Abstract
Phytochromes (phy) are red/far-red–absorbing photoreceptors that regulate the adaption of plant growth and development to changes in ambient light conditions. The nuclear transport of the phytochromes upon light activation is regarded as a key step in phytochrome signaling. Although nuclear import of phyA is regulated by the transport facilitators far red elongated hypocotyl 1 (FHY1) and fhy1-like, an intrinsic nuclear localization signal was proposed to be involved in the nuclear accumulation of phyB. We recently showed that nuclear import of phytochromes can be analyzed in a cell-free system consisting of isolated nuclei of the unicellular green algae Acetabularia acetabulum. We now show that this system is also versatile to elucidate the mechanism of the nuclear transport of phyB. We tested the nuclear transport characteristics of full-length phyB as well as N- and C-terminal phyB fragments in vitro and showed that the nuclear import of phyB can be facilitated by phytochrome-interacting factor 3 (PIF3). In vivo measurements of phyB nuclear accumulation in the absence of PIF1, -3, -4, and -5 indicate that these PIFs are the major transport facilitators during the first hours of deetiolation. Under prolonged irradiations additional factors might be responsible for phyB nuclear transport in the plant.
Keywords: Arabidopsis, basic helix-loop-helix, signal transduction
Plant development is strictly regulated by the environmental light conditions. Even though light is ubiquitously present, spectral composition, intensity, and light direction varies depending on the local environment. To adapt on local light conditions plants are equipped with a highly developed repertory of photoreceptors (1, 2).
Plant phytochromes are mainly involved in detection of red and far-red light wavelengths of the spectrum. A family of five members in Arabidopsis thaliana regulates the major developmental steps in the life of the plant: germination, photomorphogenesis, and flowering (3, 4). Despite the sequence similarities and identical spectroscopic features, the two major phytochromes, phyA and phyB, show overlapping as well as unique photosensory and functional characteristics (5–7). The light-stable phyB mediates red/far-red reversible low fluence response, whereas the light-labile phyA, is involved in far-red light sensing (high irradiance response) and in responses to very weak light (very low fluence response, VLFR) (8).
Phytochromes are cytosolic proteins of 125 kDa that are translocated into the nucleus upon light activation (6, 9–11). Nuclear transport is an essential step in phytochrome signaling and a prerequisite for the interaction with transcription factors in the nucleus (12, 13). Concerning the kinetics of nuclear import, severe differences can be found between the two major plant phytochromes, phyA and phyB (6). PhyA cannot be detected in nuclei of dark-grown plants but is rapidly transported into the nucleus upon light irradiation. In contrast, low levels of phyB are present in the nucleus even in dark-grown seedlings. The nuclear accumulation of phyB does not reach its maximum until 4 h after irradiation, which is at least one order-of-magnitude slower than the nuclear transport of phyA (6). The diverse kinetics of phytochrome nuclear accumulation suggests two distinct mechanisms responsible for the nuclear transport of phyA and phyB.
Recent studies showed that the nuclear transport of phyA depends on the two paralogous proteins far red elongated hypocotyl 1 (FHY1) and fhy1-like (FHL) (13–16). Both proteins interact in a light-dependent manner with phyA and bear a functional nuclear localization signal (NLS) and nuclear exclusion signal (13, 14, 17–20). In the absence of FHY1 and FHL phyA remains in the cytoplasm and nuclear accumulation of phyA is no longer detected after light irradiation (13). In vitro studies and the fact that an NLS fused to the phyA can complement the fhy1-mutant phenotype revealed a “piggy-back” mechanism as basis of the phyA nuclear transport (16, 21). The recognition of the phyA-FHY1/FHL complex by the nuclear import machinery is mediated by the NLS of FHY1 and FHL. It was reported that a functional NLS and a phyA-binding-site of FHY1 are necessary and sufficient to promote the light-dependent nuclear transport of phyA (16). PhyB nuclear transport seems not to be affected in the absence of FHY1 and FHL (13).
Until now there has been no evidence for a similar mechanism involved in the nuclear import of phyB. Sequence analysis pointed to a bipartite NLS located in the C terminus of the phyB molecule (22). In vivo localization studies showed that the C-terminal half of phyB that lacks the chromophore binding domain indeed has the ability to localize in the nucleus (23, 24). Based on these results, a model of a light-dependent unmasking of an NLS that is located in the C terminus of phyB was proposed as a mechanism of light-dependent nuclear import (24). This hypothesis does not take into consideration that the N-terminal half of phyB also showed in vivo nuclear localization (23). However, no light-dependency has been observed for the nuclear localization of this N-terminal half, although this fragment showed normal spectral activity (23).
We recently showed that a unique in vitro system consisting of isolated nuclei of the green algae Acetabularia is suitable to study the nuclear import of the plant photoreceptor phyA (21). This system is independent of exogenously added ATP or cytosolic factors. Nuclear import in as well as nuclear export out of manually isolated nuclei of Acetabularia showed the same characteristics as in vivo. Because no functional phytochrome has been identified in Acetabularia up to now (25–27), the system brings ideal prerequisites to investigate nuclear transport of phytochromes.
