Abstract
The initial success seen in adult cardiac surgery with the application of available robotic systems has not been realized as broadly in pediatric cardiac surgery. The main obstacles include extended set-up time and complexity of the procedures, as well as the large size of the instruments with respect to the size of the child. Moreover, while the main advantage of robotic systems is the ability to minimize incision size, for intracardiac repairs, cardiopulmonary bypass is still required. Catheter-based interventions, on the other hand, have expanded rapidly in both application as well as the complexity of procedures and lesions being treated. However, despite the development of sophisticated devices, robotic systems to aid catheter procedures have not been commonly applied in children. In this article, we describe new catheter-like robotic delivery platforms, which facilitate safe navigation and enable complex repairs, such as tissue approximation and fixation, and tissue removal, inside the beating heart. Additional features including the tracking of rapidly moving tissue targets and novel imaging approaches are described, along with a discussion of future prospects for steerable robotic systems.
Keywords: heart surgery, image guided, interventional cardiology, minimally invasive, pediatric, robotics
Current status of robotically assisted pediatric cardiac surgery
Over the last two decades robotically assisted surgical procedures have been introduced into the field of cardiac surgery. In contrast to conventional open heart surgery, robotic surgery offers the advantages of minimal trauma to neighboring structures, while the use of robotic tools provides the surgeon with the ability to operate precisely in limited spaces. Currently, the da Vinci® Surgical System is the only US FDA-approved system for intracardiac procedures. It consists of teleoperated surgical arms with a surgical console, a patient-side cart, four interactive robotic arms, a high-definition high-magnification 3D endoscopic vision system and proprietary EndoWrist® instruments (Intuitive Surgical, Inc., Sunnyvale, CA, USA). These 7 degree of freedom instruments, which possess small mechanical wrists, are designed to function as the surgeon’s forearm and wrist, with dexterity provided at the operative site through the entry ports. A computer system causes the robot arms to mimic the surgeon’s hand motions that are applied through the surgical console while also enabling the features of tremor elimination, motion scaling and motion indexing.
Since the first report of a robotically assisted cardiac surgical procedure by Carpentier et al. in 1998 [1], the da Vinci system has been used mostly in adult patients undergoing coronary revascularization or mitral valve repair [2,3]. There is limited experience with robotically assisted procedures in children. For extracardiac procedures, there are reports of successful robotically assisted patent ductus arteriosus ligation and vascular ring divisions, among other thoracic noncardiac procedures [4–7]. The conclusions of many of these reports, however, is that due to the large instrument size and need for entry port sites that are relatively far apart to avoid interference between the robotic arms, use of this system in children less than approximately 30 kg is quite difficult. For these reasons, most surgeons who have utilized the da Vinci system in children believe that a robotic approach is comparable but has no major advantages over nonrobotic thoracoscopic instruments using video-assisted techniques. For intracardiac repairs, the use of the da Vinci system has been limited to a small series of adult-size patients undergoing atrial septal defect (ASD) closure. For example, Torracca et al. have used the da Vinci surgical system for the repair of ASD in seven patients [8]. In their report, five patients had ASDs, whereas the other two patients had a patent foramen ovale (PFO) with atrial septal aneurysm. Argenziano et al. and Wimmer-Greinecker et al. reported a totally endoscopic ASD repair procedure using the da Vinci system in 17 and ten patients, respectively [9,10]. Baird et al. reported closure of the ASD using the da Vinci system and hypothermic fibrillatory arrest [11]. In all of these reports, the operative times and cardiopulmonary bypass (CPB) times were substantially longer than for the conventional open-heart approach due to extended set-up time and complexity of the procedure, which remains one of the main obstacles to widespread acceptance of such a technique. In addition, in most of the series 8 mm instruments were used, which have a larger working area and therefore limited the use of the robotic system in younger patients. Recently, a 5-mm instrument set has been introduced; however, there is limited experience with these instruments. In addition, Intuitive Surgical has announced a single-port instrument set, which is not yet available on the market for cardiac procedures, but is currently undergoing feasibility studies in adult laparoscopic procedures [12].
Despite the reduced invasiveness of the robotically assisted cardiac procedures, there is still a need for the use of CPB and its inherent risks, albeit low, for cardiac and noncardiac complications [13]. Furthermore, since in most of these procedures, bypass is achieved by peripheral vessel cannulation, the small size of children’s vessels with respect to cannula size introduces the added risk of permanent vessel damage and its impact on limb growth [14].
Catheter-based robotic interventions
Concurrent with developments in surgical robotic technology, catheter-based percutaneous procedures have also evolved and have become much more widespread. Development of multiple devices that can be delivered via catheter and development of delivery techniques have facilitated the application of this technology. Since a transcatheter technique for ASD closure was introduced in 1974 by Mills and King [15], this less-invasive approach has gradually become a routine procedure. In children, catheter-based techniques are now used for a wide variety of interventions including other types of septal defect closure, angioplasty with or without stents, valvuloplasty and, more recently, delivery of stent-mounted valves [16].
Although, robotically assisted catheter-based interventions are not widely used in pediatric interventional cardiology practice, there are procedures in adults where robotic systems are utilized. Currently, there are two robotic catheter technologies available, electromechanically based systems and magnetically controlled systems. Hansen Medical Inc. (Mountain View, CA, USA) offers the Sensei® X Robotic Navigation System designed for electrophysiology interventions, while their novel Magellan™ Robotic system is a platform for peripheral vascular interventions. The Sensei X system consists of a surgeon’s workstation, a remote catheter manipulator and a steerable guide catheter [17]. The workstation has a visualization module, which incorporates real-time imaging and 3D electroanatomical mapping and a manually controlled input device for controlling catheter motion. The remote slave catheter robot is mounted on the operating table. The Artisan Extend™ Control Catheter (Hansen Medical, Mountain View, CA, USA) consists of an articulating inner guide 10.5 cm in length, a steerable multidirectional inner guide (11.5 F outer diameter), and steerable unidirectional outer guide sheath (14 F outer diameter). The system accommodates catheters that are 8 F or smaller in diameter and have a minimum usable length of 115 cm. The system may be delivered over an independent guide wire, but does not depend on that wire to maneuver. The system provides 6 degrees of freedom and allows transmission of the surgeon’s movements to the catheter tip, while the outer guide provides stability. The Magellan System works with the novel NorthStar™ catheter (Hansen Medical, Inc.), which is designed to ensure simultaneous distal tip control of a catheter and a sheath, enabling more precise device deployment. The tip of the sheath is articulated up to 90° in any direction, while the tip of the leader is articulated in up to 180° in any direction. The system may also accommodate devices 6 F or smaller. An initial experimental study on a porcine model, where the iliac artery, bilateral renal arteries and the superior mesenteric artery were cannulated, demonstrated that the system exhibits greater stability in comparison to a conventional manual catheter and provides less trauma to the vessels intima [18].
The NIOBE® Magnetic Navigation System (Stereotaxis, St Louis, MO, USA) is operated by a magnetic field created by two computer-controlled 0.08 T permanent magnets [19–21]. The magnets are mounted on articulating arms that are enclosed within a stationary housing, with one magnet on either side of the patient table. By changing the positions of these magnets with respect to the patient, deflection of the magnetic tip of the catheter can be precisely controlled. This requires the use of proprietary magnetic intravascular guide wires (TITAN® and Pegasus™ [Stereotaxis]), which are advanced manually. The system accommodates diagnostic and ablation catheters up to 8 F in diameter, with up to 120° bend radius.
Recently, a magnetically controlled system that utilizes a technology of dynamically shaped magnetic fields was introduced (Catheter Guidance Control and Imaging, Magnetec, Los Angeles, CA, USA). The system consists of eight coil-core electromagnets arranged semispherically around a standard fluoroscopy table, which generates a shaped (‘lobed’) dynamic magnetic field within the region of the patient’s heart. Each coil is independently controlled and a computer calculates the instantaneous current values for the eight coils in real time, which enables catheter movement in 6 degrees of freedom. The Catheter Guidance Control and Imaging active sheath may be operated in a manual magnetic mode, when a surgeon navigates the tip of the catheter using a joystick-type controller, or in an automated mode, when a computer plans a path toward the target and then guides the catheter tip until it makes a firm and continuous tissue contact. In a feasibility study in a porcine model, the system demonstrated encouraging results, delivering precise continuous contact with target at a force up to 23 g [22].
