Abstract
To optimize perception, neurons in the visual system adapt to the current environment. What determines the durability of this plasticity? Longer exposures to an environment produce longer-lasting effects, which could be due to either (i) a single mechanism controlling adaptation that gains strength over time, or (ii) long-term mechanisms that become active after long-term exposure. Using recently developed technology, we tested adaptation durations an order of magnitude greater that those tested previously, and used a “deadaptation” procedure to reveal effects of a unique long-term mechanism in the longest adaptation periods. After 4 h of contrast adaptation, human observers were exposed to natural images for 15 min, which completely cancelled perceptual aftereffects of adaptation. Strikingly, during continued testing this deadaptation faded, and the original adaptation effects reappeared. This pattern strongly suggests that adaptation was maintained in a distinct long-term mechanism, whereas deadaptation affected a short-term mechanism.
Keywords: orientation, deprivation, adult neuroplasticity
The visual system continually adapts to the current environment to improve its function. Neurons in the retina and visual cortex, for example, decrease their sensitivity after prolonged exposure to a high contrast environment (for reviews, see refs. 1 and 2). Such adaptation is hypothesized to increase the efficiency of neural coding by bringing responses down from ceiling levels and allowing neurons to respond differentially to the high contrast values that are the most likely stimuli in that environment. Because adaptation effects are both large and common in the natural world, understanding them is a key component of any account of visual function (3).
Effects of contrast adaptation are evident almost immediately after onset of an adapting stimulus but get stronger and longer-lasting as the adaptation period grows in duration. This duration scaling law has been observed for both firing rates of retinal ganglion cells and contrast detection measurements of human observers (4, 5).
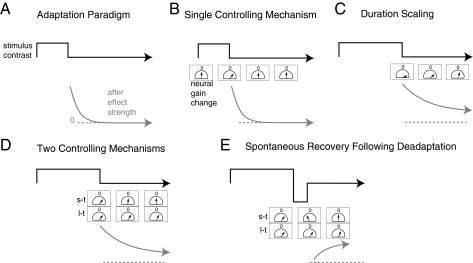
How the visual system produces these temporal dynamics remains the subject of debate (Fig. 1). One theory proposes that a single neural mechanism controls contrast adaptation at multiple time-scales (putting aside extremely rapid effects that occur within 100–200 ms; refs. 5 and 6). As the adapting period grows longer, this mechanism increases its confidence in its estimates of the current environment. Increased confidence, in turn, produces stronger adaptation effects and also increases the evidence required to convince the system that the environment has changed back to its original state, yielding longer-lasting aftereffects.
Fig. 1.
Two theories of contrast adaptation and how to test between them. (A) Adapting to a change in contrast produces an aftereffect. (B) Adaption is controlled by a neural mechanism whose output signal adjusts the responsiveness, or gain, of neurons. After adaptation, mechanism output is proportional to the behavioral aftereffect and decays over time from an initial value back to baseline, indicated by “0.” The meter icon is meant to capture this decay, by an analogy to a parking meter or other analog timer. (C) As adaptation duration increases, effects become longer lasting, which can be accounted for by a single mechanism that increases its output as adaptation continues. (D) An alternative account proposes that a second mechanism (l-t, long-term) with a longer time constant becomes active after long adaptation durations. Neural responsiveness is adjusted to the sum of signals of the two mechanisms. (E) For the two-mechanism case, adaptation followed by rapid deadaptation may cause opposing signals from the two mechanisms. Decay will affect the short-term mechanism first, leading to spontaneous recovery of adaptation due to the long-term mechanism.
Alternatively, contrast adaptation might be controlled by multiple neural mechanisms, each tuned to a different preferred timescale (7, 8). As the adapting period grows longer, mechanisms tuned to longer timescales exert increased control over adaptation. The dynamics of such mechanisms would most naturally operate at similar long time scales and would produce long-lasting aftereffects. The experiments reported here test between these two alternatives, using behavioral measurements of long-term contrast adaptation in human observers.
We tested whether long-term human contrast adaptation is controlled by the same neural mechanisms that mediate shorter-term adaptation, or whether it is controlled by distinct mechanisms. We first measured adaptation over an unprecedentedly wide range of durations, from a few minutes to 8 h in length, using recently developed “altered reality” technology (9) that enables study of adaptation durations longer than those previously investigated in the laboratory. Our results showed substantial growth in the strength and duration of effects as a function of adapting time.
We then borrowed a procedure from the classical learning literature, and recent motor adaptation literature, to test for the existence of unique long-term mechanisms of contrast adaptation (10, 11). Effects of 4 h of adaptation were rapidly cancelled by reexposing subjects to images of the natural world for 15 min (Fig. 1E). When subjects were then returned to the testing environment, this deadaptation wore off rapidly, leading to spontaneous recovery of the original adaptation effect. These results strongly suggest the operation of at least two independent mechanisms controlling adaptation, a short-term one that was affected by the viewing of natural images and a long-term one that was not.
