Abstract
Ductal growth of the mammary gland occurs in two distinct stages. The first round of branching morphogenesis occurs during embryogenesis, and the second round commences at the onset of puberty. Currently, relatively little is known about the genetic networks that control the initial phases of ductal expansion, which, unlike pubertal development, proceeds independent of hormonal input in female mice. Here we identify NF-κB downstream of the TNF-like ligand ectodysplasin (Eda) as a unique regulator of embryonic and prepubertal ductal morphogenesis. Loss of Eda, or inhibition of NF-κB, led to smaller ductal trees with fewer branches. On the other hand, overexpression of Eda caused a dramatic NF-κB–dependent phenotype in both female and male mice characterized by precocious and highly increased ductal growth and branching that correlated with enhanced cell proliferation. We have identified several putative transcriptional target genes of Eda/NF-κB, including PTHrP, Wnt10a, and Wnt10b, as well as Egf family ligands amphiregulin and epigen. We developed a mammary bud culture system that allowed us to manipulate mammary development ex vivo and found that recombinant PTHrP, Wnt3A, and Egf family ligands stimulate embryonic branching morphogenesis, suggesting that these pathways may cooperatively mediate the effects of Eda.
Keywords: hypohidrotic, ectodermal dysplasia, Edar
Mammary gland development is initiated during embryogenesis, but is completed only on pregnancy to support lactation. Similar to other skin appendages, such as hair and teeth, embryonic mammary gland development is guided by autocrine and paracrine signals mediating tissue interactions between the ectodermal epithelium and the underlying mesenchymal cells, whereas morphogenesis during puberty and pregnancy relies on hormonal cues as well (1–4). In mice, mammary gland development begins at embryonic day (E) 10.5 and proceeds from placode to bud stage by E14 similarly in both sexes. However, as a result of androgen receptor (AR) activation in mesenchymal cells of male embryos at E14, the connection of the bud to the surface epidermis is severed. Consequently, male mice have either no ductal system or only a very rudimentary system, and no nipples (2). Specification of the secondary mammary mesenchyme also begins at around E14 and gives rise to the fat pad, which supports the development of mammary glands throughout postnatal life (5). At E16, the distal tip of the bud invades the fat pad precursor tissue, and the first bifurcation of the primary sprout is detected at late E16 or early E17. By birth, a ductal tree with 10–15 branches has formed. Postnatal growth of the mammary gland is proportional to the rest of the body until the onset of puberty, when ovarian activity leads to rapid acceleration in ductal growth and branching (1–4).
Molecular regulation of hormone-dependent mammary gland morphogenesis during puberty and pregnancy has been thoroughly studied over the past several decades (3). In contrast, relatively little is known about factors driving hormone-independent embryonic and prepubertal ductal outgrowth and branching, although parathyroid hormone-related protein (PTHrP) and the canonical Wnt pathway are known to be involved (1, 2). PTHrP, expressed in the mammary epithelium, is necessary for downgrowth of the mammary bud, and deficiency in PTHrP or its mesenchymally expressed receptor PTHR1 leads to a failure in primary sprout formation (2, 6). The effects of PTHrP are at least partly mediated by Bmp signaling, as demonstrated by the ability of exogenous Bmp4 to rescue the phenotype of PTHrP null mammary buds ex vivo (7). Mice deficient in the Wnt coreceptor Lrp6 exhibit a stunted, branchless primary duct and a minuscule fat pad at birth (8). In addition, Egf receptor (Egfr) null mice have fewer branches and shorter ductal trees at birth (9). The ligands involved in these features remain elusive, however (10).
Ectodysplasin-A1 (Eda-A1, hereinafter Eda), a member of the TNF superfamily, is essential for ectodermal organogenesis (11). Mutations in Eda, its receptor Edar, or the signal mediator Edaradd cause hypohidrotic ectodermal dysplasia (HED) syndrome in both humans and mice. HED in humans is characterized by sparse hair, severe oligodontia, absence or reduced number of sweat glands, as well as defects in other exocrine glands (11, 12). Intriguingly, transgenic overexpression of Eda under the keratin 14 promoter (K14-Eda mice) leads to the formation of supernumerary mammary placodes that give rise to ectopic glands in adults (13, 14). The Eda pathway was also recently implicated in pubertal mammary gland development (15). Besides this, little is known about the role of the Eda pathway in mammary gland development.
