Abstract
We assessed HIV prevalence and associated behaviors and risk factors among men who have sex with men (MSM) in Beijing, China. Five hundred MSM were recruited for a biological and behavioral survey using respondent-driven sampling (RDS) in 2009. Serologic specimens were tested for markers of HIV and syphilis infection. A computer-assisted personal interview (CAPI) administered questionnaire gathered information including demographic characteristics, sexual behaviors, HIV testing, and social norms concerning condom use. The adjusted HIV prevalence was 8.0%, syphilis 22.0%. HIV testing and disclosure was low; only 39.3% had HIV tested in the past 12 months, 49.7% knew their own HIV status and 22.8% knew their last male partner's HIV status. HIV infection was associated with syphilis, ever having sex with a woman, not knowing the HIV status of the most recent male partner, and never buying condoms in the past 12 months. Stronger endorsement of positive social norms around condom use strongly and predicted lower prevalence of HIV infection. Compared to surveys of similar design in the recent past, HIV continues to spread rapidly among Beijing's MSM. Our results identify points of intervention that, if addressed in time, may still alter the course of the epidemic including the promotion of HIV testing and partner disclosure, syphilis control and particularly changing social norms around condom use.
Introduction
In China, men who have sex with men (MSM) are growing as a category of HIV transmission.1–8 According to the latest estimation conducted jointly by Ministry of Health of China, World Health Organization, and Joint United Nations Programme on HIV/AIDS (UNAIDS) in 2009, there were approximately 740,000 people living with HIV/AIDS in China at the end of 2009, among whom male–male sexual transmission accounted for 14.7%. Of the estimated 48,000 new HIV infections that took place in 2009, homosexual transmission accounted for 32.5%—a substantial increase compared with the 2007 estimate, where 12.2% were infected through homosexual transmission.9,10 To date, transmission of HIV among MSM has not been well-examined in China, and no behavioral theory-driven prevention intervention has been widely implemented among MSM in China to date. This serious problem underscores the needs for rigorous epidemiologic studies and data-driven HIV prevention interventions. Few epidemiologic and surveillance surveys use measures with enough detail to accurately characterize the specific behaviors that drive HIV transmission and can serve as the basis for the design of effective interventions in China. Moreover; few surveys use rigorous sampling methods, rather they rely primarily on convenience samples.
Since the introduction of voluntary counseling and testing (VCT) for the general population and specific testing interventions for MSM, increasing numbers of MSM have tested for HIV and learned their HIV status.11 Emerging data from around the world point to widespread sexual harm reduction practices among MSM based on knowing their own and their partners' serostatus. “Serosorting” or “seroadaptation,” for example, entails selecting partners, limiting roles in anal sex, or engaging in condom use on the basis of concordant HIV status thus reducing the risk for HIV transmission or acquistion.12–17 However, these methods to reduce HIV infection risk have not been formally evaluated for their efficacy; some may be ineffective, some may increase risk. Their very presence and applicability in the Chinese context have not been studied.
A challenge in conducting research among MSM is they are a stigmatized, hidden population in all areas of the world, difficult to access and therefore many surveys rely on convenience sampling. The respondent-driven sampling (RDS) method is a recruitment/sampling approach using long chains of referral for obtaining a more inclusive and, purportedly, more representative sample from hidden populations.18,19 RDS has been used successfully among MSM in China.22,36 For the present survey to obtain more reliable estimates of HIV and syphilis prevalence and more detailed behavioral data, we used RDS recruitment and analysis among the MSM population in Beijing, China.
Methods
Participants
A cross-sectional study was conducted among MSM from September 2009 to October 2009 in Beijing. Participants were eligible if they were male, 18 years age or older, a Beijing resident, had sex with another man in the past 12 months (sex can be defined as oral, anal, or mutual masturbation), had a valid study recruitment coupon, had not previously participated in the survey, and were able to provide written informed consent. The study was approved by the Committees for Human Research of the National Center for AIDS of the China Center for Disease Control and Prevention, Vanderbilt University and the University of California San Francisco.
Recruitment
Study participants were recruited using respondent-driven sampling (RDS). Seven MSM were selected to function as recruiter “seeds” and were diverse with respect to the types of venues that they frequented (e.g., bars, parks, bathhouse, and the Internet). Seeds were evaluated for their commitment to the goals of the study and motivation to recruit three eligible peers in their social network. Seeds were each asked to recruit up to three participants, who in turn were asked to recruit a subsequent wave of up to three participants, and so on, until our target sample size was reached and equilibrium was achieved on key variables (age, educational attainment, marital status, occupation, residence, income, anal sex role, number of male sex partners). Equilibrium is achieved when the composition of the sample reaches stability and does not change as recruitment continues to the point sample size is achieved. Equilibrium was achieved in four waves of recruitment or less for all key variables. All subsequent participants had to be a member of the recruiter's social network and meet the eligibility criteria for the study. Each participant was given three recruitment coupons/cards with study information to hand to potential recruits. To keep track of social networks, each card had a number code that connected participants back to the initial seeds. Participants were compensated 30 Yuan (CNY) for their participation in the study, as well as 20 Yuan (CNY) for each eligible participant they recruited who subsequently completed a study interview.