Results
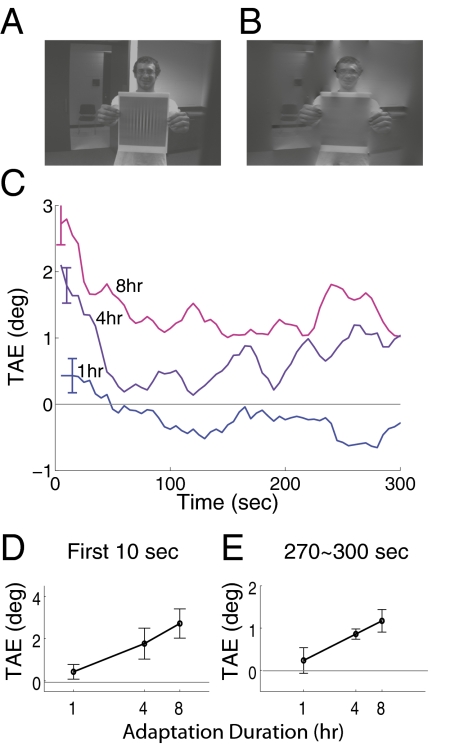
Our initial experiment tested whether contrast adaptation continued to grow in strength over very long adapting durations. Subjects viewed the natural world with most vertical energy removed for 1, 4, or 8 h; this contrast deprivation was achieved by using a novel display comprised of a head-mounted video camera that fed into a laptop computer that, in turn, drove a head-mounted display (9). Real-time image processing on the laptop removed most first-order vertical information (Fig. 2 A and B). Adaptation strength was measured as a tilt aftereffect (TAE), where subjects adjusted the orientation of two 45° components of a plaid pattern until its checks appeared square (Methods; see Fig. 5).
Fig. 2.
Long-term adaptation. Subjects adapted to images of the world (A) that had vertical energy removed (B) for 1, 4, or 8 h. (C) Plots average tilt aftereffects measured after adaptation. Error bars in all figures plot one SEM, corrected for the within-subjects design (34). (D) Shows tilt aftereffects averaged within the first 10 s of testing. (E) Shows aftereffects averaged within the final 30 s of testing. Effects of deprivation grew in strength and length as a function of the adapting duration.
Fig. 5.
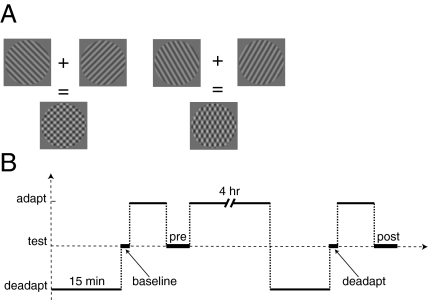
Methods. (A) The plaid stimulus used to test for a tilt aftereffect. After adaptation, the component gratings appeared tilted toward the deprived orientation, causing the checks to appear elongated vertically. Subjects adjusted the gratings until the checks appeared square. (B) The deadaptation protocol. Subjects were given pre- and post-tests after 8 min of adaptation that either preceded or followed 4 h of adaptation and 15 min of viewing unfiltered images (deadaptation). A “baseline” test measured initial tilt judgments, and a “deadapt” test measured aftereffects immediately after the deadaptation.
Both the strength and duration of aftereffects rose as the adaptation period grew from 1 to 8 h (Fig. 2 C–E). Depriving subjects of vertical produced a positive TAE, indicating that the two component gratings of the test pattern appeared to be tilted toward vertical. Adaptation likely increased the responsiveness (gain) of neurons tuned to vertical, causing the population response to diagonal gratings to be biased toward vertical (9). Note that these results are the opposite of the traditional TAE, where exposure to a high contrast vertical grating causes diagonal gratings to appear tilted away from vertical, interpreted as arising from a decrease in gain of vertical neurons.
Longer adaptation durations produced stronger adaptation effects. Fig. 2D shows the average aftereffect magnitude from the first 10 s of the posttest time series. Magnitudes increased approximately linearly with the logarithm of adaptation duration [t(6) = 2.82, P < 0.05].
Longer adaptation durations also produced longer lasting effects. The TAE was greatest immediately after adaptation and decayed rapidly thereafter. Both exponential and power functions fit the initial decay reasonably well, in line with prior reports of the decay of contrast adaptation (4, 5). Strikingly, for both the 4- and 8-h durations, the decay asymptoted to a positive effect. Fig. 2E shows the average aftereffect magnitude for the late interval, 270–300 s of the posttest timeseries, where magnitudes of the 4- and 8-h aftereffects, but not the 1-h effect, were reliably greater than zero [t(6) = 2.62, 3.06, 0.47; P < 0.04, P < 0.03, P > 0.5].
