Abstract
Enabling long-range transport of molecules, tubules are critical for human body homeostasis. One fundamental question in tubule formation is how individual cells coordinate their positioning over long spatial scales, which can be as long as the sizes of tubular organs. Recent studies indicate that type I collagen (COL) is important in the development of epithelial tubules. Nevertheless, how cell–COL interactions contribute to the initiation or the maintenance of long-scale tubular patterns is unclear. Using a two-step process to quantitatively control cell–COL interaction, we show that epithelial cells developed various patterns in response to fine-tuned percentages of COL in ECM. In contrast with conventional thoughts, these patterns were initiated and maintained by traction forces created by cells but not diffusive factors secreted by cells. In particular, COL-dependent transmission of force in the ECM led to long-scale (up to 600 μm) interactions between cells. A mechanical feedback effect was encountered when cells used forces to modify cell positioning and COL distribution and orientations. Such feedback led to a bistability in the formation of linear, tubule-like patterns. Using micro-patterning technique, we further show that the stability of tubule-like patterns depended on the lengths of tubules. Our results suggest a mechanical mechanism that cells can use to initiate and maintain long-scale tubular patterns.
Keywords: tubulogenesis, biomechanics, morphogenesis
To enable long-range bulk transport of liquid and gas, tubules are the most commonly used form of tissue architecture in our bodies. Examples where tubules are used include lung, mammary gland, blood vessels, salivary gland, and kidney (1–4). Tubule formation requires aligning the positions of individual cells (of size approximately 10–20 microns) over long spatial scales (from hundreds of microns to centimeters, i.e., the size of organs). Although current studies focus on how genetics and morphogens control tubule formation (5–10), ECM molecules are also known to be important in the patterning of tubular structures (11–15). In particular, normal epithelia are surrounded by two ECM components: basement membrane (BM) and type I collagen (COL) (16, 17), with COL fibers frequently found around epithelial tubules, e.g., the milk ducts in the mammary gland (18). Accordingly, adding collagenase or stimulating COL expression in the ECM perturbs epithelial tubular growth (13, 19, 20). Nevertheless, how cell–COL interactions contribute to the initiation or the maintenance of long-scale tubular patterns is unclear.
Here, we quantitatively study how cells change their morphology in response to the presence of COL in the ECM and how such morphogenetic changes lead to the formation of long-scale tubular patterns. Previous studies showed that breast epithelial cells developed long-scale tubules (length≥400 μm) in COL gels (21, 22), whereas they formed globular acini (diameter approximately 100 µm) in BM gels (23, 24). We thus developed a two-step process to control cell–COL interactions. COL was introduced into the ECM during the development of acini. By doing so, we find that epithelial cells could develop long-scale (up to 600 μm) mechanical interactions in the presence of COL, leading to linear, tubule-like pattern formation. We then used a micro-patterning technique to quantify how cells formed and stabilized the long-scale linear, tubule-like patterns.
Results
Cells Developed Direct Branching in Response to COL.
To quantify the response of epithelial acini to the presence of COL in the ECM, we used a two-step approach adopted from the “on-top” assay (25). The human mammary gland (MCF-10A) cells (24) were seeded on BM gels [i.e., the first layer of ECM (ECM1)] to form globular acini, as described in reference (25). A layer of ECM (ECM2) containing BM and COL was then created on top of acini (Fig. 1A), with rigidity compatible to that of ECM1 (170 ± 40 Pa, SI Text).
Fig. 1.
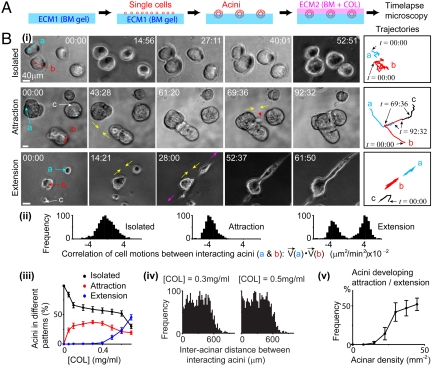
Cells developed direct branching in response to COL. (A) Experimental setup. (B) (i) Represented acinar morphogenesis at [COL] (Top) approximately 0; (Middle) 0.3 mg/mL; and (Bottom) 0.5 mg/mL. ECM2 gelled at t = 00:00. The represented single-cell motions in acini (“a,” “b,” and “c”) are displayed on the right. Directions of acinar branching are indicated by yellow and pink arrows. Red arrowhead indicates branching cells. Time is in hours and minutes. Scale bar: 40 μm. Initial cell density: 30 ± 5 mm-2. (ii) Correlations of cell motions in i. Time windows to compute correlations: t = 20:00 to 50:00 in Top, t = 44:00 to 69:00 in Middle, and t = 15:00 to 61:00 in Bottom. All cells in acini “a” and “b” were paired to compute correlations (N > 200). Data were normalized to the maximum. (iii) The distributions (mean ± SEM) of acini (N = 200) at different patterns (after 24 h of ECM2 gelification) depended on [COL]. Initial cell density: 30 ± 5 mm-2. (iv) Histograms of the initial distances between interacting acini (N = 100) show long-scale interactions. Data were normalized to the maximum. (v) The percentage (mean ± SEM) of acini (N = 200) developing attraction or extension (after 24 h of ECM2 overlay) depended on initial acinus density. [COL] = 0.5 mg/mL.
