Abstract
The histone coactivator-associated arginine methyltransferase 1 (CARM1) is a coactivator for a number of transcription factors, including nuclear receptors. Although CARM1 and its asymmetrically deposited dimethylation at histone H3 arginine 17 (H3R17me2a) are associated with transcription activation, the mechanism by which CARM1 activates transcription remains unclear. Using an unbiased biochemical approach, we discovered that the transcription elongation-associated PAF1 complex (PAF1c) directly interacts with H3R17me2a. PAF1c binds to histone H3 tails harboring dimethylation at R17 in CARM1-methylated histone octamers. Knockdown of either PAF1c subunits or CARM1 affected transcription of CARM1-regulated, estrogen-responsive genes. Furthermore, either CARM1 knockdown or CARM1 enzyme-deficient mutant knockin resulted in decreased H3R17me2a accompanied by the reduction of PAF1c occupancy at the proximal promoter estrogen-responsive elements. In contrast, PAF1c knockdown elicited no effects on H3R17me2a but reduced the H3K4me3 level at estrogen-responsive elements. These observations suggest that, apart from PAF1c's established roles in directing histone modifications, PAF1c acts as an arginine methyl histone effector that is recruited to promoters and activates a subset of genes, including targets of estrogen signaling.
Keywords: estrogen receptor-α, histone arginine methylation, PRMT
Coactivator-associated arginine methyltransferase 1 (CARM1), also known as PRMT4, is the first protein arginine methyltransferase identified as a coactivator for steroid receptors (1). It was later found to be a coactivator for a variety of transcription factors and is a multifunctional protein engaged in a variety of cellular processes, including gene expression, coupling of transcription with mRNA splicing, regulation of protein stability, and functioning in embryonic development (2). Loss of CARM1 in the mouse embryo leads to abrogation of the estrogen response and reduced expression of some estrogen receptor-α (ERα) target genes (3). Recently, CARM1 was shown to be a unique coactivator of ERα that can simultaneously block cell proliferation and induce differentiation through global regulation of ERα-regulated genes in breast cancer cells (4). The methyltransferase activity of CARM1 is required for its ability to regulate transcription of pS2 (TFF1), a standard ERα target gene (5). Moreover, the enzyme-dead CARM1 knockin mice have defects similar to those seen in their knockout counterparts (6). These observations suggest that the enzymatic activity of CARM1 is indispensable for the majority of CARM1's in vivo functions. Several putative mechanisms have been proposed to explain CARM1 activator function, including histone H3 methylation at arginine 17 (R17), methylation of other key transcription coactivators, and recruitment of chromatin remodeling factors. However, it is unclear which mechanisms drive CARM1 transcription activation function.
CARM1 was originally characterized as a histone arginine methyltransferase because of its ability to dimethylate arginines on histone H3. Subsequent biochemical analyses showed that histone H3 can be modified at R2, R17, and R26 (7). A recent study showed that asymmetrically dimethylated histone H3 at R2 (H3R2me2a) was mainly deposited by PRMT6 (8). ChIP analysis identified elevated levels of H3R17me2a at the estrogen-responsive pS2 promoter (5). Furthermore, kinetic ChIP analysis revealed that CARM1 was recruited in a cyclic manner, occurring at 40-min intervals upon treatment with 17β-estradiol (E2), and recruitment of CARM1 correlated with an increase in the H3R17me2a mark (9). These studies suggest that H3R17me2a likely accounts for CARM1's coactivator function in transcriptional regulation. Because many protein domains have been characterized to specifically bind to modified histone marks, effector molecules may exist that read H3R17me2a marks to mediate transcriptional activation. In support of this hypothesis, a recent study identified one effector protein, TDRD3, as a “reader” for H3R17me2a and H4R3me2a using a protein domain microarray approach (10). TDRD3 contains a tudor domain that is typical for mediating methyl-specific binding. Furthermore, TDRD3 functions as a coactivator in estrogen-responsive elements (ERE)-luciferase reporter assays and endogenous TDRD3 was detectable at the pS2 promoter in ChIP assays (10). Despite these observations, it is unclear how TDRD3 promotes transcriptional activation.
To further elucidate the mechanism by which CARM1 enzymatic activity and H3R17me2a mediate transcription activation, we took an unbiased biochemical approach to identify effector proteins that directly interact with the H3R17me2a mark using peptide pull-down assays from HeLa nuclear extract. We unexpectedly identified the transcription elongation-associated PAF1 complex (PAF1c) in direct association with H3R17me2a and found this association is required for complete activation of CARM1-regulated ERα target genes. Furthermore, PAF1c was detected at EREs, and its occupancy was abrogated by either knockdown of CARM1 or introduction of a CARM1 enzyme-deficient mutant, which was accompanied by the attenuation of H3R17me2a. This study presents a unique mechanism by which H3R17me2a functions to recruit PAF1c to facilitate transcription activation and encrypt a means for PAF1c's recruitment to the promoters of target genes.
Results
Identification of Human PAF1c as a Binding Partner for the Histone H3 Tail Containing the Asymmetrically Dimethylated Arginine 17.