Here we use this system to test the nuclear transport characteristics of full-length phyB as well as N- and C-terminal phyB fragments in vitro and show that the nuclear import of phyB can be reconstituted by transport factors that bear an NLS and interact with phyB. We additionally show that in vivo nuclear accumulation of phyB is reduced in the pifq background that is lacking the bHLH transcription factors phytochrome-interacting factor (PIF)1, -3, -4, and -5 (28). We thus propose that nuclear transport of phyB requires transport facilitators, as for example the PIF proteins, and is not accomplished by an intrinsic NLS.
Results
To test nuclear transport in vitro, fluorescent-labeled phytochromes were incubated for 1.5 h together with the isolated nuclei of Acetabularia at room temperature. The samples were either kept in darkness or were irradiated with red or far-red light. Before microscopic analysis, the nuclei were washed with fresh buffer to remove the proteins that remained in the surrounding buffer.
We first tested whether the ability to accumulate actively in the nucleus is an intrinsic feature of the phyB molecule itself. Therefore, we purified phyB-GFP-TAP fusion protein from 6-wk-old light-grown transgenic plants. Difference spectra of the purified phyB-GFP-TAP showed that it was spectroscopically active (Fig. 1B). Because of its ability to form homodimers, endogenous phyB was copurified by the phyB-GFP-TAP out of the wild-type plants (Fig. 1A).
Fig. 1.
PIF3 acts as a transport facilitator for phyB nuclear import in vitro. (A) phyB-GFP-TAP was purified from plants. Lane 1 shows the analysis of the purified proteins on a silver-stained SDS-polyacrylamide gel and lane 2 shows the corresponding immunoblot analysis using a polyclonal antibody against phyB. M, Marker bands are denoted in kilodaltons. (B) The purified phyB-GFP-TAP shows spectral activity. The difference absorbance spectra were recorded after irradiation with saturating far-red (Pr-Pfr) and red (Pfr-Pr) light. (C) Isolated Acetabularia nuclei were incubated with phyB-GFP-TAP. Nuclear transport of phyB-GFP-TAP was only induced in the presence of PIF3, but not when phyB-GFP-TAP was added solely to the system. Incubations took place either under continuous red light (R), far-red light (FR), or in darkness (D). (DIC images are shown Left, fluorescence images are shown Right). (Scale bars, 20 μm.)
When phyB-GFP-TAP was added to isolated nuclei of Acetabularia and incubated under red light, we could not detect phyB-GFP-TAP import into the nucleus (Fig. 1C). We thus conclude that phyB does not contain a functional NLS, irrespective of whether it is in the Pr- or Pfr-form.
Hence, we tested whether the import of phyB is, like in the case of phyA (21), also dependent on transport facilitators. Such a transport facilitator should posses an NLS and should be able to interact with phyB in a light-dependent manner. Because these properties have already been shown for the basic helix–loop–helix transcription factor PIF3 (29), we tested whether PIF3 can induce nuclear transport of phyB in our in vitro test system. A GST-tagged PIF3 was expressed in Escherichia coli and purified via glutathione-Sepharose. For nuclear transport studies the GST-tag was proteolytically removed. When PIF3 was added together with phyB-GFP-TAP to the isolated nuclei, a nuclear import of phyB could be detected (Fig. 1C). PIF3-induced nuclear import of phyB occurred under irradiation with red light and far-red light, as well as after preirradiation with RG9 light and subsequent incubation in darkness (Fig. 1C).
Previous studies analyzed the phyB–PIF3 interaction in details and could show that both the N and C termini of phyB can interact with PIF3 (29, 30). We therefore tested if PIF3 is also able to facilitate the import of these two large phytochrome fragments. The phyB-fragments phyBN (amino acids 1–450) and PHYBC (amino acids 594–1172) were expressed in E. coli as GST-fusion-proteins and purified on glutathione-affinity columns (Material and Methods). PhyBN was reconstituted in vitro with the chromophore phycocyanobilin to obtain spectral activity and in case of PHYBC the GST-tag was proteolytically removed before labeling with fluorochromes. As expected, neither phyBN nor PHYBC accumulated in the isolated nuclei when they were added solely to the in vitro assay (Fig. 2). When we tested the influence of PIF3 on the nuclear transport of the fluorescent-labeled phyBN and PHYBC we observed that PIF3 induced the nuclear import of both phyB-fragments (Fig. 2). Additionally we could detect a clear light-dependency of the nuclear import of phyBN in the presence of PIF3. PIF3 induced nuclear transport of phyBN under red light but not under far-red light (Fig. 2).
Fig. 2.
PIF3 as well as the artificial protein NLS-APB facilitated the nuclear transport of phyB-fragments. Isolated Acetabularia nuclei were incubated with N- or C-terminal phyB-fragments that had been labeled with Alexa Fluor 488. Neither phyBN nor PHYBC were imported in to isolated nuclei when they were added solely to the system. Nuclear transport of both fragments was induced in the presence of PIF3, as well as the artificial protein NLS-APB. For the nuclear transport of phyBN a light dependency was observed. When a point mutation (G767 to R) was introduced in the C-terminal phyB-fragment, PIF3 did not induce nuclear transport of PHYBC-G767R. Incubations took place either under continuous red light (R), far-red light (FR), or in darkness (D). (DIC images are shown Left, fluorescence images are shown Right). (Scale bars, 20 μm.)