Several lines of evidence indicate that many of the effects of Eda are mediated by transcription factor NF-κB (11). In unstimulated cells, NF-κB is kept inactive by binding to IκB proteins, most commonly IκBα, in the cytosol (16). On stimulation, IκBα is phosphorylated and degraded thereby releasing NF-κB and allowing target gene expression. Studies in cultured cell lines, identification of similarities in Eda null and IκBαΔN mice expressing the transdominant superrepressor IκBα, and analysis of NF-κB reporter expression in Eda−/− embryos have pinpointed NF-κB as the key mediator of Eda signaling (11, 17, 18). Here we identify Eda as a unique mesenchymal factor regulating embryonic and prepubertal mammary gland morphogenesis through activation of Edar/NF-κB in the epithelium, and provide evidence suggesting that PTHrP, Egf ligands amphiregulin (areg) and epigen (epgn), and Wnt10a and Wnt10b might cooperatively mediate the effects of Eda.
Results
Ectodysplasin Regulates Embryonic and Prepubertal Branching Morphogenesis.
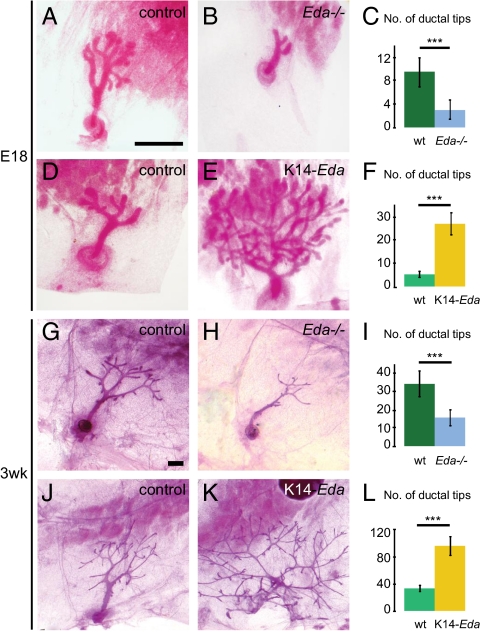
In WT embryos, a small ductal tree with an average of nine branches formed in the fourth (inguinal) mammary gland by E18. At the same stage, Eda null embryos displayed a 68% reduction of branches (Fig. 1 A–C). The mean total ductal length was decreased by 71% (from 3.07 mm ± 0.92 to 0.89 mm ± 0.35; P = 2.8 × 10−8). K14-promoter–driven overexpression of Eda had the opposite effect and led to a striking fivefold increase in the number of end buds compared with littermate controls (Fig. 1 D–F).
Fig. 1.
Embryonic and prepubertal branching correlates with the amount of ectodysplasin. Whole-mount stained fourth inguinal mammary glands of female Eda−/−, K14-Eda, and their control mice at E18 (A–F) and 3 wk of age (G–L), and quantification of epithelial branching, expressed as the average number of ductal tips ± SD per mammary gland in each genotype (C, F, I, and L). (A and G) B6CBA controls for Eda−/− mice shown in B and H. (D and J) C57BL/6 littermate controls of K14-Eda mice shown in E and K. (C) Number of ductal tips of control (n = 14) and Eda−/− (n = 10) glands at E18. (F) Number of ductal tips of control (n = 9) and K14-Eda (n = 12) glands at E18. (I) Number of ductal tips of 3-wk-old control (n = 28) and Eda−/− (n = 18) mice. (L) Number of ductal tips of 3-wk-old control (n = 13) and K14-Eda (n = 20) mice. n, number of glands analyzed. ***P < 0.001. (Scale bars: 500 μm.)