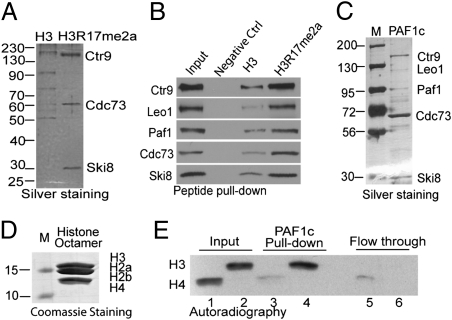
To identify proteins that recognize asymmetrically dimethylated arginine 17 at histone H3 (H3R17me2a), we took an unbiased biochemical approach using biotinylated histone tail pull-down from HeLa nuclear extract, according to previously published methods (11–13). Two histone H3 N-terminal tail peptides encompassing amino acids 1–20, with or without asymmetrically dimethylated R17 (H3R17me2a), were used for pull-down assays. Equal amounts of biotinylated peptides preimmobilized on streptavidin magnetic beads were incubated with HeLa nuclear extract followed by acid elution (12). Eluted proteins were immediately neutralized, loaded on SDS/PAGE, and silver-stained. Fig. 1A showed that three bands were specifically associated with H3R17me2a tail compared with the nonmodified H3 tail. This experiment was repeated on a large scale, and the bands were Coomassie-stained, excised, and analyzed by MALDI-TOF MS. Three bands were identified as hCtr9, hCdc73, and hSki8, all of which belong to the same multisubunit PAF1c. The matched peptides sequences are shown in Fig. S1. The PAF1c was originally identified in yeast as a RNA polymerase II (RNAPII)-associated complex mediating transcription elongation (14). The human PAF1c (hPAF1c) has been shown to be involved in multiple steps in transcription, including control of H2B ubiquitination and H3K4me3, transcription initiation and elongation, and pre-mRNA processing (15). Because hPAF1c is composed of five subunits (hCtr9, hLeo1, hPaf1, hCdc73, and hSki8), we speculated that the whole complex (including two missing subunits, hLeo1 and hPaf1) bound to the H3R17me2a tail. Their absence in silver staining (Fig. 1A) could be because of inefficient elution or poor staining by silver. The antibodies for individual subunits were used to detect their presence in eluted fractions from 3 biotinylated peptide pull-downs. The results showed that although all PAF1c subunits are strongly detected in H3R17me2a tail pull-down, much weaker PAF1c binding was detected with the nonmodified H3 tail, and no binding was detected with negative control peptide pull-downs (Fig. 1B).
Fig. 1.
The RNAPII-associated PAF1c displays binding specificity to asymmetrically dimethylated histone H3R17me2a. (A) Immobilized histone H3 tail (amino acids 1–20, Left) and the identical peptide carrying asymmetric dimethylated arginine 17 (Right) were used to identify specific effectors for the H3R17me2a mark in HeLa cell nuclear extract. Three proteins were specifically pulled down with H3R17me2a and later identified to be hCtr9, hCdc73, and hSki8 by in-gel digestion and mass spectrometry analyses. (B) Western blots showed that biotinylated H3R17me2a peptide selectively pull down PAF1c subunits, including Ctr9, Leo1, Paf1, Cdc73, and Ski8. (C) The PAF1c was affinity-purified from HEK293-Flag-Cdc73 cells and silver-stained. (D) Coomassie staining of the reconstituted histone octamers. (E) PAF1c preferentially interacts with CARM1 methylated histone octamers, which were reconstituted from bacterially expressed histone H3, H2A, H2B, and H4. Reconstituted histone octamers were in vitro methylated by CARM1 or PRMT1 in the presence of 3H-AdoMet and incubated with PAF1c immobilized on anti-Flag resin. Equal volume of input, bound, and flow-through of PRMT1-methylated 3H-histone H4R3me2 (lanes 1, 3, and 5) and CARM1-methylated 3H-histone H3R17me2 (lanes 2, 4, and 6) were loaded. Autoradiography shows that more CARM1-methylated 3H-histone H3R17me2 was found bound to PAF1c on the beads compared with PRMT1-methylated 3H-histone H4R3me2.
To identify the PAF1c subunit responsible for direct interaction with the histone H3R17me2a tail, individual PAF1c subunits were recombinantly expressed as Flag-tagged proteins in Sf9 insect cells and purified (Fig. S2A). Purified subunits were individually used for pull-down assays. Western blots showed that Paf1 subunit elicited the strongest binding, whereas all other subunits displayed either weak (Cdc73) or negligible binding to histone H3 tails (Fig. S2B). This result suggested that the Paf1 subunit is the main component in PAF1c that interacts with the histone H3 tail. The observation that Paf1 unexpectedly binds to both nonmodified and H3R17me2a tails indicates that other subunits in the complex may contribute to the specificity of Paf1.
The recently identified H3R17me2a effector molecule TDRD3 was also found to interact with H4R3me2a, a site modified by PRMT1 on histone H4 (10). To investigate if PAF1c interacts with the PRMT1-modified histone mark, we reconstituted histone octamers from bacterially expressed core histones (Fig. 1D) and in vitro methylated histone octamers by PRMT1 in the presence of 3H-AdoMet for binding assays. Similarly, histone octamers were methylated by CARM1 as a positive control. The in vitro methylation of reconstituted histone octamers by PRMTs generated 3H-labeled octamers uniquely carrying either asymmetric dimethylation of R17 on histone H3 (H3R17me2a) mediated by CARM1 or asymmetric dimethylation of R3 on histone H4 (H4R3me2a) mediated by PRMT1. PAF1c was purified from Flag-Cdc73 stably-expressing HEK293 cells, as shown by silver staining (Fig. 1C), and preimmobilized on anti-Flag M2 resin. Equal amounts of preimmobilized PAF1c were incubated with 3H-H3R17me2a or 3H-H4R3me2a histone octamers in parallel. The input, flow-through, and bead-bound samples were resolved on SDS-PAGE, and the dried gel was exposed to an X-ray film to detect 3H-labeled methylated octamers in each fraction. The autoradiography showed that the efficiency of octamer methylation by CARM1 and PRMT1 were similar, as indicated by the input samples containing equivalent radioactivity (Fig. 1E, compare lane 1 with lane 2). However, PAF1c strongly interacted with the 3H-H3R17me2a histone octamers methylated by CARM1 (Fig. 1E, compare lane 4 with lane 3). Consistently, more PRMT1-methylated 3H-H4R3me2a histone octamers were detected in the flow-through (Fig. 1E, compare lane 5 with lane 6). Our results demonstrated that, unlike TDRD3, PAF1c preferentially binds to CARM1-methylated histone octamers over those methylated by PRMT1. Moreover, PAF1c not only interacts with histone H3 tail peptides but also histone octamers carrying asymmetric dimethylation at R17 of histone H3.
