Abstract
A Jurassic salamander, Beiyanerpeton jianpingensis (gen. et sp. nov.), from a recently found site in western Liaoning Province, China is the earliest known record of Salamandroidea. As a Late Jurassic record of the group, it extends the range of the clade by ~40 Ma. The Late Jurassic taxon is neotenic and represented by exceptionally preserved specimens, including fully articulated cranial and postcranial skeletons and bony gill structures close to the cheek region. The fossil beds, consisting of dark-brown volcanic ash shales of the Upper Jurassic Tiaojishan (Lanqi) Formation (Oxfordian), underlie trachyandesite rocks that have yielded a SHRIMP zircon U-Pb date of 157 ± 3 Ma. The fossiliferous beds are substantially older than the Jehol Group, including the Yixian Formation (40Ar/39Ar dates of 122–129 Ma), but slightly younger than the Middle Jurassic Daohugou horizon (40Ar/39Ar date of 164 ± 4 Ma). The early fossil taxon shares with extant salamandroids derived character states, including: separated nasals lacking a midline contact, angular fused to the prearticular in the lower jaw, and double-headed ribs on the presacral vertebrae. In contrast to extant salamandroids, however, the salamander shows a discrete and tooth-bearing palatine, and unequivocally nonpedicellate and monocuspid marginal teeth in large and presumably mature individuals. The finding provides insights into the evolution of key characters of salamanders, and also provides direct evidence supporting the hypothesis that the split between Cryptobranchoidea and Salamandroidea had taken placed before the Late Jurassic Oxfordian time. In this aspect, both paleontological and molecular data now come to agree.
Keywords: Urodela, phylogeny, fossil record, molecular clock
Salamanders are a diverse group of modern amphibians, with high ecological diversity and extreme morphological conservatism (1–4). Among the 10 extant families, the Hynobiidae and Cryptobranchidae are classified in the suborder Cryptobranchoidea, and the remaining families (except for the Sirenidae) in another suborder, Salamandroidea. Phylogenetic relationships of the Sirenidae remain contentious, and recent phylogenetic analyses place the family in either basal or crownward positions (5–8). The Cryptobranchoidea have their earliest record from the Middle Jurassic in Asia (9–13), and the Salamandroidea have an Early Cretaceous record from Europe (14). Iridotriton was described as a putative salamandroid from the Upper Jurassic Morrison Formation (15), but our evaluation of the available data suggest that it is probably a cryptobranchoid (see SI Appendix for details). Within the phylogenetic framework of the Urodela, the split of the Salamandroidea from the Cryptobranchiodea is a major cladogenetic event in the evolution of salamanders, but the timing for this major event is still in dispute (8, 16–18), largely because of lack of early fossil evidence. New molecular data (8) point to a substantially earlier age for this split than does the existing fossil record (14) before the finding of the fossils reported in this article.
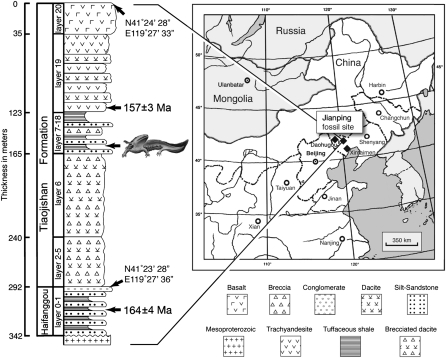
The salamandroid described herein is based on the specimens collected from the Guancaishan site near the county town of Jianping in western Liaoning Province, China (Fig. 1). With a radiometric date of 157 ± 3 Ma from the overlaying rocks (12), the fossil beds of the Upper Jurassic Tiaojishan (Lanqi) Formation consist of volcanic ash deposited in a lake during Oxfordian time (19). Thus, this Late Jurassic fossil taxon documents the earliest known record of the Salamandroidea, predating a previously Early Cretaceous record (Valdotriton, 116–114 Ma) by some 40 Ma (14). In contrast to the salamander fossils from the nearby Daohugou site, specimens from Jianping are all preserved with genuinely fossilized skeletons rather than preserved as natural molds or impressions resulting from postmortem dissolution of the skeletons. The superb preservation of skeletal details sheds new light on the evolution of key characters, including palatal structures and marginal teeth. In addition, a phylogenetic study incorporating this important fossil taxon supports the hypothesis that the divergence of the Salamandroidea from their sister clade Cryptobranchoidea likely took place before the Late Jurassic on the Eurasian continent (8).
