Abstract
Toll-like receptor 3 (TLR3) mediates antiviral response by recognizing double-stranded RNA. Its cytoplasmic domain is tyrosine phosphorylated upon ligand binding and initiates downstream signaling via the adapter TIR-containing adaptor inducing interferon–β (TRIF). However, the kinase responsible for TLR3 phosphorylation remains unknown. We show here that Bruton's tyrosine kinase (BTK)-deficient macrophages failed to secrete inflammatory cytokines and IFN-β upon TLR3 stimulation and were impaired in clearing intracellular dengue virus infection. Mutant mice were also less susceptible to d-galactosamine/p(I:C)-induced sepsis. In the absence of BTK, TLR3-induced phosphoinositide 3-kinase (PI3K), AKT and MAPK signaling and activation of NFκB, IRF3, and AP-1 transcription factors were all defective. We demonstrate that BTK directly phosphorylates TLR3 and in particular the critical Tyr759 residue. BTK point mutations that abrogate or led to constitutive kinase activity have opposite effects on TLR3 phosphorylation. Loss of BTK also compromises the formation of the downstream TRIF/receptor-interacting protein 1 (RIP1)/TBK1 complex. Thus, BTK plays a critical role in initiating TLR3 signaling.
Keywords: innate immunity, Pattern Recognition Receptors, signal transduction
Toll-like receptors (TLRs) recognize pathogen-associated molecular patterns and activate signaling pathways that induce the expression of host immune and inflammatory genes (1). All TLRs contain extracellular leucine-rich domains for ligand recognition and a cytoplasmic Toll/IL-1R (TIR) domain for signaling. Most TLRs signal via the adapter myeloid differentiating factor 88 (MyD88) with the exception of TLR4, which also uses a few other adapters and TLR3, which uses solely TIR-containing adaptor inducing interferon-β (TRIF) (2). Hence, TLR3 provides a unique system to study innate signaling.
TLR3 is involved in antiviral response. Its engagement by viral double-stranded RNA (dsRNA) or the synthetic analog polyinsosinic: polyribocytidylic acid (p(I:C)) triggers the secretion of IFN-β and other inflammatory cytokines such as IL-6 and TNF-α. Mechanistically, TLR3 stimulation leads to the recruitment of TRIF and subsequent activation of the transcription factor IFN regulatory factor-3 (IRF3) (3). The N-terminal of TRIF binds tumor necrosis factor receptor-associated factor 3 (TRAF3)-TANK-binding kinase 1 (TBK1) complex that leads to IRF3 phosphorylation, dimerization, and translocation to nucleus to induce IFN-β expression (4). The C-terminal of TRIF binds receptor-interacting protein 1 (RIP1) and induces the ubiquitination of RIP1 (5). TLR3 signaling also activates ERK, JNK, p38 MAPKs, and phosphoinositide 3-kinase (PI3K) (6). MAPKs activate the AP-1 family of transcription factors, which induces proinflammatory cytokine genes (7). On the other hand, TLR3-induced PI3K signaling contributes to the full activation of IRF3 (8). TRIF signaling leading to TNF-α production also acts in a feed-forward manner to further and fully activate NFκB signaling (9, 10). Although the major signaling pathways associated with TLR3/TRIF activation have been well studied, how TLR3 signaling is initiated remains to be determined.
There are five tyrosine residues in the TIR domain of TLR3 and tyrosine phosphorylation of TLR3 is important for its signaling (11). In particular, TLR3 phosphorylation at Tyr759 and Tyr858 is critical for the subsequent induction of NFκB, IRF3, and AP-1 (12). Phosphorylation of Tyr759 is also important for PI3K recruitment and AKT activation, which is required for the full activation of IRF3 (8). However, the protein tyrosine kinase responsible for TLR3 phosphorylation remains unknown and is of major interest in the field.
Bruton's tyrosine kinase (BTK), a member of the TEC family of cytoplasmic tyrosine kinases, plays a critical role in B-cell receptor signaling (13). It contains N-terminal pleckstrin homology (PH), Tec-homology, and Src-homology (SH) 2 domains, a C-terminal SH3 domain, and multiple tyrosine residues. Mutations in BTK lead to X-linked agammaglobulinemia (XLA) in human and X-linked immunodeficiency in mice (14). These diseases are characterized by defects in B-lymphopoiesis and impairment in humoral responses to T-cell–independent antigens (15). XLA patients also suffer from recurrent bacterial and viral infections (16), suggesting that BTK might play a role in innate immunity. In support of this hypothesis, several studies have shown BTK phosphorylation in certain TLR responses, e.g., TLR2 or -4 in human monocytes and macrophages (17, 18) and TLR2, -4, -7, and -8 in dendritic cells (19). Our previous study also indicated a role for BTK in TLR9 signaling in B cells (20). These TLRs signal predominantly via MyD88 (21) and these findings suggest that BTK is involved in MyD88-mediated signaling. Although BTK had been shown to associate with MyD88, Mal, and IRAK1 (22) and identified as the kinase that phosphorylated Mal (23), its targets in most TLR responses are unknown. It is also not known whether BTK has a role in TLR3 signaling and whether BTK can phosphorylate any TLR.