Estrogen-Enhanced Cooccupancy of PAF1c and H3R17me2a at EREs of ERα Target Genes.
Given that H3R17me2a peptide selectively binds to PAF1c in vitro, we next examined if PAF1c is recruited to ERα target genes that harbor the H3R17me2a mark in vivo. pS2, PTGES, and IGFBP4 genes were selected for ChIP analyses because they were previously shown as CARM1-regulated ER target genes (1, 16) and contain defined EREs. In agreement with past studies, H3R17me2a was enriched at EREs of selected genes upon 17β-estradiol (E2) treatment (Fig. 2 A, E, and I). Notably, Paf1 (Fig. 2 B, F, and J) and Cdc73 (Fig. 2 C, G, and K) occupancies at EREs were also enhanced by E2 treatment. To validate the specificity of Cdc73 antibody used in ChIP experiments, a MCF7-tet-on-shCdc73 cell line was constructed and used for ChIP assays using anti-Cdc73 antibody (Fig. 2M). E2-induced recruitment of Cdc73 to EREs was found decreased after knockdown of Cdc73 in MCF7 cells (Fig. 2N). Taken together, these results suggest that intact PAF1c is recruited to EREs in an E2-enhanced manner, and the recruitment of PAF1c is accompanied by the increase of H3R17me2a mark.
Fig. 2.
E2 induces cooccupancy of PAF1c and H3R17me2a at the EREs of several ERα-target genes: pS2, PTGES, and IGFBP4. Cells were treated with or without E2 for 45 min followed by ChIP and quantitative PCR assays using the indicated antibodies. PAF1c occupancy at the ERE regions (B, F, and J for Paf1; C, G, and K for Cdc73) of ERα target genes coincides with the presence of the H3R17me2a mark (A, E, and I). (D, H, and L) IgG control for pS2, PTGES, and IGFBP4 ERE regions. (M) MCF7-tet-on-shCdc73 cell line was generated and knockdown efficiency of Cdc73 was shown in Western blots. (N) Cdc73 occupancy at pS2 ERE region was decreased after knockdown of Cdc73 in MCF7-tet-on-shCdc73 cells. Error bars represent SD (n = 3).
Recruitment of hPAF1c to EREs Is CARM1-Dependent.
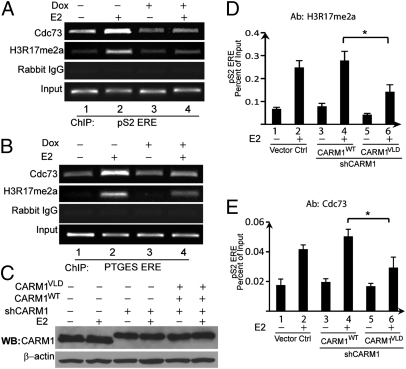
To determine if PAF1c is recruited to EREs via recognition of H3R17me2a mark, we used a CARM1 inducible knockdown cell line (4) for ChIP assays because CARM1 is the only reported enzyme to create H3R17me2a mark. MCF7-tet-on-shCARM1 cells were treated with DMSO, E2, doxycycline (Dox), or Dox+E2 and subjected to ChIP assays. E2 treatment induced H3R17me2a accumulation at the ERE of pS2, whereas E2-induced H3R17me2a level was greatly reduced when CARM1 was knocked down by Dox treatment (Fig. 3A, compare lane 2 with lane 4). A similar observation was made with the PTGES gene under identical treatment conditions (Fig. 3B). These results confirm that CARM1 is responsible for writing the H3R17me2a mark in response to E2. Using this cell-line model, we found that Cdc73 E2-induced occupancy at the EREs of pS2 or PTGES was greatly abrogated after CARM1 knockdown (Fig. 3 A and B, compare lane 2 with lane 4). Because CARM1 has been implicated in transcription elongation and splicing processes, complete loss of CARM1 may indirectly affect transcription regardless of histone H3R17 dimethylation. To address this possibility, we replaced wild-type CARM1 with an enzyme-deficient CARM1 mutant by coinfecting MCF7 cells with lentiviruses encoding CARM1shRNA and FlagCARM1VLD, a well-characterized CARM1 mutant defective for methyltransferase activity (1). As shown in Fig. 3C, endogenous CARM1 was successfully knocked down and the CARM1VLD mutant was expressed. As a control, we also restored wild-type CARM1 under the endogenous CARM1 knockdown background. ChIP assays were performed 5 d after viral infection and E2 or vehicle treatment of cells for 45 min. The results showed that E2-enhanced H3R17me2a level (Fig. 3D) and Cdc73 recruitment at ERE of pS2 (Fig. 3E) were significantly impaired in CARMVLD compared with CARM1WT-expressing cells. These data suggest that PAF1c occupancy at EREs is CARM1-dependent, most likely mediated by the H3R17me2a mark.
Fig. 3.