Measures
After providing informed consent, participants completed a CAPI administered questionnaire in a separate room of the Beijing CDC clinic. Trained health professionals conducted the interviews. Each study participant was assigned a unique and confidential identification code for the questionnaire and specimens. Questions included demographic information (e.g., age, ethnicity, education, marital status, occupation, residence, income, and health insurance status), sexual behaviors (e.g., age of the first sex with male and female partners, self-identified sexual orientation, role in anal sex, and the number and types of male and female sex partners in the past 6 months), HIV testing information, and drug using information. We also asked partner by partner sexual behavior (i.e. an assessment of sexual behavior on a partner by partner basis as opposed to an aggregate assessment across all partners), condom use, and HIV status awareness questions for up to three male partners and two female partners within the prior 6 months. A five-item condom use social norms scale was adapted to the China context from previously validated instruments from North America through development with focus group discussions, key informant interviews and pilot testing in the field.20,21 Items in the scale included such questions as “My friends always use condoms when having anal sex with new partners”, with a 6-point Likert-like scale to record responses (social norms score ranges from 1 to 6 for answering questions of strongly disagree, somewhat disagree, slightly disagree, slight agree, somewhat agree, strongly agree, respectively). The internal reliability of the scale was tested by the Cronbach α (0.925) and assessed for its linear relationship with HIV infection. The complete CAPI administered questionnaire was also pilot tested among MSM volunteers in the real-life survey setting.
Laboratory testing
Serologic specimens collected from participants were tested for syphilis (rapid plasma reagin [RPR] test, Shanghai Rongsheng, China) with confirmation of positive tests by the Treponema pallidum particle assay (TPPA) test (Fujirebio inc., Japan), and HIV-1 antibody (enzyme-linked immunosorbent assay [ELISA], Vironostika HIV Uni-Form plus O, bioMerieux, Holland) with confirmation by Western blot confirmation (HIV Blot 2.2 WBTM, Genelabs Diagnostics).
Data analysis
Survey data were analyzed to produce population point estimates using RDSAT software version 5.6 (www.respondentdrivensampling.org), which adjusted for personal network size and homophily in recruitment. Homophily is a mathematical metric between −1 and 1 that represents the tendency of individuals in a group to form social bonds with others similar to them. A value of 0 means that the formation of social bonds is independent of group membership. A value of 1 indicates that no social bonds with outsiders exist. A value of −1 means all social bonds are formed with people outside of the group. We calculated the crude and RDSAT-adjusted univariate analyses for the characteristics of MSM in the sample. Bivariate associations between selected variables and HIV were conducted using RDSAT-generated weights on the HIV infection outcome imported into in SAS version 9.1 (SAS, Cary, NC).
Variables significant at a level of p<0.10 in bivariate analyses were considered candidates for multivariate models. Multivariate logistic regression models were constructed (using RDSAT-generated HIV result weights) to select independent factors for HIV infection, while controlling for potential confounding factors. Both adjusted odds ratio (AOR) and confidence interval (CI) were obtained for each explanatory variable in the final model.
Results
A total of 501 participants were recruited for the study in 2 months; 43.4% (501/1154) of the distributed coupons were actually returned and the holder interviewed. Figure 1 shows the fewest and the highest number of waves achieved was 2 and 13, respectively. One participant was referred to the VCT clinic due to ineligibility (younger than 18 years old). All 500 eligible participants completed the questionnaire and provided blood samples. Table 1 outlines the characteristics of the sample. The median age was 29 years. Most were Han (96.0%) and received a high school (35.6%) or above education (32.9%). Only 4.8% received a primary school or no education. Most men were unmarried (72.8%) and had fulltime jobs (82.5%). The majority (61.2%) of the participants reported having two or more sexual partners in the past 6 months. By self-reported sexual orientation, 61.7% identified as homosexual, 34.7% as bisexual, and only 1.7% as heterosexual. Although most of the participants had known locations for HIV test (89.0%), only 39.3% had HIV tested in the past 12 months. About half (49.7%) of participants know their own HIV status and only 22.8% knew their last male partner's HIV status. Very few (0.1%) acknowledged noninjection drug use; none injected drugs. The adjusted prevalence of HIV was 8.0% (95% CI 4.6–12.2%), 22.0% (95% CI 16.8–27.0%) for syphilis, and 4.2% (95% CI 1.8–7.2%) for HIV and syphilis coinfection.
FIG. 1.
The spread from the various initial seeds.
Table 1.
