Abstract
The steep adolescent decline in the slow wave (delta, 1–4 Hz) electroencephalogram (EEG) of nonrapid eye movement (NREM) sleep is a dramatic maturational change in brain electrophysiology thought to be driven by cortical synaptic pruning. A perennial question is whether this change in brain electrophysiology is related to sexual maturation. Applying Gompertz growth models to longitudinal data spanning ages 9–18 y, we found that the timing of the delta decline was significantly (P < 0.0001) linked to timing of pubertal maturation. This timing relation remained significant when sex differences in the timing of the delta decline were statistically controlled. Sex differences and the relation to the timing of puberty jointly explained 67% of the between-subject variance in the timing of the delta decline. These data provide a demonstration of a temporal relation between puberty and an electrophysiological marker of adolescent brain development. They can guide research into whether the neuroendocrine events of puberty are mechanistically linked to cortical maturation or whether, instead, the two maturational processes are parallel but independent programs of human ontogenesis.
Keywords: fast Fourier transform, Tanner stage
In an ongoing longitudinal study of adolescent sleep EEG, we identified the age range 12–16.5 y as a critical period of late brain maturation (1). Centrally recorded slow wave (delta, 1–4 Hz) EEG power in nonrapid eye movement (NREM) sleep declines by >60% in this 4.5-y range. We (2) and others (3, 4) have proposed that the decline in delta power reflects the cortical synaptic pruning discovered by Huttenlocher (5). The decline in delta power also bears importantly on the physiology of sleep regulation because NREM slow wave EEG seems to reflect a recuperative process (6, 7); it increases with prior waking duration and declines across the night.
A recurrent question is whether the dramatic changes in sleep electrophysiology during adolescence are related to the concurrent physical changes of puberty. Previous studies have found lower levels of visually scored slow wave sleep (8) or delta power (3) in more sexually mature subjects, but these studies did not control for age. In 2006, we analyzed the data then available from our longitudinal study to examine sleep EEG changes in relation to both age and pubertal maturation (9). We reported a strong relation between the rate of the delta decline and the rate of progression through the Tanner stages of pubertal maturation. However, both rates were strongly related to age. When age was statistically controlled, the relation between delta power and Tanner stage became nonsignificant.
Our 2006 article included data only from ages 9–11 and 12–14 y and evaluated the rate of delta decline but not its timing. We are now able to analyze the timing of the relation between the decline in NREM delta EEG power and pubertal maturation in an expanded longitudinal dataset, which now includes ages 9–18 y. These analyses show a highly significant relation between the timing of the delta decline and the timing of pubertal development. We also demonstrate that delta power declines earlier in girls than in boys and that this sex difference does not account for the timing relation between pubertal development and electrophysiological brain maturation.
The data presented below are from longitudinal sleep EEG recordings of 67 children in two age cohorts. One cohort (n = 30, 15 female) entered the study at approximately age 9 y and was studied for 7 y. The second cohort (n = 37, 19 female) entered the study at approximately age 12 y and was studied for 6 y. Thus, the study spanned ages 9–18 y with the two cohorts overlapping across ages 12–16 y. We recorded all-night sleep EEG semiannually with ambulatory devices. The subjects slept at home in their own beds on their current school-night sleep schedules. Average power in delta (1–4 Hz) and theta (4–8 Hz) power during the first 5 h of nonrapid eye movement (NREM) sleep was calculated with fast Fourier transform (FFT) analysis. Within a month of the EEG recordings, a physician performed a physical examination that included Tanner stage ratings (10) of breast development in girls, genital development in boys (Tannerb/g), and pubic hair growth in both sexes (Tannerph).
