Abstract
Mutations in Drosophila merry-go-round (mgr) have been known for over two decades to lead to circular mitotic figures and loss of meiotic spindle integrity. However, the identity of its gene product has remained undiscovered. We now show that mgr encodes the Prefoldin subunit counterpart of human von Hippel Lindau binding-protein 1. Depletion of Mgr from cultured cells also leads to formation of monopolar and abnormal spindles and centrosome loss. These phenotypes are associated with reductions of tubulin levels in both mgr flies and mgr RNAi-treated cultured cells. Moreover, mgr spindle defects can be phenocopied by depleting β-tubulin, suggesting Mgr function is required for tubulin stability. Instability of β-tubulin in the mgr larval brain is less pronounced than in either mgr testes or in cultured cells. However, expression of transgenic β-tubulin in the larval brain leads to increased tubulin instability, indicating that Prefoldin might only be required when tubulins are synthesized at high levels. Mgr interacts with Drosophila von Hippel Lindau protein (Vhl). Both proteins interact with unpolymerized tubulins, suggesting they cooperate in regulating tubulin functions. Accordingly, codepletion of Vhl with Mgr gives partial rescue of tubulin instability, monopolar spindle formation, and loss of centrosomes, leading us to propose a requirement for Vhl to promote degradation of incorrectly folded tubulin in the absence of functional Prefoldin. Thus, Vhl may play a pivotal role: promoting microtubule stabilization when tubulins are correctly folded by Prefoldin and tubulin destruction when they are not.
Keywords: folding, chaperone, Gim, E3 ubiquitin ligase
Eukaryotes have a complex molecular machinery that promotes the folding and assembly of the actin and tubulin subunits of microfilaments and microtubules (MTs). Several protein complexes and ancillary proteins are involved in assembling αβ-tubulin dimers: chaperonin containing tailless protein (CCT), the prefoldin complex, phosducin-like CCT regulatory proteins, and five cofactors [reviewed by Lundin et al. (1)]. Prefoldin is a hexameric protein complex (2) thought to bind to partially folded tubulin and actin molecules from the ribosome (3–5).
One component of the prefoldin complex also interacts with the tumor suppressor Von Hippel Lindau (Vhl) protein and is known as Von Hippel Lindau binding protein 1 (VBP1) (6). The Vhl protein is a multifunctional adapter protein that influences multiple transcriptional pathways (for review, see refs. 7 and 8), as well as the functions of the collagen IV and fibronectin extracellular matrix and MTs. Its best-characterized function is in a complex with Cullin2 as an E3 ubiquitin-protein ligase that targets hypoxia-inducible factor α (HIF1α) for destruction (9, 10). Vhl interacts with the CCT to mediate the formation of the Vhl-ElonginB/C-Cullin2 complex (VBC) (11–15). Therefore, the interaction between Vhl and the prefoldin complex could be relevant for the folding of the VBC. However, Vhl also interacts with cytoplasmic MTs: in mitosis to influence spindle orientation (16) and in interphase to inhibit catastrophe and promote rescue (17, 18). Such an effect could account for the role of Vhl in stabilizing MTs in Drosophila follicle cells to maintain the integrity of this epithelium (19). Vhl is also required with the GSK-3β protein kinase to maintain the stability of the ciliary axoneme (20–22).
Genetic studies first identified prefoldin in yeast through mutants that could still fold tubulin but more slowly (hence the name GIM: genes involved in microtubule biogenesis) (23). Loss of prefoldin in Caenorhabditis elegans is lethal because of a high demand for tubulin in mitotic cells in the embryo (24). In plants, prefoldin 6 has been shown to be required for normal MT dynamics and organization (25). Knockout of Prefoldin 1 or mutation in Prefoldin 5 of mice lead to a variety of defects characteristic of tubulin functions in cilia or in the CNS (26, 27).
We now identify merry-go-round (mgr), a Drosophila gene identified over two decades ago, as encoding the prefoldin 3(Pfdn3)/VBP1/Gim2 subunit. We show that the characteristic monopolar mitotic spindles of this mutant arise because of diminished levels of tubulin subunits. We also show that mgr mutants cannot stabilize tubulin following overexpression of a tubulin transgene. Finally, our studies show that Mgr can physically interact with Vhl. Moreover, depletion of Vhl rescues the destabilization of tubulin resulting from loss of Mgr. This finding leads us to suggest that the E3 ubiquitin-protein ligase properties of Vhl may be required for the degradation of incorrectly folded tubulin, suggesting that Vhl can also contribute to MT dynamics through the regulation of tubulin degradation.
Results
Mgr Is a Subunit of the Highly Conserved Gim Complex/Prefoldin.