Crude and Adjusted Demographic, Behavioral, and HIV Prevalence Indicators of MSM in Beijing, 2009 (n=500)
| Variable | Homophily | Equilibrium samplea% | Crudeb,c% (n) | Adjusted % (95% CI) |
|---|---|---|---|---|
| Age (median 29, range 18–71) | ||||
| 18–25 | 0.126 | 34.9 | 34.6 (173) | 36.4 (29.3–43.2) |
| >25 | 0.181 | 65.1 | 65.4 (327) | 63.6 (56.8–70.7) |
| Ethnicity | ||||
| Han | −0.024 | 93.7 | 93.6 (468) | 96.0 (93.9–97.7) |
| Others | 0.024 | 6.3 | 6.4 (32) | 4.0 (2.3–6.1) |
| Education | ||||
| No education/elementary primary school | 0.118 | 5.6 | 5.8 (29) | 4.8 (2.2–8.4) |
| Middle school | 0.096 | 27.0 | 26.8 (134) | 26.7 (21.1–32.8) |
| High school | 0.015 | 32.8 | 32.6 (163) | 35.6 (29.0–42.4) |
| College or above | 0.339 | 34.6 | 34.8 (174) | 32.9 (25.7–39.8) |
| Current marital status | ||||
| Married | 0.203 | 22.5 | 20.8 (104) | 27.2 (20.4–36.2) |
| Unmarried | 0.387 | 77.5 | 79.2 (396) | 72.8 (63.8–79.6) |
| Occupation/work status | ||||
| Employed | 0.226 | 85.3 | 85.4 (427) | 82.5 (76.5–88.0) |
| Not employed | 0.104 | 9.2 | 9.2 (46) | 9.0 (5.5–12.9) |
| Student | 0.082 | 5.5 | 5.4 (27) | 8.6 (3.9–13.6) |
| Monthly income in the past 12 months (CNY) | ||||
| None | 0.011 | 11.8 | 11.6 (58) | 15.7 (10.4–21.5) |
| <1000 | 0.036 | 9.3 | 9.4 (47) | 8.0 (4.7–11.7) |
| 1000–2999 | −0.018 | 54.2 | 54.2 (271) | 56.8 (50.3–62.8) |
| 3000–4999 | 0.006 | 15.9 | 16.0 (80) | 12.4 (9.5–16.7) |
| ≥5000 | 0.208 | 8.8 | 8.8 (44) | 7.0 (3.6–11.5) |
| Beijing permanent residents | 0.145 | 17.2 | 18.2 (91) | 12.0 (8.5–16.1) |
| Have health insurance | 0.121 | 46.1 | 47.2 (236) | 45.6 (39.5–52.7) |
| Sexual orientation | ||||
| Homosexual | 0.154 | 68.2 | 69.2 (346) | 61.7 (55.4–68.4) |
| Heterosexual | −0.999 | 1.1 | 1.0 (5) | 1.7 (0.2–3.4) |
| Bisexual | −0.259 | 30.1 | 29.4 (147) | 34.7 (29.3–41.1) |
| Do not know | 0.236 | 0.5 | 0.4 (2) | 1.8 (0–4.1) |
| Self-reported role in anal sex with males | ||||
| Insertive | −0.162 | 26.7 | 27.1 (133) | 29.6 (24.5–36.2) |
| Both insertive and receptive | 0.145 | 59.7 | 59.4 (291) | 53.7 (46.4–59.6) |
| Receptive | −0.367 | 13.6 | 13.5 (66) | 16.7 (11.7–22.8) |
| Had male sexual partner in the past 6 months | 0.251 | 97.6 | 97.6 (488) | 96.7 (93.6–98.9) |
| Type of the most recent male partner in the past 6 months | ||||
| Regular partner | 0.199 | 63.5 | 63.3 (309) | 60.8 (53.6–67.7) |
| Casual partner | 0.100 | 36.5 | 36.7 (179) | 39.2 (32.3–46.4) |
| Number of male sexual partners >1 in the past 6 months | 0.368 | 69.8 | 71.4 (357) | 61.2 (54.5–68.5) |
| Unprotected insertive anal sex with the most recent male partner in the past 6 months | −0.075 | 36.2 | 36.6 (127) | 34.8 (28.3–42.3) |
| Unprotected receptive anal sex with the most recent male partner in the past 6 months | −0.013 | 40.8 | 40.4 (115) | 40.5 (31.5–48.1) |
| Ever had sex with female | 0.051 | 56.8 | 56.6 (283) | 61.2 (54.2–67.8) |
| Had female sexual partner in the past 6 months | −0.147 | 21.1 | 20.8 (104) | 25.8 (19.4–32.2) |
| Unprotected sex with female sexual partner in the past 6 months | −0.099 | 76.2 | 72.1 (75) | 79.6 (65.8–97.8) |
| Discussed HIV status with the most recent male partner | 0.100 | 51.1 | 52.0 (253) | 48.6 (42.4–56.5) |
| Self-known the HIV status | −0.004 | 68.4 | 64.7 (320) | 49.7 (41.9–56.4) |
| Known the HIV status of the most recent male partner | 0.041 | 25.7 | 26.4 (129) | 22.8 (18.3–28.2) |
| Told the most recent male partner your HIV status | 0.064 | 47.1 | 48.4 (236) | 45.6 (38.7–52.3) |
| Known where to get HIV test | 0.502 | 93.8 | 94.2 (470) | 89.0 (84.2–94.0) |
| Ever had a HIV test | 0.399 | 64.4 | 66.8 (334) | 51.4 (43.3–58.6) |
| Had a HIV test in the past 12 months | 0.283 | 52.6 | 53.2 (266) | 39.3 (33.6–46.0) |
| Received free condoms/lubricant in the past 12 months | 0.401 | 87.2 | 87.2 (436) | 78.0 (72.4–84.6) |
| Bought condoms in the past 12 months | 0.066 | 50.9 | 49.8 (249) | 52.0 (44.9–58.4) |
| Non-injection drug use in the past 12 months | −0.991 | 0.9 | 0.8 (4) | 0.1 (0–0.1) |
| HIV positive | 0.058 | 7.4 | 7.2 (36) | 8.0 (4.6–12.2) |
| Syphilis positive | 0.023 | 22.1 | 22.0 (110) | 22.0 (16.8–27.0) |
| Both HIV and Syphilis positive | −1.0 | 3.0 | 3.0% (15) | 4.2 (1.8–7.2) |
Composition of the sample when the sample reached stability.
Subgroups do not always add up to totals due to missing data.
Composition of the sample at the end of data collection.
