Abstract
Impaired immunity is a fundamental obstacle to successful allogeneic hematopoietic cell transplantation. Mature graft T cells are thought to provide protection from infections early after transplantation, but can cause life-threatening graft-vs.-host disease. Human CMV is a major pathogen after transplantation. We studied reactivity against the mouse homologue, murine CMV (MCMV), in lethally irradiated mice given allogeneic purified hematopoietic stem cells (HSCs) or HSCs supplemented with T cells or T-cell subsets. Unexpectedly, recipients of purified HSCs mounted superior antiviral responses compared with recipients of HSC plus unselected bulk T cells. Furthermore, supplementation of purified HSC grafts with CD8+ memory or MCMV-specific T cells resulted in enhanced antiviral reactivity. Posttransplantation lymphopenia promoted massive expansion of MCMV-specific T cells when no competing donor T cells were present. In recipients of pure HSCs, naive and memory T cells and innate lymphoid cell populations developed. In contrast, the lymphoid pool in recipients of bulk T cells was dominated by effector memory cells. These studies show that pure HSC transplantations allow superior protective immunity against a viral pathogen compared with unselected mature T cells. This reductionist transplant model reveals the impact of graft composition on regeneration of host, newly generated, and mature transferred T cells, and underscores the deleterious effects of bulk donor T cells. Our findings lead us to conclude that grafts composed of purified HSCs provide an optimal platform for in vivo expansion of selected antigen-specific cells while allowing the reconstitution of a naive T-cell pool.
Keywords: graft-vs.-host reactions, hematopoietic stem cell transplantation, immune reconstitution, lymphopenia induced proliferation, M45-tetramer
Hematopoietic stem cells (HSCs) are the only cells capable of sustaining lifelong blood formation and thus are the only indispensable component of a graft for hematopoietic cell transplantation (HCT) (1). The current clinical practice of HCT uses mixed cellular grafts that contain rare HSCs, multipotent progenitors, and mature blood cells. Acute graft-vs.-host disease (GVHD), a complication caused by graft donor T cells, has a mortality rate of 10% to 20% (2–4) and could be eliminated by transplantation of pure HSCs. However, because donor T cells are thought to provide protection against life-threatening infections that occur in the posttransplant period, such as those caused by CMV (5, 6), pure HSC transplantations have not been vigorously pursued.
Major strides have been made in the isolation, genetic manipulation, activation, and/or expansion of T-cell clones that target defined antigens. Such clones have been developed to protect recipients against infectious pathogens or cancer cells. However, to date, the achievement of stable and persistent engraftment of these typically small populations has been challenging.
In this study, we examined the use of grafts composed of pure HSCs plus defined lymphocyte subsets to provide protective immunity to allografted mice challenged with murine CMV (MCMV). We compared the degree of antiviral protection provided by the transfer of unselected bulk mature donor T cells vs. transferred donor CD8+ subsets. We further assessed the antiviral potency of new cells arising from HSCs after transplantation, and residual host T cells. As T-cell function depends on an organized microenvironment, we also examined lymphoid tissues for non–T-cell populations and innate lymphoid cells (ILCs). ILCs are required during embryonic development of lymphoid organs and have been implicated as important for lymphoid tissue repair following infection (7). Their role after lymphoablation and HCT has not been studied. Contrary to conventional expectations, we found that antiviral immune protection was superior in recipients of pure HSCs compared with mice given T-cell–replete grafts. Furthermore, pure HSCs, but not grafts containing bulk T cells, gave rise to a variety of immature and naive T cells as well as ILCs.
Results
Early Infection, a Model for MCMV-Reactivation Posttransplant.
As depicted in Fig. S1, BALB.B mice (H2b, CD45.2+) were lethally irradiated and transplanted with minor antigen (miAg)-mismatched FACS-purified cKit+Thy1.1lolineage−Sca-1+ (KTLS)-HSCs from GFP-marked C57BL/6 (B6) donors (B6.GFP; H2b, CD45.2+) or HSCs plus a threshold lethal dose of 2.5 to 4 × 106 splenic total T cells (ToTCs; i.e., “bulk T cells”) from CD45.1+ allelic B6 donors (B6.CD45.1). Two weeks after transplantation, recipients were infected with a sublethal dose of MCMV RM427+ (8). This 2-wk time point was chosen to mimic early post-HCT reactivation, as the virus requires time to replicate sufficient DNA copies to cause active disease.
BALB.B recipients of B6 HSC alone had no signs of GVHD and showed stabilization of their body weights after transplantation, as did congenic B6.CD45.2 control recipients given identical doses of (B6.GFP) HSCs plus B6.CD45.1 ToTC. In contrast, allografted BALB.B recipients of B6 HSC+ToTCs developed signs of GVHD, including weight loss, ruffled fur, and diarrhea. Infection with sublethal dose MCMV did not significantly impact weight loss or mortality during the period of observation (Fig. S2).