Robotic catheter applications include electrophysiologic procedures for arrhythmia ablation, peripheral vascular interventions, coronary interventions and, more frequently, transcatheter valve interventions [17–23]. Lumsden et al. reported successful robot-assisted stenting of a stenosis at the pulmonary artery anastomosis in a 72-year-old patient following lung transplantation. A Sensei system was used and it was reported to have markedly enhanced stability and facilitated successful navigation of the balloon catheter to the stented site [24]. Other studies demonstrated a reduction in procedure times, increased precision in targeting and increased catheter tip stability in comparison with standard manual navigation [25]. In addition, surgeon comfort and ergonomics were significantly increased while at the same time fluoroscopy exposure for the surgeon was reduced [17].
To date, however, most of these interventions are fundamentally device deployment or tissue ablation rather than tissue reconstructive procedures, which are still in the realm of the cardiac surgeon. This is principally due to the limitations of current robotic catheter design, which include limited ability for significant force application, especially in a lateral direction from the axis of the catheter and stable position control that is sufficient for tissue manipulation. These limitations impair the surgeon’s ability to manipulate, plicate, approximate and remove tissue as performed during complex repairs in open heart surgery.
Beating-heart intracardiac image-guided robotic surgery
Beating-heart intracardiac surgical repair of congenital and acquired defects has a long history and has evolved greatly over the decades. Initial beating heart approaches to intracardiac disease included finger fracture of calcified mitral valves, use of a rubber ‘well’ to repair septal defects and the introduction of a cardioscope for guiding intracardiac repairs. With the introduction of CPB, beating heart approaches were mostly abandoned, since CPB permitted precise repairs in a still and bloodless field. However, in light of the deleterious effects of CPB, there has been an ongoing interest in developing techniques to perform the same types of repairs currently performed as open procedures, but with the heart beating. Several reports, mostly in adults, have described new methods for repairing septal defects and reconstructing regurgitant valves inside a beating heart. Investigators in Thailand recruited 76 patients to undergo placement of an ASD patch with blind suture fixation followed by intra-atrial stapling under transesophageal echocardiography guidance [26]. Beating-heart insertion of artificial chords to treat mitral valve prolapse has been described and the system is currently undergoing Phase I trials in Europe [27]. Laboratory efforts have included direct image-guided approaches including optical imaging with an endocardioscope in eight dogs for septal defect repair [28] and transesophageal echocardiography-guided mitral valve suturing in a porcine model. Our group has reported beating-heart ASD and ventricular septal defect closure under image guidance in swine models [29,30]. An originally developed patch delivery device and handheld anchor delivery system were utilized for defect closure, and real-time 3D echocardiography and video-assisted cardioscopy was used for intraoperative imaging and instrument navigation. After these initial attempts, it has become evident that, in order to bring such an approach to clinical practice and also utilize it in various repairs, two major developmental efforts are required. First, new sets of instruments and devices need to be developed that provide tactile feedback, limit interference with imaging tools and provide steerability. Secondly, real-time, high-resolution imaging, which also provides tissue and instrument tracking, is required for safe navigation inside cardiac chambers.
Instruments & devices
Challenges for robotic solutions
The potential value of robotics in beating-heart intracardiac surgery is to provide a tool delivery platform that possesses the stability, stiffness and dexterity necessary to perform beating-heart interventions with the efficacy of open-heart procedures. Existing surgical robotic systems are ill suited to this task for several reasons [31]. First, their instruments possess straight shafts and are of relatively large diameter. Furthermore, they utilize the tools and techniques of conventional surgery. These techniques, such as suturing, are well suited to situations where there is an open workspace adjacent to the surgical site in which to perform complex motions of the robot hands to manipulate tools.
For intracardiac surgery, however, the workspace available to a robot consists of the comparatively small internal volumes of the cardiac chambers. In addition, navigation through the heart, while avoiding damage to delicate fast-moving structures, such as valves and valvar apparatus, favors the use of robots comprised of curved rather than straight components. Thus, the challenges of intracardiac surgery suggest that new approaches are needed. These approaches should involve new robot architectures that meet the size constraints of intracardiac surgery as well as new tools that are matched to the capabilities of these robots. In addition, new interventional techniques must be developed that, instead of mimicking existing open-heart surgery, leverage the advantages of robotics. These advantages include providing precise repeatable motion control, integrated imaging and other types of sensing and improved ergonomics.
Concentric tube robots
Concentric tube robots represent a new type of robot architecture that has been developed for minimally invasive surgery [32–40] and, in particular, for surgery inside the beating heart [38,40–42]. Comprised of concentrically combined precurved elastic metal tubes, their shape consists of a continuous curve along their length that is actively controlled by rotating and translating the constituent tubes at the robot’s base. While the stiffness of these robots is substantially higher than that of catheters, their steerability enables safe navigation through the vasculature and cardiac chambers. The innermost tube of the robot serves as a lumen for deploying and controlling tools at the robot tip.
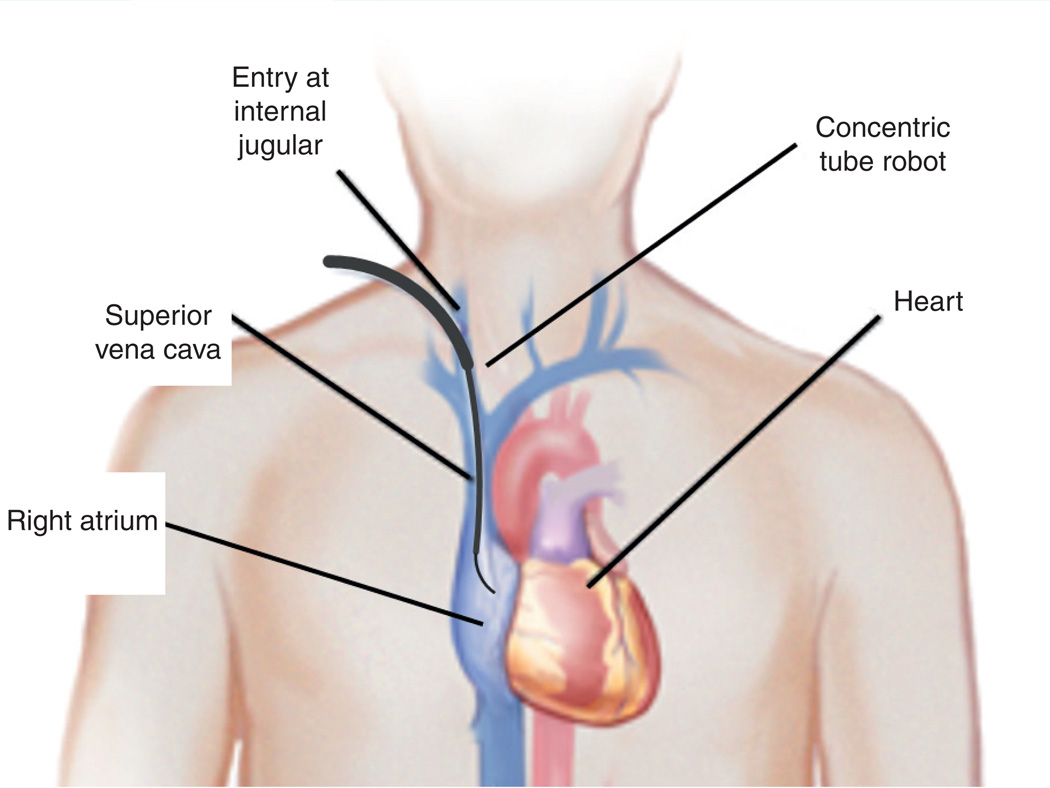
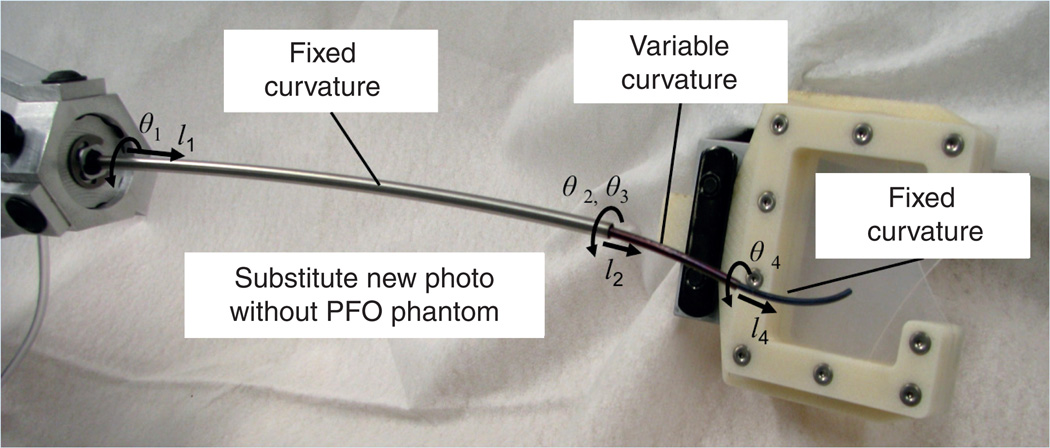
While existing medical robots employ a single robot design for all surgeries, the tube set comprising a concentric tube robot is designed for a specific procedure [33,40]. These tube sets snap into a motorized drive system (common to all procedures) in a manner similar to existing robotic catheters (e.g., Hansen Medical, Inc.). These tube sets can be designed to be either disposable or sterilizable. For the purpose of designing robots for new procedures inside the heart, design algorithms have been created that utilize anatomical images to define workspace constraints, while the steps of the surgical task provide the set of tip configurations (positions and orientations) that the robot must reach [40]. For example, Figure 1 provides a schematic for PFO closure indicating the path for the robot to navigate from the internal jugular vein to the right atrium [38]. The corresponding 7-degree of freedom robot design is shown in Figure 2. In practice, robot designs are often comprised of two parts in which the proximal portion of the robot is responsible for navigation to the surgical site through telescopic extension and the distal portion of the robot is responsible for manipulating tools and tissue at the surgical site. In the design of the robot in Figure 2, a single proximal section is responsible for navigation and it can be locked in position once the robot has entered the right atrium. The two distal sections are actively controlled during the PFO closure to manipulate the septal tissue and to deploy the closure device.