Results
The data here confirm our previous report (1) on the maturational trend of NREM delta power. Delta power declined slightly between ages 9 and 12 y and then dropped steeply until age 16.5 y, when its rate of decline markedly slowed. This pattern of decline was fit with a Gompertz function by using SAS nonlinear mixed effect analysis. Although delta power declined in every subject, there were large individual differences in the age trends (Fig. 1A). Mixed effect analysis revealed significant between-subject differences (P < 0.0001) in several Gompertz function parameters: the upper asymptote, the change from the upper to lower asymptotes, and the age at which delta power declined most rapidly. The Gompertz equation includes a fourth parameter, the relative rate of the decline. Allowing this term to vary between subjects prevented nonlinear mixed effect analysis from converging. Structural equation modeling (SEM) sacrifices precision in modeling age effects but is able to treat all four Gompertz parameters as random. SEM analyses showed that the relative rate of decline also differed significantly between subjects. There was no covariance between the relative rate of decline and the other terms. Notably, a delay in maturation was not associated with a more rapid decline.
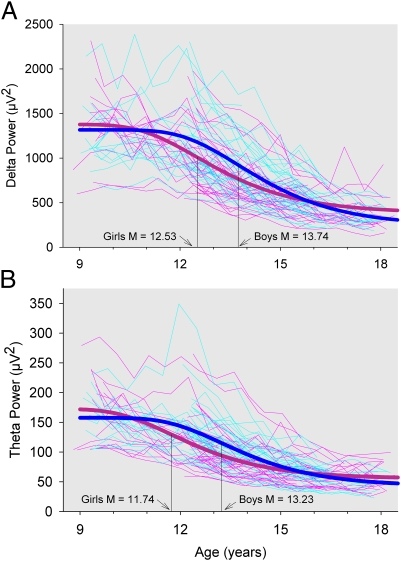
Fig. 1.
(A) Delta power at each semiannual recording is plotted against age for each male (faint blue) and each female (faint pink) subject. The delta power decline across adolescence was fit with a Gompertz equation, 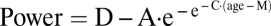 . The age of steepest decline, M, differed significantly (P < 0.0001) between individual subjects and, on average, occurred earlier in girls (heavy pink) than in boys (heavy blue). (B) Theta power at each semiannual recording is plotted against age using the same format as in Fig. 1A. The age of steepest decline for theta differed significantly (P < 0.0001) between individual subjects, occurred earlier in girls than in boys, and occurred earlier than the age of steepest decline for delta.
. The age of steepest decline, M, differed significantly (P < 0.0001) between individual subjects and, on average, occurred earlier in girls (heavy pink) than in boys (heavy blue). (B) Theta power at each semiannual recording is plotted against age using the same format as in Fig. 1A. The age of steepest decline for theta differed significantly (P < 0.0001) between individual subjects, occurred earlier in girls than in boys, and occurred earlier than the age of steepest decline for delta.
The between-subject variation in the timing of the delta decline enabled us to explore factors related to this timing difference. Specifically, we could evaluate sex differences in the timing of the delta decline and determine whether a delay in the decline was significantly associated with a delay in pubertal maturation.
Fig. 1A shows that the age of most rapid delta power decline in girls occurred at 12.53 (± SE 0.19) y, which was 1.21 (±0.25) y earlier than that for boys. This sex difference in age of most rapid delta power decline was highly significant (t64 = 4.78; P < 0.0001) and explained 32% of its between-subject variance. Neither the upper asymptote of the delta curve (t64= −0.50; P = 0.62) nor the change from the upper to lower asymptotes (t64 = 0.85; P = 0.40) differed significantly between boys and girls despite the significant between-subject variability in these coefficients. In addition, the relative rate of decline did not differ significantly between boys and girls (P = 0.13).
Using pubertal timing measures extracted from the Tanner stage ratings (10), we tested whether the timing of the decline in delta power was related to the timing of pubertal maturation. We found a robust relationship between this maturational change in sleep electrophysiology and pubertal development. Specifically, the age of most rapid delta power decline was significantly (P < 0.0001) related to the age of most rapid pubertal development for both the Tannerph and Tannerb/g measures of pubertal development. A 1-y delay in age of most rapid increase in Tannerph was associated with a 0.86 (±0.12) y delay in the age of most rapid decline of delta power. Similarly, a 1-y delay in the age of most rapid increase in Tannerb/g was associated with a 0.62 (±0.12) y delay in the age of most rapid delta decline. Analyzing the relation of the delta decline to both pubertal timing measures simultaneously revealed that the timing of the delta decline was more strongly related to the Tannerph timing measure (P = 0.020) than to the Tannerb/g timing measure (P = 0.90); therefore, we used the Tannerph timing measure for subsequent analyses. The relation of the delta decline to Tannerph timing accounted for 50% of the between-subject variance in the timing of the delta power decline. The age of most rapid increase in Tannerph significantly (t66 = 11.1; P < 0.0001) preceded the age of most rapid decline in delta power by an average of 1.08 (±0.10) y.