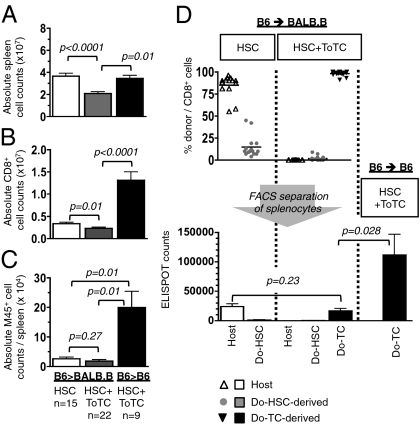
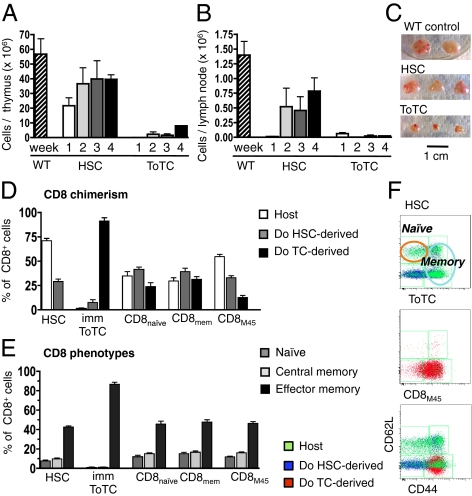
Two weeks after MCMV infection (4 wk posttransplantation), organs were harvested for analysis. BALB.B recipients of pure B6 HSCs had spleens with significantly higher cellularity and higher absolute CD8+ cell numbers compared with BALB.B recipients given B6 HSC+ToTCs (Fig. 1 A and B). However, no differences were noted between these allogeneic groups in the absolute number of MCMV-specific CD8+ cells per spleen (Fig. 1C), as determined by tetramer staining against the MCMV M45 peptide (HGIRNASFI-COOH). Compared with the allogeneic groups, congenic B6 controls given B6 HSC+ToTCs grafts had faster immune recovery and better antiviral responses, as evidenced by higher absolute splenocyte numbers (Fig. 1A) and better CD8+ cell reconstitution (Fig. 1B). M45-tetramer staining confirmed the virus specificity of this CD8+ cell response (Fig. 1C), revealing significantly higher numbers of virus-specific CD8+ T cells per spleen.
Fig. 1.
Early posttransplantation anti-MCMV response. BALB.B or B6 mice transplanted with B6 HSCs or HSC+ToTCs were infected 2 wk after HCT with a sublethal dose of 1 × 105 pfu MCMV RM427+. Tissues were harvested 2 wk postinfection. (A) BALB.B recipients of B6 HSC+ToTCs had significantly lower mean absolute cell numbers per spleen compared with BALB.B recipients of B6 HSCs or B6 recipients of B6 HSC+ToTCs. (B) BALB.B recipients of pure B6 HSCs or B6 recipients of B6 HSC+ToTCs had significantly higher absolute CD8+ cell numbers per spleen than BALB.B recipients of B6 HSC+ToTCs. (C) Absolute numbers of M45-tetramer+ splenocytes were equivalent in BALB.B recipients of B6 HSCs or HSC+ToTCs, but higher in B6 recipients given B6 HSC+ToTCs. (D) Separation of splenocytes by FACS into populations derived from host, donor HSCs, or transferred ToTCs, based on GFP expression or CD45 allele type. BALB.B recipients of B6 HSCs were mixed CD8+-chimeras (host exceeding donor). No residual host, few donor HSC-derived, and mainly cotransferred donor CD8+ cells were detectable in BALB.B recipients of B6 HSC+ToTCs (Upper). Antiviral IFN-γ responses to MCMV peptide, assessed by ELISPOT, revealed better responses from residual host CD8+ populations in B6 HSC recipients vs. transferred donor T cells in B6 HSC+ToTC recipients. Shown for comparison is the robust response of B6 donor T cells coinfused into B6 recipients.
The source of MCMV reactive cells was determined by FACS-sorting splenocytes according to their donor/host derivation. Fig. 1D shows the origins of the CD8+ cells and the IFN-γ response when exposed to the MCMV M45-peptide in an enzyme-linked immunosorbent spot (ELISPOT) assay. T cells present in BALB.B recipients of HSCs were primarily residual host type, whereas the T-cell pool of BALB.B recipients given B6 HSC+ToTCs was predominantly derived from expanded postthymic donor T cells (Fig. 1D, Upper). Unexpectedly, the residual host T cells in HSC recipients mounted robust IFN-γ responses. These responses were superior to those mediated by transferred allogeneic T cells, although this difference did not reach statistical significance (Fig. 1D, Lower). Of note, whereas adoptively transferred B6 donor T cells did not display strong anti-M45 reactivity in allogeneic BALB.B recipients, they provided excellent IFN-γ responses when transplanted into congenic B6 control recipients (Fig. 1D).
Late MCMV Infection.
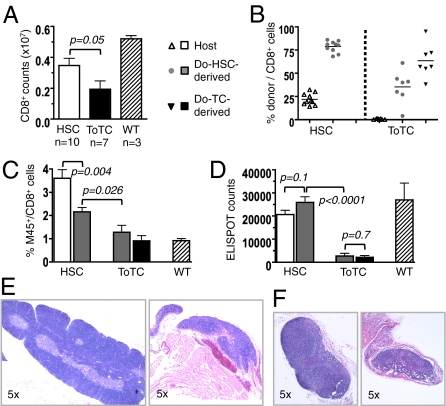
Following a 2-mo recovery period after HCT, we tested the ability of allogeneic cotransferred vs. newly generated HSC-derived T cells to respond to MCMV as a “novel pathogen.” BALB.B recipients of B6 HSC or B6 HSC+ToTC grafts were infected at 8 wk posttransplantation with a sublethal MCMV dose. Absolute CD8+ T-cell counts per spleen determined 2 wk postinfection were significantly higher in BALB.B mice given B6 HSCs alone compared with HSC+ToTCs (Fig. 2A). In HSC recipients, the contribution of CD8+ cells arising from donor HSCs increased from a median of 10% at 4 wk posttransplantation (Fig. 1D) to 80% (Fig. 2B). Tetramer staining (Fig. 2C) and ELISPOT (Fig. 2D) revealed that these HSC-derived cells made robust anti-MCMV responses. The residual host CD8+ cells present in pure HSC recipients continued to make superior antiviral responses compared with all other populations in allografted mice. In HSC+ToTC recipients, most of the CD8+ cells were from cotransferred cells (median, 57%), whereas new T-cell development from the donor HSCs was suppressed (Fig. 2B). Antiviral responses of cotransferred mature donor T cells were uniformly poor, and demonstrated the worst anti-M45 activity of all studied CD8+ populations in FACS-tetramer and ELISPOT analysis. Moreover, in BALB.B recipients of pure HSCs, newly generated T cells originating from donor HSCs had a significantly stronger antiviral reactivity compared with T cells developing from HSCs in a GVHD-affected environment in recipients of HSC+ToTCs (Fig. 2 B and C).