The recruitment of PAF1c to proximal promoter EREs of ERα target genes is CARM1- and H3R17me2a-dependent. MCF7-tet-on-shCARM1 cells were pretreated with or without 0.5 μg/mL Dox for 4 d before E2 or vehicle treatment for 45 min. The occupancy of PAF1c subunit Cdc73 at EREs of pS2 (A) and PTGES (B) was analyzed using ChIP assays. When CARM1 is knocked down with Dox treatment, E2 stimulated H3R17me2a and PAF1c occupancy at EREs of pS2 and PTGES were greatly reduced (compare lane 4 with lane 2). (C) Western blots showed the expression levels of CARM1WT and CARM1VLD were comparable to the endogenous CARM1 in MCF7 cells when endogenous CARM1 was efficiently silenced. (D and E) ChIP analyses showed that expression of the enzyme-defective CARM1VLD mutant was insufficient to restore H3R17me2a level (D, *P < 0.01) and recruit PAF1c to pS2 ERE (E, *P < 0.01). In contrast, CARM1WT can fully rescue the function of endogenous CARM1 (histograms 3 and 4). Error bars represent SD (n = 3).
PAF1c and CARM1 Coordinately Regulate a Common Set of ERα Target Genes.
We had previously identified CARM1-regulated ERα target genes using microarrays (4). To establish the functional role of PAF1c in CARM1-regulated transcription, we attempted to knockdown PAF1c components and measure the effects on mRNA levels of CARM1-regulated ER targets. Knocking down Ctr9 was previously reported to affect expression of other PAF1c subunits in yeast and human cells (17, 18), and Ctr9 and Paf1 were shown as scaffold proteins for the formation of PAF1c (19). Therefore, we generated a Dox-inducible Ctr9 knockdown cell line, MCF7-tet-on-shCtr9, which allows transient knockdown of Ctr9 to minimize cellular toxicity associated with the loss of functional PAF1c. To examine if Ctr9 knockdown interferes with PAF1c integrity, MCF7-tet-on-shCtr9 cells were infected with lentiviral Flag-tagged Ski8 to immunoprecipitate PAF1c with anti-Flag affinity resin. This cell line was treated with Dox or vehicle for 5 d to knock down Ctr9, and then both whole-cell lysates and anti-Flag immunoprecipitated PAF1c were subjected to Western blots. In accompaniment with Ctr9 knockdown, the endogenous Paf1 level was significantly reduced in whole lysate (Fig. S3, lanes 1 and 2) as well as in Flag-Ski8 immunoprecipitated PAF1c (Fig. S3, lanes 3 and 4). Interestingly, despite no detectable loss of endogenous Leo1 expression, the presence of Leo1 in PAF1c was diminished, whereas Cdc73 level was moderately affected (Fig. S3). Our findings are in agreement with previous reports that Ctr9 plays a pivotal role in PAF1c assembly (19), and further indicate that Leo1 might directly interact with Paf1 in PAF1c. Thus, the MCF7-tet-on-shCtr9 cell line is validated as a good model for elucidating the function of PAF1c in E2-dependent gene expression.
Because the MCF7-tet-on-shCARM1 cell line (4) was established from the same parental MCF7-tet-on clone as MCF7-tet-on-shCtr9, we compared expression of ERα target genes in both cell lines simultaneously. The Ctr9 and CARM1 knockdown efficiencies were confirmed by Western blots showing that 5 d of Dox treatment was sufficient to robustly decrease both protein levels by 90% (Fig. 4 A and B). To demonstrate that loss of CARM1 does not affect steady-state levels of any of the PAF1c subunits, we performed Western blots of PAF1c subunits in MCF7-tet-on-shCARM1 cell line. The results showed that PAF1c subunit levels were not altered by the loss of CARM1 (Fig. S4). The Ctr9 and CARM1 inducible knockdown cells were treated under four conditions and harvested for mRNA, followed by quantitative RT-PCR (qRT-PCR) analysis. pS2, PTGES, IGFBP4, EGR3, and MYC genes were selected for analyses because they were found to be CARM1-dependent E2 target genes (4). Fig. 4 showed that E2 induced expression of all these genes by more than twofold, and induction was inhibited over 50% by either Ctr9 or CARM1 knockdown (Fig. 4 C–G). Moreover, the trends for these ERα target genes in MCF7-tet-on-shCARM1 and MCF7-tet-on-shCtr9 cell lines under the four treatment conditions were similar (Fig. 4 C–G). These findings were further confirmed in MCF7 cells transiently transfected with siRNAs targeting Cdc73 or Paf1 subunits (Fig. S5). We also examined PAF1c dependency of three E2-induced, CARM1 nonregulated genes (4) by qRT-PCR in two inducible cell lines. Slc7a5 and Cyclin D1 were found not to be regulated by either CARM1 or PAF1c (Fig. S6 A and B), whereas TGF-α was found to be regulated by PAF1c but not by CARM1 (Fig. S6C). These data imply that PAF1c shares both common and distinct sets of genes from those of CARM1. Collectively, our data strongly support that PAF1c and CARM1 coregulate at least a subset of genes in the estrogen pathway.
Fig. 4.
Expression of several selected ERα target genes were similarly affected by knockdown of CARM1 or Ctr9. MCF-tet-on-shCtr9 and MCF7-tet-on-shCARM1 cell lines were treated with vehicle, E2 (5 h), Dox (5 d), or Dox (5 d) + E2 (5 h) in parallel. Endogenous Ctr9 (A) and CARM1 (B) were knocked down by 90% after 5 d of Dox treatment. The mRNA levels of pS2 (C), PTGES (D), IGFBP4 (E), MYC (F), and EGR3 (G) genes were analyzed by qRT-PCR in both cell lines after the indicated treatment. The attenuation of estrogen response was observed with all examined ER target genes in both Ctr9 and CARM1 knockdown cells after Dox induced silencing of Ctr9 or CARM1, respectively. Error bars represent SD (n = 3).