At age 3 wk, just before the onset of puberty, Eda−/− mice exhibited a 54% reduction in the number of ductal termini (Fig. 1 G–I), compared with the almost threefold increase seen in K14-Eda mice (Fig. 1 J–L). This indicates that the extent of ductal branching was strongly correlated with the level of Eda expression.
Eda Overexpression Induces Precocious Branching Morphogenesis.
The highly escalated branching of K14-Eda mammary glands prompted us to study the course of branching morphogenesis during embryogenesis. Edar is expressed in the mammary bud epithelium (19). Before and at the onset of branching morphogenesis, Eda transcripts were localized in the mesenchyme, whereas prominent epithelial expression was observed in K14-Eda embryos (Fig. S1). NF-κB reporter mice expressing β-galactosidase under an NF-κB–responsive element displayed high Eda-dependent NF-κB activity (18) (Fig. S2). We used this NF-κB reporter mouse to visualize developing mammary glands.
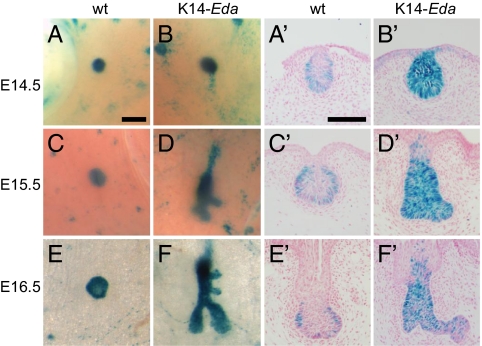
The WT mammary rudiment was relatively quiescent between E13 and E16 (2, 4) (Fig. 2 A, C, and E). At E13.5–E14.5, both WT and K14-Eda mammary buds appeared fairly similar (Fig. 2 A and B and Fig. S2). At E15.5, K14-Eda mammary buds had already elongated and formed first branches (Fig. 2D and Fig. S2). At E16.5, the WT mammary buds had sprouted, but between four and six branches had already formed in K14-Eda embryos (Fig. 2 E and F and Figs. S2 and S3). Sectioning of whole-mount stained mammary glands confirmed that Eda-dependent NF-κB activity was confined to the epithelium and was enriched in the basal cells (Fig. 2 A′, C′, and E′). Increased and ectopic NF-κB activity was observed throughout the epithelium of K14-Eda embryos (Fig. 2 B′, D′, and F′).
Fig. 2.
Transgenic overexpression of Eda induces precocious branching morphogenesis and ectopic activation of NF-κB. (A–F) Fourth inguinal mammary glands of female K14-Eda and their littermate control embryos were analyzed for NF-κB reporter activity by LacZ whole-mount staining at E14.5 (A and B), E15.5 (C and D), and E16.5 (E and F). (Scale bar: 200 μm.) (A′–F′) Sections of whole-mount stained specimens. (Scale bars: 100 μm.)
K14-Eda Male Mammary Buds Escape Androgen-Mediated Destruction.
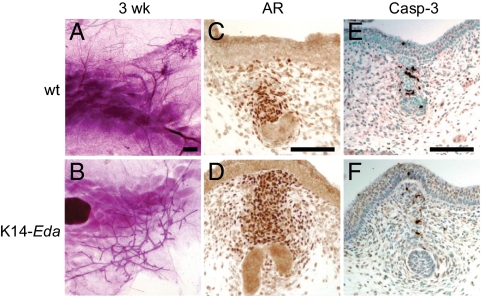
Intriguingly, nipples were observed in K14-Eda males (Fig. S4 A and B). The ductal tree of the fourth inguinal gland of K14-Eda males was highly similar to that of female transgenic mice at E18 (Fig. S4 C and D) and 3 wk of age (compare Figs. 3B and 1K), with 81.6 ± 11.4 ductal tips in the males (n = 12) and 96.5 ± 14.3 in the females (n = 20), whereas no or very rudimentary ductal structures were observed in control males (Fig. 3A). Further development of the K14-Eda male ductal system was modest, however (Fig. S4 E and F). Interestingly, the majority of K14-Eda male mammary glands developed as blind ducts (Fig. 3B and Fig. S4F).