To narrow down the requirements for a protein that can promote the transport of phyB into the nucleus, we designed a synthetic protein consisting of the SV40 large T-antigen NLS and phyB-binding domain of PIF3 (APB) (31). When the purified synthetic protein NLS-APB was added together with fluorescent-labeled phyBN and PHYBC to the in vitro nuclear transport assay, we could observe exactly the same results as for PIF3. NLS-APB induced nuclear transport of PHYBC as well as phyBN, the latter in a light-dependent manner (Fig. 2). Regarding the nuclear transport of phyB, the NLS and the phyB-binding domain seem to be sufficient for PIF3 function.
Earlier studies in Arabidopsis showed that nuclear complex formation of phyB depends on PIF3 (32). In contrast to the situation in vivo, phyB did not show accumulation in nuclear complexes in any of the analyzed Acetabularia nuclei. Although PIF3 and NLS-APB clearly mediated nuclear import of phyB, we never observed nuclear speckles in the isolated nuclei from Acetabularia (Figs. 1C and 2).
To further test the specificity of our findings, we used a mutated phyB for the import assays. It was shown previously that the point-mutation G767-to-R in the C-terminal half of phyB, which is impaired in PIF3-binding, does not localize in the nucleus in vivo (23, 30, 33). Surprisingly, this mutated phyB was capable of transducing the phyB signal when fused to an NLS (23). We analyzed whether the ability to bind PIF3 can directly be linked to the loss of nuclear accumulation of the mutated phyB. We therefore expressed a C-terminal fragment of phyB that carried the point-mutation G767-to-R (PHYBC-G767R) as a GST fusion-protein in E. coli. The purified protein GST-PHYBC-G767R was labeled with Alexa Fluor 488 and incubated together with isolated nuclei of Acetabularia. GST-PHYBC-G767R did not accumulate in the isolated nuclei and nuclear transport could also not be induced in the presence of PIF3, which is in contrast to the wild-type fragment (Fig. 2). Thus, the G767-to-R point-mutation clearly prevents the PIF3 induced nuclear import of the C-terminal phyB fragment.
Even though PIF3 did mediate nuclear transport of phyB in our in vitro system, previous in vivo localization studies of phyB-YFP fusion proteins in pif3 mutants could still detect phyB in the nucleus (32). If PIF3 is involved in nuclear import of phyB in vivo, the nuclear transport of phyB might not be dominantly regulated by a single factor. We therefore analyzed nuclear accumulation of phyB-YFP in the pifq background that is not only lacking PIF3 but also PIFs 1, 4, and 5 (28). Dark-grown seedlings that expressed the transgene PHYB-YFP in the pifq and wild-type background were irradiated with red light before we measured nuclear accumulation of phyB-YFP. All pictures were taken with identical exposure time and used for subsequent quantification of the fluorescence intensity (Material and Methods). The quantitative data indicated that under nonsaturating conditions (weak or strong R, 4.5 h, 13 or 43 μmol⋅m−2⋅s−1, 656 nm) the nuclear import of phyB-YFP was clearly reduced when we compared two independent lines expressing phyB-YFP in the pifq background to the control plants (Fig. 3). In parallel, we performed the quantification of nuclear phyB-YFP with seedlings that were kept for 12 h in white light instead of red light. In contrast to the nonsaturating conditions, the phyB-YFP levels in nuclei of pifq showed only a slight reduction of nuclear accumulation compared with wild-type plants (Fig. 3A).
Fig. 3.
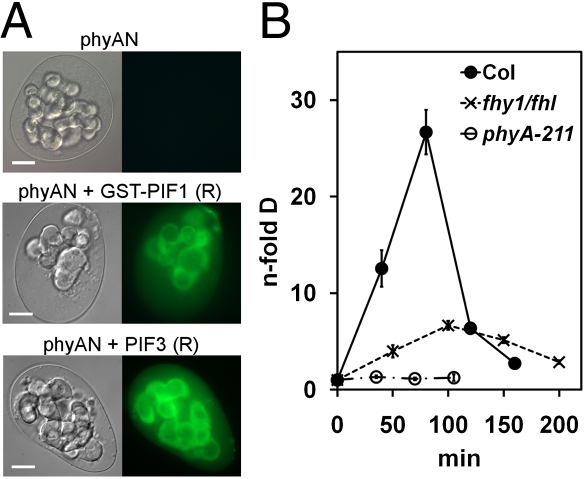
Quantification of phyB-YFP nuclear accumulation in the pifq and wild-type background. Nuclear import of phyB-YFP was analyzed in 5-d-old etiolated seedlings expressing P35S::PHYB-YFP in the pifq (two independent lines pifq-03 and pifq-11) and wild-type background by quantification of epifluorescence microscopy pictures. (A) Mean gray value of nuclei was analyzed after irradiation using either saturating (12-h white light) or nonsaturating (weak R: 4.5 h, 13 μmol⋅m−2⋅s−1 or strong R: 4.5 h, 43 μmol⋅m−2⋅s−1) conditions. Nuclear fluorescence is given relative to the corresponding wild-type control. (B) Quantification of phyB-YFP accumulation in the nuclei after irradiation with strong red light (43 μmol⋅m−2⋅s−1) measured over time. n ≥ 18; error bars, SEM.