Figure 1. Concentric tube robot entering the beating heart via the internal jugular vein.
Figure 2. Robot used for patent foramen ovale closure.
Design consists of three telescoping sections.
PFO: Patent foramen ovale.
Note that the middle section in this design is of variable curvature. In general, each telescoping section is designed to have either fixed or variable curvature [33]. A fixed curvature section relaxes to the shape of its precurvature when it is extended from the preceding section. By contrast, a variable curvature section can take on a continuous range of curvatures usually ranging between zero (straight) and a maximum value.
The robot in Figure 2 has been successfully employed by our group for PFO closure in an in vivo porcine trial [38]. In these procedures, 3D real-time echocardiography was used to navigate the robot toward the target and x-ray fluoroscopy was utilized when the tools (please see below) were then used for the PFO closure. An important advantage of the robot in comparison to manual devices, such as catheters, is its ability to hold its configuration between commanded motions. This capability enabled the surgeon to perform imaging studies without any risk that the robot would change its position or contact configuration with the tissue. Furthermore, no adverse effects from having the robot inside the heart (e.g., arrhythmias) were observed.
Intracardiac tools
Two fundamental maneuvers performed in reconstructive surgery are tissue approximation and tissue removal. The two procedures alone or combined, account for a large number of cardiac surgical interventions. Tissue approximation, such as direct PFO closure, or valve reconstruction is a major component of surgical procedures. Similarly, relief of obstruction, at the outflow tract of a ventricle, for example, is a component of many of congenital heart procedures.
Tissue approximation
Fundamentally, tissue approximation is the act of grasping one piece of tissue and moving it next to another piece of tissue in order to alter the anatomy of an organ or blood vessel.
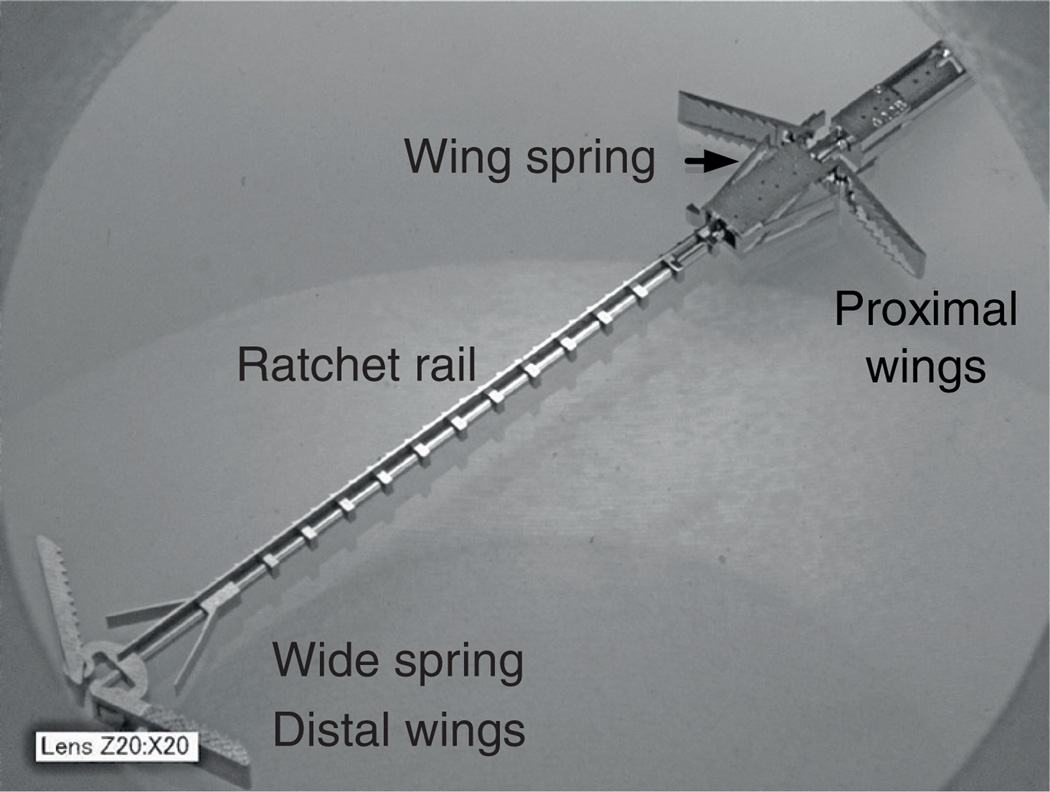
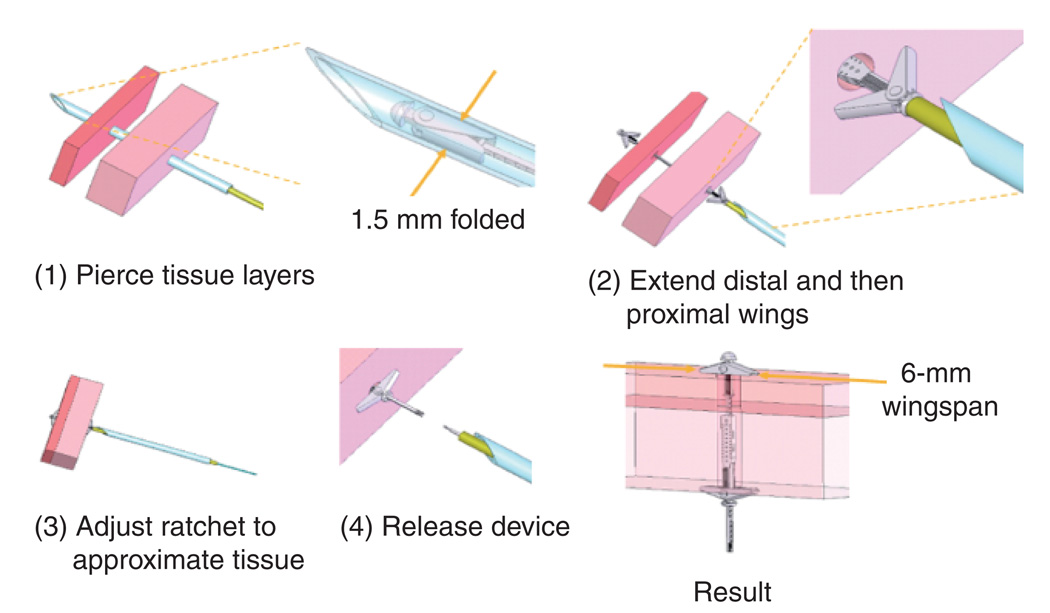
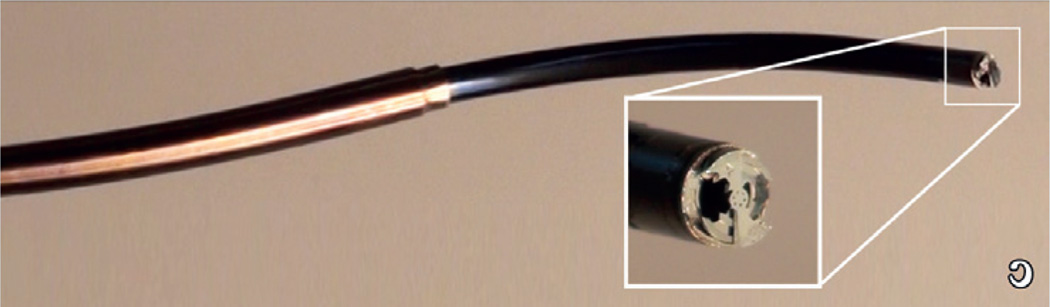
In open-heart surgical procedures, the grasping is usually performed with standard surgical instrument, such as forceps or hooks, and the two tissue sections fixed together by sutures or clips. Currently, there are no catheter-based instruments or techniques for tissue approximation other than clips or staples that snap together, trapping tissue in between the jaws of the clip. One specific example of tissue approximation is during PFO closure. Current approaches to closure include open-heart surgery, and catheter-based deployment of an occluder device. However, experience with device closure, shows that serious complications, such as major hemorrhage, cardiac tamponade, the need for surgery, pulmonary embolism and death occur in 1.5% of patients, and minor complications (arrhythmia, device fracture or embolization, air embolism, femoral hematoma and fistula) in another 7.9% [43]. Results with open-heart surgery indicate a significantly lower risk of complications and no recurrence at 23 months of follow-up [44]. A device and technique of PFO closure that mimics surgical closure was developed (Figure 3) and tested in large animal studies [42]. The device is manufactured fully assembled using a metal microelectromechanical systems fabrication process that can produce hundreds of the device on a single wafer. It is comprised of two pairs of expanding spring-loaded wings that are used to pull the tissue layers together. The wing pairs are attached by a ratcheting mechanism that enables the tissue layer approximation distance to be adjusted with submillimeter accuracy. Device deployment is illustrated schematically in Figure 4. The two tissue layers are first pierced at the location where approximation is desired. During robotic deployment, a sharpened stylet inserted through the robot was used in place of the cannula. Not shown in the figure, the robot produces appropriate overlap of the septum secundum and primum by first piercing the secundum and then dragging it laterally to achieve the desired overlap with the septum primum. The robot then punctures the second layer and proceeds with device deployment.