Fig. 2.
Late CD8+ cell recovery and response to MCMV. BALB.B recipients of B6 HSCs or B6 HSC+ToTCs were infected 8 wk after HCT with a sublethal dose (1 × 105 pfu) of MCMV RM427+. WT controls were similarly infected. Tissues were harvested 2 wk postinfection. (A) Higher absolute CD8+ T-cell numbers per spleen in HSC vs. HSC+ToTC recipients. (B) FACS chimerism analysis of spleens revealed mixed donor/host CD8+-chimerism in HSC recipients; the CD8+ pool in HSC+ToTC recipients comprised primarily cotransferred donor cells, few HSC-derived donor cells, and no host cells. (C) In HSC recipients, the percentage of M45-tetramer+ cells was higher among residual host cells compared with donor HSCs. In recipients of HSC+ToTCs, the cotransferred CD8+ T-cell pool had the lowest percentage of M45-tetramer+ cells, and their HSC-derived donor CD8+ cells had lower percentages of M45 reactivity vs. HSC-derived CD8+ cells in HSC recipients. (D) ELISPOT analysis of FACS-sorted donor and host populations. In HSC recipients, ELISPOT activity of donor and host-derived CD8+ cells was equivalent to that in WT controls. HSC+ToTC recipients had significantly lower IFN-γ responses. (E) H&E staining (magnification of 5×) of thymuses of representative HSC (Left) and HSC+ToTC (Right) recipients 8 wk post-HCT showed normal morphology for HSC recipients, but atrophy, hypocellularity, and loss of follicular structure for HSC+ToTC recipients. (F) H&E staining (magnification of 5×) of peripheral lymph nodes showed normal morphology for HSC recipients (Left), but decreased size, atrophy, hypocellularity, and disrupted morphology in HSC+ToTC recipients (Right) at 8 wk post-HCT.
The immune deficiency in HSC+ToTC recipients was associated with abnormal morphology and severe hypocellularity of thymuses (Fig. 2D) and peripheral lymph nodes (Fig. 2E). In contrast, recipients of HSCs alone had normal appearing lymphoid organs.
T-Cell Subsets from Immunized Donors.
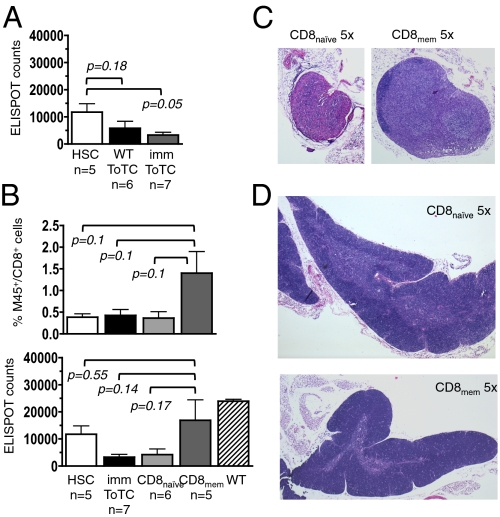
In clinical HCT, CMV-seropositive donors are thought to provide better antiviral protection for recipients than virus-naive donors (9). To model this setting, donor B6 mice were infected with a sublethal dose of live virus at 4 and 2 wk before HCT. These time points of vaccination were chosen to allow the establishment of immunological memory in the donor with a subsequent boost to achieve a higher yield of anti-MCMV reactive cells. Two weeks after transplantation, BALB.B recipients of B6 HSC or B6 HSC+ToTCs from naive or immunized donors were infected with MCMV. Fig. 3A shows that donor immunization did not improve the antiviral response in mice given HSC+ToTCs.
Fig. 3.
Anti-MCMV responses of separated cotransferred T-cell subsets. ToTCs or naive (CD8naive; CD62L+CD44−) or memory (CD8mem CD62L+/−CD44+) CD8+ T cells from MCMV-immunized B6 donors were cotransferred with B6 HSCs into allogeneic BALB.B mice. Recipients were infected with a sublethal dose of MCMV (1 × 105 pfu) 2 wk after HCT, and lymphoid organs harvested for M45-tetramer and ELISPOT assay 2 wk postinfection. (A) ELISPOT revealed that donor immunization with MCMV did not improve the antiviral reactivity of cotransferred ToTCs in allogeneic hosts. (B) M45-tetramer analysis showed the highest proportion of MCMV-reactive CD8 T cells was in recipients of HSC+CD8mem (Upper). ELISPOT analysis confirmed the highest counts of IFN-γ–secreting CD8+ T cells in recipients of HSC+CD8mem cells (Lower). (C) H&E staining of representative peripheral lymph nodes (magnification of 5×) and (D) thymuses (magnification of 5×) showed that lymph nodes of HSC+CD8naive recipients (C, Left) were decreased in size with evidence of hypocellularity, indicative of subclinical GVHD, and had thymuses that showed cortical thinning (D, Top). Representative lymph node (C, Right) and thymus (D, Lower) of a recipient of HSC+CD8mem shown in the bottom row demonstrated no histologic abnormalities.
