GTPases of the Rab family, which comprises more than 60 members in mammals, have emerged as key regulators of virtually all transport steps between intracellular compartments. By interacting with a diverse range of effector proteins, such as lipid kinases and phosphatases, molecular motors, tethering factors, and scaffolding proteins, they regulate the formation of transport carriers from donor membranes, their movement along cytoskeletal tracks, and their targeting to acceptor membranes (1). Like other GTPases, Rab proteins function as molecular switches, cycling between an inactive GDP-bound form and an active GTP-bound form. Because Rabs display high affinities for both GDP and GTP and very low intrinsic GTPase activity, the GDP/GTP cycle must be regulated by guanine nucleotide exchange factors (GEFs) that promote GDP release and by GTPase-activating proteins (GAPs) that stimulates GTP hydrolysis.
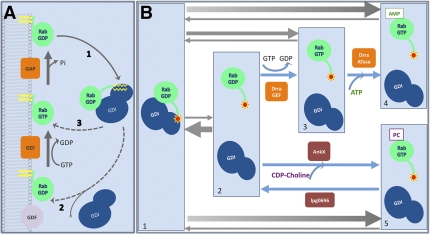
A salient feature of Rab proteins is a cycle of association/dissociation to/from membranes that is superimposed to the GDP/GTP cycle (Fig. 1A). Rabs therefore mainly exist as two pools, one membrane and GTP-bound and one cytosolic and GDP-bound (estimated depending on the Rab to range between 10% and 40% at the steady state). The stable insertion of Rabs in the outer leaflet of intracellular membranes is mediated by one or, in most cases, two geranylgeranyl lipid moieties that are attached to cysteine residue(s) present at their C terminus. This irreversible posttranslational modification occurring on newly synthesized Rabs involves a protein named REP (Rab escort protein), which presents the Rab proteins to Rab geranylgeranyl transferase (RabGGTase). Cytosolic Rabs are bound to the protein GDI (GDP Dissociation Inhibitor; two GDIs exist in mammals, GDIα/GDI-1 and GDIβ). GDI binds to Rabs with very high affinity (Kd in the nanomolar range) when they are prenylated and in the GDP state, which explains that GDI functions as a chaperone during the Rab cycle, extracting them from membranes after GAP-induced GTP hydrolysis (Fig. 1A, pathway 1) and solubilizing them in the form of RabGDP–GDI complexes. In a study in PNAS, Oesterlin et al. (2) address the question of Rab membrane attachment mechanism—the less well understood Rab cycle step.
Fig. 1.
(A) The Rab GTPase cycle. (B) Rab modifications by Legionella proteins dramatically change Rab–GDI complex affinity. A Rab protein prenylated with a fluorescently labeled farnesyl moiety (red star) forms a high-affinity complex with GDI (B, 1); the intrinsic dissociation rate of the complex is very low (B, 1 and 2), and equilibrium is shifted toward RabGDP–GDI complex (gray arrows). DrrA GEF activity converts Rab to the GTP-bound form (B, 2 and 3) snatching a small fraction of the RabGDP spontaneously released from GDI. The GTP loaded Rab has low affinity to GDI, and the equilibrium shifts toward complex dissociation. The activated Rab is recognized as a substrate by DrrA ATase domain that adenylylates the GTPase (B, 3 and 4). AnkX also relies on the free Rab pool generated by intrinsic Rab–GDI complex dissociation. The phosphocholine transferase modifies the Rabs (B, 2 and 5), rendering them incompetent to rebind to GDI and shifts the equilibrium to the complex dissociation state, whereas the lpg0696 dephosphocholination activity reverses the process (i.e., 5, then 2, in B).
