Abstract
neuralized (neur) is a neurogenic mutant of Drosophila in which many signaling events mediated by the Notch (N) receptor are disrupted. Here, we analyze the role of neur during eye development. Neur is required in a cell-autonomous fashion to restrict R8 and other photoreceptor fates and is involved in lateral inhibition of interommatidial bristles but is not required for induction of the cone cell fate. The latter contrasts with the absolute requirement for Suppressor of Hairless and the Enhancer of split-Complex for cone cell induction. Using gain-of-function experiments, we further demonstrate that ectopic wild-type and truncated Neur proteins can interfere with multiple N-controlled aspects of eye development, including both neur-dependent and neur-independent processes.
The Drosophila eye has been a particularly useful model system for studies of cell–cell interactions during formation of a biological pattern. The adult eye consists of some 800 identical ommatidia; each is a complete unit eye containing 20 cells, including 8 photoreceptors and 12 accessory cells. The proper commitment of cell fates in the eye depends on both inhibitory and inductive cell–cell interactions mediated by multiple signaling cascades (1).
The Notch (N) receptor is directly involved in the determination of all cell types in the Drosophila eye, in addition to having other functions in controlling growth and polarity of the eye (2–6). N has two opposing functions with regard to early eye neurogenesis. It first promotes R8 photoreceptor differentiation by enhancing expression of the proneural gene for photoreceptors, atonal (7, 8). R8 is the first cell fate to differentiate in the eye and is required for recruitment of all other photoreceptors; N clones lack photoreceptors (8, 9). N is subsequently involved in restriction of the R8 fate, because a temporally restricted reduction in N function results in the differentiation of clusters of ectopic R8 cells (10). Other experiments performed during larval and pupal development further demonstrated that N is involved in the determination of outer photoreceptors, cone cells, pigment cells, and bristles (2). N thus appears to be required for all stages of ommatidial assembly.
A central “core” of proteins involved in the N pathway appears to be deployed in a wide variety of settings of N-pathway activity in invertebrates as well as vertebrates (11). The basic scaffold of the N pathway, as it is most often used, appears to be as follows. Interaction of the transmembrane ligand Delta with the transmembrane receptor Notch results in the release of a proteolytic fragment including the intracellular domain of Notch (NIC) (12–14). NIC then translocates to the nucleus, where it acts as a coactivator for the sequence-specific DNA-binding protein Suppressor of Hairless [Su(H)] (15, 16). This complex activates transcription of various target genes, which include multiple members of the Enhancer of split-Complex [E(spl)-C] (17–21).
Mutations in neuralized (neur) result in a variety of developmental defects that closely resemble those of N and other N-pathway mutants, suggesting that it also acts in this pathway (22–25). neur encodes a protein of unknown function, consisting of two copies of a novel protein domain (the “neuralized homology repeat”) and a C-terminal RING domain (26–28). In this report, we investigate the role of neur and find that it regulates a subset of N-controlled processes during eye development.
Methods
Fly Stocks.
Su(H)Δ47, P{B}/CyO was obtained from Francois Schweisguth, Ecole Normale Superieure, Paris (29). FRT82B, neurIF65/TM6C, and FRT82B, neurA101/TM6C were previously described (25). FRT82B E(spl)b32.2, P{gro+}/TM6B was obtained from Christos Delidakis, University of Crete (30, 31). FRT82B ubi-GFP(nls) and eyFLP gl-lacZ; FRT82B cl P{w+}/TM6B (32) were obtained from the Bloomington Stock Center, Bloomington, IN. GMR-Gal4 was constructed by Matthew Freeman, MRC Laboratory of Molecular Biology, Cambridge, U.K. (33), and ey-Gal4 was a gift of Tom Serano, University of California, Berkeley. UAS-neur, UAS-neurΔRF, and UAS-neur RING transgenic flies were previously described (25).
Generation of Mutant Clones and Eyes.
To generate mutant clones, 24–48 h after egg laying, larvae from a cross of ywhsFLP/Y; FRT82B, neur/TM6B, Tb and FRT82B, ubi-GFP(nls) flies were subjected to two 1-hour heat shocks at 38°C, separated by 4–6 h rest at room temperature. They were then returned to 25°C for subsequent development, and female Tb+ larvae were selected for analysis.
For eyFLP-mediated recombination, we selected Tb+ individuals from a cross of eyFLP, gl-lacZ; FRT 82B, cl, P{w+}/TM6B and w/Y; FRT82B neur/TM6C or E(spl)b32.2, P{gro+}/TM6B flies. To obtain Su(H) mutant discs, Tb+ individuals from a stock of Su(H)Δ47, P{B}/SM-TM6B, Tb maintained at 18°C were selected. All animals used in misexpression experiments by using the Gal4/UAS system were reared at 25°C. To facilitate misexpression of multiple copies of various neur transgenes, we constructed recombinant stocks containing two copies of each type of UAS-neur transgene as well as stocks containing both GMR-Gal4 and each of the UAS-neur transgenes individually.