Contrasting the negative results from the use of bulk T cells, cotransplantations of HSCs plus T-cell subsets from immunized mice yielded better responses. CD8+ cells from immunized B6 donors were divided into naive (CD44−CD62L+) and memory (CD44+CD62L+/−) subsets (Fig. S3 A and B). Neither subpopulation caused overt GVHD, as assessed by weight (Fig. S3C) and organ histology (Fig. S4). Memory CD8 cells (CD8mem) mounted higher levels of anti-CMV reactivity than naive CD8+ (CD8naive) cells, as determined by tetramer staining and ELISPOT assay (Fig. 3B). Histologic studies suggested that subclinical graft-vs.-host reactions damaged lymphoid tissues and may have contributed to immune impairment in the latter group. Lymph nodes (Fig. 2C) and, to a lesser extent, thymuses (Fig. 3D) were atrophic and hypocellular in recipients of CD8naive but not CD8mem cells.
Effect of Pharmacologic Immune Suppression.
In patients, pharmacologic immune suppression is used to prevent GVHD. We therefore tested if cyclosporine A (CSA), a drug commonly used in humans for this purpose, could attenuate the negative effects of alloreactive T cells on lymphatic tissues and thereby paradoxically enhance immune function. Cohorts of BALB.B recipients of B6 HSCs vs HSC+ToTCs were treated daily with CSA after transplantation. Fig. S5A shows that weight curves of HSC recipients decreased in the presence of the drug, whereas weights of HSC+ToTC recipients with or without CSA were identical, suggesting that, in the latter group, the negative effects of the drug were counterbalanced by attenuating GVHD. CSA did not improve spleen cellularity in HSC+ToTC recipients (Fig. S5B), nor did it significantly alter the proportion of HSC-derived CD8+ cells (Fig. S5C), the proportion or numbers of M45-tetramer+ CD8+ (CD8M45) cells, or the anti-MCMV reactivity as assessed by ELISPOT (Fig. S5 D–F). Thus, pharmacologic GVHD prophylaxis with CSA did not improve the immune function of bulk donor T cells in our model.
Lymphopenia-Induced Proliferation.
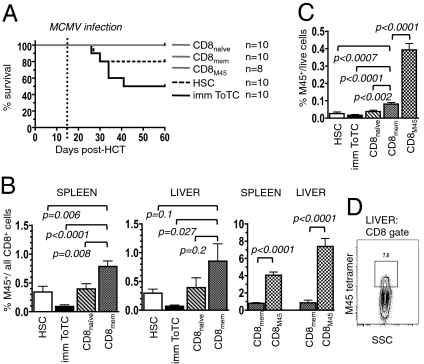
As we observed that functional immunity of allogeneic donor T cells was better if transferred as segregated subsets rather than as a bulk population, we sought to further delineate the antiviral potency of the different T-cell subsets against an increased dose of MCMV. Splenocytes from immunized B6 donors were FACS-separated into CD8naive, CD8mem, or CD8M45 T cells (Fig. S3) and cotransplanted with HSCs into BALB.B mice. Recipients were infected 2 wk posttransplantation with a threshold-lethal dose of MCMV. Survival curves revealed 50% lethality for recipients of HSC+ToTCs, and 20% mortality for HSC recipients. Mice given HSC+CD8+ T-cell subsets were without evidence of GVHD and were protected from virus lethality (Fig. 4A). Six weeks postinfection, the surviving mice were killed for organ analysis. Spleens and livers (the latter is a major target organ of MCMV) of HSC+CD8mem recipients contained significantly higher proportions of M45-tetramer+ cells compared with recipients of HSCs alone, HSC+ToTCs, or HSC+CD8naive cells. Mice given HSC+ToTCs had the lowest levels of CMV-reactive cells. By far the highest proportion of M45-tetramer+ cells was achieved in recipients of HSCs plus 6000 FACS-sorted M45-tetramer+ cells. Remarkably, this MCMV-specific population underwent massive expansion constituting a median of 4% and 7.4% of CD8+ cells in spleen and liver, respectively (Fig. 4B). Fig. 4C shows the proportion of M45-tetramer+ cells of all splenocytes was 20-fold higher in recipients of HSC+CD8M45 vs. HSC+ToTCs. Of note, HSC+CD8mem recipients also had significant expansion of the M45-tetramer+ cells, but the amount of M45-tetramer+ cells in the spleens and livers of HSC+CD8M45 recipients was four- to eightfold higher than in this former group. These observations highlight the importance of competitive reconstitution when T cell clones encounter an environment permissive for homeostatic expansion after transplantation.
Fig. 4.
Expansion and antiviral potency of HSC-derived vs. cotransferred T-cell subsets. B6 HSCs, HSC+ToTCs, HSC+CD8naive, HSC+CD8mem, or HSC+CD8M45 T cells were transplanted into BALB.B mice. Recipients were infected with 5 × 105 pfu MCMV at 2 wk after HCT. Tissues were harvested for FACS analysis at 6 wk postinfection. (A) Kaplan–Meier survival curves revealed 50% mortality for HSC+ToTC and 20% mortality for HSC recipients. No mortality occurred in HSC+CD8+-subset recipients. (B and C) Compiled FACS results display the content of M45-tetramer+ CD8 cells of (B), all CD8+ cells in spleen and liver, and (C) of all live cells in the liver. Percent M45-tetramer+ cells among splenocytes was highest in HSC+CD8M45 recipients, followed by HSC+CD8mem recipients. (D) Representative FACS plot showing M45-tetramer+ CD8+ cells in the liver of a recipient of HSC+CD8M45.
HSC Grafts Enhance Lymphoid Reconstitution and de Novo Regeneration of T Cells.
Given the poor function of cotransferred bulk donor T cells, we assessed the development and fate of donor T cells by CD8 subtype in the context of regenerating nonconventional lymphoid cells and lymphoid tissues. Lethal radiation caused marked reduction in lymphoid organ cellularity at wk 2 posttreatment in BALB.B recipients of pure B6 HSCs and B6 HSC+ToTC grafts (Fig. 5 A and B). Although cellularity increased rapidly in the following weeks in the group given HSCs alone, absolute cell counts remained severely decreased in recipients of HSC+ToTC grafts. In fact, hypocellularity persisted in these latter mice beyond 4 wk, and was associated with a markedly decreased size of the thymuses (Fig. 5C).