Adaptation to Deprivation Is a Form of Contrast Adaptation.
Does deprivation engage unique mechanisms of adaptation? It seems likely that this adaptation is controlled by the same mechanism involved in “traditional” contrast adaptation, where subjects view a high contrast pattern and tilt aftereffects are in the opposite direction of those reported here. A simple account of both these and our results is that the same controlling mechanism either increases or decreases neural gain relative to baseline. During deprivation, this mechanism increases gain so that any small bit of contrast in the environment is faithfully transmitted. During traditional adaptation, the same mechanism decreases gain so that neurons are not near saturation and can accurately signal small changes in the (high) level of contrast in the environment.
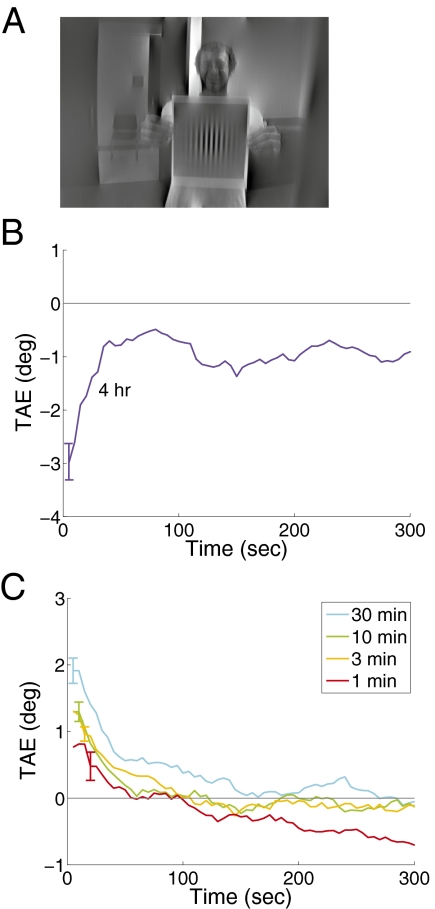
To test whether similar mechanisms are engaged by the two paradigms, we replicated the previous experiment, but increased contrast, exposing subjects for long durations to contrasts double that found in the natural environment. Four hours of exposure to high contrast at a single orientation produced robust aftereffects (Fig. 3B) that decayed to a nonzero asymptote [t(7) = 3.12, P < 0.02]. Note the aftereffect now plots negative, because adaptation decreased sensitivity to the adapted orientation.
Fig. 3.
Contrast enhancement and short-term adaptation. (A) Shows an example adapting image with vertical energy enhanced. (B) Shows tilt aftereffects measured after 4 h of adaptation to such images. Enhancement generated long lasting adaptation of the opposite sign from deprivation. (C) Shows tilt-aftereffects measured after short-term deprivation. These effects were reliable and grew with adapting duration.
If the same mechanism that controls effects of deprivation also controls traditional contrast adaptation, then deprivation should produce effects over the shorter-term timescales used in the more traditional experiments. To test this possibility, we deprived subjects for shorter durations, including 1, 3, 10, and 30 min. Adaptation was visible for all durations tested (Fig. 3C). Effects were statistically reliable in the first 10 s of all conditions tested and grew stronger with adapting duration. Overall, effects of adaptation in this experiment were stronger than in our original experiment. This difference was likely due to testing variations—for efficiency, subjects for short durations were adapted and tested in up to six sessions daily with as little as 30 min between them. It seems likely however, that some adaptation carried over from session to session inflating the overall values; indeed, effects were bigger for the last two repetitions than the first two [t(6) = 4.88, P < 0.01]. Nevertheless, it is clear that contrast deprivation can produce adaptation at short durations and that effects grow stronger as durations lengthen.
Separate Short- and Long-Term Mechanisms.
Do the same mechanisms control both short- and long-term adaptation? To answer this question, we borrowed a paradigm from the classical learning literature (10, 11). If an animal is taught to form a strong, long-term association between a stimulus and a behavior, and then is rapidly conditioned to learn a short-term association between a different behavior and that same stimulus, the animal will initially exhibit the second behavior when cued with the stimulus. Over time, however, the long-term association will “spontaneously recover” and the animal will revert to the first behavior. The simplest explanation of this pattern of results is that the long-term association remained intact while the short-term association was learned and then forgotten, and so spontaneous recovery argues strongly that the two associations were controlled by separate mechanisms.