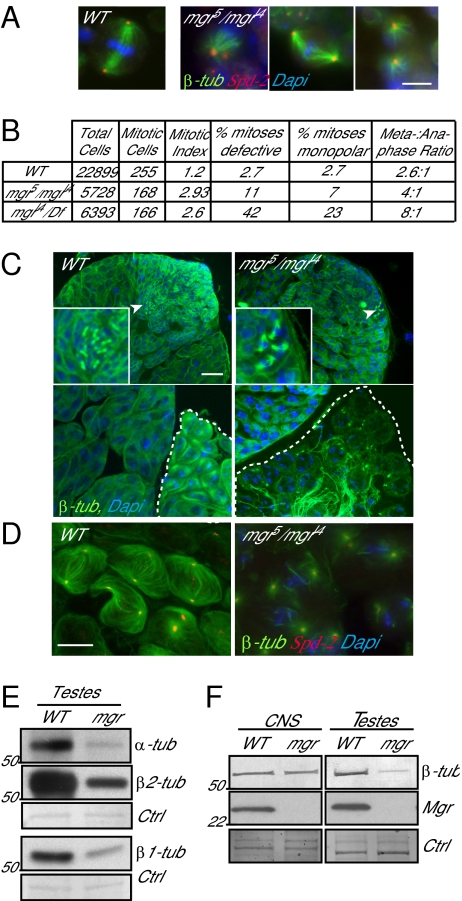
The mgr gene was originally recovered as an X-ray–induced mutant resulting in lethality late in development associated with circular mitotic figures in larval neuroblasts (28). We confirmed this phenotype by immunostaining the CNS to reveal MTs and centrosomal antigens in several mutant alleles of mgr and by counting proportions of cells at different stages of mitosis (Fig. 1 A and B, and Fig. S1 A and B). The prometaphase-like cells in such preparations could have bipolar spindles that may lack one or both centrosomes or monopolar spindles having one or two centrosomes at the single pole (Fig. 1A and Fig. S1 A and B). These centrosomes were positive for several known Drosophila centrosomal antigens (Fig. S1B). The mitotic index was elevated some twofold over that in wild-type larval brains and the ratio of metaphase:anaphase figures was elevated by two- to threefold over wild-type, depending upon the allelic combination (Fig. 1B). Taken together, these data are indicative of a delay in progression through the mitotic cycle. Previous phase-contrast images of spermatocytes of mgr mutants suggested the absence of meiotic spindles (28). To determine whether this suggestion was correct, we used an antibody recognizing both the β1-tubulin isoform and the β2-tubulin male germ-line–specific isoform to immunostain the testes of mgrl4/mgr5 transheterozygotes, a strong hypomorphic mutant combination that shows pharate lethality but gives some sterile adults. This process revealed that mgrl4/mgr5 had sufficient MTs to permit the premeiotic mitosis (arrowheads and magnification in Fig. 1C). However, mature (white dashed outlined cysts) but not young primary spermatocyte cysts had reduced MTs (Fig. 1C and Fig. S1C) such that the meiotic spindles were either absent or highly abnormal (Fig. 1D). Western blotting with antibodies specific for α-tubulin, β1-tubulin, and β2-tubulin (29) showed that the levels of all three tubulins were reduced in mgrl4/mgr5 mutant testes (Fig. 1E), thus accounting for the spindle defects.
Fig. 1.
The mgr mutant flies have microtubule-based abnormalities. (A) Representative mitotic spindles from squashed preparations of wild-type (Oregon R) and mgr (mgr5/mgrl4) mutant third-instar larval brains stained to reveal microtubules (β-tubulin, green), centrosome (Spd-2, red), and DNA (Dapi, blue). (Right column, Left and Right) Images of mgr neuroblasts show examples of monopolar spindles; (Center) a bipolar spindle of abnormal morphology. (Scale bar, 5 μm.) (B) Table indicating the mitotic defects observed in wild-type, mgr5/mgrl4 (compared with wild-type, mitotic defects P = 0.001; monopolar spindles P = 0.021), and mgrl4/Df (compared with wild-type P < 0.0001 for both mitotic defects and monopolar spindles); P values from χ2 analysis. Mitotic defects comprised monopolar and disorganized spindles (at least five independent brains scored). (C) Testes from wild-type and mgr (mgr5/mgrl4) mutant flies stained to reveal microtubules (β-tubulin, green) and DNA (Dapi, blue). (Scale bar, 10 μm.) (Upper) Young cysts (spermatogonia) in the apical region: arrowheads indicate mitotic cysts, shown in the Inset at 3× magnification; (Lower) Late primary spermatocytes with impaired microtubule network particularly in meiosis (compare outlined cysts). (D) Meiosis I spindles from wild-type and mgr stained to reveal microtubules (β-tubulin, green), centrosomes (Spd-2, red), and DNA (Dapi, blue). (Scale bar, 10 μm.) Of the meiotic spindles observed in mgr mutant testes, 100% were abnormal compared with wild-type where no abnormalities were observed (at least five testes scored and >208 complete cysts observed, P value from Student t test < 0.0001). (E) Western blots of the ubiquitous α- and β1-tubulin and the testes specific β2-tubulin isoform in testes protein extracts from wild-type and mgr mutant flies, showing that all three tubulin levels are reduced in mgr mutants. Amido black staining is the loading control (Ctrl). (F) Western blot of β-tubulin and Mgr in wild-type and mgr CNS and testes protein extracts, showing the absence of Mgr and the differential depletion of tubulin according to the tissue. Amido black staining is the loading control (Ctrl).
To understand why reduction in levels of the mgr gene product might have such an effect on tubulin levels, we set out to identify the mgr gene product. Small deficiencies [Df(3R)thoR1, Df(3R)pros235, and Df(3R)pros640] (30), which each fail to complement mgr, placed the mgr gene to 86E4 in a region containing 14 predicted genes (Fig. S2 A and B). Using a combination of RNAi to identify mitotic phenotypes resulting from the knockdown of each of these genes (Fig. S2 C–F) together with the sequencing of candidate genes from chromosomes carrying mgr mutant alleles, we identified the predicted gene CG6719 as mgr. We confirmed its identity by showing that lethality of mgrl4/mgr1 mutant, a combination showing pharate lethality, could be rescued with a CG6719 transgene. In addition to the mitotic phenotype, RNAi-mediated knockdown of mgr led to a reduction of cytoplasmic MTs in interphase cells (Fig. S2G).
