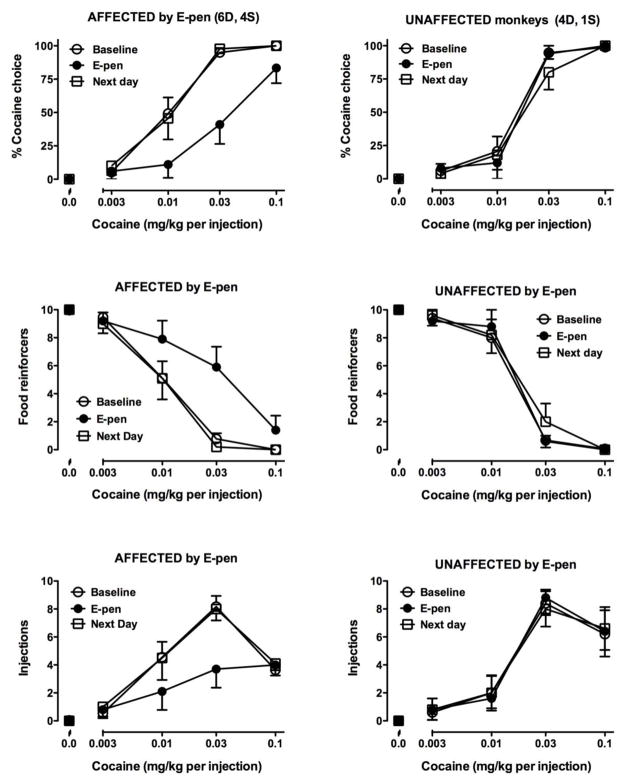
Following three days in the enlarged living space, the cocaine choice dose-effect curve was shifted to the right in 9 of the 15 monkeys tested and to the left in a tenth monkey (Fig. 3, left panels, filled symbols; Table 2). The curve was not affected in the remaining five subjects (Fig. 3, right panels, filled symbols; Table 2). In the 10 affected monkeys, a significant difference was observed when average baseline ED50 values (0.012 ± 0.002 mg/kg) were compared to ED50 values obtained after exposure to the enlarged pen (0.043 ± 0.009 mg/kg; t(9)=3.501, p<0.01). Examination of the reinforcers earned indicated that monkeys decreased drug injections received as well as increased food reinforcers received, indicating that changes in choice following exposure to the enlarged pen were not simply due to an increase in food reinforcers earned. As observed in Experiment 1A, whether or not a monkey was affected by the manipulation was unrelated to the social rank of the monkey. When self-administration was examined on the next day, after monkeys had again been housed with their social groups overnight, choice curves had completely returned to baseline in all monkeys (Fig. 3, open squares). ED50 values on the day after monkeys were returned to their social groups were not significantly different from baseline ED50 values. In follow-up studies, three monkeys were retested in the enlarged pen over the following weekend and four monkeys were retested after one week in the enlarged pen (indicated by subscripted letters c and d, respectively, in Table 2); there were no changes in cocaine choice.
Figure 3.
Cocaine self-administration in monkeys that spent 3 days in the enlarged pen. See Fig. 1 legend for details.
Discussion
Exposure to stress and enrichment has been shown to alter the reinforcing effects of cocaine in laboratory animals. Typically, subjects’ living conditions are homogeneous within and across studies. Questions remain as to whether subjects with a more diverse and ethologically valid range of experiences with respect to living environment, including social interactions, are similarly affected by brief exposure to stress and enrichment. The present studies were designed to characterize the effects of brief exposure to events hypothesized to be a stressor (a rubber snake) or enrichment (pre-session food treats or enlarged living space) on cocaine choice in socially housed male cynomolgus monkeys—a population that consists of members who, as a consequence of their position in the social dominance hierarchy, are exposed chronically to either environmental enrichment (dominant monkeys) or social stress (subordinate monkeys). Of particular interest was the assessment of whether monkeys of different ranks would be differentially sensitive to these effects.
As might be expected from clinical experience in which a given intervention, manipulation or pharmacological treatment is not universally effective, not all monkeys were sensitive to the manipulations examined in these studies. Moreover, it is important to note that the monkeys affected by one particular manipulation were not necessarily the same monkeys affected by the other manipulations, again reflecting the clinical reality that individuals differ in their response to various environmental circumstances and treatments. Thus, it was not the case that a subset of monkeys was generally more sensitive than others to environmental manipulations. The precise characteristics (genetic, neurobiological, temperamental, etc.) that may determine whether an individual was affected by a particular environmental manipulation remains to be elucidated.