MSM, men who have sex with men; CI, confidence interval.
Table 2 presents the crude and RDSAT-adjusted results of bivariate associations of demographic, sexual risk, and the condom use social norms scale. Significant bivariate predictors of HIV infection were age (older than 25 years), married, lived in Beijing less than 1 year, ever had sex with a woman, had more than one male sexual partner in the past 6 months and syphilis-positive. HIV infection was significantly less likely among MSM who had health insurance, had a regular male partner in the past 6 months, discussed HIV status with the last male partner, known the HIV status of the last male partner, self-reported HIV status to the last male partner and bought condoms in the past 12 months.
Table 2.
Crude and Adjusted Bivariate Associations Between Selected Variables and HIV Infection among MSM, Beijing, 2009 (using RDSAT-generated weights)
| Variable | Crude % (n)a | Adjusted % (95% CI) | p |
|---|---|---|---|
| Age | |||
| 18–25 | 6.4 (11) | 4.1 (1.2–8.3) | 0.019 |
| >25 | 7.6 (25) | 10.2 (5.3–15.9) | |
| Current marital status | |||
| Married | 11.5 (12) | 13.7 (4.4–23.4) | 0.005 |
| Unmarried | 6.1 (24) | 5.9 (2.8–9.8) | |
| Lived in Beijing less than 1 year | |||
| Yes | 13.1 (11) | 13.7 (5.1–24.1) | 0.017 |
| No | 6.0 (25) | 6.6 (3.4–10.6) | |
| Have health insurance | |||
| Yes | 3.8 (9) | 5.5 (1.6–10.7) | 0.029 |
| No | 10.2 (27) | 10.2 (5.7–15.8) | |
| Sexual orientation | |||
| Homosexual | 7.2 (25) | 8.5 (4.2–13.2) | 0.595 |
| Others | 7.1 (11) | 7.9 (2.9–13.8) | |
| Role in anal sex with males | |||
| Insertive | 6.0 (8) | 6.2 (1.4–12.0) | <0.001 |
| Equally | 8.9 (26) | 10.4 (5.5–17.4) | |
| Receptive | 3.0 (2) | 3.4 (0–9.3) | |
| Type of the most recent male partner in the past 6 months | |||
| Regular partner | 5.5 (17) | 5.5 (2.4–9.8) | 0.020 |
| Casual partner | 10.1 (18) | 11.4 (4.7–19.3) | |
| Unprotected insertive anal sex with the most recent male partner in the past 6 months | |||
| Yes | 6.3 (8) | 8.7 (1.3–16.0) | 0.623 |
| No | 7.3 (16) | 6.5 (2.7–11.6) | |
| Unprotected receptive anal sex with the most recent male partner in the past 6 months | |||
| Yes | 7.8 (9) | 10.7 (2.4–18.6) | 0.694 |
| No | 10.0 (17) | 8.7 (3.2–13.8) | |
| Ever had sex with female | |||
| Yes | 9.2 (26) | 10.8 (5.8–17.1) | 0.004 |
| No | 4.6 (10) | 3.4 (0.8–7.1) | |
| Had female sexual partner in the past 6 months | |||
| Yes | 9.6 (10) | 15.0 (5.0–28.9) | 0.003 |
| No | 6.6 (26) | 5.8 (3.1–9.1) | |
| Unprotected sex with female sexual partner in the past 6 months | |||
| Yes | 3.4 (1) | 34.1 (0–61.9) | 0.050 |
| No | 12.0 (9) | 20.2 (0–100) | |
| Discussed HIV status with the most recent male partner | |||
| Yes | 4.7 (12) | 3.9 (1.6–7.3) | 0.002 |
| No | 9.8 (23) | 12.3 (6.0–19.2) | |
| Self–known the HIV status | |||
| Yes | 5.3 (17) | 8.1 (3.8–13.4) | 0.591 |
| No | 8.0 (14) | 7.5 (2.8–12.8) | |
| Known the HIV status of the most recent male partner | |||
| Yes | 1.6 (2) | 1.0 (0–2.3) | 0.014 |
| No | 9.2 (33) | 10.1 (5.6–15.4) | |
| Told the most recent male partner your HIV status | |||
| Yes | 4.2 (10) | 4.0 (1.4–7.1) | 0.003 |
| No | 9.9 (25) | 11.5 (5.6–18.0) | |
| Level of HIV risk for self-perception | |||
| Great | 28.6 (18) | 28.7 (15.1–43.7) | <0.001 |
| Moderate | 7.6 (7) | 13.1 (4.0–24.7) | |
| Small | 3.5 (10) | 4.3 (1.1–7.2) | |
| None | 1.7 (1) | 1.5 (0–5.4) | |
| Ever had a HIV test | |||
| Yes | 7.5 (25) | 10.3 (5.1–15.3) | 0.250 |
| No | 6.6 (11) | 7.2 (2.6–12.5) | |
| Had a HIV test in the past 12 months | |||
| Yes | 6.0 (16) | 7.3 (3.2–11.0) | 0.370 |
| No | 8.6 (20) | 9.5 (4.6–15.4) | |
| Received free condoms/lubricant in the past 12 months | |||
| Yes | 7.3 (32) | 8.3 (4.2–10.8) | 0.291 |
| No | 6.3 (4) | 32.6 (6.1–49.8) | |
| Bought condoms in the past 12 months | |||
| Yes | 5.2 (13) | 4.7 (2.1–8.5) | 0.005 |
| No | 9.2 (23) | 11.1 (5.8–17.4) | |
| Syphilis status | |||
| Positive | 13.6 (15) | 19.1 (8.4–31.5) | <0.001 |
| Negative | 5.4 (21) | 4.8 (2.4–7.6) | |
| Condoms use social norms | AOR | ||
| (Cronbach α=0.925, median 18, range 5–20) | (95% CI) | Wald χ2=26.409 | <0.001 |
| 0.8 (0.7–0.9) | |||
Subgroups do not always add up to totals due to missing data.