Indirect Immunofluorescence.
We used the following primary antibodies: rabbit α-Atonal (1:2,000; gift of Yuh Nung Jan, University of California, San Francisco), rabbit α-Boss and mouse α-Boss (both used at 1:2,000; gift of S. Larry Zipursky, University of California, Los Angeles), mouse α-Elav (ascites, 1:2,000), rat α-Elav (1:10), mouse α-Prospero MR1A (1:4; gift of Chris Doe, University of Oregon), Mab323 (1:3; gift of Sarah Bray, University of Cambridge), mouse α-β-galactosidase (1:100; Developmental Studies Hybridoma Bank), rabbit α-β-galactosidase (1:3,000, Cappel), and mouse α-Cut (1:100, DHSB). Cy2-, FITC-, and RRX-conjugated secondary antibodies were purchased from Jackson ImmunoResearch and used at a dilution of 1:200.
Results
neur Is Essential for Drosophila Eye Development.
We investigated the role of neur in eye development by using the amorphic allele neurIF65 and the enhancer trap neurA101, which is a neur hypomorph (22, 34). We used the hsFLP/FRT system (35) and the eyFLP/FRT cell lethal system (32) to generate mutant clones and eyes, respectively.
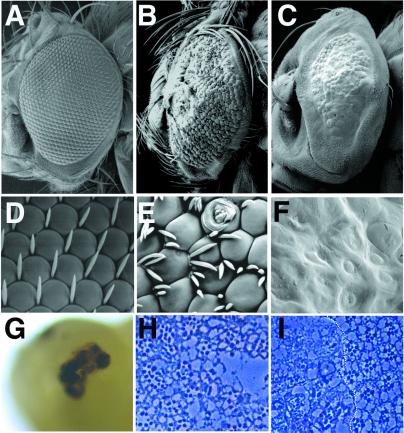
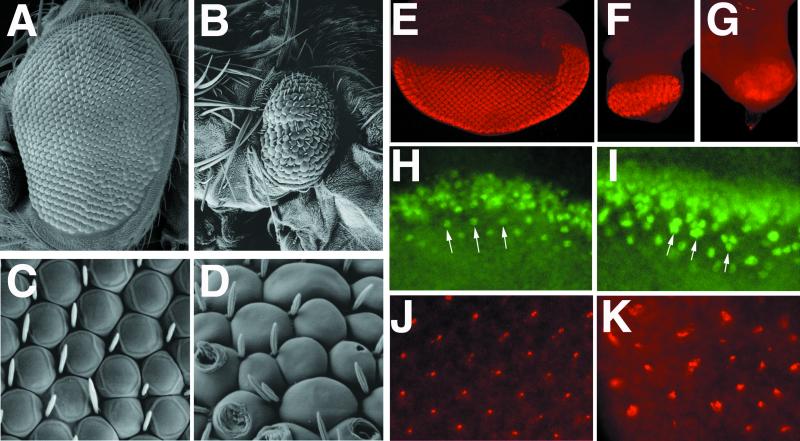
Flies containing eyes that were nearly homozygous for neurA101 were relatively healthy, although their eyes were extremely abnormal. These eyes contained ommatidia of variable sizes (including many that were larger than normal), frequent tufting of interommatidial bristles, and glazed or scarred regions (Fig. 1 A, B, D, E). Tangential sections through these eyes revealed ommatidia containing variable numbers of rhabdomeres and frequent fusion of ommatidial clusters (Fig. 1H). Flies carrying eyes homozygous for neurIF65 died during pupal development; large necrotic patches in the eye were visible by 45 h after puparium formation (Fig. 1G). Occasional escapers were obtained when they were reared at 18°C. Their ommatidia were poorly defined, lenses were not properly secreted, and all external interommatidial bristle structures were missing (Fig. 1 C and F). Sections through these mutant clones revealed that the arrangement and morphology of the rhabdomeres were severely disrupted in a cell-autonomous fashion (Fig. 1I).
Figure 1.
Phenotypes of neur mutant eyes. (A–F) Scanning electron micrographs (SEM) of adult eyes; (A–C) ≈×150; (D–F) ≈×5,000. (A, D) Wild-type eyes. (B, E) neurA101 mutant eyes generated with ey-FLP. Note tufting of interommatidial bristles, irregular sizes of ommatidia, pitting, and scarring of ommatidia. A strong degree of head macrochaetae tufting around the perimeter of the eye is also apparent. (C, F) neurIF65 mutant eyes generated with ey-FLP. Eyes are bald and smooth; ommatidia lack definition and lenses. Note that head macrochaetae are also absent. (G) Pupal head from an animal aged 45 h after puparium formation containing neurIF65 mutant eyes; a large necrotic patch at the position of the developing eye is present. (H, I) Tangential plastic sections through a neurA101 mutant eye generated by using ey-FLP (H) and an eye containing a neurIF65 mutant clone generated by using hs-FLP (I, mutant clone is left of the dotted line). neurA101 ommatidia frequently have too many rhadomeres, whereas neurIF65 clones have extremely disrupted rhabdomere morphology and numbers; compare with the normal stereotyped arrangement of rhabdomeres in wild-type ommatidia (I, right of the dotted line).
neur Is Required Autonomously for Lateral Inhibition During Photoreceptor Development.