An issue that we considered in the experimental design was how to characterize a manipulation as enriching or stressful. One option was to collect blood samples and measure cortisol concentrations. However, these measures do not robustly correlate with the magnitude of stressor in socially housed monkeys (e.g. Sapolsky 2005). In fact, we recently reported that in monkeys forming new social groups, cortisol concentrations were elevated in future subordinate monkeys, but only during the first 3 days of social housing; once hierarchies stabilized, baseline cortisol concentrations were higher on average in dominants (Czoty et al. 2009). A second option was to operationally define an event as enriching if the result was a rightward shift in the cocaine dose-response curve (e.g. Bowling et al. 1993; Bardo et al. 1996) and stressful if the event resulted in shifts to the left (e.g. Goeders 2002; Miczek et al. 2008). In Experiment 1A, we hypothesized that pre-session food treats would be enriching, but surprisingly the cocaine dose-response curve was shifted to the left in 5 monkeys, suggesting that the novel object and treats were stressful. Negus (2003) reported leftward shifts in cocaine dose-response curves following pre-feeding, but the amount of food given to the rhesus monkeys in that study (100 g) was much higher than the 10 M&Ms given to the cynomolgus monkeys in the present study, so the leftward shifts observed in this study were not likely due to food satiation.
We hypothesized that introducing a toy snake in the monkey’s home cage would elicit stress responses (Kalin et al. 2001) and when given the opportunity to choose between cocaine and food, the cocaine dose-response curve would shift leftward. For five of 12 monkeys tested, the cocaine dose-response curve shifted leftward, as hypothesized. What was most surprising was that four of these monkeys were dominant animals. The remaining four subordinate monkeys showed no effect. There are several possible reasons for the rank-related effects of the toy snake on cocaine self-administration. It may be that dominant animals are more vigilant and consequently more sensitive to possible threats (Kaplan et al. 1987). A second possibility is that the red bucket, which was previously associated with treats, had stronger positive conditioning effects in subordinate animals, which attenuated the effects of the toy snake. In partial support of this hypothesis, in two of the four subordinate monkeys not affected by the toy snake, the bucket and treats shifted the cocaine dose-response curve to the right.
In the final experiment, we hypothesized that exposure to the enlarged pen would be enriching in all monkeys, resulting in rightward shifts in the cocaine dose-response curves as observed in other studies that have exposed animals to environmental enrichment (e.g., Green et al. 2002). Of the three experiments, this study produced the most consistent results, with nine of 13 monkeys showing rightward shifts in the cocaine dose-response curve following a weekend in the enlarged pen. Some previous studies did not find significant changes in behavior when monkeys were moved to a larger living space (Crockett et al. 1995; Line et al. 1989). In such studies, however, the increase in available living space was generally modest (e.g., ~50% increase) compared to the present study and others in which the living space was at least doubled (Draper and Bernstein 1963; Paulk et al. 1977; Kitchen and Martin 1996; Kaufman et al. 2004). Under these conditions, decreases in stereotyped and other abnormal behaviors as well as increases in foraging and other normal behaviors were observed.
One potential confound in the design of the Experiment 2 is that the test subject spent the three-day period removed from his social group, which might be hypothesized to affect dominant and subordinate monkeys differently. For example, for subordinate monkeys, the relief from social stress due to the absence of higher-ranked monkeys for several days may have provided an effect additive to the enrichment provided by the larger living space. The converse could be hypothesized for dominants: the absence of pen-mates removed the opportunity to exert dominance and the enrichment associated with that rank (e.g., high frequency of grooming, first access to resources, etc.), which could mitigate any enrichment derived from the larger pen. Although the potential interaction between the absence of the social group and the increase in living space was not explicitly addressed with the present experimental design, the consistency of effects in dominant and subordinate monkeys suggests that the absence of pen-mates did not significantly influence the outcome. It remains possible that such an effect resulted in the subordinates being slightly more likely to be affected in this experiment, as 67% of subordinates tested showed rightward shifts compared to only 55% of dominant monkeys. To address this potential interaction, future studies should assess the effect of cage size per se by relocating the entire social group to a larger cage. It is also worth pointing out that effects on cocaine choice were not due to time away from the self-administration conditions, since most studies were only conducted Monday-Friday and we have not noticed any consistent differences in cocaine choice on Monday compared to other days of the week.