The Rab membrane attachment step couples, in fact, two processes: the targeting of different Rabs to specific membranes and their activation (GDP/GTP exchange), with both processes being intimately linked. Given the high affinity of GDI for prenylated RabGDP and consequently the low intrinsic rate of GDI dissociation, certainly the crucial event of this step is the dissociation of RabGDP–GDI complexes. Two main mechanisms have been proposed to explain GDI displacement. Based on the observations that nucleotide exchange occurs shortly after binding of purified Rab5 and Rab9–GDI complexes to endosomal membranes using in vitro or semi-in vitro assays (3, 4), the first mechanism suggests the existence of membrane-associated proteins termed GDF (GDI Displacement Factor) that favor the release of GDI (Fig. 1A, pathway 2). One protein with a GDF activity has been identified, Yip3/PRA1, and shown to achieve the dissociation of Rab9–GDI complexes and to facilitate Rab9 membrane insertion (5). However, it is difficult to generalize this mechanism, as Yip3 remains the only GDF identified so far. A second mechanism postulates that nucleotide exchange plays a central role in GDI release from RabGDP (Fig. 1B, pathway 3). It is based on the study of DrrA/SidM, an effector of Legionella pneumophila. Legionella effectors reorient the host cell vesicle-trafficking pathway to grab the host ER-derived vesicles necessary for the formation and maintenance of a specialized vacuolar compartment called Legionella-containing vacuole (LCV) (6, 7). DrrA/SidM specifically localizes, immediately after infection, to the LCV membrane, where it can efficiently recruit Rab1, a Rab regulating the early secretory pathway (8). Schoebel et al. first showed that the DrrA GEF domain is sufficient to displace GDI from Rab1GDP–GDI complex by catalyzing nucleotide exchange to GTP (9). Wu et al. then demonstrated, by using a semisynthetic farnesyl-NBD Rab7 probe, that the affinity of GDI for Rab7GTP is approximately three orders of magnitude lower than the affinity for Rab7GDP. Based on these results, the authors proposed that GEFs are necessary and sufficient for Rab membrane association (10). However, the GEFs cannot interact directly with RabGDP–GDI complexes, as available crystal structures of Rab–GEF and Rab–GDI complexes show that GEFs and GDI bind to overlapping sites and thus would have to compete for Rab binding (9, 11). The GEF-mediated Rab membrane attachment mechanism then implies that the dissociation rate of the RabGDP–GDI is high enough to present enough RabGDP molecules to GEFs for generating a sufficient number of active Rabs that will interact with their effectors.
Oesterlin et al. (2) demonstrate the existence of other mechanisms that can lower the affinity of GDI for RabGDP and thus stabilize the dissociated RabGDP. This study is again based on the biochemical properties of Legionella effectors. In addition to a GEF domain, DrrA/SidM contains a N-terminal domain that possesses adenosine monophosphorylation (AMPylation or adenylylation) activity toward a conserved tyrosine residue (Tyr77) in the switch II region of Rab1b, a region involved in binding to different protein partners (12). The active GTP-bound form of Rab1 is a preferable substrate for DrrA/SidM-catalyzed adenylylation, suggesting that Rab1 activation is coupled to its modification. The recently reported identification of SidD with deadenylylation activity toward Rab1-AMP (13) confirms that AMPylation is a regulated reversible modification like many other posttranslational modifications. The other Legionella effector studied by Oesterlin et al. (2) is AnkX/Lpg0695. AnkX is a Fic domain-containing protein that was predicted to function as an AMPylating toxin (14), but it was proven later that this protein is, in fact, a phosphocholine transferase (15). AnkX modifies Rab1 and Rab35 on a serine residue adjacent to the conserved tyrosine that is the adenylylation site for DrrA/SidM (15). This modification blocks early secretory events involving vesicular transport on microtubules after exit from the ER and inhibits endocytic pathways (16).