Figure 3. Metal microelectromechanical systems tissue approximation device.
Figure 4. Tissue approximation device deployment sequence.
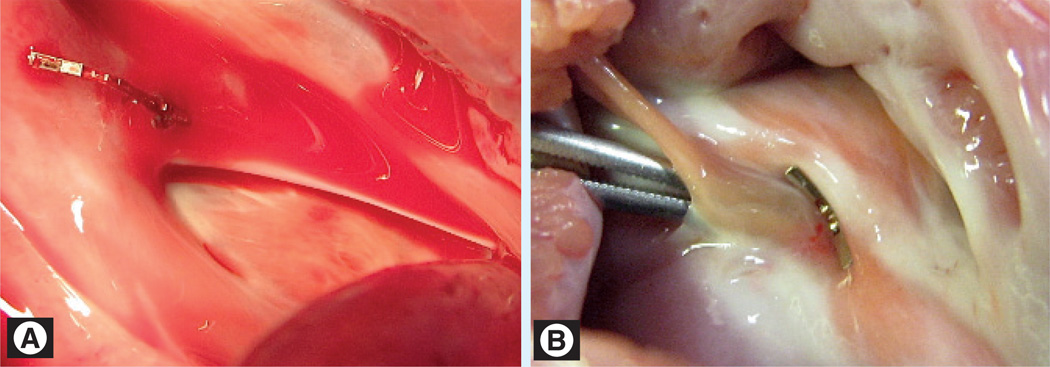
Successful PFO closure using the device has been demonstrated in porcine in vivo trials [38]. Post-mortem views from the right and left atrium are shown in Figure 5. Of note is how little foreign material is exposed to the blood on the left atrial side of the septum, in comparison to existing occlusion devices.
Figure 5. Implanted approximation device.
(A) Right atrial view. (B) Left atrial view.
Tissue removal
Abnormal tissue growth or the presence of abnormal tissue structures that interfere with normal heart function is one of the more common indications for surgical intervention in children. Examples of these are subaortic membrane, supravalve mitral membrane and abnormal muscle bundles in the right ventricle (RV), such as in the double-chambered RV. Treatment of the obstruction consists of either plastic deformation of the obstructing tissue by balloon dilatation, in catheter-based techniques, or partial to complete removal via direct open-heart surgery. Balloon dilatation with or without cutting blades is effective in enlarging narrowed vessels and in some types of valvular obstruction or stenosis since it depends on creating a tear in the tissue [45]. Its major limitation, however, has been finding the right balance between dilating or tearing the abnormal tissue as opposed to the normal tissue that comprises the structure of the valve or subvalve area of the heart. Thus the efficacy is largely dependent on the material properties of the abnormal tissue versus the normal tissue. For this reason, balloon dilatation of valvular obstruction has achieved success primarily where the abnormal tissue is fibrous and can undergo plastic deformation (i.e., can be stretched or torn), whereas the normal tissue is more elastic. This remains an important limitation, since often the characteristics of the normal and abnormal tissue are similar and therefore the only currently available form of treatment is open surgical removal of the abnormal tissue.
In children, obstructions in the right or left ventricular outflow tract account for one of the more common causes of heart muscle hypertrophy and subsequent dysfunction [46]. Open-heart surgery to remove abnormal tissue or, in severe cases, to replace the abnormal structure, is often the only option. The abnormal obstructing tissue in children is usually elastic, making simple balloon dilatation ineffective, since inelastic deformation is nearly impossible to achieve without damage to normal valve structures. In the RV, the obstruction is most often from abnormal ventricular muscle that creates both dynamic and fixed obstruction. In the left ventricle, the abnormal tissue is fibroelastic and balloon dilatation has been shown to be ineffective. The surgical procedure involves removal of the abnormal muscle and fibroelastic tissue.
An alternative approach that mimics surgical tissue removal is under development. It utilizes the concentric tube robot described above, with an integrated tissue removal tool as shown in Figure 6 [41]. To remove abnormal obstructions from the RV outflow tract, a navigation route similar to that shown in Figure 2 can be employed. In this case, the robot enters the heart percutaneously from the right internal jugular vein and passes through the tricuspid valve into the RV. From there, the robot can be steered to the RV outflow tract and the cutting tool can be employed to sculpt away excess tissue. The cutting tool provides integrated irrigation and aspiration in order that the morselized debris can be transported out of the heart through the lumen of the robot. Irrigation using a heparized normal saline solution facilitates transport while minimizing both blood loss and device clogging due to emboli formation.
Figure 6. Metal microelectromechanical systems tissue removal device.
Both irrigation and aspiration are incorporated into the design to remove tissue debris through the robot lumen.
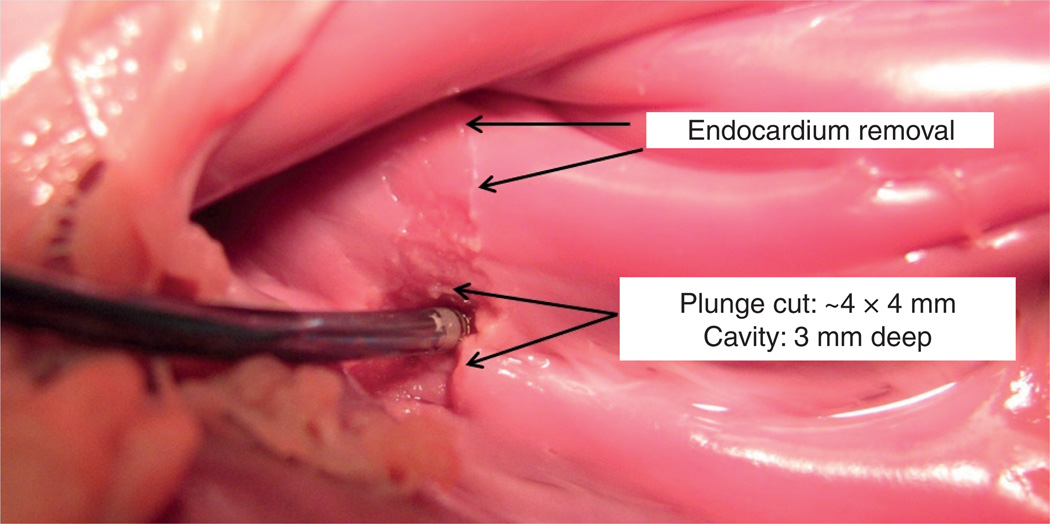
Figure 7 shows results from ex vivo experiments on two types of tissue [41]. Near the top, removal of the fibrous endocardial surface layer was performed with a gentle sweeping motion along the surface exposing the underlying myocardium. Removal of myocardial tissue is also possible, as shown. In this case, a cavity was milled into the tissue by plunging the tool roughly at a normal angle to the surface and sweeping it in a small circular pattern as it descended into the tissue. While the majority of cutting debris was aspirated through the robot lumen, a downstream embolization filter may need to be deployed into the main pulmonary artery to collect any particulate emboli that may be dislodged by the process of tissue removal.