MSM, men who have sex with men; CI, confidence interval; AOR, adjusted odds ratio.
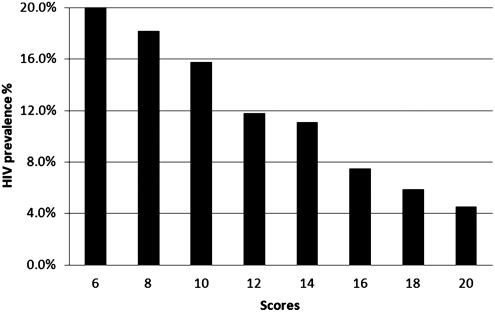
Table 3 presents adjusted results of multivariable logistic regression for HIV infection. In multivariable analysis, increased risk of HIV infection was associated with being syphilis-positive (AOR 4.7, 95% CI 2.3–9.8), having ever had sex with a woman (AOR 2.7, 95% CI 1.1–6.8), not knowing HIV status of the last male sexual partner (AOR 7.9, 95% CI 1.0–60.9), and not buying condoms within the past 12 months (AOR 2.2, 95% CI 1.0–4.8). Decreased risk of HIV infection was strongly and significantly associated with higher score on the condom use social norms scale (AOR 0.8 per point, 95% CI 0.7–0.9). Figure 2 illustrates the HIV prevalence with the condom use social norms scores among Beijing MSM.
Table 3.
Adjusted Multivariable Logistic Regression for HIV Infection Among MSM, Beijing, 2009 (Using RDSAT-Generated Weights)
| Variable | AOR (95% CI) | Wald χ2 | p |
|---|---|---|---|
| Current syphilis positive | 4.7 (2.3–9.8) | 17.184 | <0.001 |
| Ever had sex with female | 2.7 (1.1–6.8) | 4.732 | 0.030 |
| Unknown the most recent male sexual partner's HIV status in the past 6 months | 7.9 (1.0–60.9) | 3.947 | 0.047 |
| Condoms use social norms scores | 0.8 (0.7–0.9) | 17.008 | <0.001 |
| Never buy condoms in the past 12 months | 2.2 (1.0–4.8) | 3.918 | 0.048 |
MSM, men who have sex with men; AOR, adjusted odds ratio; CI, confidence interval.
FIG. 2.
HIV prevalence among men who have sex with men (MSM) with condom use social norms scores in Beijing.
Discussion
Our 2009 survey found HIV prevalence among MSM in Beijing at 8.0%, continuing a progressive increase from 0.4% in 2004 to 5.8% in 2006 determined by previous surveys in Beijing using identical RDS methods.22 HIV prevalence among MSM in Beijing is still rising. Our finding is consistent with China's current HIV prevalence trends due to increasing sexual transmission over the earlier predominant injection related mode, with high syphilis prevalence, and with homosexual transmission increasing rapidly in many major urban areas of the country.2,9,46 In our survey, only about half (51.4%) of participants had ever tested for HIV. Although this was higher than previous surveys conducted in Beijing, it was still lower than in Western countries.23–26 Awareness of one's HIV-positive status has been a key to preventing onward HIV transmission among MSM from the perspectives of changing behavior after testing and prevention with positives interventions.27 Moreover, identifying HIV-infected MSM is a necessary first step in linking them to HIV care for the benefits to their own health and also for the potential of lowering viral loads and therefore infectivity through anti-retroviral treatment.15 We also found in our survey that only a minority (22.8%) of MSM knew the serostatus of their most recent partner and this lack of knowledge was associated with being HIV-infected. These low levels of knowing one's own serostatus and even lower levels of knowing one's partners' serostatus speak to difficulty of achieving a level of “serosorting” or “seroadaption” as measured among MSM in the West.12,14,28–32 Therefore, the multiple health and preventive benefits of HIV testing and serostatus disclosure have not been realized for MSM in China.
Our study is also the first to show that HIV prevalence was strongly associated with condom use social norms among Chinese MSM. Given the strong correlation we found between a preventive effect and increasing score, interventions to change social norms around condom use may affect sexual behavior among MSM when using condoms.33,34 Interventions of this type might utilize peer based health education and counseling.47,48 In addition, our finding also showed that persons buying condoms in the past 12 months had a lower HIV prevalence compare to those who never bought condoms. Our study and the previous studies among Chinese MSM showed high proportion of unprotected sex with male and female.4,35,36 Therefore condom promotion and distribution may both be effective in preventing sexually acquired HIV infection in China.
Two findings bear further examination. In bivariate analysis, having female partners and being in Beijing less than 1 year were associated with higher HIV prevalence. The former finding was also held significance in the multivariate analysis. The relationship between having female partners, sexual orientation and identity, and is likely to be complex and may be rapidly changing with respect to HIV risk in China.37–40 Although evidence is not likely to prove that having sex with a woman would be of higher risk of acquiring HIV than with another MSM, particularly given the prevalence of HIV among other populations of women in China, there is the concern of HIV transmission to women and other sexual networks.41,42 In the Chinese context, due to marriage, family, parenthood and other traditional concepts, many MSM had to get married with homosexual orientation, so they may both have sex with men and with women who are their wives and often the mother of their child.43,44 A comprehensive HIV control strategy for MSM should also pay attention to prevent HIV transmission to women and children.