Acini were found to develop three morphogenetic patterns in the change of COL concentrations in ECM2 ([COL]; Fig. 1B, i–iii; see SI Text for the definitions of patterns). At low [COL] (< 0.1 mg/mL), most acini (> 99%) formed isolated structures (Fig. 1 B, i, Top and iii and Movie S1). At intermediate [COL] (approximately 0.3 mg/mL), acini (approximately 40%) formed pairwise attraction by branching cells to reach each other [Fig. 1 B, i, Middle (red arrowhead) and iii and Movie S2]. Such attraction was found mostly between two acini at one time (> 99%). At high [COL] (approximately 0.5 mg/mL), a single acinus (approximately 25%) could interact with multiple acini at one time. In particular, the interacting acini formed extension by branching cells to two opposite directions [Fig. 1 B, i, Bottom (yellow and pink arrows) and iii and Movie S3]. Such extension led to the formation of long-scale, linear, and tubule-like patterns (average length: 0.5–1 mm, Fig. S1) that morphologically resembled those observed in tubular organs in situ (5).
Cell motions in these three patterns were different. When acini formed isolated structures, no correlated cell motions were found between adjacent acini (Fig. 1 B, i,trajectories and ii, Left). When acini attracted each other, their cells moved collectively to each other (Fig. 1 B, i trajectories and ii, Center). When acini formed extension, their cells moved collectively in the same or the opposite directions (Fig. 1 B, i trajectories and ii, Right). For simplicity, we hereafter named COL-dependent, direct cell motions as “direct branching.”
Interestingly, we found that acini did not always develop direct branching with the nearest acinus. For example, acinus “c” in Fig. 1B, i, Middle did not attract the nearest acinus (the one right above it). Instead, acini could develop attraction with others that were 600 μm away (Fig. 1B, iv). Such spatial scale was longer than the persistence length of COL fibers (10–20 μm) (26, 27). We also found that direct branching did not require preformed acini but instead could occur at single cells (Fig. S2). Moreover, direct branching depended on the initial acinus/cell density in a nonlinear manner. Only systems with acinus/cell densities above a certain level exhibited direct branching (Fig. 1B, v and Fig. S2G). These data suggest that direct branching is a long-range (approximately 600 µm) and cell interaction-dependent (i.e., nonautonomous) process.
COL-Dependent Direct Branching Was Not Primarily Mediated by Persistent Focal Adhesion Kinase (FAK) Up-Regulation or Diffusible Biochemical Factors Secreted by Cells.
To understand how COL-dependent direct branching occurred, we examined three possibilities. First, exposing cells to COL might up-regulate integrin and FAK activities. Previous studies, however, showed that normal epithelial cells in soft BM or COL gels (rigidity approximately 170 Pa) exhibited low FAK activity, as indicated by the absence of sustained, localized FAKpY397 (22, 23). To examine whether COL-dependent direct branching involved FAK activation, we performed immuno-staining of FAKpY397. Cells were found to exhibit diffusive rather than localized FAKpY397 in the absence or presence of direct branching (Fig. S3A), suggesting that direct branching did not primarily involve persistent up-regulation of FAK activity.
Second, we examined whether direct branching was mediated by autocrine/paracrine-based mechanisms. If so, diffusive factors secreted by cells were expected to pass through media or ECM to influence cells at distant locations. However, establishing a liquid- or solid-phase connection (i.e., through media or ECM) between branched acini/cells and unbranched acini/cells did not induce direct branching at unbranched acini / cells (SI Text, Fig. S3 B–D), suggesting that direct branching was not primarily mediated by diffusible factors secreted by cells.
COL-Dependent Direct Branching Associated with COL Reorganization and Required Cell Contractility.
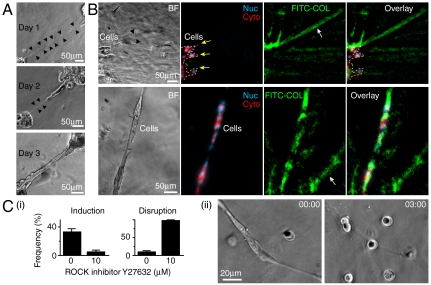
We next examined the third possibility: whether COL-dependent direct branching resulted from the inhomogeneity of COL in ECM2. COL fibers produce second harmonic generation (SHG), an optical signal that can be detected by ultrafast two-photon microscopy (28, 29). Because ECM2 was created by the self-assembly of BM and COL molecules, we expected a random distribution of initial orientations of COL fibers in ECM2. This was confirmed by SHG imaging (Fig. S4). After acini were overlaid by ECM2 for 1 d, however, local deformation of ECM was found between interacting acini [Fig. 2A, Top (black arrowheads)]. Such deformation correlated with the orientation of direct branching (Fig. 2 A, Middle and Bottom).
Fig. 2.
COL-dependent direct branching associated with COL reorganization and required cell contractility. (A) Linear ECM deformation (outlined by black arrows in day 1 and 2 after ECM2 overlay) arose between interacting acini. (B) Collagen alignment and condensation (white arrow) occurred around and away from branched cells (after 36 h of ECM2 overlay). Yellow arrows and white lines indicate cell boundaries. Nucleus (Nuc): H2B-CFP. Cytoplasm (Cyto): mCherry. Collagen: FITC-conjugated type I collagen (FITC-COL, 5% in total COL, total [COL] = 0.5 mg/mL). BF, bright field. (C) (i) Inhibiting ROCK (Left) prevented the initiation and (Right) caused the disruption of direct branching. Data are mean ± SEM (N = 50 for each case). (ii) Represented image of branch disruption after ROCK inhibition [by Y27632 (20 μM) at t = 00:00].
To see if COL was reorganized with ECM deformation, a small amount (∼5% of total COL) of FITC-conjugated COL (FITC-COL) was introduced into ECM2. Following the formation of direct branching, FITC-COL condensation and realignment was found around and away from branched cells (Fig. 2B). Interestingly, the spatial scale of COL condensation and realignment was much longer (> 400 μm) (Fig. 2B) than the persistent length of COL fibers. Such spatial scale was compatible with the lengths of the linear tubule-like patterns (Fig. S1) and the range within which acini attracted each other (Fig. 1B, iv). Moreover, COL condensation and realignment was not found in cell-free systems or in systems treated with Rho-associated kinase (ROCK) inhibitor (Y27632, 10 μM), suggesting that COL condensation and realignment required cell contractility.