Figure 7. Ex vivo example of tissue removal in the outflow tract of the pulmonary valve.
Robotically assisted motion compensation tools
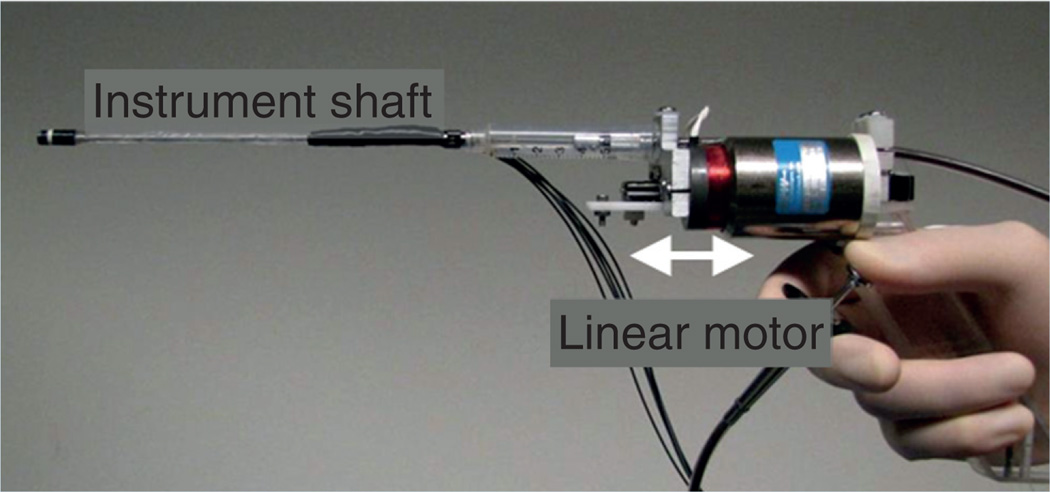
For interacting with rapidly moving structures such as valves, tool tip stabilization may not be adequate and a different approach may be required to avoid collision with delicate cardiac structures. One option is to capture and stabilize the tissue, such as a valve leaflet and if possible, to immobilize or dampen its movements. This approach is used in several tools for beating-heart mitral valve repair including MitraClip® (Abbott Laboratories. Abbott Park, IL, USA), NeoChord DS 1000 device (NeoChord Inc., Minnetonka, MN, USA), among others [27,47]. An alternative approach is for the instrument to move in coordination with the moving tissue, also called motion cancellation, which involves tracking tissue movement using image-based tracking algorithms and robotically moving the instrument tip so as to physically couple its motion relative to the tissue. The complexity of such a system depends on several factors, including the precision of image based tissue tracking, the motion profile of the tissue (i.e., how far and how rapidly it is moving in 3D space) and the ability of the instrument positioner to move at the same rate in all three directions. Such a device was developed (Figure 8) and tested in an animal model where the movement is predominantly in one direction, such as with valve leaflets or valve annulus [48].
Figure 8. Handheld 1 degree of freedom motion compensation Instrument.
The device, a motion compensation instrument (MCI), is a 1 degree of freedom robotic tool that is operated by an algorithm based on the real-time 3D echocardiography imaging. The system identifies and tracks the position of the tissue target directly in front of the tool and a linear motor moves the instrument shaft according to the target motion (Supplementary Video see online www.futuremedicine.com/doi/suppl/10.2217/FCA.12.20). This device was tested in an in vivo beating-heart animal model and was shown to minimize collisions with tissue and was able to track the complex motion of the mitral annulus in real time, giving the surgeon precise control of the relative movement of the instrument tip with respect to the target tissue.
In these studies we found that one of the limitations of image-based tracking is that once the surgical instrument tip comes into contact with the tissue target, the algorithm can no longer separate tissue movement from the instrument tip and, therefore, cannot control the instrument accurately. To address this limitation, a second-generation MCI was developed that, in addition to the image-based tracking, utilizes a force-control tracking algorithm [49]. A force sensor, which was placed on the tip of the MCI, reads the force that the surgeon applies to the target tissue in real time. The force control algorithm thus enables maintenance of constant force against the tissue, which significantly increases the safety of the procedure.
Imaging
For intracardiac surgery without CPB, imaging of the surgical field must meet the highest performance standards in order to provide a high-resolution image in real time. For these procedures to succeed, the surgeon must be able to navigate to the surgical site, perform the required task and then confirm adequate and accurate completion of the task.
3D echocardiography
Real-time 3D echocardiography is an extremely useful imaging modality for guiding beating-heart intracardiac interventions, given its relatively large field of view and its ability to image the surgical tool and the tissue structures simultaneously [50–54]. One challenge, however, is that medical instruments and robotic tools produce imaging artifacts that can make it difficult to clearly visualize the instrument as well as nearby tissue. Most surgical instruments are made of hard materials with smooth surfaces that produce a variety of image artifacts when ultrasound waves bounce off their surfaces [55,56]. The most common artifacts can be grouped into three types: shape-smoothing artifacts in which fine details of instrument shape are obscured, dropout artifacts for which parts of the instrument appear and disappear as it is moved, and topological artifacts which alter the shape of instruments [56]. These three types of artifacts combine to obscure both the instrument’s geometric details, as well as its relative location with respect to tissue.
A variety of solutions to the artifact problem have been introduced. These include instrument modification, image processing techniques, active tracking sensors and fiducial markers. Instrument modification involves the application of coatings or surface modifications to reduce the specularity of reflections or to increase absorption [55–59]. Image processing methods apply search techniques to locate an instrument in an image [60–64]. Tracking sensors can also be placed on the surgical tool to detect instrument position and by registering the position relative to the ultrasound image provide real-time information as to the position of the tool within the image [65]. Fiducial markers on the instruments that are strongly echogenic can also be used to enable the instrument position and orientation to be detected using image-based algorithms from the marker image [66,67].
Other imaging modalities & image fusion
X-ray fluoroscopy, and cineangiography, are still considered as the most common imaging modality in pediatric image-guided cardiac interventions, despite the potential harmfulness of x-rays [68–70]. They provide high resolution at a fast frame rate. The recent introduction of rotational angiography and C-arm computed tomography hold great promise for overcoming the well-known limitations of this modality, such as the absence of real-time 3D information and lack of comprehensive representation of soft tissue, without additional contrasting. Despite the fact that the 3D reconstruction is not performed in real time, it provides significant additional information for the surgeon, especially in percutaneous valvular interventions [71].
The purpose of image fusion is to provide the surgeon with additional anatomical information that may be obtained with only one of the modalities, but if combined yields a better understanding of the anatomy and device as well as instrument navigation. Real-time echocardiography or x-ray fluoroscopy information may be successfully displayed with preoperative computed tomography or MRI patient data. Images are aligned based on anatomic features or man-made objects specifically introduced into the image, known as fiducials. This approach is successfully utilized in arrhythmia ablation, transcathter valve interventions and other procedures [72,73]. Multimodality displays, that are able to simultaneously represent these complex 2D and 3D anatomical data from various imaging modalities and also incorporate real-time instrument positioning as well as patient functional information, are built in to modern robotic consoles.
These techniques can enhance the surgeon’s ability to ‘see’ the robot tool position. This improved estimate of robot position can be calculated in real time during a procedure and superimposed on the live image to provide an augmented reality display for the interventionalist [74]. Alternatively, it can be employed for image-based control of robot motion. In this way, portions a surgical task can be automated [75–77], or relative motion between the tool and the tissue can be controlled [48]. These techniques create an integrated experience for the surgeon in which they perceive the imaging and robotic systems as extensions of their own sensing and actions. This is extremely important for decision making during image-guided interventions, with the absence of direct visualization and haptic feedback.