We next investigated the possibility that the delta–puberty relationship was simply a manifestation of earlier development in girls, i.e., that the delta–puberty timing relationship depended on the sex difference described above. We tested this possibility by simultaneously analyzing the maturational pattern of delta power in relation to both sex and pubertal timing. The results showed that the age of most rapid delta power decline was significantly and independently related to both sex (P < 0.0001) and the timing of pubertal maturation (P < 0.0001). Together, the sex differences and the relation to pubertal maturation explained a remarkable 67% of the between-subject variance in the timing of the delta decline. Table 1 summarizes these results. To further test the independence of sex and puberty timing effects, we analyzed the relation of the timing of the delta decline to the timing of puberty separately in girls and boys. For both girls and boys, a 1-y delay in age of most rapid increase in Tannerph was significantly (P = 0.0016 and 0.014, respectively) associated with a delay (0.78 and 0.47 y respectively) in the age of most rapid delta decline.
Table 1.
Timing of the adolescent delta power decline: Sex differences and relation to pubertal timing
| Model | Delta M, y | Delta M adjustment | Adjustment significance | Between-subject M variance | Variance explained, % |
| Gompertz | 13.08 | — | — | 0.84 | · |
| Gompertz + Sex | 12.53 | 1.21 | P < 0.0001 | 0.57 | 32 |
| Gompertz + Tannerph M | 13.11 | 0.85 | P < 0.0001 | 0.42 | 50 |
| Gompertz + Tannerb/g M | 13.04 | 0.62 | P < 0.0001 | 0.51 | 39 |
| Gompertz + Sex + Tannerph M | 12.64 | — | — | 0.28 | 67 |
| Sex | — | 0.90 | P < 0.0001 | — | — |
| Tannerph M | — | 0.71 | P < 0.0001 | — | — |
The Gompertz equation fit to the age-related change in delta power declined most rapidly (delta M) at 13.08 y. Delta M differed significantly between sexes, with girls’ delta M at 12.53 y and boys’ delta M 1.21 y later. Delta M was also significantly related to the timing of pubertal maturation, with delta M occurring 0.85 y later for every year later in Tannerph M (age of most rapid Tanner stage increase) and 0.62 y later for every year later in Tannerb/g M. The timing of the delta decline was significantly related to both sex and pubertal timing when sex and Tannerph M effects were evaluated simultaneously.
Although most studies on sleep recuperation or “homeostasis” have focused on delta (1–4 Hz) NREM EEG, it has become recognized in recent years that NREM theta (4–8 Hz) behaves similarly with respect to prior waking duration and age. Thus, theta (4–8 Hz) EEG power during NREM sleep also declines steeply across adolescence. However, its decline begins earlier than the decline in delta power, a difference we have attributed to maturation in different brain circuits (1). We next tested for a relation between the timing of the decline in NREM theta power to the timing of pubertal maturation. As with the delta data, we fit a Gompertz equation to the decline in theta EEG power by using nonlinear mixed effect analysis. The most rapid decline for theta power occurred significantly earlier than that for delta power (12.4 vs. 13.1 y, 95% confidence intervals do not overlap), confirming our previous report of earlier theta than delta power decline (1). As with delta power, the timing of the theta power decline (Fig. 1B) differed significantly between subjects (t64 = 7.24; P < 0.0001), differed between sexes (t64 = 4.33, P < 0.0001), and was related to the timing of pubertal maturation (t64 = 5.81; P < 0.0001). A 1-y delay in age of most rapid increase in Tannerph was associated with a 0.90 (±0.15) y delay in the age of most rapid decline of theta power. A simultaneous analysis similar to that for delta showed that the age of most rapid theta power decline was significantly and independently related to both sex (P < 0.0001) and the timing of pubertal maturation (P < 0.0001). The relations to sex and pubertal timing explained 71% of the between-subject variance in the age of most rapid theta power decline. The age of most rapid increase in Tannerph significantly (t66 = 4.4; P < 0.0001) preceded the age of most rapid decline in theta power by an average of 0.45 (±0.10) y.