Fig. 5.
HSC grafts support hematopoietic reconstitution and permit regeneration of T-cell subsets. (A) Absolute thymocyte numbers 2, 3, 4, and 7 wk after HCT. Rapid restoration of thymic cellularity in HSC recipients, but prolonged lymphoablation in HSC+ToTC recipients. (B) Absolute cell counts per lymph node at 2, 3, 4, and 7 wk after HCT. Rapid recovery of cellularity in HSC recipients, but persistent hypocellularity in HSC+ToTC recipients. (C) Severe thymic atrophy 3 wk after HCT in HSC+ToTC recipients compared with normal morphology in HSC recipients and WT mice. (D) Chimerism analysis of splenic CD8+ cells revealed that residual host and HSC-derived donor T cells contributed to the CD8+ cell pool in recipients of HSC and HSC+CD8+-subsets. In HSC+ToTC recipients, nearly all CD8+ cells originated from transferred donor T cells. (E) CD8+ cells in HSC and HSC+CD8+-subset recipients were mixtures of naive (CD62L+CD44−) and memory phenotypes (central memory, CD62L+CD44+; effector memory, CD62L−CD44+). CD8+ cells in HSC+ToTC recipients were predominantly effector memory cells. (F) Representative FACS plots illustrate that, in HSC recipients, donor HSCs gave rise to naive, central, and effector memory cells. In HSC+ToTC recipients, CD8+ cells were almost exclusively effector memory cells. HSC+CD8M45 gave rise to an expanded M45-tetramer+ effector memory population, plus a mixed HSC-derived population representing all phenotype subtypes.
At 8 wk posttransplantation, CD8+ T cells in HSC recipients were mixed donor/host type (Fig. 5D), and comprised naive (CD62L+CD44−), central memory (CD62L+CD44+), and effector memory (CD62L−CD44+) cells (Fig. 5 E and F), a profile comparable to that of WT mice. In contrast, CD8+ T cells in HSC+ToTC recipients were almost exclusively derived from cotransferred T cells (Fig. 5D). Nearly all T cells in these mice demonstrated an effector memory phenotype, and there was a profound paucity of naive T cells (Fig. 5 E and F). The CD8+ T-cell profile of mice that received HSC+CD8+ subsets resembled that of HSC recipients and WT controls, not HSC+ToTC recipients, as they retained host T cells, and had CD8+ cells derived from expanded cotransferred effector memory cells as well as naive and central memory cells arising from donor HSCs (Fig. 5 D–F).
Immature Cell Populations.
Thus far our data suggest that cotransferred donor T cells expand and persist; however, despite engraftment, these cells do not function to protect against the MCMV pathogen. Moreover, the activities of these transferred T cells resulted in disrupted recovery of normal lymphoid organ architecture. These results were in contrast to those obtained with pure HSC transplantations, following which many lymphoid populations arose. Given the robust reconstitution of lymph nodes and thymuses in the HSC recipients, we studied if HSCs themselves give rise to nonconventional populations that can participate in lymphoid organ reconstruction, and how this process is obscured by expanding mature T cells.
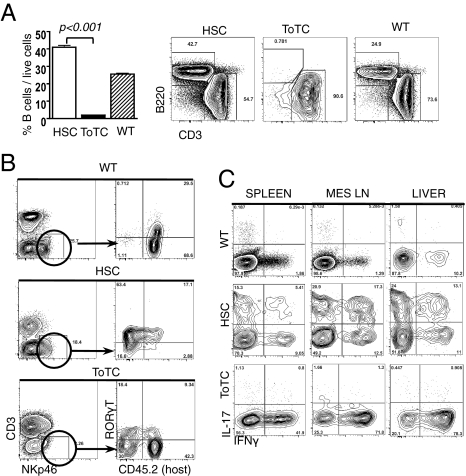
Beginning as early as 2 wk posttransplantation, BALB.B recipients of B6 HSCs exhibited high levels of B cells in blood and lymph nodes, which were largely absent in HSC+ToTC recipients (Fig. 6A). The mesenteric lymph nodes of HSC recipients showed a distinctive and robust regeneration with donor HSC-derived CD4+CD8+CD3− T cells and single-positive mature host-derived T cells (Fig. S6). The immature CD4+CD8+CD3− double-positive cells were not present in HSC+ToTC recipients or WT mice. Cells with features in common with ILCs (10–12) were also noted in the lymph nodes and livers of BALB.B recipients of pure B6 HSCs, but not HSC+ToTC recipients. Recipients of pure HSCs had NKp46+RORγt+CD3− NK-like cells (13, 14) present at levels comparable to those in WT mice that were primarily derived from donor HSCs (Fig. 6B). IL-17A secretion, another hallmark of ILCs (15, 16), was detectable at high levels 2 wk posttransplantation in the livers and mesenteric nodes of HSC recipients, exceeding levels in WT mice. Cells that secreted IL-17A only and IL-17A plus IFN-γ were identified (Fig. 6C). Residual host CD4+ T cells were determined to be the major source of this IL-17A. IL-17A was not detected in recipients of HSC+ToTCs; rather, their donor T cells were characterized by high levels of IFN-γ (Fig. S7).
Fig. 6.
Reconstitution of lymphoid organs in BALB.B recipients of B6 HSCs or HSC+ToTCs. (A) Representative FACS plots at 3 wk after HCT show rapid B-cell regeneration in peripheral lymph nodes of HSC recipients, compared with severe B-cell lymphopenia in HSC+ToTC recipients. (B) A donor and host-derived NK-like population (NKp46+CD3−RORγt+) was detected in HSC recipients and WT controls, but not HSC+ToTC recipients. (C) Representative FACS plots of CD4+ gated cells show IL-17A+ and IL-17A+IFN-γ+ double-positive cells, measurable in spleen, mesenteric lymph nodes, and liver of HSC recipients. Only marginal IL-17A but high IFN-γ expression (40–80%) in HSC+ToTC recipients. WT controls had low cytokine levels.