Fig. 1.
Map and geologic section showing the geographic location and stratigraphic position of the fossil site (Tiaojishan Formation) in relation to Daohugou (Haifanggou Formation) locality in Inner Mongolia (geologic section modified from ref. 12). The nearby Lower Cretaceous Xintaimen site is also shown in the map. Solid diamond denoting fossil localities.
Systematic Paleontology
Amphibia Linnaeus, 1758; Lissamphibia Haeckel, 1866; Caudata Scopoli, 1777; Urodela Dumèril, 1806; Suborder Salamandroidea Noble, 1931; Beiyanerpeton jianpingensis gen. et sp. nov.
Etymology.
Beiyan (ancient place name, meaning Northern Yan State) + herpeton (Greek: creeping animal); Jianping, county town nearby the type locality.
Holotype.
Peking University Paleontological Collection (PKUP) V0601, a nearly complete skeleton exposed in ventral view.
Referred Specimens.
PKUP V0602-0606, and several unnumbered specimens; all preserved as articulated cranial and postcranial skeletons.
Type Locality and Horizon.
Guancaishan, near Jianping, Liaoning Province, China; Upper Jurassic Tiaojishan (Lanqi) Formation.
Diagnosis.
A Late Jurassic neotenic salamandroid diagnosed by the following unique combination of character states: Skull relatively wider than long, both anterodorsal and anteromedial fenestrae developed; sensory canal present on external surface of premaxilla and maxilla; prefrontal present; lacrimal retained; palatine present as a discrete and dentate element; marginal teeth nonpedicellate, simple peg-like and significantly shorter than vomerine teeth; posterolateral wings of parasphenoid grooved for internal carotid artery; operculum separate from stapes; hypobranchial I and II well ossified; basibranchial I and II co-ossified; angular lost by fusion with prearticular; presacral vertebrae 15 in number; vertebral centrum short, strongly constricted, with prominent subcentral keel; phalangeal 2-2-3-2/3 in manus, and 2-2-3-4-3 in pes.
Description and Comparison.
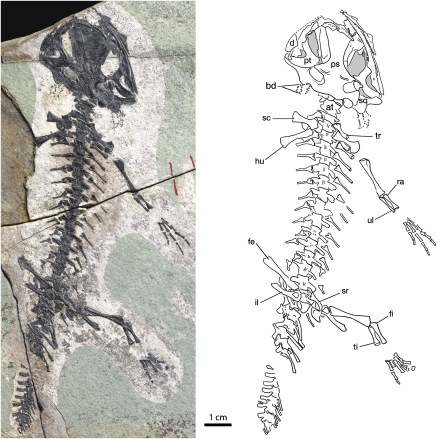
As best shown by the holotype (PKUP V0601), the salamandroid is a medium-sized salamander with a standard snout-vent length of ~100 mm (Fig. 2). Bony structures of gill rakers (branchial denticles) are preserved in all large specimens known for the taxon, giving clear indication of the gill structure for suction feeding as a neotenic feature of the Late Jurassic salamander. The tail is roughly the same length as the trunk, as shown in PKUP V0602. Caudal vertebrae are dorsoventrally expanded, reflecting a laterally compressed tail, which, in keeping with the presence of gill structures and lack of ossification of the mesopodium, implies an aquatic habitat of the salamander.
Fig. 2.
Holotype of Beiyanerpeton jianpingensis, gen. et sp. nov. (holotype PKUP V0601): a nearly complete skeleton in ventral view. Anatomical abbreviations used in figures: adf, anterodorsal fenestra; at, atlas; bd, branchial denticle; d, dentary; fe, femur; fi, fibula; fr, frontal; hb, hypobranchial; hu, humerus; il, ilium; lac, lacrimal; mx, maxilla; na, nasal; op, operculum; pa, parietal; pal, palatine; prf, prefrontal; pm, premaxilla; pra, prearticular; ps, parasphenoid; pt, pterygoid; qu, quadrate; ra, radius; sc, scapulocoracoid; se, sphenethmoid; sm, septomaxilla; sq, squamosal; sr, sacral rib; st, stapes; ti, tibia; tr, trunk rib; ul, ulna; vo, vomer.