Fig. 3.
Mammary ducts of K14-Eda males escape androgen-dependent destruction. (A and B) Whole-mount stained fourth inguinal mammary glands of control and K14-Eda male mice at 3 wk of age. (Scale bar: 500 μm.) (C–F) Expression of AR (C and D) and activated caspase-3 (E and F) in control and K14-Eda male mammary buds at E15.5. (Scale bars: 100 μm.)
In male embryos, androgens secreted by testes activate mesenchymally expressed AR at E14 (2, 4, 20). This leads to condensation of mesenchymal cells around the stalk of the bud and triggers apoptosis in the entire mammary anlage (4, 21). No differences in testis histology, AR expression, activated caspase-3 staining, or mesenchymal condensation in the stalk region were observed between control and K14-Eda males at E14.5–E15.5 (Fig. 3 C–F and Fig. S5). However, ductal tips of transgenic glands had invaded through the primary, AR-positive mammary mesenchyme by E15.5, and, consequently, only rare apoptotic cells were detected in the most distal structures (Fig. 3 E and F).
NF-κB Mediates Eda-Induced Ductal Growth and Branching.
To address the function of NF-κB in embryonic and prepubertal development, we exploited the IκBαΔN mouse strain (17). Expectedly, NF-κB reporter expression was abolished in the mammary epithelium of IκBαΔN embryos (Fig. S6). The number of ductal tips was diminished in IκBαΔN embryos by 48% at E18 (Fig. 4 A–C) and by 47% at age 3 wk (Fig. 4 D–F). IκBαΔN mice are known to be growth-retarded (17), and an average 25% reduction in weight was observed at age 3 wk. A highly significant difference between mutant and control mice also was seen when the number of ductal termini was normalized to weight, as well as when IκBαΔN mice were compared with weight-matched controls (Fig. 4F). Eda-induced branching was fully suppressed in the IκBαΔN background at E18 (Fig. 4 G–J) and 3 wk of age (Fig. 4 K–N), demonstrating the need for NF-κB downstream of Eda in ductal morphogenesis.
Fig. 4.
NF-κB regulates embryonic and prepubertal ductal development and is essential for Eda-induced accelerated branching morphogenesis. (A and B) Whole-mount stained fourth inguinal mammary glands of female IκBαΔN and littermate control mice at E18. (C) Quantification of epithelial branching, expressed as the average number of ductal tips ± SD per fourth mammary gland in control (n = 13) and IκBαΔN (n = 18) mice at E18. (D and E) Whole-mount stained fourth inguinal mammary glands of female IκBαΔN and their littermate control mice at 3 wk of age. (F) Average number of ductal tips ± SD per fourth mammary gland of 3-wk-old IκBαΔN mice (n = 18) and littermate controls (n = 13), of 3-wk-old mice normalized to weight (control, n = 13; IκBαΔN, n = 10), and of 3-wk-old IκBαΔN mice (n = 8) compared with weight-matched controls (n = 8). (G–N) Whole-mount stained fourth inguinal mammary glands of female control, K14-Eda, IκBαΔN, and K14-Eda;IκBαΔN compound mutants at E18 (G–J) and at 3 wk of age (K–N). n, number of glands analyzed. **P < 0.01; ***P < 0.001. (Scale bars: 500 μm.)
PTHrP, areg, epigen, Wnt10a, and Wnt10b Are Likely Transcriptional Targets of Eda.