Oesterlin et al. (2) use a fluorescence-based assay to explore the ability of DrrA
Oesterlin et al. address the question of Rab membrane attachment mechanism—the less well understood Rab cycle step.
and AnkX to displace GDI from NBD-farnesyl-Rab:GDI complexes in vitro. They show that recombinant DrrA and AnkX can efficiently adenylylate and phosphocholinate in vitro Rab1b and Rab35, respectively. Both modifications drastically lower their affinities for GDI by several orders of magnitude and could thus efficiently displace GDI from Rab1b/Rab35–GDI complexes. However, GDI displacement by adenylylation is dependent on DrrA-mediated Rab1 activation, which makes it unlikely that such a modification is meaningful in vivo to promote GDI dissociation from RabGDP–GDI complexes during membrane insertion (Fig. 1B). In contrast, the AnkX catalytic activity does not depend on the nucleotide status of Rab1b or Rab35. Even though phosphocholination does not accelerate the intrinsic rate of GDI dissociation from RabGDP–GDI complexes (as also observed for GEF-mediated nucleotide exchange), phosphocholination would prevent RabGDP from rebinding to GDI, which may favor its membrane insertion (Fig. 1B). Phosphocholination could however affect the interaction with GEFs, as shown for phosphocholinated Rab35 and its GEF connecdenn (15). However, interestingly, Legionella also expresses a dephosphorylcholinase, Lgp0696, that can reverse AnkX-mediated modification of Rab1 (17), and thus possibly permits binding of Rab1 to its GEF(s).
Posttranslational modifications of Rab GTPases represent a tentative mechanism to explain, at least in part, how Rab GTPases can be targeted to membranes. Evidence exists that phosphorylation can affect the membrane/cytosol distribution of a few Rabs (18–20). However, it remains to be established whether posttranslational modifications can be considered as a general regulatory mechanism. Interestingly, a human protein containing a Fic domain, termed HYPE (huntingtin yeast-interacting protein E) has been recently shown to possess the ability to AMPylate Rho GTPases, as bacterial Fic domain proteins do (21).
Another issue is to explain how Rabs, which are all geranylgeranylated by the same enzyme and interact with only one or two different GDIs, are targeted to specific membranes. It has long been thought that this specificity relies on the existence of a targeting signal within the C-terminal hypervariable domain of Rab GTPases (22). However, this view has been challenged by several studies showing that this is not the case for several Rabs and that membrane targeting involves multiple Rab regions (23, 24). In light of the results discussed here, it seems more and more likely that Rab targeting relies on specific GEFs and/or specifically localized enzymes that catalyze posttranslational modifications.
Footnotes
The authors declare no conflict of interest.
See companion article on page 5621.
References
- 1.Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91:119–149. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oesterlin LK, Goody RS, Itzen A. Posttranslational modifications of Rab proteins cause effective displacement of GDP dissociation inhibitor. Proc Natl Acad Sci USA. 2012;109:5621–5626. doi: 10.1073/pnas.1121161109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ullrich O, Horiuchi H, Bucci C, Zerial M. Membrane association of Rab5 mediated by GDP-dissociation inhibitor and accompanied by GDP/GTP exchange. Nature. 1994;368:157–160. doi: 10.1038/368157a0. [DOI] [PubMed] [Google Scholar]
- 4.Soldati T, Shapiro AD, Svejstrup AB, Pfeffer SR. Membrane targeting of the small GTPase Rab9 is accompanied by nucleotide exchange. Nature. 1994;369:76–78. doi: 10.1038/369076a0. [DOI] [PubMed] [Google Scholar]
- 5.Sivars U, Aivazian D, Pfeffer SR. Yip3 catalyses the dissociation of endosomal Rab-GDI complexes. Nature. 2003;425:856–859. doi: 10.1038/nature02057. [DOI] [PubMed] [Google Scholar]