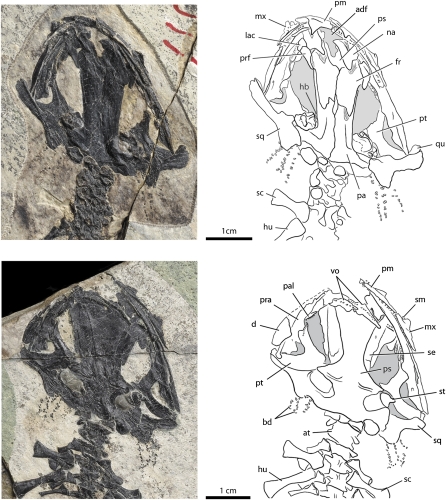
In the skull roof, the premaxillae are paired tooth-bearing elements, each of which carries ~30 monocuspid and nonpedicellate teeth. These teeth are short, thick, and loosely spaced. The premaxilla has a well-developed triangular dorsal process overlapping the nasal (Fig. 3). The partes dentalis of the premaxillae are medially in contact, but the pars dorsalis (dorsal process) along with the nasals are widely separated from one another by a large anterodorsal fenestra. The external surface of the pars dentalis carries a deep groove for the sensory canal leading to the narial opening. The paired frontals have an extensive midline sutural contact, but are anteriorly notched for the anterodorsal fenestra. Prefrontals and lacimals are present, and the former element has a posterior process that contacts the anterior extension of the parietal. The lacrimal is a small element articulated medially with the prefrontal and laterally with the dorsal process of the maxilla (PKUP V0604, V0605). Among extant salamander families, a lacrimal is present in Hynobiidae, Rhyacotritonidae, and Dicamptodontidae, but is lost in all other families (20, 21).
Fig. 3.
Skull of Beiyanerpeton jianpingensis, gen. et sp. nov. in dorsal (Upper: PKUP V0605) and palatal (Lower: holotype PKUP V0601) views.
Like other skull roof elements, the paired parietals have a smooth dorsal surface, but dorsolaterally develop a curved crest for attachment of adductor muscles. The posterior border of the parietals does not form the dorsal rim of the foramen magnum, because two sides of the otic-occipital complex contact each other below the parietals (best shown in PKUP V0604). The squamosal in dorsal view is essentially a transverse bar, but has an expanded medial end overlapping the boot-shaped posterolateral process of the parietal, and a relatively narrow lateral end above the quadrate as part of the cranio-mandibular joint.
The maxilla is short, with a pointed posterior extremity free from articulation with any bony elements. Similar to the premaxilla, the lateral surface of the maxilla also has a deep groove running horizontally towards the external narial opening. This groove may indicate the anterior extension of the infraorbital sensory canal as a branch of the Cranial Nerve V (22). The maxilla develops a low dorsal process, the anterior border of which is slightly notched for the narial opening. Close to this notch, the inner surface of the maxilla is grooved for articulation with a small septomaxilla (PKUP V0604, V0605). In those salamanders with this element present, the septomaxilla is the last cranial element to ossify ontogenetically (21), and the presence of this element indicates maturity of these individuals. The maxillary teeth are extremely short, simple and monocuspid, and the tooth row extends close to the posterior extremity of the maxilla.
In the palate, the vomers are narrow strip-like elements that are widely separated from one another by an anteromedial fenestra. The vomer lacks a posterior process that is commonly seen in more derived salamandroids, but has a small notch at its posterolateral border for the choana. Vomerine teeth are closely spaced and aligned to form a single tooth row (monostichous teeth), which is curved along the anterior border of the vomer, paralleling the marginal tooth row. The palatine as a discrete tooth-bearing element is clearly identifiable in several specimens, including the holotype. This small element is anteriorly articulated to the vomer and posteriorly to the pterygoid. The palatine carries a single tooth row, different from sirenids, which have multiple tooth rows. The parasphenoid is anteriorly notched for the anteromedial fenestra and the posterolateral wing is grooved for the internal carotid artery. The pterygoid (palatopterygoid of some other authors) is triradiate, with a sickle-shaped anterior process curved anteromedially to meet the palatine. The anterior process bears small denticles forming a single tooth row. The medial process is extremely short, contacting the lateral wing of the parasphenoid just anterior to the otic capsule. The quadrate process of the pterygoid is much wider than the anterior or the medial processes and extends posterolaterally to contact the quadrate.
Also in palatal view, hypobranchial I and II are ossified as short bar-like structures, arranged obliquely and roughly parallel to one another. Basibranchial I and II are co-ossified as a trident-shaped structure with slender arms extending anteriorly and anterolaterally (counterpart of PKUP V0601, V0602). The sphenethmoid (orbitosphenoid) forms a narrow strip covering the lateral side of the neurocranium.