In the present study, the ability of experience with the enlarged living space to decrease cocaine choice was completely and rapidly reversible. After monkeys had been reunited with their social groups for 24 hours, the rightward shift in the cocaine choice curve had completely dissipated. Thus the environmental change alone was effective, but not persistent. This outcome is perhaps not unexpected considering the high rate of relapse to cocaine use that occurs when individuals leave a relatively enriched environment (i.e., a treatment facility) and return to the environment in which drug use previously took place. We also examined whether repeated testing on weekends or allowing the monkey to remain in the enlarged pen for 1 week would enhance the initial effects, but did not observe such an outcome. Future studies will address whether the effect of environmental manipulations on the relative reinforcing strength of cocaine can be enhanced and prolonged with concurrent pharmacological treatment. Findings from the present study highlight the fact that environmental stimuli are not inherently enriching or stressful, and that the same environmental event can produce different effects across subjects. It is likely that a combined approach including pharmacotherapy and a change in environment will be required to produce lasting change in patterns of drug abuse.
Acknowledgments
This research was supported by National Institute on Drug Abuse grant R37 DA10584. The authors report no conflict of interest and would like to acknowledge the technical assistance of Michelle Icenhower and Nicholas Garrett, and the helpful contributions to experimental design provided by Dr. Jay R. Kaplan.
References
- Bardo MT, Donohew RL, Harrington NG. Psychobiology of novelty seeking and drug seeking behavior. Behav Brain Res. 1996;77:23–43. doi: 10.1016/0166-4328(95)00203-0. [DOI] [PubMed] [Google Scholar]
- Botelho S, Estanislau C, Morato S. Effects of under- and overcrowding on exploratory behavior in the elevated plus-maze. Behav Processes. 2007;74:357–362. doi: 10.1016/j.beproc.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Bowling SL, Rowlett JK, Bardo MT. The effect of environmental enrichment on amphetamine-stimulated locomotor activity, dopamine synthesis and dopamine release. Neuropsychopharmacology. 1993;32:885–893. doi: 10.1016/0028-3908(93)90144-r. [DOI] [PubMed] [Google Scholar]
- Brown KJ, Grunberg NE. Effects of environmental conditions on food consumption in female and male rats. Physiol Behav. 1996;60:293–297. doi: 10.1016/0031-9384(96)00020-0. [DOI] [PubMed] [Google Scholar]
- Cain ME, Green TA, Bardo MT. Environmental enrichment decreases responding for novelty. Behav Processes. 2006;73:360–366. doi: 10.1016/j.beproc.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockett CM, Bowers CL, Shimoji M, Leu M, Bowden DM, Sackett GP. Behavioral responses of longtailed macaques to different cage sizes and common laboratory experiences. J Comp Psychol. 1995;109:368–383. doi: 10.1037/0735-7036.109.4.368. [DOI] [PubMed] [Google Scholar]
- Czoty PW, McCabe C, Nader MA. Assessment of the reinforcing strength of cocaine in socially housed monkeys using a choice procedure. J Pharmacol Exp Ther. 2005;312:96–102. doi: 10.1124/jpet.104.073411. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Gage HD, Nader MA. Differences in D2 dopamine receptor availability and reaction to novelty in socially housed male monkeys during abstinence from cocaine. Psychopharmacology. 2010 doi: 10.1007/s00213-009-1756-4. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Gould RW, Nader MA. Relationship between social rank and cortisol and testosterone concentrations in male cynomolgus monkeys (Macaca fasciculars) J Neuroendocrinol. 2009;21:68–76. doi: 10.1111/j.1365-2826.2008.01800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Morgan D, Shannon EE, Gage HD, Nader MA. Characterization of dopamine D1 and D2 receptor function in socially housed cynomolgus monkeys self-administering cocaine. Psychopharmacology. 2004;174:381–388. doi: 10.1007/s00213-003-1752-z. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinsin ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawe S, Loxton NJ. The role of impulsivity in the development of substance abuse and eating disorders. Neurosci Biobehav Rev. 2004;28:343–351. doi: 10.1016/j.neubiorev.2004.03.007. [DOI] [PubMed] [Google Scholar]