To test whether mechanisms controlling long- and short-term contrast adaptation can be similarly separated, we adapted subjects for 4 h, and immediately deadapted them rapidly for 15 min by allowing them to view natural, unaltered images. If adaptation is controlled by separate mechanisms, then deadaptation may cause a short-term mechanism to signal for “negative” adaptation that may counteract a signal for positive adaptation from a long-term mechanism, much as animal conditioning can produce short-term associations that override long-term ones (Fig. 1D). Thus, immediately after deadaptation, there may be no behavioral aftereffect of the initial adaptation. Over continued testing, however, the short-term adaptation should fade, and the long-term adaptation should reemerge, leading to spontaneous recovery of the aftereffect.
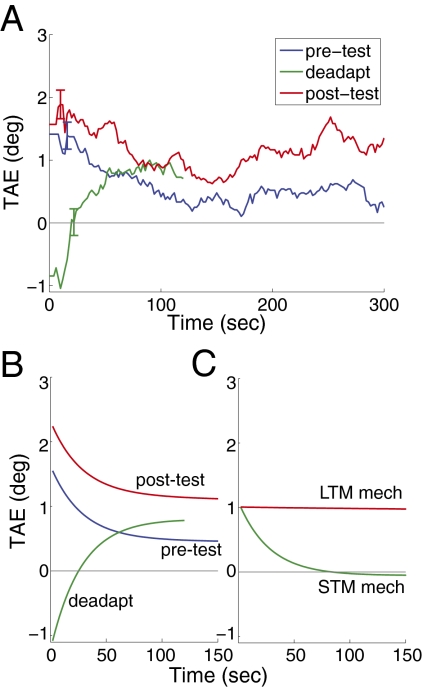
Our results showed strong evidence of spontaneous recovery (Fig. 4A). The “deadaptation test” followed 4 h of adaptation, known from experiment 1 to produce a large long-lasting aftereffect, and then 15 min of natural viewing. Deadaptation reduced the tilt aftereffect measure to near zero [t(11) = 0.26, P > 0.80 vs. zero for first 30 s of test], but strikingly, the aftereffect spontaneously recovered over time during the test [t(11) > 2.88, P < 0.02 for 90–120 s; linear trend 0–120 s, t(11) = 2.35, P < 0.04].
Fig. 4.
Results of deadaptation experiment. (A) Plots tilt aftereffect measured after an initial 8 min of adaptation (pre-test), after 4 h of deprivation and 15 min of viewing unfiltered images (deadapt), and after a final 8 min period of adaptation (post-test). The deadaptation curve shows spontaneous recovery. (B) Fits of a model to the data that contains two separate mechanisms controlling adaptation. The model accounted for >80% of the variance. (C) The decay functions for the two mechanisms identified by the model fitting. One is a short-term mechanism that decays rapidly, and the other is a longer-term mechanism.
We also tested for a second pattern of results, to provide additional evidence of separate long- and short-term mechanisms. We followed the test for spontaneous recovery with a brief 8-min adaptation period. If the short- and long-term mechanisms are distinct, then this 8-min period should be enough to fully cancel out the deadaptation, and so should produce much larger aftereffects than normally seen for 8 min of adaptation. Our results showed this predicted pattern. An initial 8 min of adaptation produced a moderate TAE that decayed quickly (Fig. 4A; “pre-test”). Eight minutes of adaptation after deadaptation produced a larger aftereffect than the pretest [“post-test”; t(11) > 2.29, P < 0.05].
Together, these results demonstrate independent control of short- and long-term adaptation. Short-term deadaptation did not permanently cancel long-term adaptation, whose ongoing strength spontaneously recovered.
Our results are consistent with an intuitive model of control of adaptation containing two mechanisms responsive to adaptation at different timescales. In this model, long-term adaptation caused both short- and long-term mechanisms to signal for increased responsiveness of vertical-preferring neurons (we assume the measured behavioral aftereffect reflects the sum of the outputs of both mechanisms). During subsequent viewing of unfiltered natural images, the high responsiveness in the vertical neurons was inappropriate (it would have led to a perceptual dominance of vertical), and so generated an error signal, which was corrected by the short-term mechanisms signaling for a decrease in neural gain. This negative signal summed with a lingering positive signal in the long-term mechanism, yielding no reliable aftereffect. Continued testing, however, allowed the negative signal to decay, because subjects saw no verticals, and let the adaptation produced by the long-term mechanism reappear. Finally, the subsequent 8-min adaptation produced additional positive signal from the short-term mechanism, which combined with the lingering positive signal from the long-term mechanism to yield a strong aftereffect.
Simple Two-Mechanism Model.
To verify this intuitive account, we fit a model to our data that contains two mechanisms controlling adaptation (11). The output of the model is the sum of the output of the two mechanisms and corresponds to a gain setting for neurons affected by adaptation, which we assume is equal to our measured tilt aftereffect. Each mechanism adjusts its output to reduce the discrepancy between the current gain setting and an optimal one for the current environment according to a simple difference equation that has two free parameters:
where xi is the output of the ith mechanism, t indexes time, and e is the difference between the current total model output and the current desired gain setting.