The otic-occipital complex of the recently identified salamander consists of the prootic, exoccipital, and opisthotic as separate elements. The dorsal exposure of the complex is limited to the posterior end of the skull, lacking an anterolateral extension between the parietal and squamosal. A stapes (columella) is clearly present with its footplate covering the fenestra ovalis and a short, thick, rod-like stylus extending laterally (Fig. 3). A stapes is present in most salamanders and loss of this element is known for all salamandrids, and some but not all plethodontids and sirenids (20, 21). In addition, a free operculum is clearly identifiable as a thin, oval, and domed plate (Fig. 3). In life, this element presumably covers part of the otic capsule, but has been taphonomically dislocated to a posterior position close to the base of the squamosal. Loss of the operculum is a phylogenetically significant feature, independently occurring in at least seven families of salamanders (SI Appendix).
The mandible consists of only two dermal elements, a derived feature of Salamandroidea: the tooth-bearing dentary and the large prearticular formed by fusion with the articular and angular. Marginal teeth, as shown in several specimens including the holotype, are extremely short, thick, loosely spaced, and clearly nonpedicellate, as none of the well-preserved teeth show a bipartite structure. The tooth crown is a simple cone without any indication of bicuspidation. The nonpedicellate and monocuspid marginal teeth are different from all extant salamanders including sirenids. Extant sirenids are highly specialized in lacking marginal teeth in greatly reduced premaxilla, maxilla, and the dentary; those monocuspid teeth covering the vomer and palatine can be weakly pedicellate or nonpedicellate (21, 23). Early fossil sirenid Habrosaurus indeed has nonpedicellate and monocuspid marginal teeth, although the teeth may be either chisel-shaped or inflated and mushroom-like (24).
The vertebral column of the Late Jurassic salamander consists of 15 presacrals, including the atlas, a single sacral, and about 30 caudals (PKUP V0601, V0602). All trunk vertebrae are amphicoelous, and ventrally have a well-developed subcentral keel. Presacral ribs are clearly bicapitate, having an expanded head and a slender shaft. The anteriormost three pairs are more robust than the others, and have both proximal and distal ends expanded. The last three-to-four pairs of ribs are significantly shorter than the others, but the sacral ribs are robust and elongate, as curved rods in articulation with the pelvic girdle. The presence or absence of spinal nerve foramina on trunk vertebrae cannot be ascertained, although the foramina can be clearly identified on the caudal vertebrae. The first two caudal vertebrae are exposed in dorsal or ventral view and show free ribs in articulation with the transverse processes. The remaining part of the caudal series is twisted at a 90° angle, and thus is exposed in lateral view. The third and more posterior caudal vertebrae bear no free ribs, but dorsally carry a high neural spine and, ventrally, an elongate chevron, forming a deep and laterally compressed tail.
The scapulocoracoid as a single ossification has a narrow and elongate scapular blade, and a thickend coracoid part, which is only slightly expanded, differing from the squared configuration in Chunerpeton from a stratigraphically slightly lower (older) horizon (9). The humerus has a low and thick delto-pectoral crest, and well-ossified epiphysis as an indication of the maturity of the individual at death. The ulna and radius are short, subequal in length; and more distally, no carpals but distal carpals and phalanges are ossified. The manus shows a phalangeal formula of 2-2-3-2 in the holotype, but 2-2-3-3 in PKUP V0602.
Both the ilium and ischium are well preserved in the pelvis, but an ossified pubis, as commonly seen in other salamanders, is lacking. The iliac shaft is elongate, more than two-thirds the length of the femur. The acetabular fossa is shallow, but has a well-developed crest to form the dorsal border of the acetabulum. The ischium is a small and thick plate, which hardly contributes to any part of the acetabulum. The femural trochanter is weakly developed as a small tubercle, different from the twig-like process in more advanced salamanders. The epipodial segment is extremely short, being roughly one-third the length of the propodial. The pes has a phalangeal formula of 2-2-3-4-3, as shown on both sides of PKUP V0602.