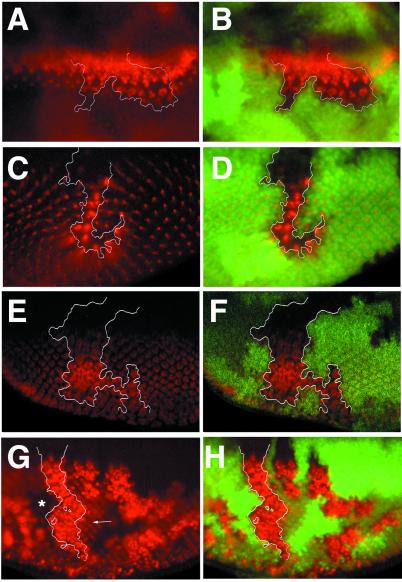
The interommatidial bristle tufting and balding phenotypes of neurIF65 and neurA101 mutant eyes, respectively, parallel their clonal phenotypes with respect to notum and head mechanosensory organs. The latter are because of defects in N-dependent lateral inhibition of sensory organ precursors and subsequent N-controlled asymmetric cell divisions in the sensory lineage (24, 25). Here we studied the requirement of neur for lateral inhibition of the R8 photoreceptor fate, a process also controlled by the N pathway (2, 36). We first examined Atonal (Ato), whose expression during eye development resolves from all nuclei just ahead of the morphogenetic furrow, to intermediate groups of ≈10 cells, to finally becoming restricted to R8. In both neurIF65 and neurA101 mutant clones and eyes, Ato was maintained in most cells of the intermediate groups in a cell-autonomous fashion (Fig. 2 A and B and data not shown). The level of Ato within neur mutant cells was comparable to, or even higher than, that in wild-type cells, suggesting that neur does not participate in the proneural phase of N activity that is required for enhancement of Ato expression (8). Differentiation of excess numbers of R8 cells in the absence of neur was confirmed by staining for the R8-specific antigen Boss; large numbers of Boss-positive cells were found in each ommatidium (Fig. 2 C and D). The discrete clusters of Boss-positive cells even in neurIF65 mutant clones suggested that not all photoreceptors differentiated as R8. Staining for the general neural marker Elav revealed substantial neural hypertrophy within neurA101 and neurIF65 eyes and clones, indicating that excess numbers of outer photoreceptors are also differentiated in the absence of neur function (Fig. 2 E and F and data not shown). We also examined the effect of neur loss of function on activity of the neur enhancer trap, which is active in most photoreceptor cells. Clones of neurA101 in the eye disc display ectopic numbers of β-galactosidase-positive cells (Fig. 2 G and H), indicative of neural hypertrophy. Thus, neur is required for lateral inhibition of photoreceptor fates in the developing eye.
Figure 2.
neur is required for lateral inhibition of photoreceptors. Clones of neurIF65 (A–F) and neurA101 (G, H) were generated with hs-FLP and are marked by the absence of nuclearly localized GFP detected in green; selected clone boundaries are outlined in white. The expression of different antigens detected in red (A, C, E, G) are shown merged with the GFP clonal marker (B, D, F, H). (A, B) Ato expression normally resolves to single presumptive R8 cells but remains expressed in large clusters within the clone and persists longer than in neighboring wild-type cells. (C, D) Boss is normally expressed in single differentiated R8 cells, but large clusters of Boss-positive cells are found within the clone. Different focal planes are shown in C and D because Boss is apically localized and the clone marker is nuclearly localized; this leads to a slight displacement in the positions of the mutant clone and the phenotypically mutant cells. (E, F) Elav is present in all photoreceptors. A large excess in Elav-positive cells is present within the clone. (G, H) Expression of β-galactosidase in neurA101 homozygous clones. A cell-autonomous increase in the number of β-galactosidase-positive cells is observed within mutant clones; compare with neighboring heterozygous tissue (G, arrow). Note that mutant cells are also homozygous for the enhancer trap and thus produce more β-galactosidase per cell than heterozygous tissue or twin-spot clones, which produce none (G, star).
neur Is Not Required for Induction of Cone Cells.