To see if cell contractility was also required for direct branching, cells were treated with ROCK inhibitor before or after the formation of direct branching. Fig. 2C shows that inhibiting ROCK prevented the formation of direct branching and caused the disruption of branches. Similar results were found by treating cells with myosin inhibitor (blebbistatin, 10 μM). Thus, both the induction and the maintenance of COL-dependent direct branching required cell contractility.
COL Mediated a Nondispersed Transmission of Traction Force in Direct Branching.
The major event in direct branching was cell motion (Fig. 1B, i), which created traction force at the ECM. The requirement of cell contractility in COL condensation and realignment suggests that such process was likely to be mediated by traction force. To see if cell motion and COL reorganization/ECM deformation mutually affected each other, we developed a geometrical assay to compute their correlations. In direct branching, we noted that cells primarily moved along the line between two interacting acini (Fig. 1
B, i trajectories and ii), with the initial width of branches compatible to the size of single cells (10–20 μm). We thus defined a central region (height = 20 μm) that enclosed the edges of two interacting acini [Fig. 3A (cyan lines)] and two peripheral regions (height = 20 μm) above and below the central region [Fig. 3A (area between cyan and pink lines)]. The motions of cells at the boundaries of the central region,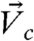 , and the motions of beads embedded in ECM2 in the central region and the peripheral regions,
, and the motions of beads embedded in ECM2 in the central region and the peripheral regions, 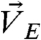 and
and 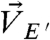 , were then used to compute the correlations (Fig. 3A, Left and SI Text).
, were then used to compute the correlations (Fig. 3A, Left and SI Text).
Fig. 3.
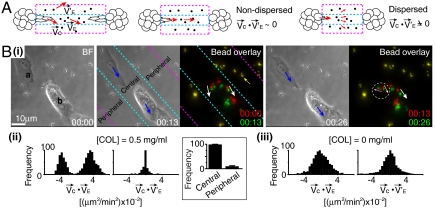
COL mediated a nondispersed transmission of traction force in direct branching. (A) (Left) Schematics to show motions (red arrows) of cells (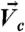 ) and beads [
) and beads [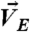 at the central region (between cyan lines) and
at the central region (between cyan lines) and 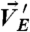 at the peripheral regions (between cyan and pink lines)]. Center and Right ECM deformation induced by cell motions was (Center) nondispersed or (Right) dispersed. (B) (i) Represented images of cell and bead motions (Δt: 13 min). Blue arrows indicate the motions of cells (“a,” “b”). Bead overlay: overlay of fluorescent images of beads at the earlier time (red) and the later time (green) to show bead displacements (white, pink, and yellow arrows). Beads in yellow indicate no movements of beads. Time is in hours and minutes. BF, bright field. (ii) and (iii) Histograms of the correlations between cell motions and bead movements in (Left) the central and (Right) the peripheral regions show distinct behaviors for [COL] = (ii) 0.5 mg/mL and (iii) 0.N = 10 × 10 × 10 (cells × beads × events). Data are normalized to the maximum in each case. Inset in (ii): Frequency (mean ± SEM) of bead motions (≥0.1 μm/ min, see SI Text) at central regions in 50 events of cell motions (≥0.1 μm/ min). [COL] = 0.5 mg/mL.
at the peripheral regions (between cyan and pink lines)]. Center and Right ECM deformation induced by cell motions was (Center) nondispersed or (Right) dispersed. (B) (i) Represented images of cell and bead motions (Δt: 13 min). Blue arrows indicate the motions of cells (“a,” “b”). Bead overlay: overlay of fluorescent images of beads at the earlier time (red) and the later time (green) to show bead displacements (white, pink, and yellow arrows). Beads in yellow indicate no movements of beads. Time is in hours and minutes. BF, bright field. (ii) and (iii) Histograms of the correlations between cell motions and bead movements in (Left) the central and (Right) the peripheral regions show distinct behaviors for [COL] = (ii) 0.5 mg/mL and (iii) 0.N = 10 × 10 × 10 (cells × beads × events). Data are normalized to the maximum in each case. Inset in (ii): Frequency (mean ± SEM) of bead motions (≥0.1 μm/ min, see SI Text) at central regions in 50 events of cell motions (≥0.1 μm/ min). [COL] = 0.5 mg/mL.
With COL in ECM2, the motions of beads were found primarily confined in the central region (Fig. 3B, ii, Inset). In particular, beads in the central region moved in the same [Fig. 3B, i (white arrows in bead overlay)] or opposite [Fig. 3B, i (pink arrow in bead overlay)] directions of cell motions [Fig. 3 B, i (blue arrows) and ii]. In addition, we often observed simultaneous, bidirectional movements of beads in small areas (< 20 μm2) of the central region [Fig. 3B, i (white and pink arrows in the white circle)]. By contrast, the motions of beads in the peripheral regions were limited [Fig. 3B, i (bead overlay, yellow arrow)] and uncorrelated with cell motions (Fig. 3B, ii).
To confirm that the patterns of bead motions reflected the displacement of COL fibers, fluorescent beads directly labeled on COL fibers through anti-COL antibody were used (see SI Text). The results revealed similar correlations between cell motions and bead movements (Fig. S5B). On the other hand, the motions of beads were found neither confined in the central region nor correlated with cell motions in the absence of COL in ECM2 (Fig. 3B, iii). The absence of correlations was also found in heat-denatured or chemically cross-linked COL gels. These data suggest that native COL fibers can mediate a nondispersed transmission (i.e., confined in the central region) of traction forces between interacting acini/cells.
Long-Scale Linear Pattern Formation Required Cell Repositioning.