Free parameters a and b control the gain and timescale of the mechanism. Large values of a make the mechanism give more weight to its past state, which makes it more sluggish, producing longer-term effects. The model also contained parameters for the optimal gains that were used to compute the error term. We assumed that our testing condition was neutral, and so we fixed its optimal gain to zero. We arbitrarily set the optimal gain for deprivation to 10 and allowed the optimal gain during deadaptation to vary as a final free parameter of the model. We then fit the model to our data, minimizing mean squared error between the model output and the aftereffects measured in all three tests.
The model fit the data well (Fig. 4B), accounting for over 80% of the total variance in the data. The parameters that best fit the data produced one mechanism with a rapid timescale and another with a slower timescale (Fig. 4C), confirming the intuitive account sketched above. The model also fit the two nonsignificant trends in our data, an initial negative aftereffect below zero after deadaptation, and a nonzero asymptote in the pretest. Future work should look at whether these trends can be reliable in some circumstances; neither alters the main implication of our results, that separate mechanisms control short- and long-term contrast adaptation.
Discussion
Adapting to removal of an orientation produced aftereffects that grew in strength and duration as the adapting period grew longer. We term this relationship the duration scaling law, and it has been confirmed in prior work that measured contrast detection, the tilt aftereffect, the motion aftereffect, and adaptation that results from viewing a face (4, 12–14). These studies used short durations, generally a few minutes in length. Our results showed that the duration scaling law in contrast adaptation holds for durations an order of magnitude longer than those tested previously, up to 8 h in length.
Our final experiment showed that especially long-lasting effects of contrast adaptation were produced by a separate mechanism from the one that controls shorter-term adaptation. After long-term adaptation and short-term deadaptation, we observed recovery of the aftereffects of adaptation. This spontaneous recovery shows that short-term adaptation can be manipulated independently from long-term adaptation and implies the existence of at least two control mechanisms with differing timescales. Our data cannot be explained by a model of adaptation that contains just a single mechanism that gains confidence as adaptation duration increases (5, 6). It remains possible, however, that each of the multiple mechanisms controlling contrast adaptation gain confidence as the duration approaches their preferred temporal interval (7).
Relatively long-term effects have also been induced by long adaptation durations in studies of binocular rivalry, color adaptation, stereo adaptation, adaptation to reduced contrast, and adaptation astigmatic lenses (15–19). Whether this adaptation arises from the same mechanism as shorter-term adaptation, or whether it arises from unique long-term mechanisms, has not been tested in any of these domains, however.
One notable exception is the McCollough effect, which is also long lasting and has recently been shown in an elegant study to have isolatable short- and long-term components (8). The McCollough effect differs in important ways from other types of adaptation, however. Most notably, it does not show the basic duration scaling effect; very long-lasting adaptation, with a decay rate of virtually zero, is produced even by very short adaptation durations. The McCollough effect also shows a large amount of eye specificity, indicating that it has a larger precortical component than the tilt aftereffect (20).
Long-lasting effects from relatively short adaptation durations have also been found in one study of the tilt aftereffect, where adaptation durations only 4 min in length led to an effect that initially decayed rapidly, but asymptoted at ≈10% of its maximum level, where it persisted for many days (21). Shorter durations of only 1.5 min failed to produce a long-lasting effect. Although suggestive of multiple mechanisms, such results could also be produced by a single one whose effects become more durable as the adapting period lengthens.
Another prior study reported spontaneous recovery of a tilt aftereffect after 30 min of adaptation and 2 min of deadaptation (22). The adaptation in this study, however, was comprised of exposure to a grating oriented 10° from vertical, whereas the deadaptation consisted of exposure to a grating oriented 20° the opposite direction from vertical. The subsequent spontaneous recovery was likely produced by separate mechanisms tuned to the two adapting orientations, with each observing the duration scaling law and combining to influence the tilt aftereffect. Separate mechanisms tuned to different orientations have long been components of most models of visual processing.
Short-term contrast adaptation also shows a “spacing” effect (22, 23). For example, five periods of adaptation, each lasting 2 min, interspersed with 1 min of recovery, produced stronger, longer-lasting aftereffects than 10 min of continuous adaptation. One interpretation of these results is that spacing produces decay in a short-term mechanism that, in turn, produces stronger input to, and adaptation in, a subsequent long-term mechanism. An alternative interpretation of spacing effects, however, is that they are produced by savings in relearning, where a second episode of adaptation produces stronger adaptation than an initial one. Spontaneous recovery is less explainable by this alternative account. In addition, the longer-term adaptation produced in this prior work decayed almost completely over 15 min of testing, and so is unlikely to explain the spontaneous recovery we observed, which followed 15 min of deadaptation. Thus, the present results identify a mechanism an order of magnitude more long term than measured before and provide stronger evidence for the existence of multiple mechanisms underlying the duration scaling law.