In this study, we provide evidence for a critical role of BTK in TLR3 signaling. We show that BTK is phosphorylated upon TLR3 engagement and is required for the secretion of inflammatory cytokines and IFN-β in macrophages. More importantly, we demonstrate that BTK is a key signaling molecule that initiates TLR3-mediated antiviral response by phosphorylating the receptor.
Results
BTK Is Activated and Required for Cytokine Production in TLR3-Activated Macrophages.
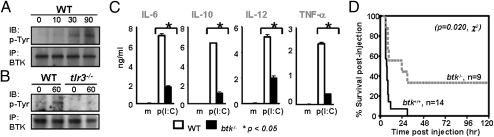
To assess whether BTK is involved in TLR3 signaling, we treated wild-type (WT) bone marrow-derived macrophages with naked p(I:C). Western blot analysis of cell lysates revealed that BTK was phosphorylated between 10 and 30 min after stimulation (Fig. 1A), indicating that BTK is activated in macrophages upon p(I:C) treatment. We further demonstrate that naked p(I:C)-induced BTK activation is primarily mediated by TLR3, as the phosphorylation and activation of BTK is abolished in tlr3−/− macrophages (Fig. 1B).
Fig. 1.
BTK is activated and required for the production of inflammatory cytokines in TLR3-stimulated macrophages. (A) BTK is phosphorylated upon p(I:C) stimulation. WT bone marrow-derived macrophages were treated with naked p(I:C) for various times and BTK was immunoprecipitated (IP) and examined via Western blot analysis with antiphosphotyrosine (pTyr) and anti-BTK antibodies. (B) Lack of BTK phosphorylation in p(I:C)-stimulated tlr3−/− macrophages. WT and tlr3−/− macrophages were treated and analyzed as above. (C) btk−/− macrophages have impaired secretion of inflammatory cytokines in response to naked p(I:C) stimulation. WT and btk−/− macrophages were nontreated or stimulated with naked p(I:C) for 6 h and their production of cytokine measured via ELISA. (D) btk−/− mice were more resistant to d-galactosamine/p(I:C)-induced sepsis. WT and btk−/− mice were challenged with d-galactosamine and p(I:C) and their survival was monitored over time.
Given that BTK is phosphorylated upon p(I:C) stimulation, we wonder if the activation of BTK by p(I:C) simulation has any functional relevance for TLR3-induced responses. As a first measure, we examined the proliferation of WT and btk−/− splenocytes to treatment with various concentrations of p(I:C) and it was apparent that btk−/− splenocytes were defective in this response (Fig. S1).
It is known that recognition of viral dsRNA by TLR3 leads to the synthesis and secretion of inflammatory cytokines (24). We next analyzed whether BTK deficiency would affect cytokine production triggered by TLR3 engagement. As shown in Fig. 1C, naked p(I:C)-stimulated btk−/− macrophages had defective production of IL-6, IL-10, IL-12, and TNF-α, as measured by ELISA, compared with similarly treated WT cells. Again, we demonstrate that the defective induction of inflammatory cytokines in btk−/− macrophages by naked p(I:C) is via TLR3. As shown in Fig. S2, real-time RT-PCR analyses indicated that the induction of cytokine genes was also defective in tlr3−/− macrophages stimulated similarly with naked p(I:C) but not with transfected p(I:C), which is sensed by RIG-I/MDA5 in the cytosol (25). We also rule out the possibility that defective cytokine production in btk−/− macrophages is due to altered expression of TLR3 or TRIF as WT and btk−/− macrophages express comparable levels of tlr3 and trif mRNA (Fig. S3).
To investigate whether the in vitro observed cytokine defects in p(I:C)-stimulated btk−/− macropahges would translate to any effect in vivo, we used a septic shock model by injecting p(I:C) and d-galactosamine into WT and btk−/− mice. In this acute inflammation model, the presence of d-galactosamine sensitizes the mice to the toxicity of p(I:C) and susceptible mice usually died within hours due to exposure to TNF-α (26). As expected, most WT mice (>90%) died within 10 h of p(I:C) injection (Fig. 1D). In contrast, only 50% of the btk−/− mice succumbed to the lethal effect of this septic shock in the first 24 h and 40% of them survived beyond 120 h after challenge. Thus, BTK deficiency diminished the lethality of p(I:C)-induced septic shock and this was consistent with the reduced production of TNF-α and other inflammatory cytokines in p(I:C)-stimulated btk−/− macrophages. We also repeated the septic-shock experiment using tlr3−/− mice alongside WT and btk−/− mice. The data indicated that tlr3−/− and btk−/− mice behaved similarly and showed statistically equivalent resistance to p(I:C)-induced sepsis compared with WT mice (Fig. S4). Collectively, the data indicated that BTK is required for TLR3-induced production of inflammatory cytokines.
BTK-Deficiency Affects TLR3-Induced Production of IFN-β and Compromises Clearance of Dengue Virus in Macrophages.