Discussion
As mentioned above, we hypothesize that the steep decline in delta power across adolescence is a marker of late brain maturation driven by cortical synaptic pruning (1, 2). Synaptic pruning could decrease delta power in two ways. Decreasing the synaptic connections among cortical neurons would decrease the size of the pool of neurons that oscillate in synchrony to produce delta waves. A decreasing population of oscillating neurons would diminish delta wave power by reducing wave amplitude. We further hypothesized that a decrease in synaptic connectivity across adolescence would reduce the intensity of neuronal activity during waking as indicated by the decline in waking cortical metabolic rate across adolescence (11). Less intense waking neuronal activity would decrease the need (substrate) for NREM recuperation, reducing the intensity (power) of the two recuperative frequencies, delta and theta. Further indirect support for this interpretation is provided by topographic differences in the timing of the delta decline (12, 13). The age of fastest NREM delta decline shows a back-to-front distribution similar to the back-to-front maturational pattern reported for structural MRI-measured cortical thinning (14). Cortical thinning has also been attributed to regional changes in synaptic density (15–17), although recent studies suggest that expansion of white matter and other architectural changes play an important role (18).
The findings here establish a clear and robust relationship between the age of most rapid pubertal (Tanner stage) maturation and the age of most rapid delta power decline. If our hypothesis that the delta decline reflects synaptic pruning is correct, these findings demonstrate that the timing of cortical synaptic pruning is strongly linked to the timing of puberty. The decline in theta EEG power presumably represents the maturation of different cortical circuits. The finding that NREM theta maturation is also significantly associated with the timing of pubertal maturation further supports a link between pubertal maturation and the maturation of brain circuitry. Although girls typically undergo pubertal maturation before boys, our analyses show that these electrophysiological-pubertal timing relations are statistically independent of this sex difference. Even within sexes, subjects with later sexual maturation show a delay in the brain maturational processes, underlying the adolescent declines in delta and theta power. Conversely, the significant sex difference even with pubertal timing statistically controlled demonstrates that the earlier pubertal maturation in girls does not fully explain the sex difference in the timing of the delta decline.
Tannerph ratings reflect maturation of the hypothalamic–pituitary–adrenal axis and production of adrenal androgens that drive axillary hair growth, whereas Tannerb/g ratings indicate maturation of the hypothalamic–pituitary–gonadal axis and production of gonadal sex hormones (19). The finding that the delta decline is more strongly related to Tannerph than to Tannerb/g raises the possibility of a stronger relation of brain maturation to the maturation of the HPA axis. This interpretation would be premature because a stronger relation to Tannerph than to Tannerb/g ratings might simply indicate that the former are more accurately or reliably judged. Both ratings were based solely on visual inspection.
The strong relations between the timing of pubertal maturation and the timing of the delta and theta power declines do not necessarily imply that the neuroendocrine events of puberty are driving the brain maturation reflected in these EEG measurements. Furthermore, the significantly earlier age of most rapid Tanner stage increase, although consistent with a causative relation to cortical maturation, does not establish causation. Both the maturation reflected in the delta decline and the developmental steps in puberty may be components of programmed sequences of late brain maturation that are not themselves mechanistically linked. One limitation of the current study is that we relied on secondary sex characteristics as markers of pubertal maturation. Our strong findings provide an impetus for undertaking the imposing task of directly assessing pubertal neuroendocrine changes in a longitudinal EEG study. Longitudinal studies of children with precocious puberty or delayed puberty would present an alternate approach to evaluating a potential mechanistic link between these neuroendocrine events and cortical maturation.