Discussion
GVHD and infections remain major complications after allogeneic HCT. Because donor T cells are thought to protect against infections and tumor recurrence, the risk that transferred alloreactive T cells mediate tissue damage, including the veiled injury of lymphatic organs, is tolerated. Evidence supporting the necessity of retaining T cells in hematopoietic grafts dates back to early clinical studies in which T-cell depletion was associated with increased infections, tumor relapse, and engraftment failures (17–19). However, in more recent T-cell depletion trials, increased frequencies of fatal viral and fungal infections, specifically CMV and Aspergillus, remained as the only major complications consistently observed (20). Notably, one study, applying the most vigorous T-cell depletion (5 logs), did not reveal more infections posttransplantation (21, 22).
Given the perception that, despite risks of GVHD, mature lymphoid cells protect against infections, our observation that functional immunity was significantly hindered, not enhanced, by bulk donor T cells was unexpected. HSC recipients exhibited accelerated lymphoid organ regeneration and superior antiviral immunity. By transplanting marked primitive HSCs into myeloablated mice, we revealed the degree to which mature donor T cells compete with and suppress cells arising de novo from donor HSCs, and regimen-resistant host T cells. Grafts of pure HSCs resulted in a balanced repertoire of T-cell phenotypes in recipients, including naive and central memory T cells. These cells provided protection against MCMV when introduced late after transplantation as a “novel” pathogen. In HSC recipients, significant contributions of surviving host T cells to immune function were also noted. In the early posttransplantation phase, virus-specific CD8+ cells arose from the host cells in numbers higher than from allografted populations, suggesting that, for humans, it would be advantageous to administer regimens that spare host T cells, yet permit pure HSCs to engraft.
Contrasting the regeneration of a competent immune system in mice that received HSCs only, the production of new T cells and development of effective anti-MCMV immunity was obscured in recipients of HSCs plus bulk mature T cells. These donor T cells home, as do all T cells, to lymphoid organs (23, 24), and there they carry out destructive graft-vs.-host reactions, compromising or eliminating these tissues wherein immune responses to exogenous antigens take place. In addition, mature donor T cells expand in response to minor antigens present at a high frequency at the time of transplantation (25, 26), and thereby suppress the production of naive T cells by rapidly saturating the lymphocyte pool (27–29). A monotonous phenotype of effector memory cells defined by loss of CD62L expression and acquisition of CD44 predominated the peripheral T-cell pool in recipients of bulk T-cell–replete grafts. Antigen activated T cells lose homing to secondary lymphoid organs by down-regulating CD62L (30) while up-regulating the expression of integrin α4β1, which enable them to home to inflamed tissues whose blood vessels express VCAM-1, the addressin for α4β1 (31–34). CD62L is the homing receptor necessary to enter secondary lymphoid organs in which productive immune responses are generated (35). Thus, the lack of CD62L expression further supports the idea that responses to novel antigens were impaired.
In addition to the better functionality and more balanced T cell pool, transplantations of purified HSCs resulted in different lymphocyte repopulation kinetics and the detection of populations not previously reported posttransplantation. Early posttransplantation, a large wave of B cells appeared in blood and lymphoid tissues of HSC recipients, but not recipients of HSC+ToTCs. Within the mesenteric lymph nodes of HSC recipients, high levels of donor T cells with an immature phenotype usually found in the thymus (CD4+CD8+CD3−) were observed. These latter cells are not found in WT mice or in recipients of HSC+ToTCs. Finally, cells with features resembling ILCs (12, 36), which included NK-like cells that expressed NKp46 and RORγt (10, 13, 14), were detected early after transplantation in the lymph nodes and livers of HSC recipients at levels near those in WT controls but were absent in recipient of HSC+ToTCs. IL-17A, a key cytokine of ILCs (15, 16), was detected early after transplantation at increased levels in lymph nodes and livers in HSC recipients, the source of which was determined to be residual host CD4+ cells. IL-17A levels were low to undetectable in HSC+ToTC recipients and WT mice. We did not detect “classical” lymphoid tissue inducer, as described in embryogenesis of lymphoid tissues (11, 12, 36). Nonetheless, given the constellation of diverse cell types and repopulation kinetics observed following transplantation of pure HSCs, we hypothesize that, in response to injury, host repair factors are activated within lymphoid tissues, which may include the induction of IL-17A–producing cells and the generation of early populations from HSC that facilitate lymphoid organ regeneration. However, when mature alloreactive cells are present, the emergence of these cells is retarded by the effects of inflammation and the dominance of the T-cell pool by effector memory cells that primarily produce IFN-γ.
A highlight of our model system is the degree to which lymphopenia-induced expansion of cotransferred antigen-specific clones can be tracked. Tremendous expansion was observed when anti-MCMV M45-tetramer+ cells were the sole mature clone cotransferred with HSCs. In contrast, M45-tetramer+ cell expansion was dampened in mice that received bulk T cells or even memory CD8+ cells. These findings relate directly to the current strategy of adoptive immunotherapy. Technologies to isolate and/or genetically modify antigen-specific T-cell clones have been established and refined. Antiviral T-cell infusions have been given posttransplantation to augment protective immunity. Tumor-specific cells have also been tested in a variety of clinical scenarios (37). However, difficulties related to the generation and isolation of sufficient numbers of these cells and the unpredictable engraftment and persistence of these cells remain as significant obstacles to broad application (38–41). Here, we graphically show that optimal expansion of T-cell clones can be achieved posttransplantation by ensuring sustained hematopoiesis via pure HSCs, which avoid the competitive expansion of irrelevant T cells. In fact, the immense capacity of even small cell numbers to expand in a lymphopenic posttransplant environment may make ex vivo cell expansion before transfer unnecessary.