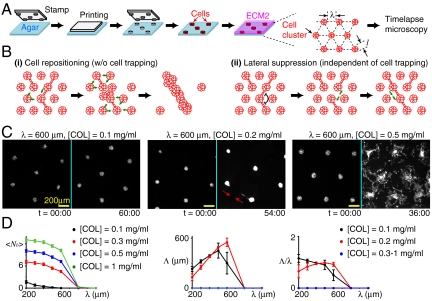
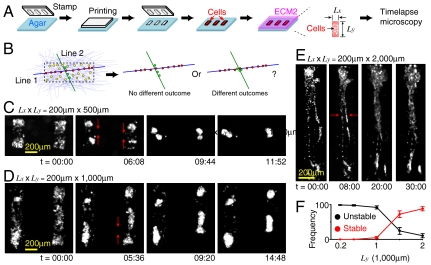
The observations above motivated us to consider a mechanical feedback loop: cells motions condensed and aligned COL fibers, which in turn affected cell motions and repositioned cells. The aligned COL fibers and repositioned cells then formed the long-scale linear patterns. To see if cell repositioning was required for the formation of long-scale (> 1 mm) linear patterns, we used a micro-patterning technique to limit cell repositioning. Circular traps were created in agarose gels to partition cells into equally separated clusters, followed by the overlay of ECM2 (Fig. 4A). We then tracked the development of long-scale linear patterns by measuring the maximal length (Λ) of linear structures, which were defined if all clusters in the structures developed branches in the same orientation.
Fig. 4.
The formation of long-scale linear patterns requires cell repositioning. (A) Experimental setup. Circular cell traps were created by PDMS stamps with diameter l and separation λ. (B) Potential models to form long-scale linear patterns (see text for details). Long-scale linear structure was defined if the number of branches (Nb) at each cluster in the structure is ≤ 2. (C) Represented images of various patterns. (Left) No branching (defined if Nb = 0). (Center) Direct branching (red arrows, defined if 0 < Nb ≤ 6). (Right) Random branching (defined if Nb > 6). Time is in hours and minutes. Scale bar: 200 μm. Fluorescence signal was from mCherry expressed in the whole cell. To enhance visualization, background scattering from the argarose gels was removed. (D) (Left) The average number of branches per trap 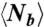 depends on λ and [COL]. (Center) and (Right) The maximal length of linear structures, Λ, and its normalization with λ show that cells could not form linear structures over three traps. Λ = 0 if there is no linear structure. For Center and Right, no significant difference was found in [COL] = 0.3–1 mg/mL. Data are represented in mean ± SEM.
depends on λ and [COL]. (Center) and (Right) The maximal length of linear structures, Λ, and its normalization with λ show that cells could not form linear structures over three traps. Λ = 0 if there is no linear structure. For Center and Right, no significant difference was found in [COL] = 0.3–1 mg/mL. Data are represented in mean ± SEM.
The micro-patterning technique allowed us to control the initial distance between clusters (λ) and [COL] in ECM2. If cell repositioning was required for the formation of long-scale linear patterns, we expected that cells failed to form long-scale linear patterns regardless of the initial conditions (Fig. 4B, i). Otherwise, we expected to see long-scale linear patterns under certain initial conditions. For example, cells might use mechanisms such as lateral suppression/inhibition to develop linear patterns, i.e., the formation of branching in one orientation suppresses branching in other orientations (Fig. 4B, ii), which does not require repositioning cells.
The experimental results supported the cell repositioning model. Specifically, we found that clusters could develop branching with each other but hardly move the entire clusters out of the trap within 36 h of ECM2 overlay. Within this period of time, three patterns were observed. These included “no-branching” (defined if the number of branches of individual clusters (Nb) = 0, Fig. 4C, Left), direct branching (Fig. 4C, Center), and “random-branching” patterns (Fig. 4C, Right; more examples can be found in Movies S4–S7). The no-branching pattern occurred when λ > 600 μm and the random-branching pattern appeared if λ ≤ 600 μm and [COL]≥0.5 mg/mL (Fig. 4D, Left). The absence of branching at λ > 600 μm was consistent with the observation that acini separated by a distance < 600 μm could interact with each other (Fig. 1B, v). No long-scale linear structures (defined as Λ≥1 mm) weres observed in all tested conditions [λ = 200–1,000 μm and [COL] = 0–1 mg/mL, (Fig. 4D, Center and Right)]. Thus, cell repositioning was required for long-scale linear pattern formation.
The Stability of Linear Patterns Depended on Their Lengths.
We next examined how the stability of linear patterns depended on their lengths. For a fixed cell density, we defined the lengths of linear patterns by the range of cells that were associated with COL fibers in a specific orientation. To determine how the length of linear patterns affected their stabilities, we created linear cell–COL structures of various lengths. Rectangular traps were generated in agarose gels, followed by a sparse seeding of cell in the traps (to allow for cell repositioning) and the overlay of COL-containing ECM2 (Fig. 5A). The spatial anisotropy of the trap allowed cells and COL fibers to form linear patterns of various lengths. For example, COL fibers and cells along the long axis of the trap [Fig. 5B (blue line and red circles)] could form longer structures than those along the short axis [Fig. 5B (green line and green circles)]. If the stability of linear patterns did not depend on their lengths, we expected no difference of outcomes between structures along the short and the long axes (Fig. 5B).
Fig. 5.
The stability of linear pattern depended on its length. (A). Experimental setup. Rectangular cell traps were created by PDMS stamps with various aspect ratios (Ly/Lx). (B) Potential outcomes in a rectangular trap covered by COL in random orientations (light purple lines). Initial cell density (yellow circles) is fixed (3–5 × 108/mL). The outcomes of two COL fibers (green and blue) and associated cells (green and red) are illustrated. (C–E) Represented images of (C) and (D) unstable and (E) stable linear structures. The instability was associated with the collapse (red arrows) of linear structures. Time is in hours and minutes. To enhance visualization, background scattering from the argarose gels was removed. (F) The stability of linear structures (mean ± SEM) increased with Ly (N = 20 and Lx = 200 μm for each case).