Results and Discussion
The assessment of these specimens as neotenic is based on a suite of diverse characters. The holotype and the referred specimens are relatively large individuals, with a snout-vent length of greater than 100 mm. These specimens show fully ossified nasals and septomaxillae, both frontals and parietals closely articulating along the midline, and well-ossified hyobranchium, including co-ossification of basibranchials I and II to form a trident-shaped structure. In the postcranial skeleton, all specimens show a well-ossified epiphysis of the long bones as well. Although all these features indicate maturity of the individual specimens (21, 25), retention of gill structures as a larval feature in these relatively large and presumably mature salamanders confirms the neotenic status of the Late Jurassic taxon.
This Late Jurassic salamander is placed in the suborder Salamandroidea because this fossil taxon shares with extant salamandroids several derived character states, including: nasals separated by anterodorsal fenestra without a midline contact; angular fused to prearticular; articular absent by fusion to prearticular; double-headed ribs associated with dorsal vertebrae; anterior process of parietal extending to midlevel of orbit; and parietal-prefrontal contact medially above orbit. Other diagnostic features of the Salamandroidea, mostly soft anatomical or genetic characters, cannot be determined for this fossil taxon, and these include: lateral wall of nasal capsule incomplete; microchromosome absent; spermathecase in cloaca present; and internal fertilization (see SI Appendix for citations).
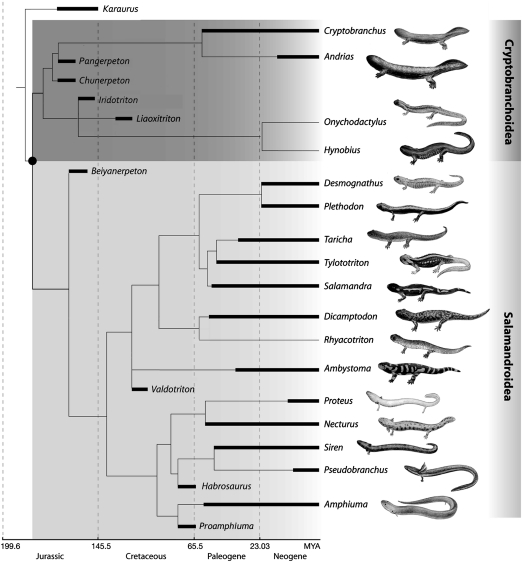
Within the phylogenetic framework of the Salamandroidea, results of the phylogenetic analysis place the Late Jurassic salamander as the most basal taxon within the clade (Fig. 4 and SI Appendix, Figs. S1 and S2). This basal position is secured for the salamander by its possession of diagnostic features of the Salamandroidea, as mentioned above, and in its lack of other features of extant salamandroids, including: dorsal process of premaxilla extending to contact frontal and separating nasals; dorsal exposure of the otic-occipital complex between the parietal and the squamosal; and absence of a squamosal-parietal contact. Forceful grouping the unique taxon with the Cryptobranchoidea or moving it to a more crownward position in the Salamandroidea requires a minimum cost of five (235 vs. 240) and four (235 vs. 239) additional steps, respectively, none of which would be a parsimonious hypothesis. The relationships within the Salamandroidea and that of the Sirenidae with other salamanders, as resulted from our morphology-based analysis (Fig. 4 and SI Appendix, Figs. S1 and S2), show topological differences from those of molecular-based studies (8, 17, 26), as have been foreshadowed by previous research (5, 6). Although resolving the molecular/morphological conflict is beyond the scope of this study, topological changes within the Salamandroidea have little impact on the phylogenetic position of the fossil taxon reported in this article.
Fig. 4.
Calibrated cladogram showing the relationships of Beiyanerpeton to other fossil and extant salamander clades, and the timing of the Salamandroidea splitting from Cryptobranchoidea (solid dot) as indicated by the fossil record. For details on phylogenetic analysis and stratigraphic range of major salamander clades see text and SI Appendix.
The fossil finding in western Liaoning provides paleontological evidence for understanding the fundamental patterns of character evolution in salamanders. One of these patterns of character evolution concerns the loss of the palatine. The presence or absence of a dentate palatine in salamanders has been in dispute: some authors (27, 28) regarded all mature salamanders as lacking a palatine as in frogs and caecilians, and other authors (21, 29) have shown that a palatine in most salamanders is present in early ontogeny, but subsequently fuses to the ptergyoid. Sirenids present an exception, as they retain the palatine as a discrete element in the mature stage (21, 24). The Late Jurassic salamandroid from western Liaoning is so far the only nonsirenid fossil taxon known with a “larval type of palatine” in seemingly mature individuals. This finding, in keeping with ontogenetic evidence, indicates that the presence of a separate palatine as a tooth-bearing element in the palate indeed occurred in early evolution of true salamanders as a primitive morphology. Temnospondyls, such as branchiosaurids and amphibamids, contenders as probable salamander ancestors, also retain a discrete but greatly reduced palatine (25, 30), supporting this interpretation. The actual condition in stem caudate Karaurus remains uncertain. Although the palate of this Middle-Late Jurassic stem-group caudate is reconstructed as lacking a palatine, the type and only known complete specimen is not exposed in palatal view.