Stimulation of macrophages through TLR3 also leads to the production of IFN-β, which is important for antiviral responses (27). Real-time RT-PCR analyses indicated that the production of IFN-β was severely compromised in p(I:C)-stimulated btk−/− macrophages compared with WT control. Furthermore, the synthesis of the TRIF-dependent chemokine, Rantes, was also affected in the absence of BTK (Fig. 2A). Other than p(I:C), which engages TLR3, LPS, which stimulates TLR4 is also known to induce via TRIF the production of IFN-β in macrophages (28). To further confirm that TRIF-dependent IFN-β production was compromised in the absence of BTK, we measured by ELISA the secretion of IFN-β from both p(I:C)- and LPS-stimulated WT and btk−/− macrophages. Indeed, our data showed that btk−/− macrophages had impaired IFN-β response when treated with these two stimuli (Fig. 2B).
Fig. 2.
BTK is required for TLR3-induced IFN-β production and inhibition of dengue virus replication. (A) WT and btk−/− macrophages were stimulated with p(I:C) for 3 h and their expression of ifn-β and Rantes mRNA quantified via real-time RT-PCR and normalized to that of actin mRNA. (B) Secretion of IFN-β by p(I:C) and LPS-stimulated WT and btk−/− macrophages was also measured via ELISA, *P < 0.05. Data shown are representative of three independent experiments. Real-time RT-PCR analyses of ifn-b mRNA induction in (C) naked p(I:C)-treated WT, tlr3−/−, and ips1−/− macrophages and (D) WT and btk−/− macrophages transfected with p(I:C). Cells were treated and analyzed as in A. Data shown are representative of two independent experiments. (E) Lack of STAT1 induction in p(I:C)-stimulated btk−/− macrophages. WT and mutant cells were treated with p(I:C) for 3 h and cell lysates were analyzed via Western blot analysis for STAT1 activation using antiphospho-STAT1 antibody. The STAT1 blot served as loading control. (F) BTK-deficient macrophages have defective control of dengue virus replication. WT and btk−/− macrophages were infected with dengue virus and at 72 h postinfection assayed for ifn-β mRNA and presence of dengue virus negative strand RNA via semiquantitative RT-PCR. The GADPH RT-PCR served as control for loading of templates.
It is known that when transfected into cells, p(I:C) is sensed by cytosolic RIG-I/MDA5, which signals via the adapter IPS-1 for IFN-β production (25). To confirm that defective IFN-β production seen in naked p(I:C)-treated btk−/− macrophages is through stimulation of TLR3 and not via cytosolic sensors, we examined ifn-b mRNA induction by naked p(I:C) stimulation in tlr3−/− and ips1−/− cells using real-time RT-PCR analyses. As shown in Fig. 2C, ifn-b mRNA was not induced in tlr3−/− macrophages, which could not sense naked p(I:C). In contrast, ips1−/− cells exhibit robust induction of ifn-b upon stimulation with naked p(I:C), and this is not surprising as they possess TLR3. This observation indicates that our manner of stimulating cells with naked p(I:C) is mediated primarily through TLR3 and not RIG-I/MDA5 pathways. We further show that the induction of ifn-b mRNA was comparable in WT and btk−/− macrophages when they were transfected with p(I:C), suggesting that BTK might not have a role in RIG-I/MDA5 signaling (Fig. 2D), in contrast to its essential role in TLR3 pathway.
Type 1 IFNs is known to signal in an autocrine manner via IFN receptors and JAK-STAT signaling to induce more IFN production (29) and consistent with the diminished secretion of IFN-β by p(I:C)-stimulated btk−/− macrophages, the phosphorylation of STAT1, which is indicative of IFN autocrine signaling, was also attenuated (Fig. 2E).
To test whether reduced production of IFN-β by TLR3-stimulated btk−/− macrophages would compromise antiviral responses, we infected WT and mutant cells with dengue viruses. The intracellular replication of dengue virus involves dsRNA intermediates and these are recognized by TLR3 (30–32). As shown in Fig. 2F, semiquantitative RT-PCR analyses indicated that btk−/− macrophages were defective in the production of IFN-β upon dengue virus infection and concomitant with this defect, the replication of dengue virus, as measured by the presence of the negative strand viral RNA, was much more pronounced in mutant compared with WT cells. (The quantification of ifn-β and dengue virus negative strand RNA in infected WT and btk−/− cells using densitometry analyses is shown in Fig. S5.) Thus, btk−/− macrophages showed defective IFN-β production to p(I:C) stimulation as well as dengue virus infection and failed to suppress intracellular dengue virus replication. We repeated the dengue virus infection experiment using WT and tlr3−/− macrophages (Fig. S6) and showed that tlr3−/− macrophages were also defective in IFN-β mRNA production and dengue virus clearance. These data together demonstrated the importance of BTK in TLR3 signaling.
BTK Is Required for the Activation of Major Signaling Pathways and Transcription Factors Downstream of TLR3.
The data above indicated that loss of BTK has profound effects on TLR3-induced responses. To unravel the role of BTK in TLR3 signaling, we examined the signaling pathways downstream of TLR3. A key pathway triggered as a consequence of TLR3 engagement is that of NFκB (33), which acts also as coactivator of IFN-β (34). NFκB transcription factors are sequestrated in the cytoplasm by inhibitory IκB subunit, which is phosphorylated and degraded upon activation. This allows NFκB to translocate into nucleus to bind promoters and activate gene expression (35).