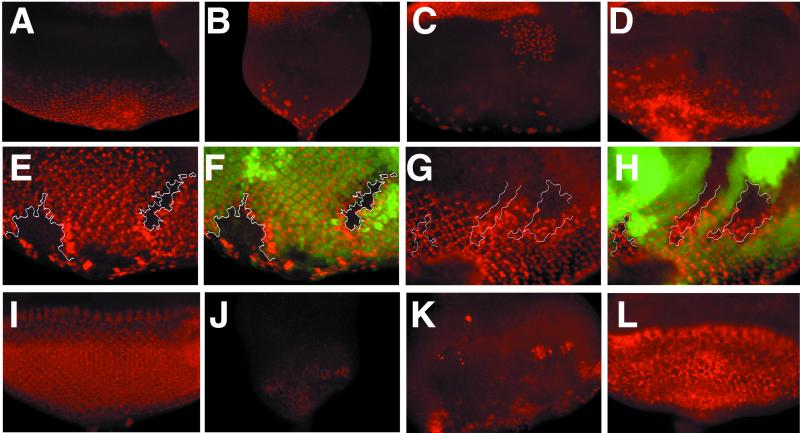
We next examined the requirement of neur for cone cell induction, a process known to depend on N and Dl (2, 37, 38). To further characterize the requirement of downstream components of the N pathway, we examined the effect of deletions of either Su(H) or the E(spl)-C on the expression of Cut and Prospero (Pros); the former is expressed by cone cells, whereas the latter is present in cone cells and R7. Su(H) and E(spl)-C mutant eyes fail to express both proteins, indicating that both are required for differentiation of R7 and cone cells (Fig. 3 A–C and data not shown). Analysis of E(spl)-C clones demonstrates that Pros (Fig. 3 E and F) and Cut (data not shown) are not expressed by mutant cells, whereas ectopic expression of both proteins is found in wild-type cells neighboring clones. This nonautonomous effect may reflect the induction of extra cone cells by E(spl)-C mutant cells, which display strong neural hypertrophy. In contrast, neur mutant clones and eyes express both antigens (Fig. 3 D, G, and H). Thus, only Su(H) and the E(spl)-C are required for cone cell induction. Finally, we examined the expression of E(spl)bHLH proteins by using Mab323, which recognizes 4 of the 7 E(spl)bHLH proteins (39). Expression of these E(spl)bHLH proteins was abolished in Su(H) discs, similar to the phenotype of N clones, but was maintained, albeit in a modified pattern, in neurIF65 mutant eyes (Fig. 3 I–L). Thus, although neur is required for N-pathway function in several settings, it is not apparently essential for the expression of at least some N-pathway target genes.
Figure 3.
Requirements of N-pathway members for cone cell induction and E(spl)bHLH expression. Eye discs from wild type (A, I); Su(H)Δ47, P{B} (B, J); eyFLP; E(spl)b32.2, P{gro+} (C, K); eyFLP; neurIF65 (D, L) larvae; eye discs containing hs-FLP induced clones of E(spl)b32.2, P{gro+} (E, F) and neurIF65 (G, H). Note that Su(H) discs are much smaller than eye discs lacking E(spl)-C or neur function. (A–D) Expression of Cut, which is found in cone cells as well as adepithelial cells on the surface of the disk; the latter can be distinguished by their larger size and their position in a different focal plane. (B) Su(H) and (C) E(spl)-C mutant discs do not express Cut in cone cells, although staining of adepithelial cells remains; (D) neur mutant discs have an altered pattern of cone cells as marked by Cut. (E–H) Staining for Pros, which is present in cone cells and R7. Mutant clones are marked by the absence of GFP and merged images are shown in F and H. (E, F) Clones of the E(spl)-C fail to express Pros in a cell-autonomous fashion, whereas excess Pros staining is observed in wild-type tissue at clone boundaries. (G, H) Clones of neurIF65 express Pros; abnormal pattern of Pros is also observed in wild-type tissue at clone boundaries. (I–L) Expression of Mab323, which recognizes 4 of the 7 E(spl)bHLH proteins. Expression is virtually absent in a Su(H) disc (J) and largely eliminated in the E(spl) mutant disc (K); the latter indicates that eyFLP-mediated recombination resulted in an eye disk that is >95% mutant. (L) Expression of Mab323 is maintained in neur mutant disc.
Misexpression of Neur and Truncated Neur Proteins Interferes with Eye Development.
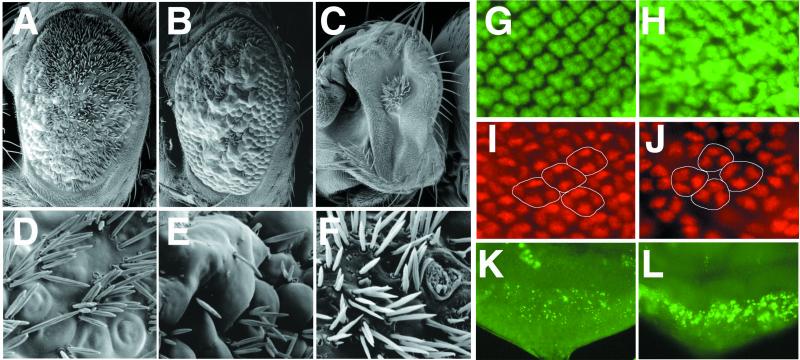
We have previously generated a series of transgenic flies in which wild-type and truncated forms of Neur can be induced by using the Gal4-UAS system (25, 40). Neur consists of two copies of a novel domain referred to as “neuralized homology repeat” and a C-terminal RING finger (27, 28). When activated by using GMR-Gal4, wild-type Neur, Neur deleted for the RING finger (NeurΔRF), and the Neur RING finger alone (Neur RING) could all cause an adult rough eye phenotype. The GMR-neur phenotype was comparable to, although slightly stronger than, that of GMR-neurΔRF when one copy of either UAS-transgene was present; both displayed mild ommatidial disarray and tufting of interommatidial bristles (data not shown). When three copies of either neur-transgene were present, clear phenotypic differences were evident. GMR-3xneur eyes showed extreme interommatidial bristle tufting and some fusion and general ommatidial disarray (Fig. 4 A and D), whereas GMR-3xneurΔRF eyes contained regions of bristle tufting and balding as well as regions of strong ommatidial fusion (Fig. 4 B and E). Eyes expressing either one or two copies of Neur RING under the control of GMR-Gal4 displayed a small eye phenotype with apparent bristle tufting and a high degree of ommatidial pitting and scarring (Fig. 4 C and F).