PAF1c Is Recruited to EREs of ERα Target Genes Downstream of the H3R17me2a Mark.
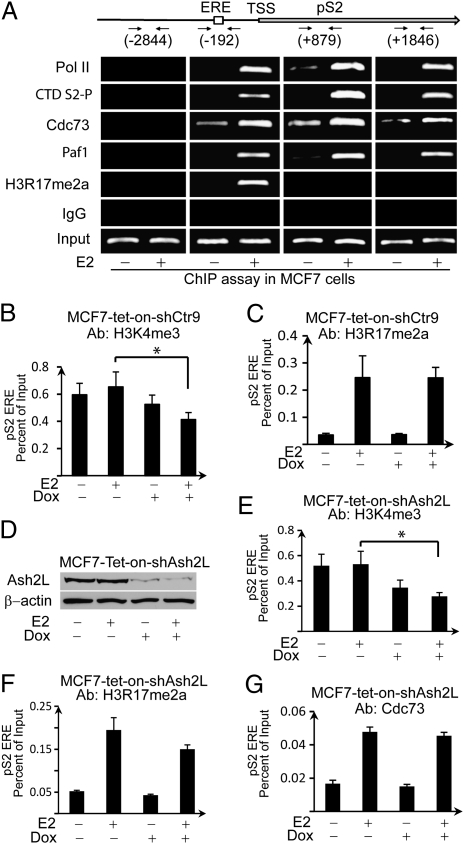
We have shown that CARM1 knockdown affects H3R17me2a and occupancy of PAF1c at the EREs of ERα target genes, indicating that PAF1c binding is a downstream event of CARM1 and H3R17me2a. The MCF7-tet-on-shCtr9 cell line allows us to further dissect the consequence of PAF1c's association with H3R17me2a. We first comprehensively examined the association of PAF1c along the pS2 gene during E2-stimulated transcriptional activation. The distribution patterns of Cdc73, Paf1, RNAPII, C-terminal domain S2-phosphorylation (CTD S2-P), and H3R17me2a on the pS2 gene were determined by ChIP assays using primers amplifying different regions of the pS2 gene: −2,844 (upstream of transcription start site), −192 (promoter ERE), +879 (upstream coding region), +1,846 (downstream coding region). CTD S2-P was primarily found at the elongation region. In contrast, the H3R17me2a mark was only detected at the ERE but not the elongation region. Consistent with the established role of PAF1c as a RNAPII-associated elongation factor, Cdc73 and Paf1 were detected at both the promoter and coding regions coinciding with total RNAPII binding patterns (Fig. 5A). These data suggest that PAF1c is involved in transcriptional elongation of ERα target genes.
Fig. 5.
Knockdown of Ctr9 did not alter the H3R17me2a level at the ERE of pS2; however, H3K4me3 level was reduced. (A) ChIP assays showed the occupancies of Cdc73, Paf1, total RNAPII, and CTD S2-phosphorylation at distal and proximal promoter and encoding regions of the pS2 gene. (B and C) Quantitative PCR of ChIP products showed that knockdown Ctr9 did not affect H3R17me2a level at the ERE region of the pS2 gene (C); however, H3K4me3 level at the ERE was significantly decreased (B, *P < 0.02). (D) Ash2L is effectively knocked down after 6-d Dox treatment in MCF7-tet-on-shAsh2L cells. (E–G) ChIP quantitative PCR results showed knockdown of Ash2L decreased H3K4me3 level at the ERE of pS2 (E, *P < 0.002), but had negligible effect on H3R17me2a level (F) and recruitment of Cdc73 (G). Error bars represent SD (n = 3).
To delineate if H3R17me2a could serve as an upstream signal for recruitment of PAF1c, we measured H3R17me2a and H3K4me3 levels at the ERE of pS2 upon Ctr9 knockdown (Fig. 5 B and C). PAF1c was previously shown to recruit Set1, a histone lysine methyltransferase responsible for H3K4me3, and thus H3K4me3 is downstream of PAF1c and serves as a positive control to show the effects of Ctr9 knockdown on transcription. MCF7-tet-on-shCtr9 cells were pretreated with or without Dox for 5 d, then treated with 20 nM E2 or vehicle for 45 min before ChIP analyses followed by qRT-PCR. Fig. 5B shows that Ctr9 knockdown indeed reduced H3K4me3 at the proximal ERE of pS2, consistent with a previous report (20). In contrast, H3R17me2a level was not affected (Fig. 5C). Although H3R17me2a level at the ERE of pS2 was unchanged, pS2 transcription was inhibited by Ctr9 knockdown (Fig. 4C). This result suggests that although H3R17me2a is typically defined as a mark of transcriptional activation, this mark alone is insufficient to activate transcription without PAF1c. PAF1c was previously reported to mediate H3K4 trimethylation by recruitment of the mixed lineage leukemia (MLL) complex via direct interaction with Ash2L, a common subunit in MLL-1 and -2 complexes (21, 22). We thus speculate that H3R17me2a recruits PAF1c, which mediates downstream H3K4 trimethylation by interaction with the MLL complex in ER-mediated gene activation. Inducible knockdown of Ash2L cells were constructed by infecting MCF7 cells with pTRIPZ-Ash2LshRNA (Fig. 5D). Western blots results showed efficient knockdown of Ash2L by Dox treatment of MCF7-tet-on-shAsh2L cells for 6 d (Fig. 5D). As expected, ChIP assays showed that knockdown of Ash2L significantly decreased the level of H3K4me3 at the ERE of pS2 (Fig. 5E), whereas the level of H3R17me2a mark and occupancy of Cdc73 at ERE of pS2 were not significantly affected (Fig. 5 F and G). These results suggest that H3K4me3 is downstream event of H3R17me2a and PAF1c recruitment in ER-mediated gene activation.