In more recent models of orientation processing, output from neurons tuned to one orientation is inhibited, or “normalized,” by an amount proportional to the pooled output of other neurons, and this normalization pool is likely affected by adaptation (24). In theory, then, deprivation of vertical could reduce the amount of inhibition received by horizontal neurons, leading them to be more active and adapt by reducing their responsiveness over time. Our prior study (ref. 9, experiment 2), and an additional control study reported in supporting information, suggest that this process did not occur to a large extent in the present paradigm, however (see SI Effects of Deadaptation upon Nondeprived Mechanisms and Fig. S1 for additional discussion).
The neural bases of long-term contrast adaptation have yet to be explored. Because it is orientation selective, the adaptation likely arises in primary visual cortex (V1) or later in the visual processing stream. Two studies have examined adaptation in V1 with inducing periods of a few tens of minutes (25, 26) and found strong effects there. V1 could provide the basis for the even longer-term effects observed here. Our previous work (9) measured detection thresholds after 4 h of adaptation and found effects still present after >10 min of testing. Because detection thresholds are often closely linked to neural activity in V1, this result is one piece of evidence that neurons there continue to adapt over long durations. Kwon et al. (19) used fMRI to measures effects of 4 h of adaptation to a 66% reduction in overall contrast, and found effects in V1. It is unclear, however, whether these arose from short- or long-term mechanisms. The cellular mechanisms of long-term adaptation also remain unexplored. In V1, short-term adaptation to high contrast produces a hyperpolarization of neurons' resting potentials (e.g., ref. 27). It is unknown whether similar mechanisms produce long-term adaptation.
Mechanisms of contrast adaptation likely exist at more than two timescales. In V1, effects appear following adapting durations lasting from milliseconds through minutes (1) and arise at different stages of neural processing (24), each of which could have its own time constant. In the retina, a rapid process that occurs within 100 ms of a stimulus differs in mechanism from longer-term adaptation (28), and similar, dissociable, very rapid adaptation is evident in motion-selective area MT, where it plays a role in motion perception (29). The prior behavioral work reviewed above is also consistent with separate shorter-term mechanisms of contrast adaptation acting over seconds and minutes (22, 23), as is additional behavioral work suggesting that contrast adaptation may be controlled by multiple mechanisms, but that was not concerned with the temporal properties of those mechanisms (30). Hence, the adaptation measured here likely represents the action of mechanisms near the long end of a continuous distribution across timescales.
Long-term visual adaptation also arises in response to naturally occurring visual challenges produced by disease and aging (e.g., refs. 31–33). Loss of central vision, for example, produces profound behavioral adaptation, although the neural bases of this adaptation remain controversial (e.g., refs. 34–38). The mechanisms of long-term adaptation identified here may be similar to those that control this natural adaptation. If so, studying long-term adaptation produced in the laboratory may lead to greater understanding of the processes by which humans adjust to visual challenges in the natural world.
Materials and Methods
For details, see SI Materials and Methods.
Subjects.
Seven observers participated in the initial deprivation experiments and eight in the enhancement experiment. Twelve observers participated in the readaptation experiment. Experimental procedures were approved by the University of Minnesota Institutional Review Board.
Stimulus Display.
The altered reality system is comprised of a camera connected to a laptop computer that feeds into a head-mounted display (9). Camera images were filtered in real-time by multiplying a second-order Butterworth filter with the captured image in the Fourier domain, and the inverse FFT of the resultant was displayed (9). Fig. 2 A and B show the original and filtered image, respectively, with the filter oriented to remove vertical energy.
Tilt Aftereffect Measurements.
Stimuli were plaid patches made up of two 1.5 cpd sine-wave gratings symmetrically tilted relative to vertical. We adopted a paradigm first introduced by Meese and Georgeson (39) in which the TAE could be investigated without presenting the adapted (vertical) orientation. The stimulus perceptually resembled a blurred square checkerboard. A TAE from adaptation to vertical causes the component grating to appear symmetrically tilted relative to 45°, which, in turn, causes the checks to appear rectangular. After adaptation, subjects were given control of the physical tilt of the gratings and adjusted them to cancel out any TAE. In our first three experiments, subject set repeated matches over time to cancel the TAE; in the readaptation experiment, a computer-controlled staircase method was used. The physical tilt required to cause the checks to appear square was recorded as our measure of the TAE. The use of a plaid greatly eases the task for subjects and does not affect measures of TAE amplitude compared with more traditional measures (39). Our prior work also reported relatively long-lasting aftereffects (at least 20 min) by using a detection task (9).