Sequencing of the mgr mutant alleles revealed them to represent a variety of deletion, frame-shift, and nonsense mutations (Fig. S2H and SI Materials and Methods). In this study we focused on two mutant alleles: mgrl4, a null mutant having a deletion in the 5′UTR; and mgr5, which because of a nonsense mutation, produces a truncated protein (not detectable by Western blot) and additionally carries a 41-bp deletion in its 3′UTR. Immunostaining on wild-type cells using the anti-Mgr antibody we generated showed that the protein localizes throughout the cytoplasm in testis, brain, and cultured cells at all cell-cycle stages (Fig. S3). Western blots on protein extracts of testes and larval CNS confirmed that the protein was not detectable in mgrl4/mgr5 mutants (Fig. 1F). The sequence of mgr revealed it to encode a subunit of the Gim/prefoldin complex; Mgr shows 37% amino acid identity with its yeast homolog PAC10 and 57% with its human homolog VBP1/Pfdn3/Gim2. Thus, the phenotypes of mgr mutants would be consistent with the improper folding and degradation of tubulin in the absence of the Prefoldin complex. The greater reductions in tubulin levels in the testes than in the CNS would account for the stronger phenotype in the spermatocytes (Fig. 1F).
Spindle Abnormalities Result from Tubulin Destabilization Following Mgr Depletion.
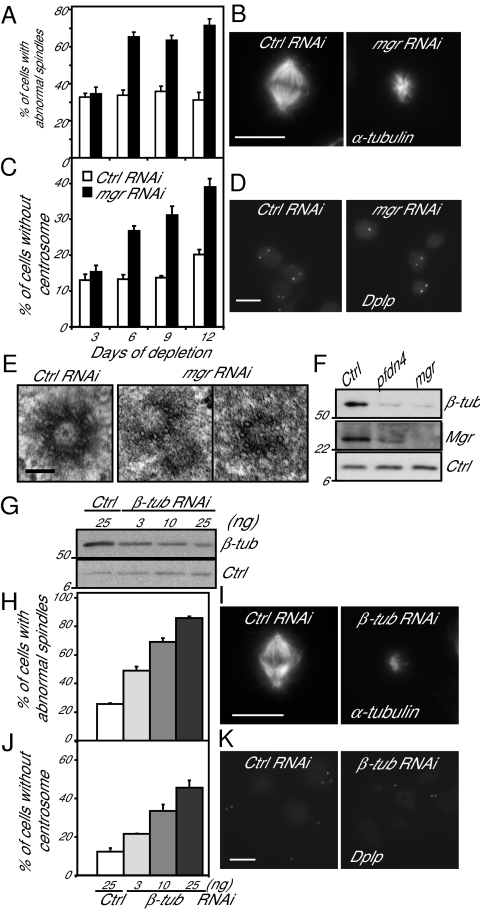
To gain further insight into how Mgr depletion affects spindle formation, we turned to RNAi in the DMEL-2 Drosophila cell line (Fig. 2). Transfection with mgr dsRNA for 6 d increased the proportion of cells with monopolar spindles similar in appearance to those seen in mgr mutant neuroblasts and greatly above the background level of mitotic abnormalities typical of this cell line (Fig. 2 A and B). Further cycles of mgr RNAi did not lead to any significant further increase in spindle abnormalities such that after 12 d, some 60% of mitotic cells had monopolar or disorganized spindles. Codepletion of BubR1 or Mad2 and Mgr led to a decrease of abnormal spindles and a restoration of a normal mitotic index compared with mgr RNAi alone (Fig. S4). These results suggest that cells are delayed in mitotic progression after Mgr depletion in response to the spindle assembly check point. To determine whether spindle abnormalities were related to centrosome defects, we counted Dplp+ punctae in interphase cells after the same period of Mgr depletion. This process revealed a progressive loss of centrosomes from cells lagging behind the appearance of spindle defects such that 40% of cells had no centrosomes by day 12 (Fig. 2 C and D). This lag in the loss of centrosomes could be because of either or both defects in centrosome duplication or segregation as a consequence of the spindle defects. To address whether Mgr depletion affected centriole structure, we carried out electron microscopy, which revealed abnormalities typical of centriole duplication/formation defects; 20% of the centrioles were incomplete and composed of singlet MTs rather than doublets or triplets, as in control cells (Fig. 2E).
Fig. 2.