Given that PAF1c and H3R17me2a occupancies at EREs were inhibited by CARM1 knockdown (Fig. 3 D and E), whereas Ctr9 knockdown did not affect H3R17me2a (Fig. 5C), our data support the sequential transcriptional activation model (Fig. S7) in which CARM1 deposits H3R17me2a at the ERE, leading to the recruitment of PAF1c, which results in H3K4me3 deposition and transcriptional elongation via RNAPII.
Discussion
CARM1 and H3R17me2a are linked to transcription activation, yet little is known about how H3R17me2a signals transcription activation. Using an unbiased biochemical approach, here we report the identification of PAF1c as a unique transcription mediator of this active histone mark. We showed that PAF1c is an effector complex that binds to H3R17me2a peptide and histone octamers methylated by CARM1 (Fig. 1). The specificity of PAF1c was demonstrated by its inability to bind to H4R3me2a containing histone octamers modified by PRMT1 (Fig. 1E) (23). Nonetheless, our in vivo ChIP assays demonstrated that PAF1c was recruited to EREs of CARM1-regulated ERα target genes (Fig. 2), and the occupancy of PAF1c on the chromatin was dependent on CARM1 and the H3R17me2a mark (Fig. 3). In contrast, knockdown of PAF1c subunits did not alter the occupancy of CARM1 and H3R17me2a at EREs, whereas the known downstream H3K4me3 mark was attenuated (Fig. 5B). These results strongly suggest that PAF1c occupancy at EREs is mediated by H3R17me2a, which functions as an activation mark because of its ability to stimulate H3K4me3 through PAF1c recruitment.
PAF1c is known to participate in multiple steps of transcription, particularly in controlling histone methylation in later steps of transcriptional regulation. PAF1c is required for both H3K4me3 and H3K36me3 during transcription elongation via interaction with the histone H2B ubiquitination complex, the Set1 methyltransferase-containing COMPASS complex, and the Set 2 methylase (15, 20). Despite the established roles of PAF1c in directing histone modifications, it remains unclear how PAF1c is recruited to its target genes. In yeast, genes encoding Paf1 and Cdc73 are not essential, and their loss results in change of only a small subset of transcripts (14, 24). Similarly, only several hundred genes are affected, either positively or negatively, by the loss of PAF1c in Cdc73-depleted HeLa cells (25). Thus, the target-gene spectrum of metazoan PAF1c remains to be determined, and the roles of PAF1c in gene expression are speculated, at least in part, to be determined by the mechanism of PAF1c recruitment to specific promoters. Studies suggest that some transcription factors directly recruit PAF1c to their target genes. For example, Cdc73 directly binds β-catenin in both Drosophila and mammalian cells to activate transcription of genes in the Wnt pathway (26). Recently, the prototypical transcriptional activator GAL4-VP16 was shown to directly bind Paf1 and recruit PAF1c to the DNA template (27). Our finding that PAF1c regulates ERα target genes expands the role of PAF1c in regulating gene expression. Furthermore, we show that the recruitment of PAF1c to EREs depends on CARM1 and H3R17me2a, constituting a unique mechanism for PAF1c's recruitment to target genes. Moreover, the newly defined role of PAF1c as an arginine methyl-histone effector distinguishes PAF1c from its established role in controlling downstream histone ubiquitination and lysine methylation.
Although we demonstrated the functional connection between PAF1c and CARM1-mediated H3R17me2a in estrogen-stimulated transcription, PAF1c may activate other transcription factors regulated by CARM1 and functionally overlap with CARM1 in other biological processes. In support of this idea, PAF1c and CARM1 are known to regulate common target genes. One such gene, β-catenin, is a gene coregulated by PAF1c (26) and CARM1 (28). Additionally, both CARM1 and the PAF1c subunits are essential for mouse embryonic development and maintenance of ES cell pluripotency. CARM1-null mice die at birth and are smaller than their wild-type littermates (3). CARM1 and H3R17/R26me2 are associated with Oct4 and Sox2 promoters in ES cells; moreover, CARM1 is required for maintaining pluripotency of ES cells (29). Coincidently, Cdc73 null mice are embryonic-lethal (30). Microarray analysis of Cdc73 knockout mouse embryonic fibroblast cells demonstrated that PAF1c directly regulates many essential genes involved in cell growth and survival (30). Moreover, a recent genome-scale RNAi screen identified PAF1c as a regulator of Oct4 expression, demonstrating an important role for PAF1c in maintaining ES cell identity (20). Finally, both PAF1c and CARM1 are involved in transcriptional regulation at multiple levels. PAF1c functions in transcription initiation, elongation (15), mRNA 3′ end cleavage (25), polyadenylation of mRNA precursors (27), and small nucleolar RNA (snoRNA) 3′ end formation (31). In addition to transcriptional activation, CARM1 methylates splicing factors (32), modulates recognition of 5′ splicing sites (33), and regulates the coupling of transcription and splicing (34). Furthermore, a recent report showed that RNAPII carboxyl-terminal domain is methylated by CARM1 (35). Mutating the CARM1 methylation site in RNAPII carboxyl-terminal domain affects snoRNA expression similar to the effect observed in CARM1−/− mouse embryonic fibroblast cells (35). Coincidently, 3′ end formation of mRNAs and snoRNAs are dependent on PAF1c (31). Collectively, PAF1c and CARM1 share overlapping genes and cellular processes, although the processes that require CARM1 methyltransferase activity and H3R17me2a for PAF1c function await elucidation.