Conclusion
Robotic systems to enable or enhance the capabilities of the surgeon to work in confined spaces and perform complex tasks have evolved significantly. Current clinically available systems have been designed primarily to mimic the surgeons maneuvers but in smaller scale and through rigid straight instruments. This confines current robotic systems to direct access to cardiac structures with the use of CPB, albeit through smaller incisions. To meet the challenges of accessing intracardiac structures in the beating heart, nonrigid systems are being developed that can steer through blood vessels, much like catheter-based interventions, but with the added functionality of providing a stable platform with the ability to manipulate tissue in a precise and controlled manner. These systems combined with enhanced imaging techniques to guide the intervention in real time may open the door to a number of tissue reconstructive interventions currently not feasible with available robotic systems or by conventional catheter-based techniques.
Future perspective
As smaller, more steerable robotic systems become available that can navigate complex trajectories to deliver specialized tools, image-guided robot-assisted interventions for true intracardiac reconstructive procedures may become a reality. These devices may enable performance of procedures inside the beating heart that would normally require an open-heart operation done under direct vision. Pediatric intracardiac interventions present an additional challenge, since the complex maneuvers required have to be performed in an even smaller space, while operating on very rapidly moving delicate targets.
From the engineering perspective, while it is not easy to predict the ideal robotic image-guided intracardiac system of the future, we believe that it should possess certain key features. The robotic platform should enter the heart percutaneously and provide ergonomic control of both tools and imaging. Essential imaging features include high-quality real-time views of the entire heart volume as well as high-fidelity tool-tip views for visualizing the tool–tissue interaction. In combination with imaging, the incorporation of touch sensing, to monitor forces applied to the tissue, will be critical for safe and effective pediatric intracardiac interventions.
Executive summary.
Current status of robotically assisted pediatric cardiac surgery
-
▪
There is limited experience with robotically assisted procedures in children. For extracardiac procedures, that includes patent ductus arteriosus (PDA) ligation, vascular ring divisions, and thoracic noncardiac procedures. For intracardiac repairs, that includes atrial septal defect closure perfomed in in adult-size patients. Large instrument size and need for entry port sites that are relatively far apart limits the use of this system in children less than approximately 30 kg. Use of cardiopulmonary bypass is required for intracardiac repairs.
Catheter-based interventions
-
▪
Robotically assisted catheter-based interventions are not widely used in pediatric interventional cardiology practice. In adults, there are two robotic catheter technologies available, electromechanically-based systems and magnetically controlled systems. Robotic catheter applications include electrophysiologic, peripheral vascular, coronary and transcatheter valve interventions. The limitations of current robotic catheter design include limited ability for significant force application, especially in a lateral direction from the axis of the catheter, which is necessary for manipulation, plication, approximation and removal of tissue as it is done during complex repairs in open-heart surgery.
Beating-heart intracardiac image-guided robotic surgery
-
▪
There has been an ongoing interest in developing image-guided techniques to perform the same types of intracardiac repairs currently done as open procedures, but without use of cardiopulmonary bypass. Two major developmental efforts are required. First, new sets of instruments and devices need to be developed that provide tactile feedback, limit interference with imaging tools, and provide steerability. Secondly, real-time, high-resolution imaging, which also provides tissue and instrument tracking, is required.
-
▪Instruments and devices:
-
–Challenges for robotic solutions
-
–Concentric tube robots
-
–Intracardiac tools
-
–Robotically-assisted motion compensation tools
-
–
-
▪Enhanced imaging:
-
–3D echocardiography
-
–Other imaging modalities and image fusion
-
–
Conclusion
-
▪
Current clinically available surgical robotic systems have been designed primarily to mimic the surgeons maneuvers but in smaller scale and through rigid straight instruments. To meet the challenges of repairing structures in the beating heart, nonrigid systems are being developed. These systems combined with enhanced imaging techniques may enable advancement of the field of beating-heart intracardiac reconstructive interventions currently not feasible with available surgical and catheter-based robotic systems.
Future perspective
-
▪
The ideal robotic image-guided intracardiac system of the future should enter the heart percutaneously and enable complex maneuvers, essential for reconstructive procedures performed in a confined space on very rapidly moving delicate targets. Essential imaging features include high-quality real-time views of the entire heart volume as well as high-fidelity tool-tip views for visualizing the tool–tissue interaction.
Supplementary Material
Acknowledgments
This work was supported in part by NIH NHLBI Awards No. 5R01HL073647 (PDN) and No. 5R01HL087797 (PD). The equipment and technology used in the study were purchased using academic funds. The authors had full control of the design of the studies, methods used, outcome measurements, analysis of data, and production of the written report.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Carpentier A, Loulmet D, Aupècle B, et al. Computer assisted open-heart surgery. First case operated on with success. C. R. Acad. Sci. III. 1998;321(5):437–442. doi: 10.1016/s0764-4469(98)80309-0. [DOI] [PubMed] [Google Scholar]
- 2.Anderson CA, Chitwood WR. Advances in mitral valve repair. Future Cardiol. 2009;5(5):511–516. doi: 10.2217/fca.09.29. [DOI] [PubMed] [Google Scholar]
- 3.Bonatti J, Schachner T, Bonaros N, Lehr EJ, Zimrin D, Griffith B. Robotically assisted totally endoscopic coronary bypass surgery. Circulation. 2011;124(2):236–244. doi: 10.1161/CIRCULATIONAHA.110.985267. [DOI] [PubMed] [Google Scholar]
- 4.Le Bret E, Papadatos S, Folliguet T, et al. Interruption of patent ductus arteriosus in children: robotically assisted versus videothoracoscopic surgery. J. Thorac. Cardiovasc. Surg. 2002;123(5):973–976. doi: 10.1067/mtc.2002.121049. [DOI] [PubMed] [Google Scholar]
- 5.Mihaljevic T, Cannon JW, del Nido PJ. Robotically assisted division of a vascular ring in children. J. Thorac. Cardiovasc. Surg. 2003;125(5):1163–1164. doi: 10.1067/mtc.2003.52. [DOI] [PubMed] [Google Scholar]
- 6.Suematsu Y, Mora BN, Mihaljevic T, del Nido PJ. Totally endoscopic robotic-assisted repair of patent ductus arteriosus and vascular ring in children. Ann. Thorac. Surg. 2005;80(6):2309–2313. doi: 10.1016/j.athoracsur.2005.05.078. [DOI] [PubMed] [Google Scholar]
- 7.Meehan JJ, Sandler AD. Robotic resection of mediastinal masses in children. J. Laparoendosc. Adv. Surg. Tech. A. 2008;18(1):114–119. doi: 10.1089/lap.2007.0092. [DOI] [PubMed] [Google Scholar]
- 8.Torracca L, Ismeno G, Quarti A, Alfieri O. Totally endoscopic atrial septal defect closure with a robotic system: experience with seven cases. Heart Surg. Forum. 2002;5(2):125–127. [PubMed] [Google Scholar]
- 9.Argenziano M, Oz MC, Kohmoto T, et al. Totally endoscopic atrial septal defect repair with robotic assistance. Circulation. 2003;9(108 Suppl. 1):II191–II194. doi: 10.1161/01.cir.0000089043.82199.2f. [DOI] [PubMed] [Google Scholar]
- 10.Wimmer-Greinecker G, Dogan S, Aybek T, et al. Totally endoscopic atrial septal repair in adults with computer-enhanced telemanipulation. J. Thorac. Cardiovasc. Surg. 2003;126(2):465–468. doi: 10.1016/s0022-5223(03)00053-9. [DOI] [PubMed] [Google Scholar]
- 11.Baird CW, Stamou SC, Skipper E, Watts L. Total endoscopic repair of a pediatric atrial septal defect using the da Vinci robot and hypothermic fibrillation. Interact. Cardiovasc. Thorac. Surg. 2007;6(6):828–829. doi: 10.1510/icvts.2007.158626. [DOI] [PubMed] [Google Scholar]
- 12.Kroh M, El-Hayek K, Rosenblatt S, et al. First human surgery with a novel single-port robotic system: cholecystectomy using the da Vinci single-site platform. Surg. Endosc. 2011;25(11):3566–3573. doi: 10.1007/s00464-011-1759-1. [DOI] [PubMed] [Google Scholar]
- 13.Menache CC, du Plessis AJ, Wessel DL, Jonas RA, Newburger JW. Current incidence of acute neurologic complications after open-heart operations in children. Ann. Thorac. Surg. 2002;73(6):1752–1758. doi: 10.1016/s0003-4975(02)03534-8. [DOI] [PubMed] [Google Scholar]
- 14.Gander JW, Fisher JC, Reichstein AR, et al. Limb ischemia after common femoral artery cannulation for venoarterial extracorporeal membrane oxygenation: an unresolved problem. J. Pediatr. Surg. 2010;45(11):2136–2140. doi: 10.1016/j.jpedsurg.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mills NL, King TD. Nonoperative closure of left-to-right shunts. J. Thorac. Cardiovasc. Surg. 1976;72(3):371–378. [PubMed] [Google Scholar]
- 16.Hijazi ZM, Awad SM. Pediatric cardiac interventions. JACC Cardiovasc. Interv. 2008;1(6):603–611. doi: 10.1016/j.jcin.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 17. Antoniou GA, Riga CV, Mayer EK, Cheshire NJ, Bicknell CD. Clinical applications of robotic technology in vascular and endovascular surgery. J. Vasc. Surg. 2011;53(2):493–499. doi: 10.1016/j.jvs.2010.06.154. ▪▪ Detailed paper on the use of the surgical- and catheter-based robotic systems in vascular applications.