Mgr or partial β-tubulin depletion result in similar microtubule-based abnormalities. DMEL-2 cells transfected with control (Ctrl) or mgr dsRNA for 3-d intervals up to a maximum of 12 d (A–F). (A) Percentage of prometaphase and metaphase cells with monopolar or disorganized spindles scored following immunostaining, as in B. Error bars = SEMs for more than three independent experiments; n > 150 metaphase cells. (B) Cells immunostained to reveal microtubules (α-tubulin) 6 d after transfection. (Scale bar, 10 μm.) (C) Percentage of cells without centrosomes scored after immunostaining, as in D. Error bars = SEMs of more than five independent experiments; n > 1,000 cells. (D) Cells immunostained to reveal centrosomes (Dplp) 6 d after transfection. (Scale bar, 10 μm.) (E) Electron micrographs of centrioles in control cells (Ctrl RNAi) and following 9–12 d of mgr RNAi. Twenty-percent of the centrioles showed an abnormal structure after Mgr depletion, whereas none were observed in the control depletion (n = 10). (Scale bar, 0.1 μm.) (F) Western blot of β-tubulin, Mgr and H2A (loading control, Ctrl) 6 d after transfection with mgr, pfdn4, or control dsRNAs. (G–K) DMEL-2 cells treated with a range of concentrations (3, 10, and 25 ng/mL) of β-tubulin dsRNA for 6 d. (G) Western blot of β-tubulin and H2A (loading control, Ctrl) following such treatment. (H) Proportion of prometaphase and metaphase cells with monopolar or disorganized spindles in relation to β-tubulin dsRNA treatment. Error bars = SEMs of more than two independent experiments; n > 100 metaphase cells. (I) Cells labeled with an anti-α-tubulin to reveal spindle microtubules in control and β-tubulin dsRNA treated cells. (Scale bar 10 μm.) (J) Percentage of cells without centrosomes in relation to β-tubulin dsRNA treament. Error bars = SEMs of more than two independent experiments; n > 200 cells. (K) Cells labeled to reveal centrosomes (Dplp) following control dsRNA and β-tubulin dsRNA treatment. (Scale bar, 10 μm.)
Immunoblotting revealed that Mgr depletion led to a reduction in β-tubulin levels by ∼70% (Fig. 2F), also apparent by immunofluorescence (Fig. S2G). Depletion of the Pfdn4 Prefoldin subunit also led not only to a similar reduction in β-tubulin, but also in Mgr levels, indicating the importance of interactions between subunits of the complex for its stability. Levels of γ-tubulin were reduced by ∼50%, whereas actin was unaffected (Fig. S5 A and B). To determine whether the spindle and centrosome defects resulting from Mgr depletion might reflect the reduction in tubulin levels, we performed partial depletion of β- and γ-tubulin (Fig. 2 G–K and Fig. S5 C–J). DMEL-2 cells were treated with varying amounts of β- or γ-tubulin dsRNA and assayed for spindle defects and centrosome numbers after 6 d. An increase in monopolar and disorganized spindles and in centrosome loss mirrored the extent of β- and γ-tubulin knockdown. However, only the β-tubulin knockdown recapitulated the small spindle size observed after mgr RNAi (Fig. S5D). Moreover, γ-tubulin knockdown leads to a population of bipolar spindles with the centrosomes at one pole only (Fig. S5E), which was not observed after mgr RNAi or partial depletion of β-tubulin. Thus, the mitotic defects that follow loss of Mgr appear to be largely a consequence of tubulin destabilization. To assess if the structural defects observed on the centrioles after mgr RNAi was a consequence of αβ- or γ-tubulin depletion, we carried out electron microscopy in cells partially depleted for 6 d for β- or γ-tubulin. These analyses failed to reveal any centriolar structural defects in either case (Fig. S5 I and J). However, the difficulties in assessing the level and timing of β- or γ-tubulin depletion that would be equivalent to that seen following mgr RNAi, together with the restricted numbers of centrioles that can be observed by electron microscopy in such experiments, makes it difficult to reach a firm conclusion about the effects on centrioles.
Levels of Free αβ-Tubulin Sensitize Mgr Activity.
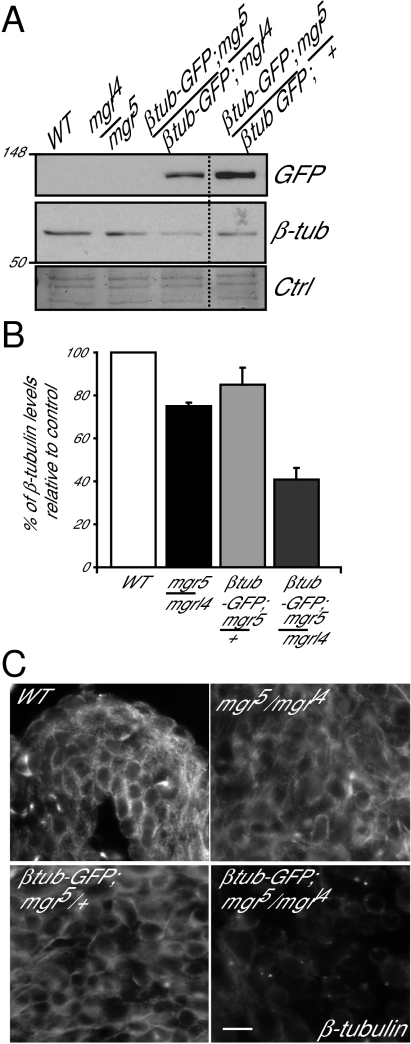
The above studies showed that MTs of different tissues present different sensitivity to reduced Mgr levels (Fig. 1). Moreover, even in mgr testes, where disruption of the MT network is most pronounced, it only occurs once primary spermatocytes have reached a late stage in their development. This result is despite the fact that Mgr protein is present throughout the wild-type testes and so ought, in principle, to be equally functional at all stages. This finding suggests that the onset of synthesis of the β2-tubulin testes specific isoform in late spermatogenesis might bring a particular requirement for Mgr (31, 32). If this were the case, we wondered whether increased synthesis of β1-tubulin from a transgene might trigger a stronger requirement for Mgr in larval neuroblasts (Fig. 3). We found that in mgrl4/mgr5 mutant neuroblasts the level of endogenous β-tubulin was only slightly reduced (by 25% compared with wild-type levels). The additional expression of β1-tubulin–GFP did not drastically affect endogenous β-tubulin levels if one wild-type copy of mgr was present (mgr5/+;β1-tubulin–GFP, a reduction of 15% compared with wild-type). However, in mgrl4/mgr5 mutants, expression of β1-tubulin–GFP leads to a further decrease in levels of endogenous β-tubulin by 60% compared with wild-type levels (Fig. 3 A and B). There was also a dramatic loss of MT staining in immunostained preparations of the larval CNS of such organisms (Fig. 3C). Taken together, these results suggest that if tubulin levels exceed a certain threshold, this overproduced tubulin may change the complex balance of folding, causing a synthetically lethal phenotype with mgr.