Arginine methylation of histone tails correlates with either transcriptional activation or repression. The specific marks, as part of histone codes, are thought to promote or prevent the docking of key transcriptional effector molecules (36, 37). PAF1c subunits are nuclear proteins directly associated with RNAPII and can regulate multiple steps of transcription. Recently, TDRD3 was found to bind both the H3R17me2a and H4R3me2a marks (10). It is plausible that multiple effector proteins exist for the H3R17me2a mark that direct this mark to divergent biological processes. Future work should determine if these two H3R17me2a effectors directly interact and whether they regulate different CARM1-coupled biological processes in cell-type and tissue-specific manners. In conclusion, we identified PAF1c as an effector complex for H3R17me2a and linked its function to the regulation of estrogen-responsive genes. This study presents a unique mechanism by which the CARM1-catalyzed H3R17me2a mark is linked to transcriptional activation via recruitment of PAF1c (Fig. S7).
Materials and Methods
Cell Culture and Estrogen Treatment.
MCF7 and HEK293 cells were maintained in DMEM supplemented with 10% (vol/vol) FBS. Three days before E2 treatment, the cells were transferred to phenol red-free DMEM containing 6xSFBS (six-time stripped FBS). The working concentration for E2 was 20 nM.
Peptide Pull-Down Assays.
Peptide pull-down was performed according to previously published methods (12). For each assay, biotinylated peptide (1 μg) were immobilized on 200 μg streptavidin coated DynaBeads (Invitrogen). HeLa nuclear extract or purified recombinant proteins were incubated with the magnetic beads immobilized with indicated peptide for 2 h at 4 °C. The peptide interaction protein was detected by Western blot or silver staining.
Description on cell lines, antibodies, peptide pull-down, qRT-PCR, ChIP assay, transfection and in vitro methylation of histone octamer are available in SI Materials and Methods. All of the primers and oligo DNA are listed in Table S1.
Supplementary Material
Acknowledgments
We thank Dr. Danny Reinberg for histone expression plasmids; Shaun V. Hernandez for technical support; Erin Shanle and Nancy Thompson for editing and comments; and Drs. Emery Bresnick and Shigeki Miyamoto for critical reading of the manuscript. This work was supported by National Institutes of Health Grant CA125387 (to W.X.), a Department of Defense Era of hope Scholar Award, Shaw Scientist Award from the Greater Milwaukee Foundation (to W.X.), and in part by NIH/NCI P30CA014520-University of Wisconsin Comprehensive Cancer Center Support.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1114905109/-/DCSupplemental.
References
- 1.Chen D, et al. Regulation of transcription by a protein methyltransferase. Science. 1999;284:2174–2177. doi: 10.1126/science.284.5423.2174. [DOI] [PubMed] [Google Scholar]
- 2.Kuhn P, Xu W. Protein arginine methyltransferases: Nuclear receptor coregulators and beyond. Prog Mol Biol Transl Sci. 2009;87:299–342. doi: 10.1016/S1877-1173(09)87009-9. [DOI] [PubMed] [Google Scholar]
- 3.Yadav N, et al. Specific protein methylation defects and gene expression perturbations in coactivator-associated arginine methyltransferase 1-deficient mice. Proc Natl Acad Sci USA. 2003;100:6464–6468. doi: 10.1073/pnas.1232272100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Dhaheri M, et al. CARM1 is an important determinant of ERα-dependent breast cancer cell differentiation and proliferation in breast cancer cells. Cancer Res. 2011;71:2118–2128. doi: 10.1158/0008-5472.CAN-10-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma H, et al. Hormone-dependent, CARM1-directed, arginine-specific methylation of histone H3 on a steroid-regulated promoter. Curr Biol. 2001;11:1981–1985. doi: 10.1016/s0960-9822(01)00600-5. [DOI] [PubMed] [Google Scholar]
- 6.Kim D, et al. Enzymatic activity is required for the in vivo functions of CARM1. J Biol Chem. 2010;285:1147–1152. doi: 10.1074/jbc.M109.035865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schurter BT, et al. Methylation of histone H3 by coactivator-associated arginine methyltransferase 1. Biochemistry. 2001;40:5747–5756. doi: 10.1021/bi002631b. [DOI] [PubMed] [Google Scholar]
- 8.Guccione E, et al. Methylation of histone H3R2 by PRMT6 and H3K4 by an MLL complex are mutually exclusive. Nature. 2007;449:933–937. doi: 10.1038/nature06166. [DOI] [PubMed] [Google Scholar]
- 9.Métivier R, et al. Estrogen receptor-alpha directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell. 2003;115:751–763. doi: 10.1016/s0092-8674(03)00934-6. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y, et al. TDRD3 is an effector molecule for arginine-methylated histone marks. Mol Cell. 2010;40:1016–1023. doi: 10.1016/j.molcel.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sims RJ, 3rd, Trojer P, Li G, Reinberg D. Methods to identify and functionally analyze factors that specifically recognize histone lysine methylation. Methods. 2006;40:331–338. doi: 10.1016/j.ymeth.2006.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wysocka J. Identifying novel proteins recognizing histone modifications using peptide pull-down assay. Methods. 2006;40:339–343. doi: 10.1016/j.ymeth.2006.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu W, et al. A transcriptional switch mediated by cofactor methylation. Science. 2001;294:2507–2511. doi: 10.1126/science.1065961. [DOI] [PubMed] [Google Scholar]