Readaptation Protocol.
This experiment had several stages (Fig. 5B) all completed in succession without breaks. Subjects: (i) Viewed unfiltered images for 15 min. (ii) Completed an initial set of tilt-aftereffect measurements, termed “baseline.” (iii) Adapted (to vertical deprivation) using the altered reality system for 8 min, the “initial adaptation”. (iv) Completed a second set of tilt aftereffect measurements, the pre-test. (v) Adapted for 4 h. (vi) Viewed unfiltered images for 15 min, the deadaptation period. (vii) Completed a third set of tilt aftereffect measurements, the deadaptation test. (viii) Adapted for a second 8-min period. (ix) Completed a final set of TAE measurements, the post-test.
Supplementary Material
Acknowledgments
This work was supported by National Science Foundation Behavioral and Cognitive Sciences Grant 1028584.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1113503109/-/DCSupplemental.
References
- 1.Kohn A. Visual adaptation: Physiology, mechanisms, and functional benefits. J Neurophysiol. 2007;97:3155–3164. doi: 10.1152/jn.00086.2007. [DOI] [PubMed] [Google Scholar]
- 2.Wark B, Lundstrom BN, Fairhall A. Sensory adaptation. Curr Opin Neurobiol. 2007;17:423–429. doi: 10.1016/j.conb.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clifford CW, et al. Visual adaptation: Neural, psychological and computational aspects. Vision Res. 2007;47:3125–3131. doi: 10.1016/j.visres.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 4.Greenlee MW, Georgeson MA, Magnussen S, Harris JP. The time course of adaptation to spatial contrast. Vision Res. 1991;31:223–236. doi: 10.1016/0042-6989(91)90113-j. [DOI] [PubMed] [Google Scholar]
- 5.Wark B, Fairhall A, Rieke F. Timescales of inference in visual adaptation. Neuron. 2009;61:750–761. doi: 10.1016/j.neuron.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grzywacz NM, de Juan J. Sensory adaptation as Kalman filtering: Theory and illustration with contrast adaptation. Network. 2003;14:465–482. [PubMed] [Google Scholar]
- 7.Kording KP, Tenenbaum JB, Shadmehr R. The dynamics of memory as a consequence of optimal adaptation to a changing body. Nat Neurosci. 2007;10:779–786. doi: 10.1038/nn1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vul E, Krizay E, MacLeod DI. The McCollough effect reflects permanent and transient adaptation in early visual cortex. J Vis. 2008;8:4.1–4.12. doi: 10.1167/8.12.4. [DOI] [PubMed] [Google Scholar]
- 9.Zhang P, Bao M, Kwon M, He S, Engel SA. Effects of orientation-specific visual deprivation induced with altered reality. Curr Biol. 2009;19:1956–1960. doi: 10.1016/j.cub.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 10.Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol Bull. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- 11.Smith MA, Ghazizadeh A, Shadmehr R. Interacting adaptive processes with different timescales underlie short-term motor learning. PLoS Biol. 2006;4:e179. doi: 10.1371/journal.pbio.0040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenlee MW, Magnussen S. Saturation of the tilt aftereffect. Vision Res. 1987;27:1041–1043. doi: 10.1016/0042-6989(87)90017-4. [DOI] [PubMed] [Google Scholar]
- 13.Hershenson M. Duration, time constant, and decay of the linear motion aftereffect as a function of inspection duration. Percept Psychophys. 1989;45:251–257. doi: 10.3758/bf03210704. [DOI] [PubMed] [Google Scholar]
- 14.Leopold DA, Rhodes G, Müller KM, Jeffery L. The dynamics of visual adaptation to faces. Proc Biol Sci. 2005;272:897–904. doi: 10.1098/rspb.2004.3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams WJ, Banks MS, van Ee R. Adaptation to three-dimensional distortions in human vision. Nat Neurosci. 2001;4:1063–1064. doi: 10.1038/nn729. [DOI] [PubMed] [Google Scholar]
- 16.Klink PC, Brascamp JW, Blake R, van Wezel RJ. Experience-driven plasticity in binocular vision. Curr Biol. 2010;20:1464–1469. doi: 10.1016/j.cub.2010.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon M, Legge GE, Fang F, Cheong AMY, He S. Adaptive changes in visual cortex following prolonged contrast reduction. J Vis. 2009;9(20):1–16. doi: 10.1167/9.2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neitz J, Carroll J, Yamauchi Y, Neitz M, Williams DR. Color perception is mediated by a plastic neural mechanism that is adjustable in adults. Neuron. 2002;35:783–792. doi: 10.1016/s0896-6273(02)00818-8. [DOI] [PubMed] [Google Scholar]