This study was subject to several limitations. First, there are limitations to RDS that have been previously noted.45 For example, seeds for RDS recruitment are purposefully selected and do not guarantee the inclusion of all networks of MSM. Beijing is a particularly large city and RDS may not be able to reach across all geographic areas and across all networks. Second, we recognize that, although data were collected by CAPI, some high-risk behaviors and self-reported HIV status may have been underreported due to discrimination or stigma, leading to potential underestimation of prevalence and associations with these variables and HIV infection. Third, the multivariate analysis was conducted by using exported weights for the dependent variable (HIV infection). However, standardized guidelines for RDS data multivariate analysis are still under development and require validation.
Conclusions
Our study showed that HIV continues in a phase of rapid growth among MSM in Beijing, yet at 8% there is still opportunity for prevention. Meanwhile, high proportion of MSM have sex with women and are not Beijing permanent residents. Our findings reinforce the need for a multidimensional approach to prevent further spread of HIV infection both among these men and to their heterosexual partners in Beijing, and to at-risk groups in other geographic areas. One area of strong appeal is to increase resources and efforts to make HIV testing opportunities more accessible and to reduce barriers to testing so that infected persons can learn their status and obtain appropriate medical care and prevention services.
Acknowledgments
Y.R., Y.X. was the PI for the study; S.F., H.L. was the lead author for the paper; Y.R., Y.X., X.H., H.F.R., W.M.F., J.S., W.M., and Y.J. contributed the design of the study; H.L., X.M., Y.S., X.H., C.L., and S.F. oversaw data collection at the study site; SF performed all the statistical analyses and all the authors contributed to the write up. All authors read and approved the final manuscript.
This study was supported by grants from the National Natural Science Foundation of China (81161120428), the National Institutes of Health (# R01 AI078933 and #5D43TW001035-13), the Ministry of Science and Technology of China (2012ZX10001-002) and Chinese State Key Laboratory for Infectious Disease Develop Grant (2011SKLID102).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Guo H. Wei JF. Yang H. Huan X. Tsui SK. Zhang C. Rapidly increasing prevalence of HIV and syphilis and HIV-1 subtype characterization among men who have sex with men in Jiangsu, China. Sex Transm Dis. 2009;36:120–125. doi: 10.1097/OLQ.0b013e31818d3fa0. [DOI] [PubMed] [Google Scholar]
- 2.Ruan Y. Jia Y. Zhang X, et al. Incidence of HIV-1, syphilis, hepatitis B, and hepatitis C virus infections and predictors associated with retention in a 12-month follow-up study among men who have sex with men in Beijing, China. J Acquir Immune Defic Syndr. 2009;52:604–610. doi: 10.1097/QAI.0b013e3181b31f5c. [DOI] [PubMed] [Google Scholar]
- 3.Xiao Y. Ding X. Li C. Liu J. Sun J. Jia Y. Prevalence and correlates of HIV and syphilis infections among men who have sex with men in Chongqing Municipality, China. Sex Transm Dis. 2009;36:647–656. doi: 10.1097/OLQ.0b013e3181aac23d. [DOI] [PubMed] [Google Scholar]
- 4.Feng Y. Wu Z. Detels R. Qin G. Liu L. Wang X. Wang J. Zhang L. HIV/STD prevalence among men who have sex with men in Chengdu, China and associated risk factors for HIV infection. J Acquir Immune Defic Syndr. 2010;53(Suppl 1):S74–80. doi: 10.1097/QAI.0b013e3181c7dd16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong FY. Huang ZJ. Wang W, et al. STIs and HIV among men having sex with men in China: a ticking time bomb? AIDS Educ Prev. 2009;21:430–446. doi: 10.1521/aeap.2009.21.5.430. [DOI] [PubMed] [Google Scholar]
- 6.Cai WD. Zhao J. Zhao JK, et al. HIV prevalence and related risk factors among male sex workers in Shenzhen, China: Results from a time-location sampling survey. Sex Transm Infect. 2010;86:15–20. doi: 10.1136/sti.2009.037440. [DOI] [PubMed] [Google Scholar]
- 7.Xu JJ. Zhang M. Brown K, et al. Syphilis and HIV seroconversion among a 12-month prospective cohort of men who have sex with men in Shenyang, China. Sex Transm Dis. 2010;37:432–439. doi: 10.1097/OLQ.0b013e3181d13eed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang H. Hao C. Huan X, et al. HIV Incidence and associated factors in a cohort of men who have sex with men in Nanjing, China. Sex Transm Dis. doi: 10.1097/OLQ.0b013e3181d13c59. (in press). [DOI] [PubMed] [Google Scholar]
- 9.Ministry of Health PsRoC. Joint United Nations Programme on HIV/AIDS, World Health Organization. 2009. Estimates for the HIV/AIDS Epidemic China. 2010 [Google Scholar]