Fig. 3.
Mgr is a sensor of a free pool of tubulin. (A) Western blot of GFP or endogenous β-tubulin in extracts of testes of wild-type or mgrl4/mgr5 flies in presence or absence of an exogenous GFP-tagged β1-tubulin transgene. Note the β1-tubulin-GFP is not detected by the β-tubulin antibody due to low levels of expression. Amido black staining is loading control (Ctrl). (B) Quantitation of the endogenous β-tubulin levels relative to the wild-type control on Western blots. Error bars = SEMs for two independent experiments. (C) Wild-type field images of brain squashes from indicated genotypes stained to reveal microtubules (β-tubulin). (Scale bar, 5 μm.)
Mgr and Vhl Cooperate to Control the Degradation of αβ-Tubulins.
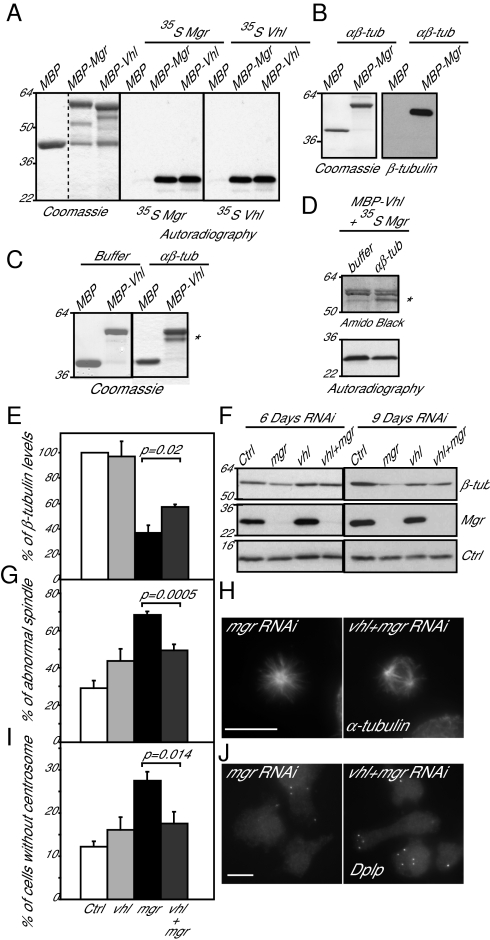
Mgr's human counterpart, which shares 57% amino acid identity, can interact with human Vhl, an E3 ubiquitin-protein ligase also able to bind the MT lattice (17). Several lines of evidence indicated that Mgr was able to interact with Drosophila Vhl and that both proteins could interact with tubulin monomers and dimers. First, beads carrying Maltose binding protein (MBP)-Mgr or MBP-Vhl fusion proteins synthesized in bacteria could bind 35S-labeled Mgr or Vhl synthesized by coupled transcription-translation in vitro (Fig. 4A). This experiment also indicated that both proteins could undertake self-self interactions. Second, both Mgr and Vhl synthesized in bacteria were able to bind 35S-labeled β1-tubulin synthesized by coupled transcription-translation in vitro (Fig. S6 A and B). Conversely β1-tubulin synthesized in bacteria would interact with 35S-labeled Vhl or Mgr (Fig. S6 C and D). Third, MBP-tagged Mgr or MBP-tagged Vhl immobilized on beads directly bound αβ-tubulin dimers (Fig. 4 B and C). Fourth, we were unable to disrupt a complex formed between bacterially expressed MBP-Vhl and 35S-labeled Mgr by adding dimeric αβ-tubulin (Fig. 4D). Instead the Vhl:Mgr complex also bound αβ-tubulin dimer. However, whereas Drosophila Vhl, like mammalian Vhl, could interact with MTs (Fig. S6 E and F), Mgr could not. This finding suggests that Mgr can interact with free, but not with polymerized, tubulin.
Fig. 4.