For simplicity, we fixed the horizontal width of the traps (Lx) at 200 μm and varied the vertical height (Ly′≥200 μm) of the traps. Stable linear patterns along the long axis were observed only if Ly > 1,000 μm. For Ly < 1,000 μm, cells predominately (> 99%) coalesced into globular aggregates (Fig. 5 C, D, and F and Fig. S6B). For Ly > 1,000 μm, cells formed linear structures along the long axis [over 24 h (Fig. 5 E and F and Fig. S6B)] with the stability slightly increasing with Ly. These data suggest that the formation of linear patterns was bistable and depended on the lengths of linear patterns.
COL-Dependent Direct Branching Required PI3K/Rac1 and Exhibited Evidence of Mechano-Transduction.
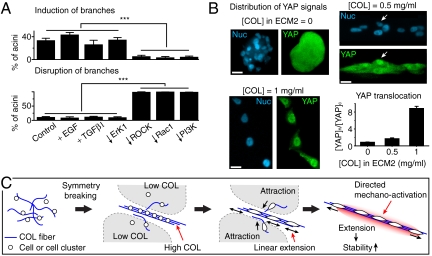
Previous studies showed that branching morphogenesis of tubular tissues can be modulated by inhibitory morphogens such as TGFβ1 (30) and stimulatory morphogens such as hepatic growth factor (HGF) and EGF. In particular, EGF up-regulates PI3K, Rac1, and ERK1 activities, which are known to be involved in cell motion and proliferation (31, 32). We showed that COL-dependent direct branching was not primarily mediated by diffusion of chemical factors in the media or ECM (Fig. S3 B–D). To further see if cells used local gradients of known morphogens such as TGFβ1 and EGF to control direct branching, we treated cells with a homogeneous stimulation of TGFβ1/EGF before or after the formation of direct branching (SI Text). If direct branching required local gradients of TGFβ1/EGF, treating cells with a homogeneous stimulation of these molecules could interfere with the initiation or the maintenance of direct branching.
We found that treating cells with TGFβ1 did not significantly suppress direct branching, whereas treating cells with EGF slightly enhanced direct branching (Fig. 6A). The enhancement by EGF was not mediated by the mitogenic effect through ERK1 but instead through PI3K/Rac1, because inhibiting ERK1 did not disable direct branching while inhibiting PI3K or Rac1 significantly suppressed the initiation and the maintenance of direct branching (Fig. 6A). Nevertheless, the ability of cells to form direct branching and linear patterns was not destroyed by the treatment of EGF.
Fig. 6.
COL-dependent direct branching required PI3K/Rac1 and exhibited evidence of mechano-transduction. (A) The percentage of acini developing branching after 24 h of ECM2 overlay ([COL] = 0.5 mg/mL) in the presence of various growth factors or inhibitors. (B) The percentage of branched acini that lose branches after 24 h treatment of various growth factors or inhibitors. In Aand B, EGF: 100 ng/mL, TGFβ1: 5 ng/mL, LY294002 (for PI3K): 20 μM; NSC553502 (for Rac1): 70 μM; and PD98095 (for ERK1): 10 μM. Control: serum-free and growth-factor-free. “***” indicates P < 0.001 in student’s t-test. (B) Represented images of YAP immuno-staining at (Upper, Left) [COL] = 0, (Upper, Right) 0.5 mg/mL, and (Lower, Left) 1.0 mg/mL. Nucleus (Nuc): H2B-CFP. Cells were exposed to ECM2 containing various [COL] for 24 h and fixed for YAP immuno-staining. Note the nuclear translocation of YAP (white arrows) in [COL] = 0.5 mg/mL. Scale bar: 20 μm. (Lower, Right) Ratios of average fluorescent intensities (mean ± SEM, subtracted by background) of YAP immuno-staining in the nucleus and the cytoplasm depended on [COL] (N = 50 for each case). (C) Proposed mechanical feedback loop for long-scale linear pattern formation. See text for details.
Despite the lack of evidence showing the involvement of diffusive factors in COL-dependent direct branching, we wondered if the output of traction force along aligned COL fibers could function as a “mechanical factor” to stimulate cells through a process known as mechano-transduction (33). Mechano-transduction involves many cell signaling events such as the translocation of Yes-associated protein (YAP) from cell cytoplasm to nucleus (34). We thus measured the ratios of immuno-stained YAP at cell nucleus and cytoplasm in the absence or presence of COL in ECM2. Nuclear translocation of YAP with a dose dependence on [COL] was observed (Fig. 6B). Such translocation required cell contractility because it was not observed when cells were treated with myosin inhibitor (blebbistatin, 10 μM). Nevertheless, YAP activation might not be crucial for COL-dependent direct branching, because we found that both YAP-overexpressing MCF-10A cells and YAP-KO MCF-10A cells exhibited similar morphogenetic response to COL as normal MCF-10A cells.
Discussion
To make decisions, biological systems often use genetic circuits or chemical signaling to form feedback loops. Here, we find evidence for a mechanical feedback loop by which cells and COL can use nondispersed transmission of force to initiate and maintain long-scale linear patterns. We show that such pattern formation is primarily mediated by force, rather than known diffusive morphogens.