Figure 4.
Effect of misexpression of Neur and truncated Neur proteins on adult eye morphology and third instar eye disc development. (A–F) SEM of adult eyes of the following genotypes: (A, D) GMR-Gal4; 3xUAS-neur; (B, E) GMR-Gal4; 3xUAS-neurΔRF; (C, F) GMR-Gal4; 2xUAS-neur RING. Misexpression of Neur leads to ommatidial fusions and extreme interommatidial bristle tufting (A, D); compare with wild-type eyes (Fig. 1 A and D). Misexpression of NeurΔRF leads to more pronounced ommatidial fusions and regions of bristle tufting as well as bristle loss (B, E). Misexpression of Neur RING results in a very small disorganized eye (C, F). (G–L) Third instar eye discs of the following genotypes: (G, I, K) wild type, (H, J) GMR-Gal4; 3xUAS-neur, (L) GMR-Gal4; 3xUAS-neur RING. (G–J) Posterior regions of the eye disc are shown at a higher magnification than in K and L. Misexpression of Neur causes a mild increase in photoreceptor numbers as marked by Elav (G, H) and decreases the number of cone cells in each ommatidia (circled) as marked by Cut from four to three (I, J). Misexpression of Neur RING results in a strong increase in cell death as marked by acridine orange staining fragments (K, L).
Although Neur and NeurΔRF easily perturbed the adoption of interommatidial bristle fates, the consequences on photoreceptor and cone cell patterning were relatively mild. In GMR-3xneur eye discs, photoreceptor patterning in the posterior of the disc was abnormal; mild neural hypertrophy and fusion of clusters were apparent (Fig. 4 G and H). In addition, we found that ommatidia contained three cone cells instead of four, as marked by Cut (Fig. 4 I and J). GMR-3xneurΔRF discs displayed a milder disruption in general photoreceptor patterning, whereas the large majority of ommatidia contained four cone cells (data not shown).
Eye discs misexpressing one or three copies of UAS-neur RING under the control of GMR-Gal4 displayed mild to severe derangement of patterning in the posterior of the eye disc as marked by Elav, Boss, and Mab323 (data not shown), and suggested a defect in cell viability. To test the possibility that misexpression of Neur RING induces cell death, we stained these disks with acridine orange, which marks dying cells. We found that GMR-3xNeur RING discs indeed have a large excess of staining fragments in the posterior of the eye disc, in approximately the same location where normal patterning is lost (Fig. 4 K and L and data not shown). Because neur mutant eye discs do not display significant ectopic cell death at the imaginal disc stage (data not shown), this phenotype elicited by the RING finger might represent a nonspecific effect.
Misexpression of NeurΔRF Anterior to the Furrow Impairs Eye Growth and Lateral Inhibition of R8 Cells.
To assess the effect of ectopic Neur on lateral inhibition of R8 cells, we used ey-Gal4, which is active anterior to the morphogenetic furrow. Misexpression of one or two copies of UAS-neur or one copy of UAS-neurΔRF with ey-Gal4 resulted in only minor defects in the overall appearance of the adult eye and did not alter the pattern of Ato and Boss (Fig. 5 A and C and data not shown). However, misexpression of two copies of UAS-neurΔRF led to a dramatic reduction in the size of the adult eye (Fig. 5 B and D). The basis of the small eyes in this genotype is distinct from that caused by misexpression of Neur RING, as the corresponding eye discs in the latter are wild-type in size (Fig. 4L). Instead, eye discs in which high levels of NeurΔRF have been misexpressed anterior to the furrow closely resemble eye discs lacking Su(H) function, which display a severe growth defect in addition to their neurogenic phenotype (Fig. 5 E–G).
Figure 5.