- 10.State Council AIDS Working Committee Office UtGoHAiC. A Joint Assessment of HIV/AIDS Prevention, Treatment and Care in China (2007) Beijing: 2007. [Google Scholar]
- 11.Wu Z. Sun X. Sullivan SG. Detels R. Public health. HIV testing in China. Science. 2006;312:1475–1476. doi: 10.1126/science.1120682. [DOI] [PubMed] [Google Scholar]
- 12.Snowden JM. Raymond HF. McFarland W. Prevalence of seroadaptive behaviours of men who have sex with men, San Francisco, 2004. Sex Transm Infect. 2009;85:469–476. doi: 10.1136/sti.2009.036269. [DOI] [PubMed] [Google Scholar]
- 13.Crawford JM. Rodden P. Kippax S. Van de Ven P. Negotiated safety and other agreements between men in relationships: Risk practice redefined. Int J STD AIDS. 2001;12:164–170. doi: 10.1258/0956462011916965. [DOI] [PubMed] [Google Scholar]
- 14.Dawson JM. Fitzpatrick RM. Reeves G, et al. Awareness of sexual partners' HIV status as an influence upon high-risk sexual behaviour among gay men. AIDS. 1994;8:837–841. [PubMed] [Google Scholar]
- 15.Janssen RS. Holtgrave DR. Valdiserri RO. Shepherd M. Gayle HD. De Cock KM. The serostatus approach to fighting the HIV epidemic: Prevention strategies for infected individuals. Am J Public Health. 2001;91:1019–1024. doi: 10.2105/ajph.91.7.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson WD. Diaz RM. Flanders WD, et al. Behavioral interventions to reduce risk for sexual transmission of HIV among men who have sex with men. Cochrane Database Syst Rev. 2008(3):CD001230. doi: 10.1002/14651858.CD001230.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Kippax S. Noble J. Prestage G, et al. Sexual negotiation in the AIDS era: negotiated safety revisited. AIDS. 1997;11:191–197. doi: 10.1097/00002030-199702000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Heckathorn D. Respondent driven sampling II: Deriving valid population estimates from Chain-referral samples of hidden populations. Soc Probl. 2002;49:11–34. [Google Scholar]
- 19.Heckathorn D. Respondent driven sampling: A new approach to the study of hidden populations. Soc Probl. 1997;44:174–199. [Google Scholar]
- 20.Kegeles SM. Hays RB. Coates TJ. The Mpowerment Project: A community-level HIV prevention intervention for young gay men. Am J Public Health. 1996;86:1129–1136. doi: 10.2105/ajph.86.8_pt_1.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kegeles SM. Hays RB. Pollack LM. Coates TJ. Mobilizing young gay and bisexual men for HIV prevention: A two-community study. AIDS. 1999;13:1753–1762. doi: 10.1097/00002030-199909100-00020. [DOI] [PubMed] [Google Scholar]
- 22.Ma X. Zhang Q. He X, et al. Trends in prevalence of HIV, syphilis, hepatitis C, hepatitis B, and sexual risk behavior among men who have sex with men. Results of 3 consecutive respondent-driven sampling surveys in Beijing, 2004 through 2006. J Acquir Immune Defic Syndr. 2007;45:581–587. doi: 10.1097/QAI.0b013e31811eadbc. [DOI] [PubMed] [Google Scholar]
- 23.Helms DJ. Weinstock HS. Mahle KC, et al. HIV testing frequency among men who have sex with men attending sexually transmitted disease clinics: implications for HIV prevention and surveillance. J Acquir Immune Defic Syndr. 2009;50:320–326. doi: 10.1097/QAI.0b013e3181945f03. [DOI] [PubMed] [Google Scholar]
- 24.Prestage G. Jin F. Zablotska IB. Imrie J. Grulich AE. Pitts M. Trends in HIV testing among homosexual and bisexual men in eastern Australian states. Sex Health. 2008;5:119–123. doi: 10.1071/sh07081. [DOI] [PubMed] [Google Scholar]
- 25.Sandfort TG. Nel J. Rich E. Reddy V. Yi H. HIV testing and self-reported HIV status in South African men who have sex with men: results from a community-based survey. Sex Transm Infect. 2008;84:425–429. doi: 10.1136/sti.2008.031500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sumartojo E. Lyles C. Choi K, et al. Prevalence and correlates of HIV testing in a multi-site sample of young men who have sex with men. AIDS Care. 2008;20:1–14. doi: 10.1080/09540120701450425. [DOI] [PubMed] [Google Scholar]
- 27.Xia Q. Molitor F. Osmond DH, et al. Knowledge of sexual partner's HIV serostatus and serosorting practices in a California population-based sample of men who have sex with men. AIDS. 2006;20:2081–2089. doi: 10.1097/01.aids.0000247566.57762.b2. [DOI] [PubMed] [Google Scholar]
- 28.Kippax S. Crawford J. Davis M. Rodden P. Dowsett G. Sustaining safe sex: A longitudinal study of a sample of homosexual men. AIDS. 1993;7:257–263. [PubMed] [Google Scholar]
- 29.Van de Ven P. Kippax S. Crawford J, et al. In a minority of gay men, sexual risk practice indicates strategic positioning for perceived risk reduction rather than unbridled sex. AIDS Care. 2002;14:471–480. doi: 10.1080/09540120208629666. [DOI] [PubMed] [Google Scholar]