Another key character seen in the recently identified salamander is its nonpedicellate and monocuspid marginal dentition. Pedicellate teeth with bicuspid crowns have been widely accepted as unchallenged synapomorphies, supporting a single origin of the Lissamphibia (salamanders, frogs, caecilians). With the exception of sirenids that possess monocuspid but weakly pedicellate or nonpedicellate teeth in mature individuals (21, 23), most extant salamanders have pedicellate and monocuspid teeth replaced by bicuspid teeth at metamorphosis, and neotenic salamanders at sexual maturity may show positional or regional variations with a mixture of monocuspid and bicuspid marginal teeth in the same individual (31). Surprisingly, the Late Jurassic salamander from western Liaoning, China, clearly shows nonpedicellate teeth that have a monocuspid crown at maturity. The tooth morphology is unknown for the stem caudate Karaurus and Marmorerpeton (14, 32), but a recent study has shown that the Middle Jurassic Kokartus as another stem caudate has indeed monocuspid and nonpedicellate teeth (33). The salamander from western Liaoning, as a basal salamandroid, provides unequivocal evidence showing that primitive crown-group salamanders may have had nonpedicellate and monocuspid teeth as the stem caudate Kokartus. Furthermore, this finding indicates that the pedicelly and bicuspid crown patterns are most likely derived features within the Caudata, and thus reopen the question on interpretation of these as synapomorphies supporting the monophyly of Lissamphibia.
The finding provides the earliest known fossil record of the suborder Salamandroidea, predating the previously known record from Europe (Valdotriton from the Cretaceous of Spain) by at least 40 Ma. Fragmentary fossils from the Middle Jurassic Kirtlington Quarry, England, have been mentioned to contain specimens of possible crown-group salamanders (14, 32), but their crown-group status cannot be sensibly confirmed or refuted, as the fossil material remains undescribed. In that context, the Late Jurassic salamander from western Liaoning also provides, so far, the most reliable paleontological evidence to estimate the timing for the split of Salamandroidea from its sister clade Cryptobranchoidea. Because the fossil beds described herein, in western Liaoning, are stratigraphically below the trachyandesite rocks that have been dated as 157 ± 3 Ma (12), the fossil occurrence indicates that the Salamandriodea/Cryptobranchoidea split, a major cladogenetic event in salamander evolution, had taken place before the Late Jurassic. This evidence, in keeping with our previous report of an early cryptobranchid from Inner Mongolia (9), rejects the purported timing of 140 Ma for this cladogenetic event (16), but supports a more recent study based on complete mitochondrial genomes for a Middle Jurassic divergence of the Salamandroidea from Cryptobranchoidea (8). With a plausible estimation of the origin of living salamanders at ~183 Ma in the Early Jurassic (8), there is still a considerable gap to be filled between this early fossil record of the Salamandroidea and the Middle Jurassic origin of the group.
Materials and Methods
The dataset constricted for the phylogenetic study comprised 105 characters coded across 26 basic taxa, with the stem caudate Karaurus designated as the outgroup (SI Appendix, Table S1). Source of character information and explanation on sampling of taxa are given in the SI Appendix. Phylogenetic analysis is performed using the Macintosh version of PAUP* 4.0 (34) under the Branch-and-Bound search option, and trace of character evolution is carried out by using MacClade 4.06 (35).
The initial analysis of the dataset found 40 most parsimonious trees (TL = 235 steps, CI = 0.506, RI = 0.727), and all of the most parsimonious trees have the taxon Beiyanerpeton placed as a basal salamandroid, as shown in the strict consensus tree (SI Appendix, Fig. S1). Because the relationships within the Cryptobranchoidea and among several salamandroid clades are poorly resolved, further analysis of the dataset is carried out after character reweighting by maximum value of rescaled consistency indices (36). The latter analysis generated 10 most parsimonious trees (TL = 9,131 steps, CI = 0.695, RI = 0.852). Both the strict and Adams consensus trees are given in the SI Appendix, and the Adams consensus tree is converted into a calibrated cladogram as presented in Fig. 4.