Misexpression of NeurΔRF anterior to the furrow inhibits eye disc growth and lateral inhibition. (A–D) SEM of ey-Gal4; 2xUAS-neur (A, C) and ey-Gal4; 2xUAS-neurΔRF; (B, D) adult eyes. (E–K) Expression of cell-type specific markers in ey-Gal4/+ (E, H, J), ey-Gal4; 2xUAS-neurΔRF (F, I, K), or Su(H) (G) discs. (E–G) Expression of Elav; misexpression of NeurΔRF results in an extremely small retinal field that displays neural hypertrophy (F), similar to the phenotype of Su(H) (G). (H, I) Expression of Ato; resolution of Ato to single R8 cells is incomplete after misexpression of NeurΔRF. A row that has resolved to single Ato-expressing cells in wild-type (H, arrows) maintains Ato in clusters of ≈3 cells (I, arrows) following misexpression of NeurΔRF. (J, K) Expression of Boss; multiple Boss-positive cells are observed in many ommatidia following misexpression of NeurΔRF.
ey-2x neurΔRF eyes also appeared to have undergone significant neural hypertrophy based on the large size of Elav-positive photoreceptor clusters, suggesting impaired lateral inhibition. Indeed, staining for Ato revealed groups of three Ato positive cells at a position where Ato expression has singularized in wild type (Fig. 5 H and I). Ato eventually disappears as it does normally; in so doing, it apparently resolves to single cells in ey-2xneurΔRF discs. To distinguish between a delayed resolution of Ato expression and a failure of lateral inhibition, we examined expression of Boss. We observed that clusters of Boss-expressing cells frequently arise in these discs, indicating that multiple R8 cells have differentiated and that lateral inhibition of this fate has indeed been compromised (Fig. 5 J and K). Thus, NeurΔRF has dominant-negative activity with respect to lateral inhibition of photoreceptor fates and is further capable of interfering with eye disc growth, a N- and Su(H)-controlled process that is neur-independent (see Fig. 3).
Discussion
neur Is Required for a Subset of N-Dependent Processes During Eye Development.
In this report, we investigated the requirement for neur during eye development and found that it is required only for a subset of N-dependent cell fate choices. Notably, we determined that neur is essential for lateral inhibition of the R8 photoreceptor fate. Thus, neur is essential for lateral inhibitory processes involving two distinct populations of imaginal disc cells, R8 cells and sensory organ precursors (refs. 24 and 25; this report). In light of these findings, it is curious that neur is dispensable for lateral inhibition during wing vein determination (25). N also mediates a variety of inductive events, and neur is required for some of these (determination of the mesectoderm) but not for others (determination of the wing margin, induction of cone cells) (ref. 41; this report). Overall, there does yet not appear to be an obvious way to categorize all Neur-dependent N-mediated processes.
Although N is known to be involved in induction of the cone cell fate (2), the precise role of the N pathway in this process in unclear. N signaling via Su(H) has recently been shown to activate expression of D-Pax2 in cone cells (37); however, cone cell development in D-pax2 mutants is abnormal but not eliminated (42). E(spl)bHLH proteins are also expressed in cone cells, and we observe that this expression [as well as other aspects of retinal E(spl)bHLH expression] is Su(H)-dependent. In addition, we find that cone cells fail to differentiate in eyes mutant for either Su(H) or E(spl)-C. These results suggest that the full canonical N pathway is required for cone cell induction. Because the requirement for E(spl)-C in cone cell induction is cell-autonomous, one possibility is that E(spl)bHLH proteins may repress the activity of another repressor of the cone cell fate. The ETS-domain repressor Yan has recently been shown to capable of directly repressing at least two genes that are expressed in cone cells (D-pax2 and prospero) and may thus be a target of E(spl)bHLH repression during cone cell induction (37, 43, 44).
Model for Neur Function.
The RING finger domains from several otherwise unrelated proteins have recently been shown to have ubiquitin ligase activity (45), suggesting a model in which Neur may directly ubiquitinate a target protein whose degradation is required for N-pathway activity. The dominant-negative activity of NeurΔRF might then be reasonably interpreted as an isoform that can bind its cognate target but is unable to mediate its degradation, resulting in a failure of N signaling. Although we have shown that endogenous Neur is required for only a subset of N-controlled processes, we find that ectopic Neur and NeurΔRF proteins are able to affect a wide variety of N-pathway-dependent processes, including those that require, and others that are independent of, endogenous neur. Examples of the latter class include the ability of Neur and NeurΔRF to interfere with lateral inhibition of wing veins and the ability of NeurΔRF to compromise formation of the wing margin and growth of the retinal portion of the eye disc (ref. 25; this report). These observations suggest that Neur affects the function of a “core” component of the N pathway. Finally, we have shown that in two different settings, during lateral inhibition of sensory organ precursors and of R8 cells, neur acts cell-autonomously. An attractive candidate target of Neur ubiquitin ligase activity that is consistent with all of these observations is Delta. Although activation of the N pathway by Delta is nonautonomous, it has been shown that Delta also autonomously interferes with the ability of a cell to activate the N pathway (46). Degradation of Delta by Neur might then autonomously potentiate the ability of a cell to receive a signal and activate the N pathway. Tests of this hypothesis are currently underway.