We also followed nuclear accumulation of phyB-YFP over time to analyze whether nuclear import of phyB was not only less sensitive to light intensity but also slower in the absence of PIF1, -3, -4, and -5. Nuclear accumulation of phyB-YFP measured 60 and 90 min after the onset of irradiation with red light was therefore compared with the level of phyB-YFP already present in the nuclei of dark-grown plants (Fig. 3B). Our measurements revealed that the steady import of phyB-YFP was clearly detectable after 60 and 90 min, when we analyzed seedlings expressing the transgene in the wild-type background. In contrast, we could not measure any increase in phyB-YFP accumulation in the nuclei of seedlings that expressed phyB-YFP in the pifq background. Even after 90-min irradiation with red light, phyB-YFP levels in the nuclei did not rise above the dark level in the absence of the four PIF proteins.
When we compared the protein level of the transgene in pifq and wild-type backgrounds, we could detect that phyB-YFP was expressed at even higher levels in the pifq background than in our control line (Fig. S1). The reduced accumulation of phyB in the nuclei of pifq seedlings can therefore not be explained by an overall reduction of phyB level in these plants. Nuclear transport of phyB-YFP was therefore clearly reduced—but not completely abolished—in the absence of the bHLH transcription factors PIF1, -3, -4, and -5.
It was hypothesized that FHY1 and FHL specifically evolved to regulate phyA nuclear transport. Because our in vitro studies propose that the nuclear transport of phyB could depend on transcription factors that interact with phyB, we were curious whether in vitro nuclear transport of phyA can be achieved independently of the already known transport helpers FHY1 and FHL. Such a transport mechanism should only have a minor influence of the in vivo situation, because the phenotype of fhy1/fhl double-mutants resemble the phyA mutant in far-red light (18). However, circumstantial evidence indicated the existence of phyA-mediated responses also in the fhy1/fhl double-mutants. These responses have been explained by a cytosolic function of phyA (15), although the required little amount of active phyA in the nucleus could have also been imported by an FHY1/FHL-independent mechanism.
To test the hypothesis in vivo, we expressed another phyA interacting PIF protein, namely PIF1 as GST-tagged fusion protein (Material and Methods) and added GST-PIF1, as well as the already used PIF3 together with labeled phyAN to isolated nuclei. PhyAN, which was not imported in the absence of FHY1, was clearly detected in the Acetabularia nuclei when either PIF3 or GST-PIF1 was added to the assay (Fig. 4A). In the presence of PIF3 we could even detect a light-dependent nuclear import of phyAN (Fig. S2). Based on our in vitro data, the induction of the nuclear transport of phyA is not only a privilege of FHY1 and FHL, but can also be obtained by the transcription factors PIF1 and PIF3.
Fig. 4.
Nuclear transport of phyAN and analysis of phyA-induced AtPRR9 expression. (A) Isolated nuclei were incubated with phyAN that had been labeled with Alexa Fluor 514. Nuclear transport of phyAN was induced in the presence of GST-PIF1 as well as PIF3 when incubated under continuous red light (R). (DIC images are shown Left, fluorescence images are shown Right). (Scale bars, 20 μm.) (B) Expression levels of AtPRR9 under VLFR-conditions in wild-type (Col), phyA-mutant (phyA-211), and fhy1/fhl double-mutant were analyzed by quantitative RT-PCR. Transcript levels normalized to ACTIN1 are given relative to dark levels. Data represent the average of two biological replicates (error bars, SEM).
We further analyzed the physiological relevance of a phyA nuclear transport independent of FHY1 and FHL. Because localization studies with GFP-tagged phyA could not detect phyA in the nucleus in an fhy1/FHL RNAi line, we decided to use a more sensitive method to study phyA responses in fhy1/fhl double-mutants. We therefore analyzed gene expression of an early responding light-inducible gene, PRR9 (pseudoresponse regulator 9). PRR9 is a member of a small family of genes encoding Arabidopsis pseudoresponse regulators and is associated to the circadian clock (34). Expression of PRR9 is rapidly induced after light activation by the phytochromes (35, 36). Real-time RT-PCR analysis in dark-grown wild-type seedlings detected a maximal induction of PRR9-expression around 80 min after a 30-s pulse-treatment with very weak red light (Fig. 4B). As expected, phyA mutants showed no reaction to this weak-light pulse treatment concerning the PRR9 expression. In contrast, fhy1/fhl double mutants showed a low but significant induction of PRR9 expression in response to the weak-light pulse. Even though no nuclear transport of phyA into the nucleus was detected in absence of FHY1 and FHL in vivo (13), we could detect a phyA-dependent gene expression in the fhy1/fhl double-mutant. Together with the import assays, these experiments support the presence of an FHY1/FHL-independent nuclear import for phyA. As in the case of phyB, the transport of phyA might also be facilitated by the PIF proteins.