- 18.Bismuth J, Kashef E, Cheshire N, Lumsden A. Feasibility and safety of remote endovascular catheter navigation in a porcine model. J. Endovasc. Ther. 2011;18:243–249. doi: 10.1583/10-3324R.1. [DOI] [PubMed] [Google Scholar]
- 19.Wood MA, Orlov M, Ramaswamy K, Haffajee C, Ellenbogen K. Stereotaxis Heart Study Investigators. Remote magnetic versus manual catheter navigation for ablation of supraventricular tachycardias: a randomized, multicenter trial. Pacing Clin. Electrophysiol. 2008;31(10):1313–1321. doi: 10.1111/j.1540-8159.2008.01183.x. [DOI] [PubMed] [Google Scholar]
- 20.Di Biase L, Fahmy TS, Patel D, et al. Remote magnetic navigation: human experience in pulmonary vein ablation. J. Am. Coll. Cardiol. 2007;50(9):868–874. doi: 10.1016/j.jacc.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 21.Miyazaki S, Shah AJ, Xhaët O, et al. Remote magnetic navigation with irrigated tip catheter for ablation of paroxysmal atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2010;3(6):585–589. doi: 10.1161/CIRCEP.110.957803. [DOI] [PubMed] [Google Scholar]
- 22. Gang ES, Nguyen BL, Shachar Y, et al. Dynamically shaped magnetic fields: initial animal validation of a new remote electrophysiology catheter guidance and control system. Circ. Arrhythm. Electrophysiol. 2011;4(5):770–777. doi: 10.1161/CIRCEP.110.959692. ▪ Report on a new magnetically controlled system robotic catheter-based system that enables precise intracardiac navigation.
- 23.Lüthje L, Vollmann D, Seegers J, et al. Remote magnetic versus manual catheter navigation for circumferential pulmonary vein ablation in patients with atrial fibrillation. Clin. Res. Cardiol. 2011;100(11):1003–1011. doi: 10.1007/s00392-011-0333-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lumsden AB, Anaya-Ayala JE, Birnbaum I, et al. Robot-assisted stenting of a high-grade anastomotic pulmonary artery stenosis following single lung transplantation. J. Endovasc. Ther. 2010;17(5):612–616. doi: 10.1583/10-3208R.1. [DOI] [PubMed] [Google Scholar]
- 25.Riga CV, Bicknell CD, Hamady MS, Cheshire NJ. Evaluation of robotic endovascular catheters for arch vessel cannulation. J. Vasc. Surg. 2011;54(3):799–809. doi: 10.1016/j.jvs.2011.03.218. [DOI] [PubMed] [Google Scholar]
- 26.Warinsirikul W, Sangchote S, Mokarapong P, Chaiyodsilp S, Tanamai S. Closure of atrial septal defects without cardiopulmonary bypass: the sandwich operation. J. Thorac. Cardiovasc. Surg. 2001;121(6):1122–1129. doi: 10.1067/mtc.2001.113324. [DOI] [PubMed] [Google Scholar]
- 27.Seeburger J, Leontjev S, Neumuth M, et al. Trans-apical beating-heart implantation of neo-chordae to mitral valve leaflets: results of an acute animal study. J. Vasc. Surg. 2011 doi: 10.1016/j.ejcts.2011.03.058. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sogawa M, Moro H, Tsuchida M, Shinonaga M, Ohzeki H, Hayashi J. Development of an endocardioscope for repair of an atrial septal defect in the beating heart. ASAIO J. 1999;45(1):90–93. doi: 10.1097/00002480-199901000-00020. [DOI] [PubMed] [Google Scholar]
- 29.Vasilyev NV, Martinez JF, Freudenthal FP, Suematsu Y, Marx GR, del Nido PJ. Three-dimensional echo and videocardioscopy-guided atrial septal defect closure. Ann. Thorac. Surg. 2006;82(4):1322–1326. doi: 10.1016/j.athoracsur.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Vasilyev NV, Melnychenko I, Kitahori K, et al. Beating-heart patch closure of muscular ventricular septal defects under real-time 3D echo guidance: a pre-clinical study. J. Thorac. Cardiovasc. Surg. 2008;135(3):603–609. doi: 10.1016/j.jtcvs.2007.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cannon JW, Howe RD, Dupont PE, Triedman JK, Marx GR, del Nido PJ. Application of robotics in congenital cardiac surgery. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2003;6:72–83. doi: 10.1053/pcsu.2003.50000. [DOI] [PubMed] [Google Scholar]
- 32. Sears P, Dupont P. A steerable needle technology using curved concentric tubes. Intel. Robots Syst. 2006:2850–2856. ▪ Engineering paper that describes in detail the design of the new concentric tube robotic system.
- 33.Dupont P, Lock J, Itkowitz B, Butler E. Design and control of concentric tube robots. IEEE Trans. Robot. 2010;26(2):209–225. doi: 10.1109/TRO.2009.2035740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rucker D, Webster R, 3rd, Chirikjian G, Cowan N. Equilibrium conformations of concentric-tube continuum robots. Int. J. Robot. Res. 2010;29(10):1263–1280. doi: 10.1177/0278364910367543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lock J, Laing G, Mahvash M, Dupont P. Quasistatic modeling of concentric tube robots with external loads. Rep US. 2010;2010:2325–2332. doi: 10.1109/IROS.2010.5651240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rucker DC, Jones BA, Webster RJ., III A geometrically exact model for externally loaded concentric-tube continuum robots. IEEE Trans. Robot. 2010;26(5):769–780. doi: 10.1109/TRO.2010.2062570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahvash M, Dupont P. Stiffness control of surgical continuum manipulators. IEEE Trans. Robot. 2011;27(2):334–345. doi: 10.1109/TRO.2011.2105410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Butler E, Folk C, Cohen A, et al. Metal MEMS tools for beating-heart tissue approximation; IEEE Int. Conf. Robot. Autom; 2011. pp. 411–416. ▪ Report on the first successful use of metal MEMS tools in image-guided intracardiac robotic procedures.
- 39.Lock J, Dupont P. Friction modeling in concentric tube robots; IEEE Int. Conf. Robot. Autom; 2011. pp. 1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bedell C, Lock J, Gosline A, Dupont P. Design optimization of concentric tube robots based on task and anatomical constraints; IEEE Int. Conf. Robot. Autom; 2011. pp. 398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gosline A, Vasilyev N, Veeramani A, et al. Metal MEMS tools for beating-heart tissue removal; IEEE Int. Conf. Robot. Autom; 2012. (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gosline A, Vasilyev NV, Butler E, et al. Percutaneous intracardiac beating-heart surgery using metal MEMS tissue approximation tools. Int. J. Rob. Res. 2012 doi: 10.1177/0278364912443718. (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khairy P, O’Donnell CP, Landzberg MJ. Transcatheter closure versus medical therapy of patent foramen ovale and presumed paradoxical thromboemboli: a systematic review. Ann. Intern. Med. 2003;139:753–760. doi: 10.7326/0003-4819-139-9-200311040-00010. [DOI] [PubMed] [Google Scholar]
- 44.Devuyst G, Bogousslavsky J, Ruchat P, et al. Prognosis after stroke followed by surgical closure of patent foramen ovale: a prospective follow-up study with brain MRI and simultaneous transesophageal and transcranial Doppler ultrasound. Neurology. 1996;47:1162–1166. doi: 10.1212/wnl.47.5.1162. [DOI] [PubMed] [Google Scholar]
- 45.Tsetis D, Morgan R, Belli AM. Cutting balloons for the treatment of vascular stenoses. Eur. Radiol. 2006;16(8):1675–1683. doi: 10.1007/s00330-006-0181-x. [DOI] [PubMed] [Google Scholar]
- 46.Margossian R. Contemporary management of pediatric heart failure. Expert Rev. Cardiovasc. Ther. 2008;6(2):187–197. doi: 10.1586/14779072.6.2.187. [DOI] [PubMed] [Google Scholar]
- 47.Goldberg SL, Feldman T. Percutaneous mitral valve interventions: overview of new approaches. Curr. Cardiol. Rep. 2010;12(5):404–412. doi: 10.1007/s11886-010-0130-9. [DOI] [PubMed] [Google Scholar]
- 48. Yuen SG, Kesner SB, Vasilyev NV, del Nido PJ, Howe RD. 3D ultrasound-guided motion compensation system for beating heart mitral valve repair. Med. Image Comput. Comput. Assist. Interv. 2008;11(Pt 1):711–719. doi: 10.1007/978-3-540-85988-8_85. ▪ Engineering paper that describes in detail the design and an in vivo testing of the new motion compensation robotic system.