- 6.Swanson MS, Isberg RR. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect Immun. 1995;63:3609–3620. doi: 10.1128/iai.63.9.3609-3620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kagan JC, Roy CR. Legionella phagosomes intercept vesicular traffic from endoplasmic reticulum exit sites. Nat Cell Biol. 2002;4:945–954. doi: 10.1038/ncb883. [DOI] [PubMed] [Google Scholar]
- 8.Murata T, et al. The Legionella pneumophila effector protein DrrA is a Rab1 guanine nucleotide-exchange factor. Nat Cell Biol. 2006;8:971–977. doi: 10.1038/ncb1463. [DOI] [PubMed] [Google Scholar]
- 9.Schoebel S, Oesterlin LK, Blankenfeldt W, Goody RS, Itzen A. RabGDI displacement by DrrA from Legionella is a consequence of its guanine nucleotide exchange activity. Mol Cell. 2009;36:1060–1072. doi: 10.1016/j.molcel.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Wu Y-W, et al. Membrane targeting mechanism of Rab GTPases elucidated by semisynthetic protein probes. Nat Chem Biol. 2010;6:534–540. doi: 10.1038/nchembio.386. [DOI] [PubMed] [Google Scholar]
- 11.Rak A, et al. Structure of Rab GDP-dissociation inhibitor in complex with prenylated YPT1 GTPase. Science. 2003;302:646–650. doi: 10.1126/science.1087761. [DOI] [PubMed] [Google Scholar]
- 12.Müller MP, et al. The Legionella effector protein DrrA AMPylates the membrane traffic regulator Rab1b. Science. 2010;329:946–949. doi: 10.1126/science.1192276. [DOI] [PubMed] [Google Scholar]
- 13.Neunuebel MR, et al. De-AMPylation of the small GTPase Rab1 by the pathogen Legionella pneumophila. Science. 2011;333:453–456. doi: 10.1126/science.1207193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roy CR, Mukherjee S. Bacterial FIC proteins AMP up infection. Sci Signal. 2009;2:pe14. doi: 10.1126/scisignal.262pe14. [DOI] [PubMed] [Google Scholar]
- 15.Mukherjee S, et al. Modulation of Rab GTPase function by a protein phosphocholine transferase. Nature. 2011;477:103–106. doi: 10.1038/nature10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan X, Lührmann A, Satoh A, Laskowski-Arce MA, Roy CR. Ankyrin repeat proteins comprise a diverse family of bacterial type IV effectors. Science. 2008;320:1651–1654. doi: 10.1126/science.1158160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan Y, Arnold RJ, Luo Z-Q. Legionella pneumophila regulates the small GTPase Rab1 activity by reversible phosphorylcholination. Proc Natl Acad Sci USA. 2011;108:21212–21217. doi: 10.1073/pnas.1114023109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bailly E, et al. Phosphorylation of two small GTP-binding proteins of the Rab family by p34cdc2. Nature. 1991;350:715–718. doi: 10.1038/350715a0. [DOI] [PubMed] [Google Scholar]
- 19.van der Sluijs P, et al. Reversible phosphorylation—dephosphorylation determines the localization of rab4 during the cell cycle. EMBO J. 1992;11:4379–4389. doi: 10.1002/j.1460-2075.1992.tb05538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fitzgerald ML, Reed GL. Rab6 is phosphorylated in thrombin-activated platelets by a protein kinase C-dependent mechanism: Effects on GTP/GDP binding and cellular distribution. Biochem J. 1999;342:353–360. [PMC free article] [PubMed] [Google Scholar]
- 21.Worby CA, et al. The fic domain: Regulation of cell signaling by adenylylation. Mol Cell. 2009;34:93–103. doi: 10.1016/j.molcel.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chavrier P, et al. Hypervariable C-terminal domain of rab proteins acts a targeting signal. Nature. 1991;353:769–712. doi: 10.1038/353769a0. [DOI] [PubMed] [Google Scholar]
- 23.Beranger F, Paterson H, Powers S, de Gunzburg J, Hancock JF. The effector domain of Rab6, plus a highly hydrophobic C terminus, is required for Golgi apparatus localization. Mol Cell Biol. 1994;14:744–758. doi: 10.1128/mcb.14.1.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ali BR, Wasmeier C, Lamoreux L, Strom M, Seabra MC. Multiple regions contribute to membrane targeting of Rab GTPases. J Cell Sci. 2004;117:6401–6412. doi: 10.1242/jcs.01542. [DOI] [PubMed] [Google Scholar]