Discussion
Although the mechanism of the nuclear transport of phyA was intensively studied in recent years (13–16, 21), the underlying mechanism of phyB nuclear import remained less understood. Until now, an intrinsic NLS in the phyB molecule itself was proposed (22–24). The results of our in vitro studies strongly indicate that phyB does not contain an intrinsic NLS, neither in the C- nor N-terminal half. As has been previously shown, for phyA, the import of phyB can be reconstituted in vitro by the addition of transport-helper proteins. We therefore propose that nuclear transport of all phytochromes is based on the cytosolic interaction with transport facilitators that bear an NLS (e.g., transcription factors). The higher affinity to the Pfr-form could be the driving force for the light-dependent accumulation in the nucleus. For phyA, with concentrations almost two orders-of-magnitude higher than those of the other phytochromes (3), the phyA specific FHY1-transport-system evolved that interacts with N terminus of phyA (13, 14, 37).
Based on our in vitro and in vivo analysis of the nuclear transport of phyB, we conclude that members of the PIF family might not only play a role in phytochrome signal transduction but could also be involved in nuclear transport of phytochromes. We showed that PIF3 was able to induce nuclear import of phyB and phyB fragments in vitro. Based on the fact that the synthetic protein NLS-ABP was also able to induce nuclear transport of phyBN and PHYBC in the in vitro assay, additional members of the PIF-family (PIF1, -4, -5, -6, and -7) that interact with phyB via the APB-domain (31, 38, 39) are potential candidates for additional factors that could be involved in phyB nuclear transport. This hypothesis is additionally supported by our in vivo analysis of phyB-YFP transport in the pifq compared with wild-type plants. Nuclear accumulation of phyB-YFP was reduced in pifq when we irradiated for 4.5 h with red light and not measurable at all when we analyzed the nuclear accumulation within the first 90 min of irradiation (Fig. 3). Our measurements of phyB nuclear import in the absence of PIF1, -3, -4, and -5 indicate that these PIFs are the major transport facilitators during the first hours of deetiolation. However, under prolonged irradiations the PIFs might not be exclusively responsible for phyB nuclear transport in the plant; other phyB interacting factors might be involved in this process as well.
The finding that PIF3 and potentially also other PIFs can facilitate the nuclear transport of phyB was also strongly supported by the results of the analysis of the G767-to-R mutant of phyB. It has previously been shown that this mutation affects PIF3 binding (30). Consistent with this finding, we could not induce nuclear import of the mutated phyB fragment by adding PIF3 to our in vitro assay. In Arabidopsis mutants, the phenotype of the G767-to-R point mutation can be rescued by a fusion of an NLS to the protein (23). It is therefore likely that the mutant phyB protein lacks the ability to bind to transport facilitators. It is not known whether the point-mutation G767-to-R also influences the binding to other PIF proteins, but a mutation of the PIF binding site in the phyB-molecule would explain the severe phenotype concerning the nuclear import of phyB compared with the pif3 mutant.
The interaction between PIF3 and the phytochromes is not restricted to phyB. Several publications showed that phyA is also able to interact with members of the PIF-family, namely PIF1 and PIF3 (40–42). Consequently, the results of our in vitro transport assay indicate that both PIF proteins were able to induce nuclear transport of the N-terminal phyA fragment. The finding of an FHY1-independent transport protein for phyA is also supported by the PRR9 expression data. The slight induction of PRR9 expression observed in the fhy1/fhl double-mutant could be because of small amounts of phyA in the nucleus that are not detectable by fluorescence microscopy. Because the fhl mutant contains no FHL transcript and the theoretical protein would lack the phyA binding side (18), we currently assume that FHL is really absent in the fhy1/fhl double-mutant. Hence, we suggest that phyA responses, especially those that need only few molecules of active phyA in the nucleus, could be accomplished by a phytochrome import mechanism independent of FHY1 and FHL. However, we cannot principally exclude that residual amounts of both proteins facilitate the import of phyA into the nucleus.
Our in vitro and in vivo data clearly show that phyB import into the nucleus does not require an intrinsic NLS. Hence, transport facilitators were identified that could accomplish light-dependent import of phyB into the nucleus. In contrast to the nuclear transport of phyA, the identified putative transport facilitators also induced import of the inactive phyB. This finding could explain the nuclear accumulation of phyB in dark-grown plants. A light-independent interaction between PIFs and the C-terminal part of phyB—as we could demonstrate in vitro by analyzing C-terminal phyB fragments and as has been shown by Ni et al. (29)—might be one explanation for light-independent nuclear transport of phyB in the dark-grown plant. However, we cannot exclude that additional, up-to-now unidentified transport facilitators that interact with the inactive phyB, are responsible for this light-independent nuclear accumulation of phyB.