Supplementary Material
Acknowledgments
We thank Richard Fox and Nadia Frobisch for reading and commenting on an early version of the manuscript and two anonymous referees who improved the manuscript significantly. Specimen photography was done by Mick Ellison (American Museum of Natural History). Rendering of the line drawings of the specimens and the illustration of the cladogram was done by Kapi Monoyios (University of Chicago). This research was supported by Grants 40532008 and 41072007 from the National Natural Science Foundation of China, and Topsun Corporation of China; a grant from the John Simon Guggenheim Memorial Foundation (to N.H.S.); the National Science Foundation of the United States of America (N.H.S.); and the Biological Sciences Division of the University of Chicago (N.H.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 5557.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009828109/-/DCSupplemental.
References
- 1.Cannatella DC, Hillis DM. Amphibian relationships: Phylogenetic analysis of morphology and molecules. Herpetol Monogr. 1993;7:1–7. [Google Scholar]
- 2.Wake DB, Larson A. Multidimensional analysis of an evolving lineage. Science. 1987;238(4823):42–48. doi: 10.1126/science.238.4823.42. [DOI] [PubMed] [Google Scholar]
- 3.Wake DB. Homoplasy: The result of natural selection or evidence of design limitations? Am Nat. 1991;138:543–567. [Google Scholar]
- 4.Shubin N, Wake DB. Phylogeny, variation, and morphological integration. Am Zool. 1996;36(1):51–60. [Google Scholar]
- 5.Gao K-Q, Shubin NH. Late Jurassic salamanders from northern China. Nature. 2001;410:574–577. doi: 10.1038/35069051. [DOI] [PubMed] [Google Scholar]
- 6.Wiens JJ, Bonett RM, Chippindale PT. Ontogeny discombobulates phylogeny: Paedomorphosis and higher-level salamander relationships. Syst Biol. 2005;54(1):91–110. doi: 10.1080/10635150590906037. [DOI] [PubMed] [Google Scholar]
- 7.Weisrock DW, Harmon L, Larson A. Resolving deep phylogenetic relationships in salamanders: Analyses of mitochondrial and nuclear genomic data. Syst Biol. 2005;54:758–777. doi: 10.1080/10635150500234641. [DOI] [PubMed] [Google Scholar]
- 8.Zhang P, Wake DB. Higher-level salamander relationships and divergence dates inferred from complete mitochondrial genomes. Mol Phylo Evol. 2009;53:492–508. doi: 10.1016/j.ympev.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Gao K-Q, Shubin NH. Earliest known crown-group salamanders. Nature. 2003;422:424–428. doi: 10.1038/nature01491. [DOI] [PubMed] [Google Scholar]
- 10.Chen W, et al. Isotope geochronology of the fossil-bearing beds in the Daohugou area, Ningcheng, Inner Mongolia. Geol Bull Chin. 2004;23:1165–1169. [Google Scholar]
- 11.Zhang Y, et al. 2005 Ar-Ar and SHRIMP U-Pb age evidence of the Daohugou fossil-bearing beds in Ningcheng, Inner Mongolia, NE China. Geochimica et Cosmochimica Acta Supplement, vol. 69, issue 10, Goldschmidt Conf Abstract: p. A328. [Google Scholar]
- 12.Liu Y, Liu Y, Ji S, Yang Z. U-Pb zircon age for the Daohugou Biota at Ningcheng of Inner Mongolia and comments on related issues. Chin Sci Bull. 2006;51:2634–2644. [Google Scholar]
- 13.Yang W, Li S. Geochronology and geochemistry of the Mesozoic volcanic rocks in Western Liaoning: Implications for lithospheric thinning of the North China Craton. Lithos. 2008;102(1–2):88–117. [Google Scholar]
- 14.Evans SE, Milner AR. A metamorphosed salamander from the Early Cretaceous of Las Hoyas, Spain. Philos Trans R Soc Lond, B. 1996;351:627–646. [Google Scholar]
- 15.Evans SE, Lally C, Chure DC, Maisano JA. A Late Jurassic salamander (Amphibia: Caudata) from the Morrison Formation of North America. Zool J Linn Soc. 2005;143:599–616. [Google Scholar]