- 49.Yuen SG, Yip MC, Vasilyev NV, Perrin DP, Del Nido PJ, Howe RD. Robotic force stabilization for beating heart intracardiac surgery. Med. Image Comput. Comput. Assist. Interv. 2009;5761(2009):26–33. doi: 10.1007/978-3-642-04268-3_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suematsu Y, Marx GR, Stoll JA, et al. Three-dimensional echocardiography-guided beating-heart surgery without cardiopulmonary bypass: a feasibility study. J. Thorac. Cardiovasc. Surg. 2004;128(4):579–587. doi: 10.1016/j.jtcvs.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 51.Suematsu Y, Martinez JF, Wolf BK, et al. Three-dimensional echo-guided beating heart surgery without cardiopulmonary bypass: atrial septal defect closure in a swine model. J. Thorac. Cardiovasc. Surg. 2005;130(5):1348–1357. doi: 10.1016/j.jtcvs.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 52.Cannon J, Stoll J, Salgo I, et al. Real-time three-dimensional ultrasound for guiding surgical tasks. Computer Aid. Surg. 2003;2(8):82–90. doi: 10.3109/10929080309146042. [DOI] [PubMed] [Google Scholar]
- 53.Balzer J, Kuhl H, Rassaf T, et al. Real-time transesophageal three-dimensional echocardiography for guidance of percutaneous cardiac interventions: first experience. Clin. Res. Cardiol. 2008;(97):565–574. doi: 10.1007/s00392-008-0676-3. [DOI] [PubMed] [Google Scholar]
- 54.Deng J, Rodeck C. Current applications of fetal cardiac imaging technology. Curr. Opin. Obstet. Gynecol. 2006;18(2):177–184. doi: 10.1097/01.gco.0000192987.99847.a4. [DOI] [PubMed] [Google Scholar]
- 55.Huang J, Dupont P, Undurti A, Triedman J, Cleveland R. Producing diffuse ultrasound reflections from medical instruments using the quadratic residue diffuser. Ultrasound Med. Biol. 2006;32(5):721–727. doi: 10.1016/j.ultrasmedbio.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 56.Huang J, Triedman JK, Vasilyev NV, Suematsu Y, Cleveland RO, Dupont PE. Imaging artifacts of medical instruments in ultrasound-guided interventions. J. Ultrasound Med. 2007;26(10):1303–1322. doi: 10.7863/jum.2007.26.10.1303. [DOI] [PubMed] [Google Scholar]
- 57.Nichols K, Wright L, Spencer T, Culp W. Changes in ultrasonographic echogenicity and visibility of needles with changes in angles of insonation. J. Vasc. Interv. Radiol. 2003;14(12):1553–1557. doi: 10.1097/01.rvi.0000099527.29957.a6. [DOI] [PubMed] [Google Scholar]
- 58.Reading C, Charboneau J, James E, Hurt M. Sonographically guided percutaneous biopsy of small (3 cm or less) masses. Am. J. Roentgenol. 1988;151(1):189. doi: 10.2214/ajr.151.1.189. [DOI] [PubMed] [Google Scholar]
- 59.Culp W, McCowan T, Goertzen T, et al. Relative ultrasonographic echogenicity of standard, dimpled, and polymeric-coated needles. J. Vasc. Interv. Radiol. 2000;11(3):351–358. doi: 10.1016/s1051-0443(07)61429-8. [DOI] [PubMed] [Google Scholar]
- 60.Ding M, Fenster A. A real-time biopsy needle segmentation technique using hough transform. Med. Phys. 2003;30(8):2222–2233. doi: 10.1118/1.1591192. [DOI] [PubMed] [Google Scholar]
- 61.Draper KJ, Blake CC, Gowman L, Downey DB, Fenster A. An algorithm for automatic needle localization in ultrasound-guided breast biopsies. Med. Phys. 2000;27(8):1971–1979. doi: 10.1118/1.1287437. [DOI] [PubMed] [Google Scholar]
- 62.Novotny P, Cannon J, Howe R. Tool localization in 3D ultrasound images. MICCAI. 2003:969–970. [Google Scholar]
- 63.Ren H, Vasilyev N, Dupont P. Detection of curved robots using 3D ultrasound. Rep US. 2011;2011:2083–2089. doi: 10.1109/IROS.2011.6094915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ren H, Dupont P. Tubular structure enhancement for surgical instrument detection in 3D ultrasound; Conf. Proc. IEEE Eng. Med. Biol. Soc; 2011. pp. 7203–7206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leotta D. An efficient calibration method for freehand 3-D ultrasound imaging systems. Ultrasound Med. Biol. 2004;30(7):999–1008. doi: 10.1016/j.ultrasmedbio.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 66.Stoll J, Dupont P. Passive markers for ultrasound tracking of surgical instruments. Med. Image Comput. Comput. Assist. Interv. 2005;8(Pt 2):41–48. doi: 10.1007/11566489_6. [DOI] [PubMed] [Google Scholar]
- 67.Stoll J, Ren H, Dupont P. Passive markers for tracking surgical instruments in real-time 3D. IEEE Trans. Med. Imaging. 2011 doi: 10.1109/TMI.2011.2173586. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Venneri L, Rossi F, Botto N, et al. Cancer risk from professional exposure in staff working in cardiac catheterization laboratory: insights from the National Research Council’s Biological Effects of Ionizing Radiation VII Report. Am. Heart J. 2009;157:118–124. doi: 10.1016/j.ahj.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 69.Clay MA, Campbell RM, Strieper M, et al. Long-term risk of fatal malignancy following pediatric radiofrequency ablation. Am. J. Cardiol. 2008;102:913–915. doi: 10.1016/j.amjcard.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 70.Kim KP, Miller DL, Balter S, et al. Occupational radiation doses to operators performing cardiac catheterization procedures. Health Phys. 2008;94:211–227. doi: 10.1097/01.HP.0000290614.76386.35. [DOI] [PubMed] [Google Scholar]
- 71.Juergen M, Johanna A, Michael N, Frank H, Thomas S, Christian B. Rotational angiography for preinterventional imaging in transcatheter aortic valve implantation. Catheter. Cardiovasc. Interv. 2011 doi: 10.1002/ccd.23217. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 72. Govil A, Calkins H, Spragg DD. Fusion of imaging technologies: how, when, and for whom? J. Interv. Card. Electrophysiol. 2011;32(3):195–203. doi: 10.1007/s10840-011-9616-7. ▪▪ Detailed paper on image fusion technologies for electrophysiology applications.
- 73.Glöckler M, Koch A, Greim V, et al. The value of flat-detector computed tomography during catheterisation of congenital heart disease. Eur. Radiol. 2011;21(12):2511–2520. doi: 10.1007/s00330-011-2214-3. [DOI] [PubMed] [Google Scholar]
- 74. Perrin D, Vasilyev N, Novotny P, et al. Image guided surgical interventions. Curr. Probl. Surg. 2009;46(9):730–766. doi: 10.1067/j.cpsurg.2009.04.001. ▪▪ Comprehensive review on the basic concepts of the technologies utilized in image-guided surgical interventions.
- 75.Novotny P, Stoll J, Vasilyev N, et al. GPU based real-time instrument tracking with threedimensional ultrasound. Med. Image Anal. 2007;11(5):458–464. doi: 10.1016/j.media.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stoll J, Novotny P, Dupont P, Howe R. Real-time 3D ultrasound-based servoing of a surgical instrument; IEEE Int. Conf. Robot. Autom; 2006. pp. 613–618. [Google Scholar]
- 77.Novotny P, Stoll J, Dupont P, Howe R. Real-time visual servoing of a robot using three dimensional ultrasound; IEEE Int. Conf. Robot. Autom; 2007. pp. 2655–2660. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.