Regarding the role of PIF1, -3, -4, and -5 in the light-dependent nuclear transport of phyB, one would expect that the pifq mutant seedlings should react hyposensitively to red light. However, because these four PIFs also function as transcriptional repressors of photomorphogenesis in darkness, pifq seedlings actually display a constitutive photomorphogenic (cop)-like phenotype and are therefore shorter in darkness and light compared with wild-type (28, 43). However, on a molecular level, the pifq mutant was shown to be less sensitive to red light than the wild-type. Expression of rapidly light-induced genes that are not dependent on PIF1, -3, -4, and -5 in darkness were studied by Leivar et al. using whole-genome microarrays (these genes were denoted as class 3 and 6 genes in their study) (43). When comparing the gene expression in pifq to wild-type, these genes show a reduced level of induction in response to 1-h red light but no regulation by PIFs in darkness. Based on our results, the hyposensitivity of class 3 and 6 genes could be explained by the reduced light-dependent nuclear import of phyB in the pifq mutant. Even though their function as repressors of photomorphogenesis is dominating the morphological effects, we still can detect the positive role of the PIFs in phyB signal transduction on the molecular level.
Materials and Methods
For the cloning of the constructs, the purification of the proteins and the TaqMan real-time RT-PCR, see SI Material and Methods.
Generation of Transgenic Plants.
Transgenic plants expressing the P35S::PHYB-YFP in Col 0 and pifq (28) were obtained by Agrobacterium tumefaciens mediated transformation. Transformation and selection of transgenic lines was performed as previously described (6).
Isolation of Nuclei from Acetabularia acetabulum.
For the manual isolation of Acetabularia nuclei, we used cells of at least 20 mm in length that were grown and cultivated in artificial seawater Ace 27 (44, 45). For further detail, see Pfeiffer et al. (21).
Nuclear Import Assays.
The nuclear import assays were done as described previously (21). The nucleus buffer (46) was supplemented with 0.01 μg/μL of the labeled phytochrome fragments or 0.001 μg/μL of phyB-GFP-TAP. Potential transport facilitators were added in a 10-times molar excess to the phytochromes. Incubations for 1.5 h took place either under continuous red light (R, 2.5 μmol⋅m−2⋅s−1, 656 nm), far-red light (FR, 13 μmol⋅m−2⋅sec−1, 730 nm), or in darkness (D) after a 5 min RG9 pulse (40 μmol⋅m−2⋅sec−1, 756 nm). Ten to 20 nuclei were incubated in the same drop of buffer for each experiment. Import experiments were counted as positive when repeatedly more than 40% of the nuclei showed a detectable import signal after the incubation. If less than 10% of the nuclei showed such a signal in a recurrent manner, the assay was considered negative. SDS/PAGE analysis of all proteins that were used for nuclear import assays are shown in Fig. S3.
Plant Material and Growth Conditions.
The phyA-211 mutant (5), phyA-211/phyB-9 double mutant and fhl1/fhy1–3 double mutant (15) are in the Columbia ecotype background and were therefore compared with the Columbia wild-type in the real-time RT-PCR analysis. Transgenic plants expressing the P35S::PHYB-YFP in Col-0 and pifq, were used for the microscopic analysis. All seeds were imbibed for 2 d at 6 °C in the dark and germination was induced by 24 h irradiation with white light.
Quantification of Nuclear Import.
We took images of at least 18 nuclei per experiment with a specific YFP-filter set using identical exposure time. The images were transformed into grayscale and analyzed using ImageJ software (National Institutes of Health). The mean gray value per area was calculated for each nucleus.
Supplementary Material
Acknowledgments
We thank Peter Quail for providing pifq lines, Dina Mandoli for providing the Acetabularia cells, and Daniel Kirchenbauer for critical reading of the manuscript. This work was supported by the Deutsche Forschungsgemeinschaft Grant SFB 746 and Centre for Biological Signalling Studies grants (to E.S.); work in Hungary was supported by grants from the Hungarian Science Foundation for Basic Research (OTKA 81399), and in the United Kingdom by a Scottish Universities Life Science Alliance Professorial Grant (to F.N.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1120764109/-/DCSupplemental.