Fig. 6C summarizes our findings. First, we find that cells use traction force to condense/align COL fibers, which in turn influence cell motions and cell positioning. Such cell–COL interactions lead to a positive feedback loop. With a sufficient density of cells, the initial symmetry of the COL distribution and orientations can be broken, leading to the formation of high/low [COL] regions (Fig. 6C, Left). Second, we find that cells preferentially develop linear extension in ECM with high [COL], whereas they form pairwise attraction in ECM with low [COL]. Such spatial organization of the force can reposition cells to the COL condensed area, facilitating the formation of linear patterns (Fig. 6C, Center). Indeed, we show that the ability to reposition cells is required for the formation of long-scale linear patterns. Third, we find that the stability of linear pattern increases with its length. Such length dependence facilitates the extension of linear pattern (i.e., rich get richer, Fig. 6C, Right). We also find evidence of traction force-induced mechano-transduction, which can further stabilize the feedback loop by restricting cell activations along the principal axis of the linear pattern. Taken together, our findings indicate a mechanical feedback that cells can use to break symmetry and form long-scale linear (i.e., tubule-like) patterns.
There are several possible hypotheses for the length-dependent stability of linear patterns. For example, the longer the linear structure, the higher the probability that individual cells create force within the structure. Likewise, forces created by individual cells can be added and transmitted over a long distance along the aligned COL fibers; thus, the longer the structure, the more force can be created and transmitted within the structure. Because force is required for the maintenance of linear patterns, both hypotheses explain why the stability of linear pattern increases with its length. These hypotheses can be investigated in the future with models of the intercellular mechanics.
We have presented evidence showing the lack of direct involvement of diffusive factors in our system. However, the measured, traction force-induced mechano-activation can be considered as an alternative means for the creation of chemical gradients. Indeed, cells can use force to reposition themselves into linear structures first, followed by laying down chemical gradients for the detailed future development of the tubular structures. Such an orchestral process can make the patterning of tubular organs more efficient. Thus, we expect that the situation in vivo involves a combination of chemical and mechanical interactions (see below).
Nonautonomousness in Branching Initiation.
We next compare our findings with current concept of tubular pattern formation. We find that through COL, branching is induced and directed between acini, rather than spontaneously arising at individual acini. This nonautonomous behavior is different from what is observed in chemical-induced branching, i.e., individual acini form branching in direct response to morphogens (35, 36). Nonautonomous behavior has also been observed in autocrine-regulated branching processes, where individual cells secrete inhibitory morphogens (such as TGF-β) to suppress branching at each other (37). In this study, however, we find that direct branching is unlikely to be induced by secreted biochemical factors.
Mechanical Force in Branching Initiation and Pattern Formation.
We find that COL-dependent branching can be initiated by mechanical interactions between acini/cells. Such mechanical interactions appear to determine the sites of branches and create spatial anisotropy dictating the orientation of branches. This mechanism is quite different from current models where branching is induced by diffusible, biochemical factors (35, 38). Spatial inhomogeneity of mechanical stress has been reported in engineered tubule-like structures, where the mechanical inhomogeneity is correlated with the sites of branches (39). Such spatial inhomogeneity, however, was externally determined by the engineered shape of tubule-like structures (39). By contrast, the spatial anisotropy found in this study can spontaneously emerge in an initially homogeneous environment, leading to long-scale linear pattern formation.
Spatial Scales of COL-Dependent mMechanical Interaction.
One striking observation in this study is the spatial scale of COL-dependent cell–cell interaction, which is far longer than the persistence length of COL fibers. One hypothesis is that such a long scale results from the condensation and alignment of COL fibers, which is mediated by cell motions and the maximal force acini can create in our setup. The alignment of COL fibers reduces the dissipation / dispersion of force. On the other hand, the diameter of acini was found approximately 50–200 µm (even after long-term culture), and the motions of individual cells in acini were not correlated. These two factors limit the maximal force the acini can create before the onset of direct branching. In this study, we find that direct branching results from mechanical interactions between acini and requires the initial acinus density above a certain level. We therefore argue that a minimal force is required to activate cells, hence limiting the range whereby direct branching can occur. Future work is required to verify the proposed hypothesis.
Mechano-Chemical Coupling in Tubular Pattern Formation.
Here, instead of known morphogens such as TGFβ1 and EGF, we find that ROCK, PI3K, and Rac1 play primary roles in the initiation and the maintenance of linear/tubular pattern formation. Because cell repositioning is required for the formation of long-scale linear patterns, we hypothesize that the effect of PI3K/Rac1 inhibition mainly results from disabling cell protrusion, an essential element in cell motion. Cell protrusion often requires actin filament polymerization (40), which is primarily regulated by Rho GTPases such as Rac1, and their upstream regulators such as PI3K (31, 32).
Nevertheless, we do not think that mechanical force alone can generate and maintain the patterning process under most conditions. To form a positive feedback, amplification processes are often required. In this study, we do not have any explicit evidence showing where such amplifications occur. One potential model is via the traction force-induced mechano-transduction, possibly leading to regulation by extracellular biochemical factors. Our view on the mechanical force-based patterning process is that the transmission of force via the ECM enables the long-range delivery of messages created by individual cells without much dissipation, whereas biochemical pathways are required to receive, create, and amplify the messages.
The precise mechanism orchestrating tubule formation in vivo is still unknown. Using animal models and 3D cultures, it was shown that soluble factors, stromal cells, and ECM all participate coordinately in tubule formation. As we have already discussed, there are many ways that mechanical interactions can be combined with chemical-based patterning processes. For example, cells can use the diffusion of morphogens such as TGFβ to locally regulate branching sites, while using mechanical interactions to align branches at large spatial scales. Furthermore, TGFβ stimulation is known to enhance COL expression, and the aligned fibrillar form of COL can inhibit cell proliferation through activating discoidin domain receptor (19, 41). These observations provide evidence of couplings among mechanical interaction, biochemical signaling, and cell fate. Taken together, our findings and previous data argue that both biochemical and biomechanical signals mediate the interaction among cells and matrix components and are critical for robust tubular pattern formation.
Methods
Cell Culture and Cell Line Preparation.
To visualize cell motion and organization in the 3D environment, MCF-10A cells were engineered to express CFP-conjugated histone H2B (H2B-cerulean) in the nucleus and mCherry throughout the whole cell. See SI Text for more details.