In summary, pure HSC grafts have distinct advantages vs. grafts replete with T cells. Our data underscore the challenges of controlling the negative effects of bulk mature donor T-cell populations. More importantly, these studies illuminate the dynamics of lymphoid cell repopulation and show that, under the condition of lymphopenia, which exists as part of every allogeneic transplantation, pure HSCs can serve as a superior platform for delivery of antigen-specific cellular therapy.
Materials and Methods
Mice.
Congenic C57BL/6 (B6) mice (H2b; Thy1.1; B6.CD45.1, B6.CD45.2, and B6.GFP) were used as donors of HSC and lymphocytes for BALB.B hosts (H2b, Thy1.2; CD45.2).
HSC Transplantation and MCMV Infection.
BALB.B mice underwent lethal 800-cGy total body irradiation before infusion of grafts. Three thousand KTLS-HSCs were FACS-isolated from cKit-enriched bone marrow according to their cKit+Thy1.1lowLineagenegSca-1+ phenotype. In cotransfer experiments, 2.5 to 4 × 106 ToTCs (CD4++CD8+ = ToTC), 3 × 105 naive, or 1 × 105 memory CD8+ T cells, or 6,000 M45-tetramer+ CD8+ cells enriched from spleens by magnetic column separation and FACS, were coinjected with the HSCs. At 2 or 8 wk after HCT, recipients were challenged with 1 to 5 × 105 pfu of MCMV.
FACS, Tetramer, and ELISPOT Analysis.
Two weeks after MCMV infection, lymphatic organs and liver were harvested. For phenotype and chimerism analysis, cell suspensions were antibody-stained for FACS analysis. For measurement of IL-17A and IFN-γ, cells were stimulated with phorbol myristate acetate, ionomycin, and monensin for 5 h at 37 °C before staining of surface and intracellular markers. For ELISPOT assays, FACS-separated donor and host populations were incubated for 20 h together with MCMV M45 peptide in plates coated with IFN-γ antibody. A secondary IFN-γ antibody was used to visualize individual IFN-γ–secreting cells.
Detailed descriptions of cell isolations, HCT, cell stimulations, antibody staining, flow cytometry, ELISPOT, histologic analysis, and statistical calculations are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank the National Institutes of Health (NIH) tetramer facility for providing the customized M-45 tetramers. This work was supported by NIH Grants R01 HL087240, P01 CA049605, PO1 HL075462, and a grant from the Snyder Foundation (to J.A.S.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1120237109/-/DCSupplemental.
References
- 1.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 2.Hahn T, et al. Risk factors for acute graft-versus-host disease after human leukocyte antigen-identical sibling transplants for adults with leukemia. J Clin Oncol. 2008;26:5728–5734. doi: 10.1200/JCO.2008.17.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin MT, et al. Relation of an interleukin-10 promoter polymorphism to graft-versus-host disease and survival after hematopoietic-cell transplantation. N Engl J Med. 2003;349:2201–2210. doi: 10.1056/NEJMoa022060. [DOI] [PubMed] [Google Scholar]
- 4.Weisdorf D. GVHD the nuts and bolts. Hematology (Am Soc Hematol Educ Program) 2007:62–67. doi: 10.1182/asheducation-2007.1.62. [DOI] [PubMed] [Google Scholar]
- 5.Gandhi MK, Wills MR, Sissons JG, Carmichael AJ. Human cytomegalovirus-specific immunity following haemopoietic stem cell transplantation. Blood Rev. 2003;17:259–264. doi: 10.1016/s0268-960x(03)00028-6. [DOI] [PubMed] [Google Scholar]
- 6.Kontoyiannis DP, Lewis RE, Marr K. The burden of bacterial and viral infections in hematopoietic stem cell transplant. Biol Blood Marrow Transplant. 2009;15(1 suppl):128–133. doi: 10.1016/j.bbmt.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Scandella E, et al. Restoration of lymphoid organ integrity through the interaction of lymphoid tissue-inducer cells with stroma of the T cell zone. Nat Immunol. 2008;9:667–675. doi: 10.1038/ni.1605. [DOI] [PubMed] [Google Scholar]
- 8.Stoddart CA, et al. Peripheral blood mononuclear phagocytes mediate dissemination of murine cytomegalovirus. J Virol. 1994;68:6243–6253. doi: 10.1128/jvi.68.10.6243-6253.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ljungman P, et al. Donor CMV serologic status and outcome of CMV-seropositive recipients after unrelated donor stem cell transplantation: An EBMT megafile analysis. Blood. 2003;102:4255–4260. doi: 10.1182/blood-2002-10-3263. [DOI] [PubMed] [Google Scholar]
- 10.Eberl G, et al. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 11.Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+CD3- LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7:493–504. doi: 10.1016/s1074-7613(00)80371-4. [DOI] [PubMed] [Google Scholar]
- 12.van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nat Rev Immunol. 2010;10:664–674. doi: 10.1038/nri2832. [DOI] [PubMed] [Google Scholar]
- 13.Luci C, et al. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat Immunol. 2009;10:75–82. doi: 10.1038/ni.1681. [DOI] [PubMed] [Google Scholar]
- 14.Sanos SL, et al. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reynolds JM, Angkasekwinai P, Dong C. IL-17 family member cytokines: Regulation and function in innate immunity. Cytokine Growth Factor Rev. 2010;21:413–423. doi: 10.1016/j.cytogfr.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takatori H, et al. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med. 2009;206:35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marmont AM, et al. T-cell depletion of HLA-identical transplants in leukemia. Blood. 1991;78:2120–2130. [PubMed] [Google Scholar]
- 18.Martin PJ, et al. Graft failure in patients receiving T cell-depleted HLA-identical allogeneic marrow transplants. Bone Marrow Transplant. 1988;3:445–456. [PubMed] [Google Scholar]