References
- 1.Chen M, Chory J, Fankhauser C. Light signal transduction in higher plants. Annu Rev Genet. 2004;38:87–117. doi: 10.1146/annurev.genet.38.072902.092259. [DOI] [PubMed] [Google Scholar]
- 2.Franklin KA. Light and temperature signal crosstalk in plant development. Curr Opin Plant Biol. 2009;12:63–68. doi: 10.1016/j.pbi.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Clack T, Mathews S, Sharrock RA. The phytochrome apoprotein family in Arabidopsis is encoded by five genes: The sequences and expression of PHYD and PHYE. Plant Mol Biol. 1994;25:413–427. doi: 10.1007/BF00043870. [DOI] [PubMed] [Google Scholar]
- 4.Sharrock RA, Quail PH. Novel phytochrome sequences in Arabidopsis thaliana: Structure, evolution, and differential expression of a plant regulatory photoreceptor family. Genes Dev. 1989;3:1745–1757. doi: 10.1101/gad.3.11.1745. [DOI] [PubMed] [Google Scholar]
- 5.Reed JW, Nagatani A, Elich TD, Fagan M, Chory J. Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol. 1994;104:1139–1149. doi: 10.1104/pp.104.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kircher S, et al. Nucleocytoplasmic partitioning of the plant photoreceptors phytochrome A, B, C, D, and E is regulated differentially by light and exhibits a diurnal rhythm. Plant Cell. 2002;14:1541–1555. doi: 10.1105/tpc.001156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tepperman JM, Hwang Y-S, Quail PH. phyA dominates in transduction of red-light signals to rapidly responding genes at the initiation of Arabidopsis seedling de-etiolation. Plant J. 2006;48:728–742. doi: 10.1111/j.1365-313X.2006.02914.x. [DOI] [PubMed] [Google Scholar]
- 8.Nagy F, Schäfer E. Phytochromes control photomorphogenesis by differentially regulated, interacting signaling pathways in higher plants. Annu Rev Plant Biol. 2002;53:329–355. doi: 10.1146/annurev.arplant.53.100301.135302. [DOI] [PubMed] [Google Scholar]
- 9.Kircher S, et al. Light quality-dependent nuclear import of the plant photoreceptors phytochrome A and B. Plant Cell. 1999;11:1445–1456. doi: 10.1105/tpc.11.8.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamaguchi R, Nakamura M, Mochizuki N, Kay SA, Nagatani A. Light-dependent translocation of a phytochrome B-GFP fusion protein to the nucleus in transgenic Arabidopsis. J Cell Biol. 1999;145:437–445. doi: 10.1083/jcb.145.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hisada A, et al. Light-induced nuclear translocation of endogenous pea phytochrome A visualized by immunocytochemical procedures. Plant Cell. 2000;12:1063–1078. doi: 10.1105/tpc.12.7.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huq E, Al-Sady B, Quail PH. Nuclear translocation of the photoreceptor phytochrome B is necessary for its biological function in seedling photomorphogenesis. Plant J. 2003;35:660–664. doi: 10.1046/j.1365-313x.2003.01836.x. [DOI] [PubMed] [Google Scholar]
- 13.Hiltbrunner A, et al. FHY1 and FHL act together to mediate nuclear accumulation of the phytochrome A photoreceptor. Plant Cell Physiol. 2006;47:1023–1034. doi: 10.1093/pcp/pcj087. [DOI] [PubMed] [Google Scholar]
- 14.Hiltbrunner A, et al. Nuclear accumulation of the phytochrome A photoreceptor requires FHY1. Curr Biol. 2005;15:2125–2130. doi: 10.1016/j.cub.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 15.Rösler J, Klein I, Zeidler M. Arabidopsis fhl/fhy1 double mutant reveals a distinct cytoplasmic action of phytochrome A. Proc Natl Acad Sci USA. 2007;104:10737–10742. doi: 10.1073/pnas.0703855104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Genoud T, et al. FHY1 mediates nuclear import of the light-activated phytochrome A photoreceptor. PLoS Genet. 2008;4:e1000143. doi: 10.1371/journal.pgen.1000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeidler M, Zhou Q, Sarda X, Yau C-P, Chua N-H. The nuclear localization signal and the C-terminal region of FHY1 are required for transmission of phytochrome A signals. Plant J. 2004;40:355–365. doi: 10.1111/j.1365-313X.2004.02212.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Q, et al. FHL is required for full phytochrome A signaling and shares overlapping functions with FHY1. Plant J. 2005;43:356–370. doi: 10.1111/j.1365-313X.2005.02453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang SW, Jang I-C, Henriques R, Chua N-H. FAR-RED ELONGATED HYPOCOTYL1 and FHY1-LIKE associate with the Arabidopsis transcription factors LAF1 and HFR1 to transmit phytochrome A signals for inhibition of hypocotyl elongation. Plant Cell. 2009;21:1341–1359. doi: 10.1105/tpc.109.067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen Y, et al. Phytochrome A mediates rapid red light-induced phosphorylation of Arabidopsis FAR-RED ELONGATED HYPOCOTYL1 in a low fluence response. Plant Cell. 2009;21:494–506. doi: 10.1105/tpc.108.061259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfeiffer A, et al. A cell-free system for light-dependent nuclear import of phytochrome. Plant J. 2009;57:680–689. doi: 10.1111/j.1365-313X.2008.03721.x. [DOI] [PubMed] [Google Scholar]
- 22.Sakamoto K, Nagatani A. Nuclear localization activity of phytochrome B. Plant J. 1996;10:859–868. doi: 10.1046/j.1365-313x.1996.10050859.x. [DOI] [PubMed] [Google Scholar]
- 23.Matsushita T, Mochizuki N, Nagatani A. Dimers of the N-terminal domain of phytochrome B are functional in the nucleus. Nature. 2003;424:571–574. doi: 10.1038/nature01837. [DOI] [PubMed] [Google Scholar]