Reagents, Immuno-Staining, and Microscopy.
See SI Text for details.
Two-Step ECM Construction, ECM Rigidity Measurement, and Acinar/Cell Density Measurement.
Collagen and basement membrane gels were prepared and mixed using Cultrex 3-D Culture Matrix, Rat Collagen I (R&D), and BD Matrigel [basement membrane matrix, growth factor reduced (GFR), phenol red-free, BD Biosciences]. Following the measurement by Paszek et al. (23), the amounts of BM and COL in ECM2 were carefully adjusted to match the rigidity of ECM2 with ECM1 (170 ± 40 Pa) whereas COL concentrations in ECM2 were ranged from 0 to 1 mg/mL. The goal was to maintain constant rigidity of ECM such that the observed cellular responses were not induced by ECM rigidity change. See SI Text for details. To measure the initial seeding cell density or the acinus density before the creation of ECM2, samples were imaged and scanned by bright field microscope equipped with a motorized stage. The image data were collected to compute the densities.
The Involvement of Cell Contractility in COL-Dependent Direct Branching.
To examine if cell contractility is essential in the induction of direct branching, cells were spread on BM gels (ECM1) for 72 h to form acini, followed by the gelification of ECM2 ([COL] = 0.5 mg/mL) in serum-free, growth-factor-free medium (to minimize interference from other known biochemical stimulations) containing ROCK inhibitor (Y27632, 10 μM). After 24 h, the frequency of direct branching at individual acini was measured. To examine if cell contractility is required in the maintenance of direct branching, acini on BM gels (ECM1) were covered by ECM2 ([COL] = 0.5 mg/mL) for 48 h to facilitate branching formation. The system was then perfused with serum-free, growth-factor-free medium containing 10 μM Y27632, followed by timelapse microscopy.
Micro-pattering Experiment.
Circular traps were created by polydimethylsiloxane stamps in agarose gels with a fixed diameter l = 200 μm and a fixed depth = 100 μm. The clusters were arranged in hexagonal configurations, separated by distance λ = 200–1,000 μm (Fig. 5A). Cells were then covered by ECM2 with [COL] ranging from 0 to 1 mg/mL. Rectangular traps were created in agarose gels with a fixed depth = 100 μm and a constant separation between traps: 500 μm. See SI Text for more details.
Supplementary Material
ACKNOWLEDGMENTS.
This work is supported by Ellison Medical Foundation and Western Heaven Funds.
Footnotes
This Feature Article is part of a series identified by the Editorial Board as reporting findings of exceptional significance.
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1114781109/-/DCSupplemental.
References
- 1.Martin-Belmonte F, et al. Cell-polarity dynamics controls the mechanism of lumen formation in epithelial morphogenesis. Curr Biol. 2008;18:507–513. doi: 10.1016/j.cub.2008.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horowitz A, Simons M. Branching morphogenesis. Circ Res. 2008;103:784–795. doi: 10.1161/CIRCRESAHA.108.181818. [DOI] [PubMed] [Google Scholar]
- 3.Shah MM, Sampogna RV, Sakurai H, Bush KT, Nigam SK. Branching morphogenesis and kidney disease. Development. 2004;131:1449–1462. doi: 10.1242/dev.01089. [DOI] [PubMed] [Google Scholar]
- 4.Warburton D, et al. The molecular basis of lung morphogenesis. Mech Dev. 2000;92:55–81. doi: 10.1016/s0925-4773(99)00325-1. [DOI] [PubMed] [Google Scholar]
- 5.Gjorevski N, Nelson CM. Branch formation during organ development. Wiley Interdiscip Rev Syst Biol Med. 2010;2:734–741. doi: 10.1002/wsbm.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costantini F, Kopan R. Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev Cell. 2010;18:698–712. doi: 10.1016/j.devcel.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horowitz A, Simons M. Branching morphogenesis. Circ Res. 20098;103:784–795. doi: 10.1161/CIRCRESAHA.108.181818. [DOI] [PubMed] [Google Scholar]
- 8.Bridgewater D, Rosenblum ND. Stimulatory and inhibitory signaling molecules that regulate renal branching morphogenesis. Pediatr Nephrol. 2009;24:1611–1619. doi: 10.1007/s00467-008-1048-y. [DOI] [PubMed] [Google Scholar]
- 9.Sternlicht MD, Kouros-Mehr H, Lu P, Werb Z. Hormonal and local control of mammary branching morphogenesis. Differentiation. 2006;74:365–381. doi: 10.1111/j.1432-0436.2006.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yevtodiyenko A, Schmidt JV. Dlk1 expression marks developing endothelium and sites of branching morphogenesis in the mouse embryo and placenta. (Translated from eng) Dev Dyn. 2006;235:1115–1123. doi: 10.1002/dvdy.20705. in eng. [DOI] [PubMed] [Google Scholar]
- 11.Patel VN, Rebustini IT, Hoffman MP. Salivary gland branching morphogenesis. Differentiation. 2006;74:349–364. doi: 10.1111/j.1432-0436.2006.00088.x. [DOI] [PubMed] [Google Scholar]
- 12.Moore KA, et al. Control of basement membrane remodeling and epithelial branching morphogenesis in embryonic lung by Rho and cytoskeletal tension. Dev Dyn. 2005;232:268–281. doi: 10.1002/dvdy.20237. [DOI] [PubMed] [Google Scholar]
- 13.Fata JE, Werb Z, Bissell MJ. Regulation of mammary gland branching morphogenesis by the extracellular matrix and its remodeling enzymes. Breast Cancer Res. 2004;6:1–11. doi: 10.1186/bcr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bush KT, et al. TGF-beta superfamily members modulate growth, branching, shaping, and patterning of the ureteric bud. Dev Biol. 2004;266:285–298. doi: 10.1016/j.ydbio.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 15.Berdichevsky F, Alford D, D’Souza B, Taylor-Papadimitriou J. Branching morphogenesis of human mammary epithelial cells in collagen gels. J Cell Sci. 1994;107:3557–3568. doi: 10.1242/jcs.107.12.3557. [DOI] [PubMed] [Google Scholar]