Acknowledgments
We are grateful to Francois Schweisguth, Christos Delidakis, Tom Serano, Chris Doe, S. Larry Zipursky, Yuh Nung Jan, Sarah Bray, and the Developmental Studies Hybridoma Bank under the auspices of the National Institute of Child Health and Human Development for making fly stocks and antibodies available to us for these studies. We thank Adina Bailey and Amy Tang for providing useful comments on the manuscript. E.L. was supported by a Cancer Research Fund of the Damon Runyon–Walter Winchell Foundation Fellowship, DRG 1632.
Abbreviations
- N
Notch
- neur
neuralized
- Su(H)
Suppressor of Hairless
- E(spl)-C
Enhancer of split-Complex
- Ato
Atonal
- Pros
Prospero
References
- 1.Brennan C A, Moses K. Cell Mol Life Sci. 2000;57:195–214. doi: 10.1007/PL00000684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cagan R L, Ready D F. Genes Dev. 1989;3:1099–1112. doi: 10.1101/gad.3.8.1099. [DOI] [PubMed] [Google Scholar]
- 3.Go M J, Eastman D S, Artavanis-Tsakonas S. Development (Cambridge, UK) 1998;125:2031–2040. doi: 10.1242/dev.125.11.2031. [DOI] [PubMed] [Google Scholar]
- 4.Papayannopoulos V, Tomlinson A, Panin V M, Rauskolb C, Irvine K D. Science. 1998;281:2031–2034. doi: 10.1126/science.281.5385.2031. [DOI] [PubMed] [Google Scholar]
- 5.Dominguez M, de Celis J F. Nature (London) 1998;396:276–278. doi: 10.1038/24402. [DOI] [PubMed] [Google Scholar]
- 6.Cho K-O, Choi K-W. Nature (London) 1998;396:272–276. doi: 10.1038/24394. [DOI] [PubMed] [Google Scholar]
- 7.Jarman A, Grell E, Ackerman L, Jan L, Jan Y. Nature (London) 1994;369:398–400. doi: 10.1038/369398a0. [DOI] [PubMed] [Google Scholar]
- 8.Baker N E, Yu S-Y. Curr Biol. 1997;7:122–132. doi: 10.1016/s0960-9822(06)00056-x. [DOI] [PubMed] [Google Scholar]
- 9.Ready D F, Hanson T E, Benzer S. Dev Biol. 1976;53:217–240. doi: 10.1016/0012-1606(76)90225-6. [DOI] [PubMed] [Google Scholar]
- 10.Baker N E, Yu S, Han D. Curr Biol. 1996;6:1290–1301. doi: 10.1016/s0960-9822(02)70715-x. [DOI] [PubMed] [Google Scholar]
- 11.Artavanis-Tsakonas S, Rand M D, Lake R J. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 12.Rebay I, Fleming R J, Fehon R G, Cherbas L, Cherbas P, Artavanis-Tsakonas S. Cell. 1991;67:687–699. doi: 10.1016/0092-8674(91)90064-6. [DOI] [PubMed] [Google Scholar]
- 13.Struhl G, Adachi A. Cell. 1998;93:649–660. doi: 10.1016/s0092-8674(00)81193-9. [DOI] [PubMed] [Google Scholar]
- 14.Lecourtois M, Schweisguth F. Curr Biol. 1998;8:771–774. doi: 10.1016/s0960-9822(98)70300-8. [DOI] [PubMed] [Google Scholar]
- 15.Tamura K, Taniguchi Y, Minoguchi S, Sakai T, Tun T, Furukawa T, Honjo T. Curr Biol. 1995;5:1416–1423. doi: 10.1016/s0960-9822(95)00279-x. [DOI] [PubMed] [Google Scholar]
- 16.Fortini M E, Artavanis-Tsakonas S. Cell. 1994;79:273–282. doi: 10.1016/0092-8674(94)90196-1. [DOI] [PubMed] [Google Scholar]
- 17.Bailey A M, Posakony J W. Genes Dev. 1995;9:2609–2622. doi: 10.1101/gad.9.21.2609. [DOI] [PubMed] [Google Scholar]
- 18.Nellesen D T, Lai E C, Posakony J W. Dev Biol. 1999;213:33–53. doi: 10.1006/dbio.1999.9324. [DOI] [PubMed] [Google Scholar]
- 19.Lecourtois M, Schweisguth F. Genes Dev. 1995;9:2598–2608. doi: 10.1101/gad.9.21.2598. [DOI] [PubMed] [Google Scholar]
- 20.Furukawa T, Kobayakawa Y, Tamura K, Kimura K, Kawaichi M, Tanimura T, Honjo T. Jpn J Genet. 1995;70:505–524. doi: 10.1266/jjg.70.505. [DOI] [PubMed] [Google Scholar]
- 21.Lai E C, Bodner R, Posakony J W. Development (Cambridge, UK) 2000;127:3441–3455. doi: 10.1242/dev.127.16.3441. [DOI] [PubMed] [Google Scholar]