- 24.Chen M, Tao Y, Lim J, Shaw A, Chory J. Regulation of phytochrome B nuclear localization through light-dependent unmasking of nuclear-localization signals. Curr Biol. 2005;15:637–642. doi: 10.1016/j.cub.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 25.Clauss H. On the influence of red and blue light on the growth of nucleated parts of Acetabularia mediterranea. Naturwissenschaften. 1963;50:791. [Google Scholar]
- 26.Kratz RF, Mandoli DF. The roles of light and the nucleus in the regulation of reproductive onset in Acetabularia acetabulum. Planta. 1999;209:503–512. doi: 10.1007/s004250050754. [DOI] [PubMed] [Google Scholar]
- 27.Terborgh J. Effects of red and blue light on the growth and morphogenesis of Acetabularia crenulata. Nature. 1965;207:1360–1363. doi: 10.1038/2071360a0. [DOI] [PubMed] [Google Scholar]
- 28.Leivar P, et al. Multiple phytochrome-interacting bHLH transcription factors repress premature seedling photomorphogenesis in darkness. Curr Biol. 2008;18:1815–1823. doi: 10.1016/j.cub.2008.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ni M, Tepperman JM, Quail PH. PIF3, a phytochrome-interacting factor necessary for normal photoinduced signal transduction, is a novel basic helix-loop-helix protein. Cell. 1998;95:657–667. doi: 10.1016/s0092-8674(00)81636-0. [DOI] [PubMed] [Google Scholar]
- 30.Ni M, Tepperman JM, Quail PH. Binding of phytochrome B to its nuclear signalling partner PIF3 is reversibly induced by light. Nature. 1999;400:781–784. doi: 10.1038/23500. [DOI] [PubMed] [Google Scholar]
- 31.Khanna R, et al. A novel molecular recognition motif necessary for targeting photoactivated phytochrome signaling to specific basic helix-loop-helix transcription factors. Plant Cell. 2004;16:3033–3044. doi: 10.1105/tpc.104.025643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bauer D, et al. Constitutive photomorphogenesis 1 and multiple photoreceptors control degradation of phytochrome interacting factor 3, a transcription factor required for light signaling in Arabidopsis. Plant Cell. 2004;16:1433–1445. doi: 10.1105/tpc.021568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagner D, Quail PH. Mutational analysis of phytochrome B identifies a small COOH-terminal-domain region critical for regulatory activity. Proc Natl Acad Sci USA. 1995;92:8596–8600. doi: 10.1073/pnas.92.19.8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsushika A, Imamura A, Yamashino T, Mizuno T. Aberrant expression of the light-inducible and circadian-regulated APRR9 gene belonging to the circadian-associated APRR1/TOC1 quintet results in the phenotype of early flowering in Arabidopsis thaliana. Plant Cell Physiol. 2002;43:833–843. doi: 10.1093/pcp/pcf118. [DOI] [PubMed] [Google Scholar]
- 35.Ito S, et al. Characterization of the APRR9 pseudo-response regulator belonging to the APRR1/TOC1 quintet in Arabidopsis thaliana. Plant Cell Physiol. 2003;44:1237–1245. doi: 10.1093/pcp/pcg136. [DOI] [PubMed] [Google Scholar]
- 36.Ito S, et al. Molecular dissection of the promoter of the light-induced and circadian-controlled APRR9 gene encoding a clock-associated component of Arabidopsis thaliana. Biosci Biotechnol Biochem. 2005;69:382–390. doi: 10.1271/bbb.69.382. [DOI] [PubMed] [Google Scholar]
- 37.Sorokina O, et al. A switchable light-input, light-output system modelled and constructed in yeast. J Biol Eng. 2009;3:15. doi: 10.1186/1754-1611-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leivar P, et al. The Arabidopsis phytochrome-interacting factor PIF7, together with PIF3 and PIF4, regulates responses to prolonged red light by modulating phyB levels. Plant Cell. 2008;20:337–352. doi: 10.1105/tpc.107.052142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leivar P, Quail PH. PIFs: Pivotal components in a cellular signaling hub. Trends Plant Sci. 2011;16:19–28. doi: 10.1016/j.tplants.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu Y, Tepperman JM, Fairchild CD, Quail PH. Phytochrome B binds with greater apparent affinity than phytochrome A to the basic helix-loop-helix factor PIF3 in a reaction requiring the PAS domain of PIF3. Proc Natl Acad Sci USA. 2000;97:13419–13424. doi: 10.1073/pnas.230433797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al-Sady B, Ni W, Kircher S, Schäfer E, Quail PH. Photoactivated phytochrome induces rapid PIF3 phosphorylation prior to proteasome-mediated degradation. Mol Cell. 2006;23:439–446. doi: 10.1016/j.molcel.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 42.Castillon A, Shen H, Huq E. Phytochrome interacting factors: Central players in phytochrome-mediated light signaling networks. Trends Plant Sci. 2007;12:514–521. doi: 10.1016/j.tplants.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Leivar P, et al. Definition of early transcriptional circuitry involved in light-induced reversal of PIF-imposed repression of photomorphogenesis in young Arabidopsis seedlings. Plant Cell. 2009;21:3535–3553. doi: 10.1105/tpc.109.070672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hunt BE, Mandoli DF. A new, artificial seawater that facilitates growth of large numbers of cells of Acetabularia acetabulum (Chlorophyta) and reduces the labor inherent in cell culture. J Phycol. 1996;32:483–495. [Google Scholar]
- 45.Mandoli DF. What ever happened to Acetabularia? Bringing a once-classic model system into the age of molecular genetics. Int Rev Cytol. 1998;182:1–67. [Google Scholar]
- 46.Berger S, Schweiger HG. Cytoplasmic induction of changes in the ultrastructure of the Acetabularia nucleus and perinuclear cytoplasm. J Cell Sci. 1975;17:517–529. doi: 10.1242/jcs.17.3.517. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.