The hypothesis that the human brain undergoes a pervasive maturational reorganization during the second decade of life is now widely accepted. Cortical synaptic density, cortical metabolic rate and delta wave amplitude each decline by ≈50% between ages 10 and 20 y with roughly parallel age curves (20). Synaptic pruning in adolescence may be the final ontogenetic manifestation of the mechanism of overproduction and subsequent pruning of neural elements used repeatedly in earlier development of the nervous system. It may represent a sacrifice of the relative plasticity of the child's brain (e.g., ability to recover function after lesions) for increased speed and efficiency of information processing. Elucidation of the factors determining the adolescent maturation of sleep EEG could therefore shed light on the fundamental mechanisms that give rise to the adult brain. The data we present here should contribute to more focused investigations of the relations of cortical and neuroendocrine maturations.
Materials and Methods
Subjects.
At the start of the study, 70 subjects were enrolled in two age cohorts. Subjects in the C9 cohort (n = 32, 16 girls) entered the study at approximately age 9 y and were studied until age 16 y. Subjects in the C12 cohort (n = 38, 19 girls) entered the study at approximately age 12 y and were studied until age 18 y. At the time of enrollment, a parental interview screened subjects for sleep disturbances and neurologic and psychiatric illnesses. Subject retention was outstanding with 67 subjects completing the first 3 y of the study and 56 completing the entire 6 or 7 y. Only data from the 67 subjects who completed at least 3 y of the study are included in the analyses presented here. The subjects were selected from the population of a university town and likely do not reflect the demographics of the society as a whole. This group of 67 subjects had the following racial/ethnicity distribution: 79% non-Hispanic white, 7% Asian, 5% Hispanic, 3% African-American, and 7% mixed race. At age 12 (the first time point when the cohorts overlap), average body mass index was 19.9 with a SD of 2.7. Subjects received monetary compensation for participating. The UC Davis human subjects Institutional Review Board approved all procedures.
Recording Schedule.
Semiannually, all-night sleep EEG was recorded for four consecutive nights at the homes of subjects in their typical sleep environment. The first two nights, typically Wednesday and Thursday, subjects maintained their habitual weekday sleep schedules. On the final two nights, typically Friday and Saturday, subjects kept their habitual weekday bedtimes but were instructed to sleep as long as possible up to 12 h. During the 5 d before EEG recording, subjects adhered to their habitual weekday sleep schedules. Daytime napping was prohibited on these 5 d and on the four recording days. Actigraphy watches (Minimitter A16) confirmed schedule compliance. Subjects who deviated from the schedule were rescheduled for recording in the following weeks. Sleep schedules were allowed to change as children progressed through adolescence. Details on adolescent sleep duration changes in these subjects have been published (21).
EEG Recording and Analysis.
EEG electrodes applied at C3, C4, Fz, Cz, O1, and either Pz or O2 were referred to mastoid electrodes, A1 or A2. Left and right outer canthus electrodes were referred to a forehead electrode for electrooculogram recording. Signals were recorded with Grass H2O (200 Hz digitization) or Grass Aura (400 Hz digitization) ambulatory EEG recorders. The two recorder types have similar frequency response curves in the range of EEG frequencies (1–8 Hz) analyzed here (22).
Using a computer display of digitized EEG, each 20-s epoch was visually scored as waking, stage 1, NREM sleep, rapid eye movement sleep, or movement time using Rechtschaffen and Kales (23) criteria modified by collapsing stages 2, 3, and 4 into a single NREM stage. Artifacts were marked separately from vigilance state. C3/A2 or C4/A1 EEG was chosen for analysis based on which signal had fewer artifacts. All artifact free epochs scored as NREM were analyzed with FFT by using the spectral analysis component of Pass Plus (Delta Software). FFT parameters were 5.12-s Welch tapered windows with 2.62 s of overlap, producing eight windows in each 20-s epoch. To avoid effects of the change in sleep duration across adolescence, average power was calculated in the first 5 h of NREM sleep. Average power was determined for both delta (1–4 Hz) and theta (4–8 Hz) EEG. Although sleep opportunity was extended on night 3, the first 5 h of NREM sleep occurred during the habitual time in bed before the sleep extension. Therefore, each semiannual recording provide three nights of data for these analyses. Night 4 data were not used. The semiannual data point for each subject was the average of the data on the three baseline nights. Delta power was consistent from night to night. The average deviation from the three-night mean was <9% for each baseline night.