Mgr and Vhl cooperate in regulating tubulin destruction. (A) MBP, MBP-Mgr, and MBP-Vhl, affinity purified from Escherichia coli extracts (Coomassie stain) tested for binding 35S-Methionine labeled Mgr and Vhl synthesized by coupled transcription-translation in vitro (Autoradiography). (B) MBP and MBP-Mgr, affinity-purified from E. coli extracts (Coomassie stain) tested for binding purified αβ-tubulin (Western blot). (C) MBP and MBP-Vhl, affinity-purified from E. coli extracts (Coomassie stain, Right) tested for binding-purified αβ-tubulin (Coomassie stain, Left). (D) MBP-Vhl, affinity purified from E. coli extracts, and tested for binding 35S-Mgr (as in A). Excess of purified αβ-tubulin is insufficient to release the Vhl:Mgr interaction. (E–J) DMEL-2 cells treated with Control, mgr, Vhl, or mgr and Vhl dsRNA for 6 or 9 d. (E) Levels of β-tubulin in three independent experiments 9 d after transfection. (F) Western blot of β-tubulin and Mgr after such treatment. H2A is used as loading control (Ctrl). (G) Percentage of prometaphase and metaphase cells with monopolar or disorganized spindles after indicated dsRNA treatment. Error bars = SEMs of three independent experiments. n > 300 metaphase cells; (H) Mitotic cells immunostained to reveal microtubules (α-tubulin). (Scale bar, 10 μm.) (I) Percentage of cells without centrosome 9 d after indicated transfections. Error bars = SEM of three independent experiments. n > 600 cells. (J) Cells immunostained to reveal centrosomes (Dplp). (Scale bar, 10 μm.) All P values are from Student t tests.
To test whether the interaction between Vhl and Mgr played any part in the control of tubulin degradation, we asked what the consequences would be if Mgr and Vhl were to be codepleted. Vhl RNAi alone had little effect on the levels of β-tubulin in cultured cells (Fig. 4 E and F), confirming the recent finding of Duchi and colleagues (19). Moreover, Vhl depletion resulted in only a slight increase in the proportion of mitotic cells with monopolar or disorganized spindles or lacking centrosomes (Fig. 4 G–J). In contrast, Mgr depletion resulted in a clear decrease in β-tubulin levels, formation of monopolar/disorganized spindles in the majority of mitotic cells, and an increase of the number of cells without centrosomes. We found all three of these phenotypes were significantly rescued by the codepletion of Vhl (Fig. 4 E and J). Thus, the proteolytic destruction of tubulin, which takes place when tubulin cannot be correctly folded by the Prefoldin chaperone, requires the function of the Vhl protein.
Discussion
The finding that the Drosophila merry-go-round gene encodes a subunit of the Prefoldin complex has allowed us to account for aberrant structure and function of spindles and centrosomes in cells depleted of its gene product. The inability to correctly fold tubulins in Prefoldin-deficient cells leads to tubulin instability and, hence, defects that can be phenocopied by depleting β- or γ-tubulin. However, whereas β-tubulin depletion phenocopied all of the defects observed, γ-tubulin depletion only recapitulated some of them. The more dramatic phenotypes seen in Mgr-deficient cells expressing high levels of tubulin (primary spermatocytes and neuroblasts expressing a β-tubulin transgene) suggest that the Prefoldin complex is critical to maintain tubulin levels above a certain threshold of tubulin expression. This finding could be a consequence of the impact of an excess of tubulin upon its complex folding pathway. Interestingly, in mammalian cells, increased soluble tubulin, in response to a MT-destabilizing agent, leads to the rapid degradation of tubulin (33, 34). In Drosophila, tubulins in the testes are the most affected by the absence of Mgr compared with other tissues. Indeed, it may be of particular importance to regulate tubulin levels at the late stages of spermatogenesis, where the very large meiotic cells are provided with proportionally large amounts of tubulin that are used in the meiotic spindle but have a major additional purpose: the building of the sperm tail. Similarly, in the mouse, the effects of depletion or mutation of prefoldin subunits are largely restricted to the brain, where tubulin levels are also very high (26, 27). Whether this tissue specificity is a consequence of tubulin levels will be an interesting question to address. Finally, our demonstration that Vhl is required for tubulin destruction in the absence of Mgr and the ability of Vhl to interact with tubulin monomers and dimers raises the possibility that its role as an E3 ubiquitin-protein ligase could come to play in regulating tubulin levels.
The idea that Vhl and Prefoldin can cooperate in regulating protein stability was also raised by Mousnier et al. (35), who identified the prefoldin subunit VBP1 as a binding partner of the HIV-1 viral integrase and suggested this mediated the interaction of the integrase with the Cul2-Vhl E3-Ubiquitin ligase. This finding led these authors to suggest a role for prefoldin at a pivotal part of the pathway that would determine whether a protein was passed on to the CCT chaperonin for folding or to the proteasome for degradation (35). Similarly, we can speculate that prefoldin as a partner of Vhl may well serve a key role in regulating the equilibrium between tubulin targeted for destruction or for folding and incorporation into MTs. The concentration of assembly-competent tubulin must be tightly controlled because it affects cytoskeletal dynamics. Vhl might contribute to this influence by an effect on MT dynamics through interaction with MAPs on the MT lattice (17, 18) and by intervening in the regulation of tubulin folding. There is growing evidence for a critical function of Vhl in stabilizing cytoplasmic MTs (16, 17) and axonemal MTs in response to levels of soluble tubulin (36). Reciprocally, MT stability can contribute to regulating levels of proteins that are targets of the Cul2-Vhl E3-Ubiquitin ligase, such as the HIF proteins, the levels of which fall when their mRNAs accumulate in cytoplasmic P-bodies for translational repression following MT disruption (37). It will be important in future to consider the roles played by the Prefoldin complex and Vhl to understand the interrelationships between the machinery regulating tubulin levels in relation to MT stability, both in normal and tumor cells.