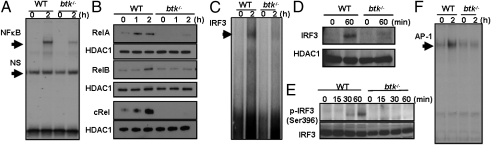
To investigate the activation of NFκB, we first used an electrophorectic mobility-shift assay (EMSA) using a consensus DNA probe and nuclear extracts from p(I:C)-stimulated WT and btk−/− macrophages. Data shown in Fig. 3A indicated that p(I:C)-induced NFκB activation was severely attenuated in btk−/− macrophages compared with WT control. The defect in NFκB activation in btk−/− macrophages was further confirmed by the lack of nuclear localization of the RelA, RelB, and cRel subunits (Fig. 3B). Thus, BTK deficiency affects NFκB activation.
Fig. 3.
BTK deficiency impairs the induction of NFκB, IRF3, and AP-1 transcription factors downstream of TLR3 signaling. (A) Lack of NFκB DNA-binding activity in p(I:C)-stimulated btk−/− macrophages. Nuclear extracts from p(I:C)-stimulated macrophages were incubated with oligonucleotide bearing NFκB consensus sequence and examined via gel shift assay. NS, nonspecific band. (B) Absence of nuclear-localized RelA, RelB, and c-Rel in p(I:C)-stimulated btk−/− macrophages. Nuclear extracts from nontreated or p(I:C)-stimulated cells were analyzed via Western blotting for the presence of various NFκB subunits using specific antibodies. The HDAC1 blots served as loading controls. (C) Gel-shift analysis of IRF3 activation in p(I:C)-treated WT and btk−/− macrophages. Nuclear extracts were analyzed via EMSA using DNA probe bearing a consensus ISRE. (D) Lack of IRF3 nuclear localization in TLR3-activated btk−/− macrophages. WT and mutant cells were stimulated with p(I:C) for various times and nuclear extracts were analyzed via Western blotting with specific antibody. HDAC1 blot served as loading control. (E) Absence of IRF3 phosphorylation in p(I:C)-stimulated btk−/− macrophages. IRF3 activation in wild-type and mutant macrophages was examined via Western blot with an antibody that recognizes phospho-Serine396 residue of IRF3. (F) Lack of induction of AP-1 transcription factor in p(I:C)-stimulated btk−/− macrophages. Nuclear extracts from p(I:C)-stimulated wild-type and mutant cells were incubated with an oligonucleotide probe bearing an AP-1 consensus sequence and analyzed via EMSA.
TLR3 engagement also activates the transcription factor IRF3, which leads to the induction of IFN-β gene transcription (36). IRF-3 is activated by serine phosphorylation, which leads to its dimerization and nuclear translocation (37) to bind gene promoters bearing IFN stimulated response element (ISRE). To investigate the stage at which BTK deficiency impacted upon the induction of IFN-β, we first examined IRF3 binding to DNA elements bearing ISRE in TLR3-activated WT and btk−/− macrophages. EMSA conducted with nuclear extracts from these cells indicated that there was a lack of IRF3 binding to ISRE in the mutant cells (Fig. 3C). Detailed studies further revealed that p(I:C)-induced IRF3 nuclear localization (Fig. 3D) was defective in TLR3-stimulated btk−/− macrophages compared with WT controls. Interestingly, IRF3 activation as indicated by serine 396 phosphorylation (Fig. 3E) was also impaired in p(I:C)-stimulated btk−/− macrophages. Thus, BTK signaling is required for TLR3-induced activation of IRF3 and our data suggest that BTK affects IRF3 activation further upstream in the signaling cascade.
Next, we examined the activation of ERK, JNK, and p38 MAPK in p(I:C)-treated btk−/− macrophages. These signaling cascades are known to increase proinflammatory gene expressions (7). As shown in Fig. 4A, p(I:C) stimulation of WT macrophages resulted in the activation of all three classes of MAPKs. In contrast, the activation of ERK and JNK was impaired, whereas the induction of p38 MAPK was reduced in p(I:C)-treated btk−/− macrophages. The defects in ERK and JNK signaling in TLR3-treated btk−/− macrophages was further confirmed by the lack of induction of AP-1 transcription factor as examined via EMSA (Fig. 3F). These data indicate that BTK plays a role in TLR3-induced activation of MAPK and AP-1.
Fig. 4.
Defective PI3K/AKT and MAPK signaling in p(I:C)-treated btk−/− macrophages. (A) TLR3-stimulated btk−/− macrophages have impaired activation of ERK, JNK, and p38 MAPK. WT and btk−/− macrophages were stimulated with p(I:C) for various times and examined via Western blot using antiphospho-ERK, antiphospho-JNK, and antiphospho-p38 antibodies. The anti-ERK, -JNK, and -p38 blots served as loading controls. (B) btk−/− macrophages have defective activation of PI3K and AKT upon p(I:C) stimulation. WT and btk−/− macrophages were stimulated with p(I:C) for various times and cell lysates were probed for PI3K activation using antiphospho-p85α antibody and for AKT activation using antiphospho-AKT(Serine 473) and antiphospho-AKT(Threonine308) specific antibodies. The anti-p85α and anti-AKT blots served as loading controls.