Pubertal Maturation.
Within 1 mo of EEG recording, subjects visited a physician for a physical examination that included Tanner stage ratings (10). The same physician performed all of the examinations across the entire study. Tanner stage ratings include two components: a five-point maturity rating based on pubic hair growth in both boys and girls and a five-point maturity rating based on breast development in girls and genital development in boys.
Statistical Analyses.
The maturational trend in delta EEG power was fit with a Gompertz equation. Gompertz equations fit growth patterns characterized by a period of stability followed by a period of rapid change toward a second asymptote. We used the form 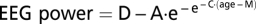 with the following terms: D = power at the upper asymptote (μV2); A = difference in power between the upper and lower asymptotes (μV2); M = age at which power is decreasing most rapidly (y); C = relative rate of decline at age M (1/y).
with the following terms: D = power at the upper asymptote (μV2); A = difference in power between the upper and lower asymptotes (μV2); M = age at which power is decreasing most rapidly (y); C = relative rate of decline at age M (1/y).
A Gompertz equation was fit to the declining delta power trend with SAS procedure NLmixed treating the D, A, and M coefficients as random, i.e., varying between subjects. NLmixed produced estimates and SDs of the four coefficients as well as estimates and significance of the between-subject variance in D, A, and M and covariances between these coefficients. NLmixed did not converge when the C coefficient was random. We used structural equation modeling to evaluate the model with all four coefficients random. Structural equation modeling is a more flexible framework for studying multivariate change but sacrifices precision in modeling age effects, and the Gompertz model is approximated through linearization (24). Sex differences in the three random coefficients were evaluated with NLmixed by adding a sex term (female = 0, male = 1) with a multiplier to each of the three coefficients and determining whether multipliers differed significantly from 0. Similarly, in the structural equation modeling analysis, the sex term was mean centered and added as a predictor of the four latent variables representing the four coefficients of the Gompertz equation.
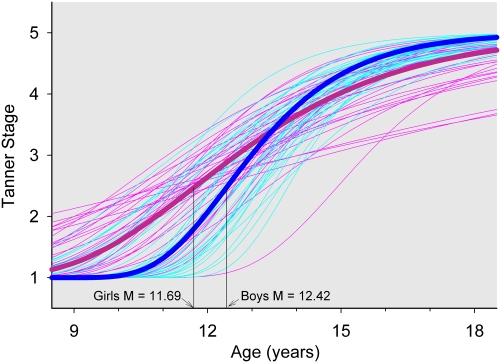
Rather than using the five-point ordinal Tanner stage ratings as a time-varying covariate, we used Tanner ratings to produce a pubertal timing measure for each subject. Independently for both pubic hair ratings and breast/genital rating, a Gompertz equation (with a positive A parameter) was fit to Tanner stage ratings. Because all people begin at Tanner stage 1 and end at Tanner stage 5, the lower asymptote and difference between asymptotes were fixed at 1 and 4, respectively. The age of most rapid decline and relative rate of decline varied significantly between subjects. The analysis produced for each subject, the subject's difference from the average age of most rapid decline. This value was used for subsequent analyses of the relation of pubertal timing to maturational change in delta power. Two tests indicate that this value is a valid measure of pubertal timing. The Gompertz pubertal timing measure was earlier in girls (Fig. 2) for both Tannerph (0.73 ± 0.28 y earlier; P = 0.012) and Tannerb/g (1.00 ± 0.31 y earlier; P = 0.0017). Also when analyzing only girls, the pubertal timing measure was significantly related to the age of menarche (Tannerph P < 0.0001; Tannerb/g P = 0.0002).
Fig. 2.
Tannerph stage Gompertz growth curves for each boy (faint blue) and each girl (faint pink) were estimated with nonlinear mixed effect analysis. The age of most rapid increase for each subject was used in subsequent analyses as a marker of the timing of pubertal maturation. The validity of this estimate is demonstrated by the earlier average maturation in girls (heavy pink) than in boys (heavy blue) and by the significant P < 0.0001 relation between the age of most rapid Tanner stage increase and the age of menarche in female subjects (see text).