- 30.McConnell JJ. Bragg L. Shiboski S. Grant RM. Sexual seroadaptation: Lessons for prevention and sex research from a cohort of HIV-positive men who have sex with men. PloS One. 2010;5:e8831. doi: 10.1371/journal.pone.0008831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Truong HM. Kellogg T. Klausner JD, et al. Increases in sexually transmitted infections and sexual risk behaviour without a concurrent increase in HIV incidence among men who have sex with men in San Francisco: A suggestion of HIV serosorting? Sex Transm Infect. 2006;82:461–466. doi: 10.1136/sti.2006.019950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Velter A. Bouyssou-Michel A. Arnaud A. Semaille C. Do men who have sex with men use serosorting with casual partners in France? Results of a nationwide survey (ANRS-EN17-Presse Gay 2004) Euro Surveill. 2009;14:47. doi: 10.2807/ese.14.47.19416-en. [DOI] [PubMed] [Google Scholar]
- 33.Miner MH. Peterson JL. Welles SL. Jacoby SM. Rosser BR. How do social norms impact HIV sexual risk behavior in HIV-positive men who have sex with men?: multiple mediator effects. J Health Psychol. 2009;14:761–770. doi: 10.1177/1359105309338976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peterson JL. Rothenberg R. Kraft JM. Beeker C. Trotter R. Perceived condom norms and HIV risks among social and sexual networks of young African American men who have sex with men. Health Educ Res. 2009;24:119–127. doi: 10.1093/her/cyn003. [DOI] [PubMed] [Google Scholar]
- 35.Jiang J. Cao N. Zhang J, et al. High prevalence of sexually transmitted diseases among men who have sex with men in Jiangsu Province, China. Sex Transm Dis. 2006;33:118–123. doi: 10.1097/01.olq.0000199763.14766.2b. [DOI] [PubMed] [Google Scholar]
- 36.Ruan S. Yang H. Zhu Y, et al. HIV prevalence and correlates of unprotected anal intercourse among men who have sex with men, Jinan, China. AIDS Behav. 2008;12:469–475. doi: 10.1007/s10461-008-9361-9. [DOI] [PubMed] [Google Scholar]
- 37.Lau JT. Wang M. Wong HN, et al. Prevalence of bisexual behaviors among men who have sex with men (MSM) in China and associations between condom use in MSM and heterosexual behaviors. Sex Transm Dis. 2008;35:406–413. doi: 10.1097/OLQ.0b013e318164467f. [DOI] [PubMed] [Google Scholar]
- 38.Ruan Y. Li D. Li X, et al. Relationship between syphilis and HIV infections among men who have sex with men in Beijing, China. Sex Transm Dis. 2007;34:592–597. doi: 10.1097/01.olq.0000253336.64324.ef. [DOI] [PubMed] [Google Scholar]
- 39.Zhang BC. Chu QS. MSM and HIV/AIDS in China. Cell Res. 2005;15:858–864. doi: 10.1038/sj.cr.7290359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu H. Yang H. Li X, et al. Men who have sex with men and human immunodeficiency virus/sexually transmitted disease control in China. Sex Transm Dis. 2006;33:68–76. doi: 10.1097/01.olq.0000187266.29927.11. [DOI] [PubMed] [Google Scholar]
- 41.Yang X. Xia G. Gender, migration, and unprotected causal and commercial sex: Individual and social determinants of HIV and STD risk among female migrants. In: Poston DL, editor; Tucker J, editor; Ren Q, et al., editors. Gender Policy and HIV in China. Vol. 22. The Netherlands: Springer; 2009. pp. 97–114. [Google Scholar]
- 42.Lin D. Li X. Stanton B, et al. Theory-based HIV-related sexual risk reduction prevention for chinese female rural-to-urban migrants. AIDS Educ Prev. 2010;22:344–355. doi: 10.1521/aeap.2010.22.4.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi KH. Gibson DR. Han L. Guo Y. High levels of unprotected sex with men and women among men who have sex with men: A potential bridge of HIV transmission in Beijing, China. AIDS Educ Prev. 2004;16:19–30. doi: 10.1521/aeap.16.1.19.27721. [DOI] [PubMed] [Google Scholar]
- 44.He Q. Wang Y. Lin P, et al. Potential bridges for HIV infection to men who have sex with men in Guangzhou, China. AIDS Behav. 2006;10(4 Suppl):S17–23. doi: 10.1007/s10461-006-9125-3. [DOI] [PubMed] [Google Scholar]
- 45.Johnston LG. Khanam R. Reza M, et al. The effectiveness of respondent driven sampling for recruiting males who have sex with males in Dhaka, Bangladesh. AIDS Behav. 2008;12:294–304. doi: 10.1007/s10461-007-9300-1. [DOI] [PubMed] [Google Scholar]
- 46.Li D. Jia Y. Ruan Y, et al. Correlates of incident infections for HIV, syphilis, and hepatitis B virus in a cohort of men who have sex with men in Beijing. AIDS Patient Care STDs. 2010;24:595–602. doi: 10.1089/apc.2010.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu J. Qu B. Guo HQ. Sun G. Factors that influence risky sexual behaviors among men who have sex with men in Liaoning Province, China: A structural equation model. AIDS Patient Care STDs. 2011;25:423–429. doi: 10.1089/apc.2010.0333. [DOI] [PubMed] [Google Scholar]
- 48.Liu H. Feng T. Liu H, et al. Egocentric networks of Chinese men who have sex with men: Network components, condom use norms, and safer sex. AIDS Patient Care STDs. 2009;23:885–893. doi: 10.1089/apc.2009.0043. [DOI] [PubMed] [Google Scholar]