Previous studies have shown that TLR3 stimulation also activates PI3K/AKT signaling, which is induced independently of TRIF and important for the full phosphorylation and activation of IRF3 (8). We found PI3K activation, as shown by phosphorylation of p85α regulatory subunit, to be attenuated in p(I:C)-stimulated btk−/− macrophages compared with WT control (Fig. 4B). As a result, the activation of the downstream AKT kinase was also defective as shown by the lack of phosphorylation of its Ser473 and Thr308 residues in p(I:C)-treated btk−/− macrophages.
Overall our data suggest that BTK plays a critical role in TLR3 signaling and is required for the activation of not only NFκB and IRF3 but also PI3K and MAPK signaling and hence account for the severe functional defects seen in p(I:C)-stimulated btk−/− macrophages and mice.
BTK Phosphorylates TLR3.
The severe phenotype of btk−/− mice and macrophages suggests that BTK might play a pivotal role in TLR3 signaling. It is currently accepted that TRIF is immediate downstream of TLR3 signaling but TRIF has not been shown to be phosphorylated (2). Our attempts to determine whether TRIF is phosphorylated also did not yield any positive results (Fig. S7). Further downstream of TRIF is the various MAPK, NFκB, and IRF3 signaling pathways (3). Thus, we wonder whether BTK would act very proximal to TLR3. It is known that the TIR domain of TLR3 contains tyrosine residues and that TLR3 is phosphorylated following p(I:C) stimulation. Furthermore, it has been shown that Tyr759 phosphorylation is critical for TLR3 signaling (11). Thus, we checked whether TLR3 phosphorylation would be compromised in the absence of BTK. We used a specific antibody that recognizes phosphorylated Tyr759 of TLR3. We also used the general antiphosphotyrosine antibody 4G10 to detect whether other tyrosine residues of TLR3 are phosphorylated in the absence of BTK. As shown in Fig. 5A, Upper, TLR3 was phosphorylated at Tyr759 in p(I:C)-stimulated WT but not btk−/− macrophages. In addition, using 4G10 antibody, we showed that the phosphorylation of all tyrosine residues of TLR3 was impaired in the absence of BTK (Fig. 5A, Lower). Thus, BTK is needed for TLR3 phosphorylation.
Fig. 5.
BTK directly phosphorylates TLR3. (A) Defective TLR3 phosphorylation in p(I:C)-stimulated btk−/− macrophages. WT and btk−/− macrophages were stimulated with p(I:C) and probed for TLR3 phosphorylation using an antibody that recognizes phospho-Tyrosine759 of TLR3 (Upper) or 4G10 antibody that recognizes all phosphotyrosine residues (Lower). The anti-TLR3 blot served as loading control. (B) BTK is required for TLR3 phosphorylation. HEK293 cells were transfected with various combinations of plasmids bearing TLR3-FLAG, BTK-HA and Lyn and were either nontreated or stimulated with p(I:C) and examined via immunoblotting (IB) for tyrosine phosphorylation of TLR3 (Left, using 4G10 antibody) or Tyrosine759 residue (Right) of the immunoprecipitated (IP) FLAG-tagged TLR3. Whole cell lysates (WCLs) were also examined for expression of the various transfected constructs. (C) BTK kinase activity is required for TLR3 phosphorylation. HEK293 cells were transfected with FLAG-TLR3 only or with BTK-HA, kinase deleted (kinase Δ) BTK-FLAG, kinase dead (K430R) BTK-HA, or constitutively active (E41K) BTK-HA and stimulated with p(I:C). FLAG-tagged TLR3 was immunoprecipitated and examined for phosphorylation using 4G10 antibody. Expression of the transfected constructs was assayed as above.
To further assess BTK's role in TLR3 phosphorylation, we transfected FLAG-tagged TLR3 together with HA-tagged BTK into HEK293T cells and examined the phosphorylation of the ectopically expressed TLR3. As shown in Fig. 5B, FLAG-tagged TLR3 was phosphorylated (Left, lanes 2 and 4) and in particular, at Tyr759 residue (Right, lanes 2 and 4) with or without p(I:C) stimulation when it was coexpressed with BTK but not with another tyrosine kinase, Lyn. This finding suggested that the presence of BTK leads specifically to TLR3 phosphorylation.
Because BTK is a multidomain protein and could act as an adapter in addition to a kinase, it remained to be seen whether BTK directly phosphorylates TLR3 or acts as a bridge to recruit another kinase to phosphorylate TLR3. To examine this question, we deleted its kinase domain (kinase Δ) or introduced point mutations that abolished (K430R) or leads to constitutive (E41K) kinase activity and coexpressed these mutants in HEK293T cells with FLAG-tagged TLR3. As shown in Fig. 5C, TLR3 phosphorylation, as indicated by 4G10 staining, was absent in the presence of the kinase-deleted or dead BTK (lanes 3 and 4) and enhanced in the presence of constitutively active BTK (lane 5). Taken together, these data indicated that BTK did not act as an adapter to recruit another kinase but acts specifically and directly to phosphorylate TLR3.
TLR3-Induced Formation of TRIF/RIP1 and TRIF/TBK1 Complex Is Impaired in the Absence of BTK.