- 16.Marjanović D, Laurin M. Fossils, molecules, divergence times, and the origin of lissamphibians. Syst Biol. 2007;56:369–388. doi: 10.1080/10635150701397635. [DOI] [PubMed] [Google Scholar]
- 17.Roelants K, et al. Global patterns of diversification in the history of modern amphibians. Proc Natl Acad Sci USA. 2007;104:887–892. doi: 10.1073/pnas.0608378104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng Y, Peng R, Kuro-o M, Zeng X. Exploring patterns and extent of bias in estimating divergence time from mitochondrial DNA sequence data in a particular lineage: A case study of salamanders (Order Caudata) Mol Biol Evol. 2011;28:2521–2535. doi: 10.1093/molbev/msr072. [DOI] [PubMed] [Google Scholar]
- 19.Gradstein FM, et al. A Geological Time Scale 2005. Cambridge: Cambridge Univ Press; 2005. [Google Scholar]
- 20.Duellman WE, Trueb L. Biology of Amphibians. New York: McGraw-Hill Book Company; 1986. [Google Scholar]
- 21.Rose CS. In: Amphibian Biology. Heatwole H, Davies M, editors. Vol 5. Chipping Norton, Australia: Surrey Beatty & Sons; 2003. pp. 1684–1781. [Google Scholar]
- 22.Vorobyeva E. In: Amphibian Biology. Heatwole H, Davies M, editors. Vol 5. Chipping Norton, Australia: Surrey Beatty& Sons; 2003. pp. 1497–1550. [Google Scholar]
- 23.Means DB. Comments on undivided teeth in Urodeles. Copeia. 1972;1972:586–588. [Google Scholar]
- 24.Gardner J. Revison of Habrosaurus Gilmore (Caudata: Sirenidae) and relationships among sirenid salamanders. Palaeont. 2003;46:1089–1122. [Google Scholar]
- 25.Schoch R. The early formation of the skull in extant and Paleozoic amphibians. Paleobiol. 2002;28:278–296. [Google Scholar]
- 26.Pyron RA, Wiens JJ. A large-scale phylogeny of Amphibia including over 2800 species, and a revised classification of extant frogs, salamanders, and caecilians. Mol Phyl Evol. 2011;61:543–583. doi: 10.1016/j.ympev.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 27.Noble GK. The Biology of the Amphibia. New York: MaGraw-Hill Book Company; 1931. [Google Scholar]
- 28.Trueb L, Cloutier R. In: Origins of the Higher Groups of Tetrapods: Controversy and Consensus. Schultze H-P, Trueb L, editors. Ithaca, London: Cornell University Press; 1991. pp. 223–313. [Google Scholar]
- 29.Gömann N, Clemen G, Greven H. Notes on cranial ontogeny and delayed metamorphosis in the hynobiid salamander Ranodon sibiricus Kessler, 1866 (Urodela) Ann Anat. 2005;187:305–321. doi: 10.1016/j.aanat.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Boy JA, Sues H-D. In: Amphibian Biology 4. Heatwole H, Carroll RL, editors. Chipping Norton, Australia: Surrey Beatty & Sons; 2000. pp. 1150–1197. [Google Scholar]
- 31.Beneski JT, Jr, Larsen JH., Jr Ontogenetic alternations in the gross tooth morphology of Dicamptodon and Rhyacotriton (Amphibia, Urodela, and Dicamptodontidae) J Morphol. 1989;199:165–174. doi: 10.1002/jmor.1051990204. [DOI] [PubMed] [Google Scholar]
- 32.Milner AR. In: Amphibian Biology 4. Heatwole H, Carroll RL, editors. Chipping Norton, Australia: Surrey Beatty & Sons; 2000. pp. 1412–1444. [Google Scholar]
- 33.Skutschas P, Martin T. Cranial anatomy of the stem salamander Kokartus honorarius (Amphibia: Caudata) from the Middle Jurassic of Kyrgyzstan. Zool J Linn Soc. 2011;161:816–838. doi: 10.1111/joa.12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swofford DL. PAUP* Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4. Sunderland, MA: Sinauer Associates; 2003. [Google Scholar]
- 35.Maddison DR, Maddison WP. MacClade 4: Analysis of Phylogeny and Character Evolution. Version 4.06. Sunderland, MA: Sinauer Associates; 2003. [Google Scholar]
- 36.Farris JS. The retention index and the rescaled consistency index. Cladistics. 1989;5:417–419. doi: 10.1111/j.1096-0031.1989.tb00573.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.