- 22.Lehmann R, Jiménez F, Dietrich U, Campos-Ortega J. Roux's Arch Dev Biol. 1983;192:62–74. doi: 10.1007/BF00848482. [DOI] [PubMed] [Google Scholar]
- 23.Hartenstein A Y, Rugendorff A, Tepass U, Hartenstein V. Development (Cambridge, UK) 1992;116:1203–1220. doi: 10.1242/dev.116.4.1203. [DOI] [PubMed] [Google Scholar]
- 24.Yeh E, Zhou L, Rudzik N, Boulianne G L. EMBO J. 2000;19:4827–4837. doi: 10.1093/emboj/19.17.4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai E C, Rubin G M. Dev Biol. 2001;231:217–233. doi: 10.1006/dbio.2000.0124. [DOI] [PubMed] [Google Scholar]
- 26.Boulianne G L, de la Concha A, Campos-Ortega J A, Jan L Y, Jan Y N. EMBO J. 1991;10:2975–2983. doi: 10.1002/j.1460-2075.1991.tb07848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Price B D, Chang Z, Smith R, Bockheim S, Laughon A. EMBO J. 1993;12:2411–2418. doi: 10.1002/j.1460-2075.1993.tb05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura H, Yoshida M, Tsuiki H, Ito K, Ueno M, Nakao M, Oka K, Tada M, Kochi M, Kuratsu J, Ushio U, Saya H. Oncogene. 1998;16:1009–1019. doi: 10.1038/sj.onc.1201618. [DOI] [PubMed] [Google Scholar]
- 29.Morel V, Schweisguth F. Genes Dev. 2000;14:377–388. [PMC free article] [PubMed] [Google Scholar]
- 30.Schrons H, Knust E, Campos-Ortega J A. Genetics. 1992;132:481–503. doi: 10.1093/genetics/132.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heitzler P, Bourouis M, Ruel L, Carteret C, Simpson P. Development (Cambridge, UK) 1996;122:161–171. doi: 10.1242/dev.122.1.161. [DOI] [PubMed] [Google Scholar]
- 32.Newsome T P, Asling B, Dickson B J. Development (Cambridge, UK) 2000;127:851–860. doi: 10.1242/dev.127.4.851. [DOI] [PubMed] [Google Scholar]
- 33.Freeman M. Cell. 1996;87:651–660. doi: 10.1016/s0092-8674(00)81385-9. [DOI] [PubMed] [Google Scholar]
- 34.Bellen H J, O'Kane C J, Wilson C, Grossniklaus U, Pearson R K, Gehring W J. Genes Dev. 1989;3:1288–1300. doi: 10.1101/gad.3.9.1288. [DOI] [PubMed] [Google Scholar]
- 35.Xu T, Rubin G M. Development (Cambridge, UK) 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- 36.Ligoxygakis P, Yu S Y, Delidakis C, Baker N E. Development (Cambridge, UK) 1998;125:2893–2900. doi: 10.1242/dev.125.15.2893. [DOI] [PubMed] [Google Scholar]
- 37.Flores G V, Duan H, Yan H, Nagaraj R, Fu W, Zou Y, Noll M, Banerjee U. Cell. 2000;103:75–85. doi: 10.1016/s0092-8674(00)00106-9. [DOI] [PubMed] [Google Scholar]
- 38.Parks A L, Muskavitch M A. Dev Biol. 1993;157:484–496. doi: 10.1006/dbio.1993.1151. [DOI] [PubMed] [Google Scholar]
- 39.Jennings B, Preiss A, Delidakis C, Bray S. Development (Cambridge, UK) 1994;120:3537–3548. doi: 10.1242/dev.120.12.3537. [DOI] [PubMed] [Google Scholar]
- 40.Brand A H, Perrimon N. Development (Cambridge, UK) 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 41.Martin-Bermundo M D, Carmena A, Jimenez F. Development (Cambridge, UK) 1995;121:219–224. doi: 10.1242/dev.121.1.219. [DOI] [PubMed] [Google Scholar]
- 42.Fu W, Noll M. Genes Dev. 1997;11:2066–2078. doi: 10.1101/gad.11.16.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu C, Kauffmann R, Zhang J, Kladny S, Carthew R W. Cell. 2000;103:87–97. doi: 10.1016/s0092-8674(00)00107-0. [DOI] [PubMed] [Google Scholar]
- 44.Lai Z-C, Rubin G M. Cell. 1992;70:609–620. doi: 10.1016/0092-8674(92)90430-k. [DOI] [PubMed] [Google Scholar]
- 45.Joazeiro C, Weissman A M. Cell. 2000;102:549–552. doi: 10.1016/s0092-8674(00)00077-5. [DOI] [PubMed] [Google Scholar]
- 46.Jacobsen T L, Brennan K, Arias A M, Muskavitch M A T. Development (Cambridge, UK) 1998;125:4531–4540. doi: 10.1242/dev.125.22.4531. [DOI] [PubMed] [Google Scholar]