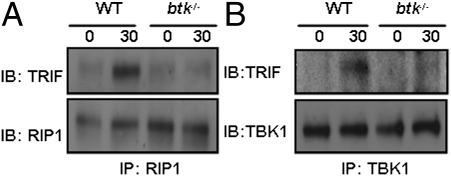
Our findings above indicate that BTK phosphorylation of TLR3 could be the initiating event in TLR3 signal transduction. It is well established that TRIF is immediately downstream of TLR3 (38) and that RIP1 (5) and TBK-1 (39) are recruited to TRIF upon induction of TLR3 signaling. Hence, we asked whether the recruitment of RIP1 and TBK1 to TRIF would be affected in the absence of BTK. Because of the nonavailability of a good anti-TRIF antibody for coimmunoprecipitation (COIP) studies, we could use only anti-RIP1 and anti-TBK1 antibodies for this purpose. We stimulated wild-type and btk−/− macrophages with p(I:C) and subjected the cell lysates to COIP with anti-RIP1 or anti-TBK1 antibodies. Our data indicated that there was increased COIP of TRIF with RIP1 (Fig. 6A) and with TBK1 (Fig. 6B) in WT macrophages upon TLR3 activation but the associations of these molecules were not apparent in btk−/− macrophages. This finding suggests that BTK acts upstream and is essential for the induced recruitment of RIP1 and TBK1 to TRIF following TLR3 phosphorylation.
Fig. 6.
TLR3-induced formation of TRIF-TBK1 and TRIF-RIP1 complex is compromised in the absence of BTK. WT and btk−/− macrophages were nontreated or stimulated with p(I:C) for 30 min and cell lysates were immunoprecipitated with (A) anti-RIP1 or (B) anti-TBK1 antibodies and immunoblotted with anti-TRIF antibody to examine association. Data shown are representative of three independent experiments.
Discussion
We show in this study that BTK plays a critical role in TLR3 signaling. We demonstrate that BTK is phosphorylated upon TLR3 engagement and its deficiency impaired the secretion of proinflammatory cytokines and IFN-β in naked p(I:C)-treated macrophages. As a result of reduced cytokine production, btk−/− mice were more resistant to d-galactosamine-sensitized p(I:C)-induced septic shock. In these aspects, the responses of btk−/− macrophages and mice to p(I:C) stimulation resemble those of tlr3−/− cells and mice. TLR3-deficient cells have also reduced production of cytokines in response to p(I:C) stimulation and tlr3−/− mice were also resistant to d-galactosamine/p(I:C)-induced septic shock (40). These observations suggest that BTK could be part of the TLR3 signaling pathway.
Engagement of TLR3 triggers the induction of multiple signaling pathways that culminate in the activation of NFκB, AP-1, and IRF3, which are critical for the production of cytokines and interferons (34). At the biochemical level, our data indicated that BTK deficiency affects TLR3 induction of these transcription factors and led us to hypothesize that BTK could act at the level of TRIF signaling. However, TRIF has not been shown to be tyrosine phosphorylated upon TLR3 engagement. Also, BTK deficiency impacts upon TLR3-induced activation of PI3K/AKT, which was shown to be independent of TRIF (8). Taking these observations into consideration, the data suggest that BTK either acts very upstream or participates in various branches of TLR3 signaling. Our systematic analyses suggest that BTK probably acts very upstream in TLR3/TRIF signaling and its deficiency simultaneously cripples, from the start, multiple branches of TLR3 signal transduction.
Our pursuit of the role of BTK in TLR3 signaling was aided by earlier findings that TLR3 harbored five tyrosine residues in its TIR domain and was phosphorylated upon ligand engagement (8). We show that tyrosine phosphorylation of the TIR domain of TLR3 and in particular Tyr759 is impaired in the absence of BTK. The phosphorylation of the Tyr759 and Tyr858 residues is thought to initiate TLR3 signaling and critical for the activation of AKT and NFκB, respectively (8, 12). Our experiments involving overexpression studies in a heterologous system further indicate that BTK directly phosphorylates TLR3 because point mutations that crippled BTK activity could not result in TLR3 phosphorylation.
Previously, c-Src was shown to be induced by dsRNA and leads to IRF-3 activation (41). However, our data indicate that Lyn, a Src family member, could not directly phosphorylate TLR3. Indeed, c-Src was shown to associate with TRAF3 (42) and this association occurred further downstream in TLR3 signaling. Taken together, our findings represent an important advance in understanding TLR3 signaling and definitively demonstrate BTK as the tyrosine kinase important for phosphorylating TLR3.
Materials and Methods
Mice, Cells, and Plasmids.
Wild-type C57BL/6 and btk−/− mice were obtained from The Jackson Laboratory and bred in our facilities. tlr3−/− mice, ips1−/− MEFs were kindly provided by Osamu Takeuchi and Shizuo Akira (Osaka University, Osaka, Japan). Experiments with mice were performed according to guidelines from the National Advisory Committee on Laboratory Animal Research. Macrophages were differentiated from bone marrow as described (43). HEK293 cells were transfected with various recombinant vectors encoding TLR3, TRIF, LYN, BTK, and various BTK mutants with HA or FLAG tag using Lipofectamine 2000 (Invitrogen). Cells were stimulated with LPS 0111:B4 (Sigma) or poly(I:C) (InvivoGen).