Acknowledgments
We thank Roxanne Sanders for the Tanner stage evaluations and we thank the subjects who participated in this study. This work was supported by US Public Health Service Grant R01 MH62521.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Campbell IG, Feinberg I. Longitudinal trajectories of non-rapid eye movement delta and theta EEG as indicators of adolescent brain maturation. Proc Natl Acad Sci USA. 2009;106:5177–5180. doi: 10.1073/pnas.0812947106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feinberg I. Schizophrenia: Caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982-1983/1983;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 3.Jenni OG, Carskadon MA. Spectral analysis of the sleep electroencephalogram during adolescence. Sleep. 2004;27:774–783. [PubMed] [Google Scholar]
- 4.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 6.Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 7.Feinberg I. Changes in sleep cycle patterns with age. J Psychiatr Res. 1974;10:283–306. doi: 10.1016/0022-3956(74)90011-9. [DOI] [PubMed] [Google Scholar]
- 8.Carskadon MA, et al. Pubertal changes in daytime sleepiness. Sleep. 1980;2:453–460. doi: 10.1093/sleep/2.4.453. [DOI] [PubMed] [Google Scholar]
- 9.Feinberg I, Higgins LM, Khaw WY, Campbell IG. The adolescent decline of NREM delta, an indicator of brain maturation, is linked to age and sex but not to pubertal stage. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1724–R1729. doi: 10.1152/ajpregu.00293.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanner JM. Growth at Adolescence. 2nd Ed. Oxford: Blackwell; 1962. [Google Scholar]
- 11.Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Ann Neurol. 1987;22:487–497. doi: 10.1002/ana.410220408. [DOI] [PubMed] [Google Scholar]
- 12.Feinberg I, de Bie E, Davis NM, Campbell IG. Topographic differences in the adolescent maturation of the slow wave EEG during NREM sleep. Sleep. 2011;34:325–333. doi: 10.1093/sleep/34.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurth S, et al. Mapping of cortical activity in the first two decades of life: A high-density sleep electroencephalogram study. J Neurosci. 2010;30:13211–13219. doi: 10.1523/JNEUROSCI.2532-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gogtay N, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- 16.Jernigan TL, Trauner DA, Hesselink JR, Tallal PA. Maturation of human cerebrum observed in vivo during adolescence. Brain. 1991;114:2037–2049. doi: 10.1093/brain/114.5.2037. [DOI] [PubMed] [Google Scholar]
- 17.Shaw P, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends Neurosci. 2006;29:148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grumbach MM, Styne DM. Puberty: Ontogeny, neuroendocrinology, physiology, and disorders. In: Wilson JD, Foster DW, Kronenberg HM, Larsen PR, editors. Williams Textbook of Endocrinology. 9th Ed. Philadelphia: Saunders; 1998. pp. 1509–1551. [Google Scholar]
- 20.Feinberg I, Thode HC, Jr, Chugani HT, March JD. Gamma distribution model describes maturational curves for delta wave amplitude, cortical metabolic rate and synaptic density. J Theor Biol. 1990;142:149–161. doi: 10.1016/s0022-5193(05)80218-8. [DOI] [PubMed] [Google Scholar]
- 21.Feinberg I, Davis NM, de Bie E, Grimm KJ, Campbell IG. The maturational trajectories of NREM and REM sleep durations differ across adolescence on both school-night and extended sleep. Am J Physiol Regul Integr Comp Physiol. 2012;302:R533–R540. doi: 10.1152/ajpregu.00532.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campbell IG, et al. Adolescent changes in homeostatic regulation of EEG activity in the delta and theta frequency bands during NREM sleep. Sleep. 2011;34:83–91. doi: 10.1093/sleep/34.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring Systems for Sleep Stages of Human Subjects. Washington, DC: Public Health Services, US Govt Print Office; 1968. [Google Scholar]
- 24.Browne M, du Toit SHC. Models for learning data. In: Collings L, Horn JL, editors. Best Methods for the Analysis of Change. Washington, DC: Am Psychol Assoc; 1991. pp. 47–68. [Google Scholar]