Materials and Methods
Briefly, testes from pupae and CNS from third-instar larvae were dissected in PBS and fixed with methanol before proceeding to immunostaining. Cultured cells were pre-extracted before fixation with paraformaldehyde and immunostaining. Depletion of proteins in cultured cells was performed by transfection of dsRNA with transfast reagent. Protein extracts were obtained after homogeneization of cells, CNS or testes in lysis buffer. Protein interactions were tested in vitro using either recombinant commercially available tubulins (Cytoskeleton), proteins produced in bacteria, or proteins translated in reticulocyte lysate.
A more detailed description of gene constructs, cell culture, immunocytochemistry, dsRNA, microscopy, protein purification, Western blot analysis, in vitro binding assays, fly genetics, and primers list can be found in the SI Materials and Methods and Table S1.
Supplementary Material
Acknowledgments
We thank Renate Renkawitz-Pohl for anti-β1- and -β2-tubulin antibodies and Matthew Savoian for the β1-tubulin construct; the E7-β-tubulin antibody was developed by Michael Klymkowsky and obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by the University of Iowa. We thank the Cancer Research Campaign and more recently Cancer Research United Kingdom for supporting this work.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. Y.Z. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1108537109/-/DCSupplemental.
References
- 1.Lundin VF, Leroux MR, Stirling PC. Quality control of cytoskeletal proteins and human disease. Trends Biochem Sci. 2010;35:288–297. doi: 10.1016/j.tibs.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Siegert R, Leroux MR, Scheufler C, Hartl FU, Moarefi I. Structure of the molecular chaperone prefoldin: Unique interaction of multiple coiled coil tentacles with unfolded proteins. Cell. 2000;103:621–632. doi: 10.1016/s0092-8674(00)00165-3. [DOI] [PubMed] [Google Scholar]
- 3.Vainberg IE, et al. Prefoldin, a chaperone that delivers unfolded proteins to cytosolic chaperonin. Cell. 1998;93:863–873. doi: 10.1016/s0092-8674(00)81446-4. [DOI] [PubMed] [Google Scholar]
- 4.Siegers K, et al. Compartmentation of protein folding in vivo: Sequestration of non-native polypeptide by the chaperonin-GimC system. EMBO J. 1999;18:75–84. doi: 10.1093/emboj/18.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansen WJ, Cowan NJ, Welch WJ. Prefoldin-nascent chain complexes in the folding of cytoskeletal proteins. J Cell Biol. 1999;145:265–277. doi: 10.1083/jcb.145.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsuchiya H, Iseda T, Hino O. Identification of a novel protein (VBP-1) binding to the von Hippel-Lindau (VHL) tumor suppressor gene product. Cancer Res. 1996;56:2881–2885. [PubMed] [Google Scholar]
- 7.Frew IJ, Krek W. pVHL: A multipurpose adaptor protein. Sci Signal. 2008;1:pe30. doi: 10.1126/scisignal.124pe30. [DOI] [PubMed] [Google Scholar]
- 8.Frew IJ, Krek W. Multitasking by pVHL in tumour suppression. Curr Opin Cell Biol. 2007;19:685–690. doi: 10.1016/j.ceb.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Maxwell PH, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 10.Lisztwan J, Imbert G, Wirbelauer C, Gstaiger M, Krek W. The von Hippel-Lindau tumor suppressor protein is a component of an E3 ubiquitin-protein ligase activity. Genes Dev. 1999;13:1822–1833. doi: 10.1101/gad.13.14.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen WJ, et al. Diverse effects of mutations in exon II of the von Hippel-Lindau (VHL) tumor suppressor gene on the interaction of pVHL with the cytosolic chaperonin and pVHL-dependent ubiquitin ligase activity. Mol Cell Biol. 2002;22:1947–1960. doi: 10.1128/MCB.22.6.1947-1960.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldman DE, Thulasiraman V, Ferreyra RG, Frydman J. Formation of the VHL-elongin BC tumor suppressor complex is mediated by the chaperonin TRiC. Mol Cell. 1999;4:1051–1061. doi: 10.1016/s1097-2765(00)80233-6. [DOI] [PubMed] [Google Scholar]
- 13.Melville MW, McClellan AJ, Meyer AS, Darveau A, Frydman J. The Hsp70 and TRiC/CCT chaperone systems cooperate in vivo to assemble the von Hippel-Lindau tumor suppressor complex. Mol Cell Biol. 2003;23:3141–3151. doi: 10.1128/MCB.23.9.3141-3151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feldman DE, Spiess C, Howard DE, Frydman J. Tumorigenic mutations in VHL disrupt folding in vivo by interfering with chaperonin binding. Mol Cell. 2003;12:1213–1224. doi: 10.1016/s1097-2765(03)00423-4. [DOI] [PubMed] [Google Scholar]
- 15.McClellan AJ, Scott MD, Frydman J. Folding and quality control of the VHL tumor suppressor proceed through distinct chaperone pathways. Cell. 2005;121:739–748. doi: 10.1016/j.cell.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 16.Thoma CR, et al. VHL loss causes spindle misorientation and chromosome instability. Nat Cell Biol. 2009;11:994–1001. doi: 10.1038/ncb1912. [DOI] [PubMed] [Google Scholar]