Statistics.
Statistical analysis was performed using an unpaired t test (Prism; GraphPad Software). P values of <0.05 were considered significant and marked with an asterisk. Survival curves (Kaplan-Meyer plots) were compared using a log-rank test. Final mortality rates were compared with χ2 test. Detailed methods are provided in SI Text.
Supplementary Material
Acknowledgments
We thank members of our laboratory for discussions. This work was supported by the Biomedical Research Council of A*STAR.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1119238109/-/DCSupplemental.
References
- 1.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 2.O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 3.Vercammen E, Staal J, Beyaert R. Sensing of viral infection and activation of innate immunity by toll-like receptor 3. Clin Microbiol Rev. 2008;21:13–25. doi: 10.1128/CMR.00022-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sato S, et al. Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-kappa B and IFN-regulatory factor-3, in the Toll-like receptor signaling. J Immunol. 2003;171:4304–4310. doi: 10.4049/jimmunol.171.8.4304. [DOI] [PubMed] [Google Scholar]
- 5.Meylan E, et al. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat Immunol. 2004;5:503–507. doi: 10.1038/ni1061. [DOI] [PubMed] [Google Scholar]
- 6.Schröder M, Bowie AG. TLR3 in antiviral immunity: Key player or bystander? Trends Immunol. 2005;26:462–468. doi: 10.1016/j.it.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Ameyar M, Wisniewska M, Weitzman JB. A role for AP-1 in apoptosis: The case for and against. Biochimie. 2003;85:747–752. doi: 10.1016/j.biochi.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Sarkar SN, et al. Novel roles of TLR3 tyrosine phosphorylation and PI3 kinase in double-stranded RNA signaling. Nat Struct Mol Biol. 2004;11:1060–1067. doi: 10.1038/nsmb847. [DOI] [PubMed] [Google Scholar]
- 9.Covert MW, Leung TH, Gaston JE, Baltimore D. Achieving stability of lipopolysaccharide-induced NF-kappaB activation. Science. 2005;309:1854–1857. doi: 10.1126/science.1112304. [DOI] [PubMed] [Google Scholar]
- 10.Werner SL, Barken D, Hoffmann A. Stimulus specificity of gene expression programs determined by temporal control of IKK activity. Science. 2005;309:1857–1861. doi: 10.1126/science.1113319. [DOI] [PubMed] [Google Scholar]
- 11.Sarkar SN, Smith HL, Rowe TM, Sen GC. Double-stranded RNA signaling by Toll-like receptor 3 requires specific tyrosine residues in its cytoplasmic domain. J Biol Chem. 2003;278:4393–4396. doi: 10.1074/jbc.C200655200. [DOI] [PubMed] [Google Scholar]
- 12.Sarkar SN, Elco CP, Peters KL, Chattopadhyay S, Sen GC. Two tyrosine residues of Toll-like receptor 3 trigger different steps of NF-kappa B activation. J Biol Chem. 2007;282:3423–3427. doi: 10.1074/jbc.C600226200. [DOI] [PubMed] [Google Scholar]
- 13.Khan WN, et al. Defective B cell development and function in Btk-deficient mice. Immunity. 1995;3:283–299. doi: 10.1016/1074-7613(95)90114-0. [DOI] [PubMed] [Google Scholar]
- 14.Satterthwaite AB, Witte ON. The role of Bruton's tyrosine kinase in B-cell development and function: A genetic perspective. Immunol Rev. 2000;175:120–127. [PubMed] [Google Scholar]
- 15.Fruman DA, Satterthwaite AB, Witte ON. Xid-like phenotypes: A B cell signalosome takes shape. Immunity. 2000;13:1–3. doi: 10.1016/s1074-7613(00)00002-9. [DOI] [PubMed] [Google Scholar]
- 16.Lindvall JM, et al. Bruton's tyrosine kinase: Cell biology, sequence conservation, mutation spectrum, siRNA modifications, and expression profiling. Immunol Rev. 2005;203:200–215. doi: 10.1111/j.0105-2896.2005.00225.x. [DOI] [PubMed] [Google Scholar]
- 17.Jefferies CA, et al. Bruton's tyrosine kinase is a Toll/interleukin-1 receptor domain-binding protein that participates in nuclear factor kappaB activation by Toll-like receptor 4. J Biol Chem. 2003;278:26258–26264. doi: 10.1074/jbc.M301484200. [DOI] [PubMed] [Google Scholar]
- 18.Liljeroos M, et al. Bruton's tyrosine kinase together with PI 3-kinase are part of Toll-like receptor 2 multiprotein complex and mediate LTA induced Toll-like receptor 2 responses in macrophages. Cell Signal. 2007;19:625–633. doi: 10.1016/j.cellsig.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Taneichi H, et al. Toll-like receptor signaling is impaired in dendritic cells from patients with X-linked agammaglobulinemia. Clin Immunol. 2008;126:148–154. doi: 10.1016/j.clim.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Lee KG, Xu S, Wong ET, Tergaonkar V, Lam KP. Bruton's tyrosine kinase separately regulates NFkappaB p65RelA activation and cytokine interleukin (IL)-10/IL-12 production in TLR9-stimulated B Cells. J Biol Chem. 2008;283:11189–11198. doi: 10.1074/jbc.M708516200. [DOI] [PubMed] [Google Scholar]