- 16.Fratzl P. Collagen: Structure and Mechanics. New York: Springer; 2008. [Google Scholar]
- 17.Paulsson M. Basement membrane proteins: Structure, assembly, and cellular interactions. Crit Rev Biochem Mol Biol. 1992;27:93–127. doi: 10.3109/10409239209082560. [DOI] [PubMed] [Google Scholar]
- 18.Hinck L, Silberstein GB. Key stages in mammary gland development: the mammary end bud as a motile organ. Breast Cancer Res. 2005;7:245–251. doi: 10.1186/bcr1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silberstein GB, Strickland P, Coleman S, Daniel CW. Epithelium-dependent extracellular matrix synthesis in transforming growth factor-beta 1-growth-inhibited mouse mammary gland. J Cell Biol. 1990;110:2209–2219. doi: 10.1083/jcb.110.6.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wicha MS, Liotta LA, Vonderhaar BK, Kidwell WR. Effects of inhibition of basement membrane collagen deposition on rat mammary gland development. Dev Biol. 1980;80:253–256. doi: 10.1016/0012-1606(80)90402-9. [DOI] [PubMed] [Google Scholar]
- 21.Dhimolea E, Maffini MV, Soto AM, Sonnenschein C. The role of collagen reorganization on mammary epithelial morphogenesis in a 3D culture model. Biomaterials. 2010;31:3622–3630. doi: 10.1016/j.biomaterials.2010.01.077. [DOI] [PubMed] [Google Scholar]
- 22.Wozniak MA, Desai R, Solski PA, Der CJ, Keely PJ. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. J Cell Biol. 2003;163:583–595. doi: 10.1083/jcb.200305010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paszek MJ, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Muthuswamy SK, Li D, Lelievre S, Bissell MJ, Brugge JS. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat Cell Biol. 2001;3:785–792. doi: 10.1038/ncb0901-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee GY, Kenny PA, Lee EH, Bissell MJ. Three-dimensional culture models of normal and malignant breast epithelial cells. (Translated from English) Nat Methods. 2007;4:359–365. doi: 10.1038/nmeth1015. in English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sivakumar L, Agarwal G. The influence of discoidin domain receptor 2 on the persistence length of collagen type I fibers. (Translated from English) Biomaterials. 2010;31:4802–4808. doi: 10.1016/j.biomaterials.2010.02.070. (in English) [DOI] [PubMed] [Google Scholar]
- 27.Stein AM, Vader DA, Jawerth LM, Weitz DA, Sander LM. An algorithm for extracting the network geometry of three-dimensional collagen gels. J Microsc. 2008;232:463–475. doi: 10.1111/j.1365-2818.2008.02141.x. [DOI] [PubMed] [Google Scholar]
- 28.Brown E, et al. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. (Translated from English) Nat Med. 2003;9:796–800. doi: 10.1038/nm879. in English. [DOI] [PubMed] [Google Scholar]
- 29.Raub CB, et al. Image correlation spectroscopy of multiphoton images correlates with collagen mechanical properties. Biophys J. 2008;94:2361–2373. doi: 10.1529/biophysj.107.120006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soriano JV, Orci L, Montesano R. TGF-beta1 induces morphogenesis of branching cords by cloned mammary epithelial cells at subpicomolar concentrations. Biochem Biophys Res Commun. 1996;220:879–885. doi: 10.1006/bbrc.1996.0499. [DOI] [PubMed] [Google Scholar]
- 31.Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 32.Ulrich TA, Lee TG, Shon HK, Moon DW, Kumar S. Microscale mechanisms of agarose-induced disruption of collagen remodeling. Biomaterials. 2010;32:5633–5642. doi: 10.1016/j.biomaterials.2011.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janmey PA, McCulloch CA. Cell mechanics: Integrating cell responses to mechanical stimuli. Annu Rev Biomed Eng. 2007;9:1–34. doi: 10.1146/annurev.bioeng.9.060906.151927. [DOI] [PubMed] [Google Scholar]
- 34.Dupont S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 35.Lu P, Werb Z. Patterning mechanisms of branched organs. Science. 2008;322:1506–1509. doi: 10.1126/science.1162783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garner OB, et al. Stage-dependent regulation of mammary ductal branching by heparan sulfate and HGF-cMet signaling. Dev Biol. 2011;355:394–503. doi: 10.1016/j.ydbio.2011.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daniel CW, Silberstein GB, Van Horn K, Strickland P, Robinson S. TGF-beta 1-induced inhibition of mouse mammary ductal growth: Developmental specificity and characterization. Dev Biol. 1989;135:20–30. doi: 10.1016/0012-1606(89)90154-1. [DOI] [PubMed] [Google Scholar]
- 38.Affolter M, et al. Tube or not tube: Remodeling epithelial tissues by branching morphogenesis. Dev Cell. 2003;4:11–18. doi: 10.1016/s1534-5807(02)00410-0. [DOI] [PubMed] [Google Scholar]
- 39.Gjorevski N, Nelson CM. Endogenous patterns of mechanical stress are required for branching morphogenesis. Integr Biol (Camb) 2010;2:424–434. doi: 10.1039/c0ib00040j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mogilner A. On the edge: Modeling protrusion. Curr Opin Cell Biol. 2006;18:32–39. doi: 10.1016/j.ceb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Vogel WF, Aszodi A, Alves F, Pawson T. Discoidin domain receptor 1 tyrosine kinase has an essential role in mammary gland development. Mol Cell Biol. 2001;21:2906–2917. doi: 10.1128/MCB.21.8.2906-2917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.