- 14.Shi X, et al. Paf1p, an RNA polymerase II-associated factor in Saccharomyces cerevisiae, may have both positive and negative roles in transcription. Mol Cell Biol. 1996;16:669–676. doi: 10.1128/mcb.16.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaehning JA. The Paf1 complex: platform or player in RNA polymerase II transcription? Biochim Biophys Acta. 2010;1799:379–388. doi: 10.1016/j.bbagrm.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bauer UM, Daujat S, Nielsen SJ, Nightingale K, Kouzarides T. Methylation at arginine 17 of histone H3 is linked to gene activation. EMBO Rep. 2002;3:39–44. doi: 10.1093/embo-reports/kvf013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mueller CL, Porter SE, Hoffman MG, Jaehning JA. The Paf1 complex has functions independent of actively transcribing RNA polymerase II. Mol Cell. 2004;14:447–456. doi: 10.1016/s1097-2765(04)00257-6. [DOI] [PubMed] [Google Scholar]
- 18.Zhu B, et al. The human PAF complex coordinates transcription with events downstream of RNA synthesis. Genes Dev. 2005;19:1668–1673. doi: 10.1101/gad.1292105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim J, Guermah M, Roeder RG. The human PAF1 complex acts in chromatin transcription elongation both independently and cooperatively with SII/TFIIS. Cell. 2010;140:491–503. doi: 10.1016/j.cell.2009.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding L, et al. A genome-scale RNAi screen for Oct4 modulators defines a role of the Paf1 complex for embryonic stem cell identity. Cell Stem Cell. 2009;4:403–415. doi: 10.1016/j.stem.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Rozenblatt-Rosen O, et al. The parafibromin tumor suppressor protein is part of a human Paf1 complex. Mol Cell Biol. 2005;25:612–620. doi: 10.1128/MCB.25.2.612-620.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steward MM, et al. Molecular regulation of H3K4 trimethylation by ASH2L, a shared subunit of MLL complexes. Nat Struct Mol Biol. 2006;13:852–854. doi: 10.1038/nsmb1131. [DOI] [PubMed] [Google Scholar]
- 23.Ruthenburg AJ, Li H, Patel DJ, Allis CD. Multivalent engagement of chromatin modifications by linked binding modules. Nat Rev Mol Cell Biol. 2007;8:983–994. doi: 10.1038/nrm2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Porter SE, Washburn TM, Chang M, Jaehning JA. The yeast pafl-rNA polymerase II complex is required for full expression of a subset of cell cycle-regulated genes. Eukaryot Cell. 2002;1:830–842. doi: 10.1128/EC.1.5.830-842.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rozenblatt-Rosen O, et al. The tumor suppressor Cdc73 functionally associates with CPSF and CstF 3′ mRNA processing factors. Proc Natl Acad Sci USA. 2009;106:755–760. doi: 10.1073/pnas.0812023106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mosimann C, Hausmann G, Basler K. Parafibromin/Hyrax activates Wnt/Wg target gene transcription by direct association with beta-catenin/Armadillo. Cell. 2006;125:327–341. doi: 10.1016/j.cell.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 27.Nagaike T, et al. Transcriptional activators enhance polyadenylation of mRNA precursors. Mol Cell. 2011;41:409–418. doi: 10.1016/j.molcel.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ou CY, et al. A coactivator role of CARM1 in the dysregulation of β-catenin activity in colorectal cancer cell growth and gene expression. Mol Cancer Res. 2011;9:660–670. doi: 10.1158/1541-7786.MCR-10-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Q, et al. CARM1 is required in embryonic stem cells to maintain pluripotency and resist differentiation. Stem Cells. 2009;27:2637–2645. doi: 10.1002/stem.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang P, et al. Parafibromin, a component of the human PAF complex, regulates growth factors and is required for embryonic development and survival in adult mice. Mol Cell Biol. 2008;28:2930–2940. doi: 10.1128/MCB.00654-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheldon KE, Mauger DM, Arndt KM. A requirement for the Saccharomyces cerevisiae Paf1 complex in snoRNA 3′ end formation. Mol Cell. 2005;20:225–236. doi: 10.1016/j.molcel.2005.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng D, Côté J, Shaaban S, Bedford MT. The arginine methyltransferase CARM1 regulates the coupling of transcription and mRNA processing. Mol Cell. 2007;25:71–83. doi: 10.1016/j.molcel.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 33.Ohkura N, Takahashi M, Yaguchi H, Nagamura Y, Tsukada T. Coactivator-associated arginine methyltransferase 1, CARM1, affects pre-mRNA splicing in an isoform-specific manner. J Biol Chem. 2005;280:28927–28935. doi: 10.1074/jbc.M502173200. [DOI] [PubMed] [Google Scholar]
- 34.Kuhn P, et al. Automethylation of CARM1 allows coupling of transcription and mRNA splicing. Nucleic Acids Res. 2011;39:2717–2726. doi: 10.1093/nar/gkq1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sims RJ, 3rd, et al. The C-terminal domain of RNA polymerase II is modified by site-specific methylation. Science. 2011;332:99–103. doi: 10.1126/science.1202663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Lorenzo A, Bedford MT. Histone arginine methylation. FEBS Lett. 2011;585:2024–2031. doi: 10.1016/j.febslet.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith E, Shilatifard A. The chromatin signaling pathway: diverse mechanisms of recruitment of histone-modifying enzymes and varied biological outcomes. Mol Cell. 2010;40:689–701. doi: 10.1016/j.molcel.2010.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.