- 21.Kaisho T, Akira S. Dendritic-cell function in Toll-like receptor- and MyD88-knockout mice. Trends Immunol. 2001;22:78–83. doi: 10.1016/s1471-4906(00)01811-1. [DOI] [PubMed] [Google Scholar]
- 22.Gray P, et al. MyD88 adapter-like (Mal) is phosphorylated by Bruton's tyrosine kinase during TLR2 and TLR4 signal transduction. J Biol Chem. 2006;281:10489–10495. doi: 10.1074/jbc.M508892200. [DOI] [PubMed] [Google Scholar]
- 23.Mansell A, et al. Suppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nat Immunol. 2006;7:148–155. doi: 10.1038/ni1299. [DOI] [PubMed] [Google Scholar]
- 24.Kawai T, Akira S. Antiviral signaling through pattern recognition receptors. J Biochem. 2007;141:137–145. doi: 10.1093/jb/mvm032. [DOI] [PubMed] [Google Scholar]
- 25.Onomoto K, Yoneyama M, Fujita T. Regulation of antiviral innate immune responses by RIG-I family of RNA helicases. Curr Top Microbiol Immunol. 2007;316:193–205. doi: 10.1007/978-3-540-71329-6_10. [DOI] [PubMed] [Google Scholar]
- 26.Dejager L, Libert C. Tumor necrosis factor alpha mediates the lethal hepatotoxic effects of poly(I:C) in D-galactosamine-sensitized mice. Cytokine. 2008;42:55–61. doi: 10.1016/j.cyto.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 27.Kopp E, Medzhitov R. Recognition of microbial infection by Toll-like receptors. Curr Opin Immunol. 2003;15:396–401. doi: 10.1016/s0952-7915(03)00080-3. [DOI] [PubMed] [Google Scholar]
- 28.Hertzog PJ, O'Neill LA, Hamilton JA. The interferon in TLR signaling: More than just antiviral. Trends Immunol. 2003;24:534–539. doi: 10.1016/j.it.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Schindler C, Brutsaert S. Interferons as a paradigm for cytokine signal transduction. Cell Mol Life Sci. 1999;55:1509–1522. doi: 10.1007/s000180050391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsai YT, Chang SY, Lee CN, Kao CL. Human TLR3 recognizes dengue virus and modulates viral replication in vitro. Cell Microbiol. 2009;11:604–615. doi: 10.1111/j.1462-5822.2008.01277.x. [DOI] [PubMed] [Google Scholar]
- 31.Liang Z, et al. Activation of Toll-like receptor 3 impairs the dengue virus serotype 2 replication through induction of IFN-β in cultured hepatoma cells. PLoS ONE. 2011;6:e23346. doi: 10.1371/journal.pone.0023346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nasirudeen AM, et al. RIG-I, MDA5 and TLR3 synergistically play an important role in restriction of dengue virus infection. PLoS Negl Trop Dis. 2011;5:e926. doi: 10.1371/journal.pntd.0000926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 34.Kim T, Kim TY, Lee WG, Yim J, Kim TK. Signaling pathways to the assembly of an interferon-beta enhanceosome. Chemical genetic studies with a small molecule. J Biol Chem. 2000;275:16910–16917. doi: 10.1074/jbc.M000524200. [DOI] [PubMed] [Google Scholar]
- 35.Gloire G, Dejardin E, Piette J. Extending the nuclear roles of IkappaB kinase subunits. Biochem Pharmacol. 2006;72:1081–1089. doi: 10.1016/j.bcp.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 36.Honda K, Taniguchi T. Toll-like receptor signaling and IRF transcription factors. IUBMB Life. 2006;58:290–295. doi: 10.1080/15216540600702206. [DOI] [PubMed] [Google Scholar]
- 37.Sato M, Tanaka N, Hata N, Oda E, Taniguchi T. Involvement of the IRF family transcription factor IRF-3 in virus-induced activation of the IFN-beta gene. FEBS Lett. 1998;425:112–116. doi: 10.1016/s0014-5793(98)00210-5. [DOI] [PubMed] [Google Scholar]
- 38.Oshiumi H, Matsumoto M, Funami K, Akazawa T, Seya T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat Immunol. 2003;4:161–167. doi: 10.1038/ni886. [DOI] [PubMed] [Google Scholar]
- 39.Hemmi H, et al. The roles of two IkappaB kinase-related kinases in lipopolysaccharide and double stranded RNA signaling and viral infection. J Exp Med. 2004;199:1641–1650. doi: 10.1084/jem.20040520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 41.Johnsen IB, et al. Toll-like receptor 3 associates with c-Src tyrosine kinase on endosomes to initiate antiviral signaling. EMBO J. 2006;25:3335–3346. doi: 10.1038/sj.emboj.7601222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnsen IB, Nguyen TT, Bergstroem B, Fitzgerald KA, Anthonsen MW. The tyrosine kinase c-Src enhances RIG-I (retinoic acid-inducible gene I)-elicited antiviral signaling. J Biol Chem. 2009;284:19122–19131. doi: 10.1074/jbc.M808233200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boone DL, et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol. 2004;5:1052–1060. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.