- 19.Yehezkel O, Sagi D, Sterkin A, Belkin M, Polat U. Learning to adapt: Dynamics of readaptation to geometrical distortions. Vision Res. 2010;50:1550–1558. doi: 10.1016/j.visres.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 20.McCollough C. Color adaptation of edge-detectors in the human visual system. Science. 1965;149:1115–1116. doi: 10.1126/science.149.3688.1115. [DOI] [PubMed] [Google Scholar]
- 21.Wolfe JM, O'Connell KM. Fatigue and structural change: Two consequences of visual pattern adaptation. Invest Ophthalmol Vis Sci. 1986;27:538–543. [PubMed] [Google Scholar]
- 22.Magnussen S, Greenlee MW. Contrast threshold elevation following continuous and interrupted adaptation. Vision Res. 1986;26:673–675. doi: 10.1016/0042-6989(86)90015-5. [DOI] [PubMed] [Google Scholar]
- 23.Magnussen S, Johnsen T. Temporal aspects of spatial adaptation. A study of the tilt aftereffect. Vision Res. 1986;26:661–672. doi: 10.1016/0042-6989(86)90014-3. [DOI] [PubMed] [Google Scholar]
- 24.Dhruv NT, Tailby C, Sokol SH, Lennie P. Multiple adaptable mechanisms early in the primate visual pathway. J Neurosci. 2011;31:15016–15025. doi: 10.1523/JNEUROSCI.0890-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dragoi V, Sharma J, Sur M. Adaptation-induced plasticity of orientation tuning in adult visual cortex. Neuron. 2000;28:287–298. doi: 10.1016/s0896-6273(00)00103-3. [DOI] [PubMed] [Google Scholar]
- 26.Sharpee TO, et al. Adaptive filtering enhances information transmission in visual cortex. Nature. 2006;439:936–942. doi: 10.1038/nature04519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carandini M, Ferster D. A tonic hyperpolarization underlying contrast adaptation in cat visual cortex. Science. 1997;276:949–952. doi: 10.1126/science.276.5314.949. [DOI] [PubMed] [Google Scholar]
- 28.Baccus SA, Meister M. Fast and slow contrast adaptation in retinal circuitry. Neuron. 2002;36:909–919. doi: 10.1016/s0896-6273(02)01050-4. [DOI] [PubMed] [Google Scholar]
- 29.Glasser DM, Tsui JM, Pack CC, Tadin D. Perceptual and neural consequences of rapid motion adaptation. Proc Natl Acad Sci USA. 2011;108:E1080–E1088. doi: 10.1073/pnas.1101141108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Müller KM, Schillinger F, Do DH, Leopold DA. Dissociable perceptual effects of visual adaptation. PLoS ONE. 2009;4:e6183. doi: 10.1371/journal.pone.0006183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baseler HA, et al. Reorganization of human cortical maps caused by inherited photoreceptor abnormalities. Nat Neurosci. 2002;5:364–370. doi: 10.1038/nn817. [DOI] [PubMed] [Google Scholar]
- 32.Delahunt PB, Webster MA, Ma L, Werner JS. Long-term renormalization of chromatic mechanisms following cataract surgery. Vis Neurosci. 2004;21:301–307. doi: 10.1017/S0952523804213025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fine I, et al. Long-term deprivation affects visual perception and cortex. Nat Neurosci. 2003;6:915–916. doi: 10.1038/nn1102. [DOI] [PubMed] [Google Scholar]
- 34.Baker CI, Peli E, Knouf N, Kanwisher NG. Reorganization of visual processing in macular degeneration. J Neurosci. 2005;25:614–618. doi: 10.1523/JNEUROSCI.3476-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baker CI, Dilks DD, Peli E, Kanwisher N. Reorganization of visual processing in macular degeneration: Replication and clues about the role of foveal loss. Vision Res. 2008;48:1910–1919. doi: 10.1016/j.visres.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masuda Y, Dumoulin SO, Nakadomari S, Wandell BA. V1 projection zone signals in human macular degeneration depend on task, not stimulus. Cereb Cortex. 2008;18:2483–2493. doi: 10.1093/cercor/bhm256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baseler HA, et al. Large-scale remapping of visual cortex is absent in adult humans with macular degeneration. Nat Neurosci. 2011;14:649–655. doi: 10.1038/nn.2793. [DOI] [PubMed] [Google Scholar]
- 38.Liu T, et al. Incomplete cortical reorganization in macular degeneration. Invest Ophthalmol Vis Sci. 2010;51:6826–6834. doi: 10.1167/iovs.09-4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meese TS, Georgeson MA. The tilt aftereffect in plaids and gratings: Channel codes, local signs and “patchwise” transforms. Vision Res. 1996;36:1421–1437. doi: 10.1016/0042-6989(95)00212-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.