- DeGrado TR, Turkington TG, Williams JJ, Stearns CW, Hoffman JM, Coleman RE. Performance characteristics of a whole-body PET scanner. J Nucl Med. 1994;35:1398–1406. [PubMed] [Google Scholar]
- Dellu F, Piazza PV, Mayo W, Le Moal M, Simon H. Novelty-seeking in rats – behavioral characteristics and possible relationship with the sensation-seeking trait in man. Neuropsychobiology. 1996;34:136–145. doi: 10.1159/000119305. [DOI] [PubMed] [Google Scholar]
- Draper WA, Bernstein WS. Stereotyped behaviour and cage size. Perceptual and Motor Skills. 1963;16:231–234. [Google Scholar]
- Drgon T, Zhang PW, Johnson C, Walther D, Hess J, Nino M, Uhl GR. Genome wide association for addiction: replicated results and comparisons of two analytic approaches. PLoS One. 2010 Jan 21;5(1):e8832. doi: 10.1371/journal.pone.0008832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadek-Michalska A, Bugajski J. Repeated handling, restraint, or chronic crowding impair the hypothalamic-pituitary-adrenocortical response to acute restraint stress. J Physiol Pharmacol. 2003;54:449–459. [PubMed] [Google Scholar]
- Goeders NE. The HPA axis and cocaine reinforcement. Psychoneuroendocrinology. 2002;27:13–33. doi: 10.1016/s0306-4530(01)00034-8. [DOI] [PubMed] [Google Scholar]
- Green TA, Gehrke BJ, Bardo MT. Environmental enrichment decreases intravenous amphetamine self-administration in rats: dose-response functions for fixed- and progressive-ratio schedules. Psychopharmacology. 2002;162:373–378. doi: 10.1007/s00213-002-1134-y. [DOI] [PubMed] [Google Scholar]
- Hall FS, Humby T, Wilkinson LS, Robbins TW. The effects of isolation-rearing of rats on behavioural responses to food and environmental novelty. Physiol Behav. 1997;62:281–290. doi: 10.1016/s0031-9384(97)00115-7. [DOI] [PubMed] [Google Scholar]
- Hall FS, Wilkinson LS, Humby T, Inglis W, Kendall DA, Marsden CA, Robbins TW. Isolation rearing in rats: pre- and post-synaptic changes in striatal dopaminergic mechanisms. Pharmacol Biochem Behav. 1998;59:859–872. doi: 10.1016/s0091-3057(97)00510-8. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB., Jr Response to novelty predicts the locomotor and nucleus accumbens response to cocaine. Synapse. 1991;9:121–128. doi: 10.1002/syn.890090206. [DOI] [PubMed] [Google Scholar]
- Howes SR, Dalley JW, Morrison CH, Robbins TW, Everitt BJ. Leftward shift in the acquisition of cocaine self-administration in isolation-reared rats: relationship to extracellular levels of dopamine, serotonin and glutamate in the nucleus accumbens and amygdala-striatal FOS expression. Psychopharmacology. 2000;151:55–63. doi: 10.1007/s002130000451. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ, Kelley AE. The primate amygdala mediates acute fear but not the behavioral and physiological components of anxious temperament. J Neurosci. 2001;21:2067–74. doi: 10.1523/JNEUROSCI.21-06-02067.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB, Adams MR, Weingand KW, Clarkson TB. Inhibition of coronary atherosclerosis by propranolol in behaviorally predisposed monkeys fed an atherogenic diet. Circulation. 1987;76:1364–1372. doi: 10.1161/01.cir.76.6.1364. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB, Clarkson TB, Lusso FM, Taub DM. Social status, environment, and atherosclerosis in cynomolgus monkeys. Arteriosclerosis. 1982;2:359–368. doi: 10.1161/01.atv.2.5.359. [DOI] [PubMed] [Google Scholar]
- Kaufman BM, Pouliot AL, Tiefenbacher S, Novak MA. Short and long-term effects of a substantial change in cage size on individually housed, adult male rhesus monkeys (Macaca mulata) Applied Animal Behav Sci. 2004;88:319–330. [Google Scholar]
- Kitchen AM, Martin AA. The effects of cage size and complexity on the behaviour anf captive common marmosets, Callithrix jacchus jacchus. Lab Animals. 1996;30:317–326. doi: 10.1258/002367796780739853. [DOI] [PubMed] [Google Scholar]
- Line SW, Morgan KN, Markowitz H, Strong S. Influence of cage size on heart rate and behavior in rhesus monkeys. Am J Vet Res. 1989;50:1523–156. [PubMed] [Google Scholar]