- 19.Kernan NA, et al. Graft failure after T-cell-depleted human leukocyte antigen identical marrow transplants for leukemia: I. Analysis of risk factors and results of secondary transplants. Blood. 1989;74:2227–2236. [PubMed] [Google Scholar]
- 20.van Burik JA, et al. Higher risk of cytomegalovirus and aspergillus infections in recipients of T cell-depleted unrelated bone marrow: Analysis of infectious complications in patients treated with T cell depletion versus immunosuppressive therapy to prevent graft-versus-host disease. Biol Blood Marrow Transplant. 2007;13:1487–1498. doi: 10.1016/j.bbmt.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 21.Wagner JE, Thompson JS, Carter SL, Kernan NA. Unrelated Donor Marrow Transplantation Trial Effect of graft-versus-host disease prophylaxis on 3-year disease-free survival in recipients of unrelated donor bone marrow (T-cell Depletion Trial): A multi-centre, randomised phase II-III trial. Lancet. 2005;366:733–741. doi: 10.1016/S0140-6736(05)66996-6. [DOI] [PubMed] [Google Scholar]
- 22.Jakubowski AA, et al. T cell depleted stem-cell transplantation for adults with hematologic malignancies: sustained engraftment of HLA-matched related donor grafts without the use of antithymocyte globulin. Blood. 2007;110:4552–4559. doi: 10.1182/blood-2007-06-093880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallatin M, et al. Lymphocyte homing receptors. Cell. 1986;44:673–680. doi: 10.1016/0092-8674(86)90832-9. [DOI] [PubMed] [Google Scholar]
- 24.Holzmann B, McIntyre BW, Weissman IL. Identification of a murine Peyer's patch—specific lymphocyte homing receptor as an integrin molecule with an alpha chain homologous to human VLA-4 alpha. Cell. 1989;56:37–46. doi: 10.1016/0092-8674(89)90981-1. [DOI] [PubMed] [Google Scholar]
- 25.Gaschet J, et al. Alterations of T cell repertoire after bone marrow transplantation: characterization of over-represented subsets. Bone Marrow Transplant. 1995;16:427–435. [PubMed] [Google Scholar]
- 26.Storek J, et al. Reconstitution of the immune system after hematopoietic stem cell transplantation in humans. Semin Immunopathol. 2008;30:425–437. doi: 10.1007/s00281-008-0132-5. [DOI] [PubMed] [Google Scholar]
- 27.Bourgeois C, Kassiotis G, Stockinger B. A major role for memory CD4 T cells in the control of lymphopenia-induced proliferation of naive CD4 T cells. J Immunol. 2005;174:5316–5323. doi: 10.4049/jimmunol.174.9.5316. [DOI] [PubMed] [Google Scholar]
- 28.Bourgeois C, Stockinger B. T cell homeostasis in steady state and lymphopenic conditions. Immunol Lett. 2006;107:89–92. doi: 10.1016/j.imlet.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Ge Q, Bai A, Jones B, Eisen HN, Chen J. Competition for self-peptide-MHC complexes and cytokines between naive and memory CD8+ T cells expressing the same or different T cell receptors. Proc Natl Acad Sci USA. 2004;101:3041–3046. doi: 10.1073/pnas.0307339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dailey MO, Gallatin WM, Weissman IL. The in vivo behavior of T cell clones: altered migration due to loss of the lymphocyte surface homing receptor. J Mol Cell Immunol. 1985;2:27–36. [PubMed] [Google Scholar]
- 31.Kina T, et al. Identification of a 107-kD glycoprotein that mediates adhesion between stromal cells and hematolymphoid cells. J Exp Med. 1991;173:373–381. doi: 10.1084/jem.173.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neuhaus H, et al. Cloning and expression of cDNAs for the alpha subunit of the murine lymphocyte-Peyer's patch adhesion molecule. J Cell Biol. 1991;115:1149–1158. doi: 10.1083/jcb.115.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyake K, Weissman IL, Greenberger JS, Kincade PW. Evidence for a role of the integrin VLA-4 in lympho-hemopoiesis. J Exp Med. 1991;173:599–607. doi: 10.1084/jem.173.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brocke S, Piercy C, Steinman L, Weissman IL, Veromaa T. Antibodies to CD44 and integrin alpha4, but not L-selectin, prevent central nervous system inflammation and experimental encephalomyelitis by blocking secondary leukocyte recruitment. Proc Natl Acad Sci USA. 1999;96:6896–6901. doi: 10.1073/pnas.96.12.6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegelman MH, van de Rijn M, Weissman IL. Mouse lymph node homing receptor cDNA clone encodes a glycoprotein revealing tandem interaction domains. Science. 1989;243:1165–1172. doi: 10.1126/science.2646713. [DOI] [PubMed] [Google Scholar]
- 36.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12:21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 37.Rosenberg SA, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heslop HE, Stevenson FK, Molldrem JJ. Immunotherapy of hematologic malignancy. Hematology (Am Soc Hematol Educ Program) 2003:331–349. doi: 10.1182/asheducation-2003.1.331. [DOI] [PubMed] [Google Scholar]
- 39.Melenhorst JJ, et al. Allogeneic virus-specific T cells with HLA alloreactivity do not produce GVHD in human subjects. Blood. 2010;116:4700–4702. doi: 10.1182/blood-2010-06-289991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turtle CJ, Riddell SR. Genetically retargeting CD8+ lymphocyte subsets for cancer immunotherapy. Curr Opin Immunol. 2011;23:299–305. doi: 10.1016/j.coi.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warren EH, et al. Therapy of relapsed leukemia after allogeneic hematopoietic cell transplantation with T cells specific for minor histocompatibility antigens. Blood. 2010;115:3869–3878. doi: 10.1182/blood-2009-10-248997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.