- 17.Hergovich A, Lisztwan J, Barry R, Ballschmieter P, Krek W. Regulation of microtubule stability by the von Hippel-Lindau tumour suppressor protein pVHL. Nat Cell Biol. 2003;5:64–70. doi: 10.1038/ncb899. [DOI] [PubMed] [Google Scholar]
- 18.Thoma CR, et al. Quantitative image analysis identifies pVHL as a key regulator of microtubule dynamic instability. J Cell Biol. 2010;190:991–1003. doi: 10.1083/jcb.201006059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duchi S, et al. Drosophila VHL tumor-suppressor gene regulates epithelial morphogenesis by promoting microtubule and aPKC stability. Development. 2010;137:1493–1503. doi: 10.1242/dev.042804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schermer B, et al. The von Hippel-Lindau tumor suppressor protein controls ciliogenesis by orienting microtubule growth. J Cell Biol. 2006;175:547–554. doi: 10.1083/jcb.200605092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thoma CR, Frew IJ, Krek W. The VHL tumor suppressor: Riding tandem with GSK3beta in primary cilium maintenance. Cell Cycle. 2007;6:1809–1813. doi: 10.4161/cc.6.15.4518. [DOI] [PubMed] [Google Scholar]
- 22.Thoma CR, et al. pVHL and GSK3beta are components of a primary cilium-maintenance signalling network. Nat Cell Biol. 2007;9:588–595. doi: 10.1038/ncb1579. [DOI] [PubMed] [Google Scholar]
- 23.Geissler S, Siegers K, Schiebel E. A novel protein complex promoting formation of functional alpha- and gamma-tubulin. EMBO J. 1998;17:952–966. doi: 10.1093/emboj/17.4.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lundin VF, Srayko M, Hyman AA, Leroux MR. Efficient chaperone-mediated tubulin biogenesis is essential for cell division and cell migration in C. elegans. Dev Biol. 2008;313:320–334. doi: 10.1016/j.ydbio.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 25.Gu Y, et al. Prefoldin 6 is required for normal microtubule dynamics and organization in Arabidopsis. Proc Natl Acad Sci USA. 2008;105:18064–18069. doi: 10.1073/pnas.0808652105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao S, et al. Subunit 1 of the prefoldin chaperone complex is required for lymphocyte development and function. J Immunol. 2008;181:476–484. doi: 10.4049/jimmunol.181.1.476. [DOI] [PubMed] [Google Scholar]
- 27.Lee Y, et al. Prefoldin 5 is required for normal sensory and neuronal development in a murine model. J Biol Chem. 2011;286:726–736. doi: 10.1074/jbc.M110.177352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.González C, Casal J, Ripoll P. Functional monopolar spindles caused by mutation in mgr, a cell division gene of Drosophila melanogaster. J Cell Sci. 1988;89:39–47. doi: 10.1242/jcs.89.1.39. [DOI] [PubMed] [Google Scholar]
- 29.Kaltschmidt B, Glätzer KH, Michiels F, Leiss D, Renkawitz-Pohl R. During Drosophila spermatogenesis beta 1, beta 2 and beta 3 tubulin isotypes are cell-type specifically expressed but have the potential to coassemble into the axoneme of transgenic flies. Eur J Cell Biol. 1991;54:110–120. [PubMed] [Google Scholar]
- 30.Kusano K, Johnson-Schlitz DM, Engels WR. Sterility of Drosophila with mutations in the Bloom syndrome gene—Complementation by Ku70. Science. 2001;291:2600–2602. doi: 10.1126/science.291.5513.2600. [DOI] [PubMed] [Google Scholar]
- 31.Kemphues KJ, Raff RA, Kaufman TC, Raff EC. Mutation in a structural gene for a beta-tubulin specific to testis in Drosophila melanogaster. Proc Natl Acad Sci USA. 1979;76:3991–3995. doi: 10.1073/pnas.76.8.3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kemphues KJ, Kaufman TC, Raff RA, Raff EC. The testis-specific beta-tubulin subunit in Drosophila melanogaster has multiple functions in spermatogenesis. Cell. 1982;31:655–670. doi: 10.1016/0092-8674(82)90321-x. [DOI] [PubMed] [Google Scholar]
- 33.Caron JM, Jones AL, Kirschner MW. Autoregulation of tubulin synthesis in hepatocytes and fibroblasts. J Cell Biol. 1985;101:1763–1772. doi: 10.1083/jcb.101.5.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huff LM, Sackett DL, Poruchynsky MS, Fojo T. Microtubule-disrupting chemotherapeutics result in enhanced proteasome-mediated degradation and disappearance of tubulin in neural cells. Cancer Res. 2010;70:5870–5879. doi: 10.1158/0008-5472.CAN-09-4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mousnier A, et al. von Hippel Lindau binding protein 1-mediated degradation of integrase affects HIV-1 gene expression at a postintegration step. Proc Natl Acad Sci USA. 2007;104:13615–13620. doi: 10.1073/pnas.0705162104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma N, Kosan ZA, Stallworth JE, Berbari NF, Yoder BK. Soluble levels of cytosolic tubulin regulate ciliary length control. Mol Biol Cell. 2011;22:806–816. doi: 10.1091/mbc.E10-03-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carbonaro M, O'Brate A, Giannakakou P. Microtubule disruption targets HIF-1alpha mRNA to cytoplasmic P-bodies for translational repression. J Cell Biol. 2011;192:83–99. doi: 10.1083/jcb.201004145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.