- Miczek KA, Yap JJ, Covington HE., 3rd Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther. 2008;120:102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Prioleau OA, Nader SH, Kaplan JR, Nader MA. Predictors of social status in cynomolgus monkeys (Macaca fascicularis) after group formation. Am J Primatol. 2000;52:115–131. doi: 10.1002/1098-2345(200011)52:3<115::AID-AJP1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Nader MA, Czoty PW. PET imaging studies of dopamine D2 receptors in monkey models of cocaine abuse: genetic predisposition versus environmental modulation. Am J Psychiatry. 2005;162:1473–1482. doi: 10.1176/appi.ajp.162.8.1473. [DOI] [PubMed] [Google Scholar]
- Negus SS. Rapid assessment of choice between cocaine and food in rhesus monkeys: effects of environmental manipulations and treatment with d-amphetamine and flupenthixol. Neuropsychopharmacology. 2003;28:919–931. doi: 10.1038/sj.npp.1300096. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Shelton SE, Kalin NH. Individual differences in the responses of naïve rhesus monkeys to snakes. Emotion. 2003;3:3–11. doi: 10.1037/1528-3542.3.1.3. [DOI] [PubMed] [Google Scholar]
- Paronis CA, Gasior M, Bergman J. Effects of cocaine under concurrent fixed ratio schedules of food and IV drug availability: a novel choice procedure in monkeys. Psychopharmacology. 2002;163:283–291. doi: 10.1007/s00213-002-1180-5. [DOI] [PubMed] [Google Scholar]
- Paulk HH, Dienske H, Ribbens LG. 1 Abnormal behavior in relation to cage size in rhesus monkeys. Journal of Abnormal Psychology. 1977;86:87–92. doi: 10.1037//0021-843x.86.1.87. [DOI] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology. 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonet V, Rouge-Pont F, Le Moal M. Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci. 2000;20:4226–4232. doi: 10.1523/JNEUROSCI.20-11-04226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PF, Rouge-Pont F, Deminiere JM, Kharoubi M, Le Moal M, Siman H. Dopamine activity is reduced in the prefrontal cortex and increased in the nucleus accumbens of rats predisposed to develop amphetamine self-administration. Brain Res. 1991;567:169–174. doi: 10.1016/0006-8993(91)91452-7. [DOI] [PubMed] [Google Scholar]
- Prather MD, Lavenex P, Mauldin-Jourdain ML, Mason WA, Capitanio JP, Mendoza SP, Amaral DG. Increased social fear and decreased fear of objects in monkeys with neonatal amygdala lesions. Neuroscience. 2001;106:653–658. doi: 10.1016/s0306-4522(01)00445-6. [DOI] [PubMed] [Google Scholar]
- Rilke O, May T, Oehler J, Wolffgramm J. Influences of housing conditions and ethanol intake on binding characteristics of D2, 5-HT1A, and benzodiazepine receptors of rats. Pharmacol Biochem Behav. 1995;52:23–28. doi: 10.1016/0091-3057(95)00093-c. [DOI] [PubMed] [Google Scholar]
- Rouge-Pont F, Piazza PV, Kharouby M, Le Moal M, Simon H. Higher and longer stress-induced increase in dopamine concentrations in the nucleus accumbens of animals predisposed to amphetamine self-administration. A microdialysis study. Brain Res. 1993;602:169–174. doi: 10.1016/0006-8993(93)90260-t. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Schenk S, Lacelle G, Gorman K, Amit Z. Cocaine self-administration in rats influenced by environmental conditions: implications for the etiology of drug abuse. Neurosci Lett. 1987;81:227–231. doi: 10.1016/0304-3940(87)91003-2. [DOI] [PubMed] [Google Scholar]
- Stairs DJ, Bardo MT. Neurobehavioral effects of environmental enrichment and drug abuse vulnerability. Pharmacol Biochem Behav. 2009;92:377–382. doi: 10.1016/j.pbb.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Sanabria F, Pentkowski NS, Neisewander JL. Anti-craving effects of environmental enrichment. Int J Neuropsychopharmacol. 2009;12:1151–1156. doi: 10.1017/S1461145709990472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner FA, Anthony JC. From first use to drug dependence; developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology. 2002;26:479–488. doi: 10.1016/S0893-133X(01)00367-0. [DOI] [PubMed] [Google Scholar]
- Yuferov V, Levran O, Proudnikov D, Nielsen DA, Kreek MJ. Search for genetic markers and functional variants involved in the development of opiate and cocaine addiction and treatment. Ann N Y Acad Sci. 2010;1187:184–207. doi: 10.1111/j.1749-6632.2009.05275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]