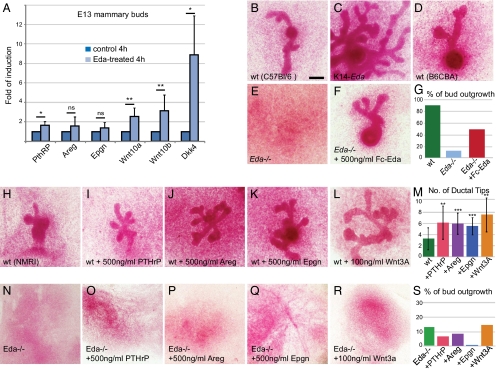
We next sought the mediators of Eda/NF-κB action in developing mammary glands. We previously performed microarray profiling of genes differentially expressed in embryonic Eda−/− skin after short exposure to recombinant Eda protein (Fc-Eda-A1) (22, 23). PTHrP (7.5-fold) and the Egfr agonists epgn (6.4-fold) and areg (4.4-fold) were among the 15 most highly up-regulated genes after 4 h of Fc-Eda-A1 treatment (23). We also considered Wnt10a and Wnt10b as promising candidates, because both genes have been identified as likely direct NF-κB target genes in hair germs (24). Quantitative RT-PCR (qRT-PCR) confirmed the rapid up-regulation of PTHrP, epgn, and areg by Fc-Eda-A1 in skin explants, along with a modest but reproducible effect on Wnt10a and Wnt10b mRNA levels (Fig. S7). Treatment of microdissected Eda−/− mammary buds with Fc-Eda-A1 led to a significant increase in the levels of Wnt10a, Wnt10b, and PTHrP (Fig. 5A). Areg and epgn were expressed at low levels, but a tendency toward up-regulation of their mRNA levels by Fc-Eda-A1 was seen (Fig. 5A). Wnt inhibitor Dkk4, a bona fide transcriptional target of Eda/NF-κB colocalizing with Edar in hair and mammary buds (22–24), was highly induced (Fig. 5A and Fig. S7).
Fig. 5.
Identification of PTHrP, Areg, Epgn, Wnt10a, and Wnt10b as potential mediators of Eda during ductal development. (A) Quantitative RT-PCR analysis of the indicated genes in E13.5 Eda−/− mammary buds (n = 8–9) treated with recombinant Fc-Eda protein for 4 h. Data are mean ± SD. (B–F) Mammary buds of E13.5 WT (C57BL/6) (B) or its littermate K14-Eda (C) embryos, Eda−/− (E) and its control (B6CBA) (D) embryos, and Eda−/− mammary buds supplemented with 500 ng/mL of Fc-Eda (F) were cultured for 5 d ex vivo, followed by visualization by carmine alum whole-mount staining. (Scale bar: 500 μm.) (G) Quantification of the frequency of successful bud outgrowths of WT (B6CBA) and Eda−/− mammary buds cultured in control medium, and Eda−/− buds cultured in medium supplemented with 500 ng/mL of Fc-Eda. (H–S) WT (NMRI) (H–M) or Eda−/− mammary buds (N–S) were cultured in the control medium (nwt = 32; nEda−/− = 144) (H and N) or in medium supplemented with 500 ng/mL of PTHrP (nwt = 27; nEda−/− = 45) (I and O), 500 ng/mL of areg (nwt = 45; nEda−/− = 119) (J and P), 500 ng/mL of epgn (nwt = 16; nEda−/− = 33) (K and Q), or 100 ng/mL of Wnt3A (nwt = 10; nEda−/− = 66) (L and R). (M) Number of ductal tips ± SD of WT mammary buds cultured in medium supplemented with factors indicated in (H–L). (S) Quantification of the frequency of successful bud outgrowths of Eda−/− mammary buds cultured in medium supplemented with factors indicated in (N–R). ns, P > 0.05; *P < 0.05; **P < 0.01; ***P < 0.001.
In situ hybridization analysis of Wnt10b expression before (E13.5) and during (E15.5) the onset of ductal morphogenesis revealed a correlation, with a reduction in Eda−/− mammary buds and an up-regulation in K14-Eda mammary buds (Fig. S8 A–F). Expression of PTHrP also was up-regulated in K14-Eda buds at E13.5, but no obvious difference between the genotypes was apparent at E15.5 (Fig. S8 G–L). PTHrP was reduced in IκBαΔN mammary buds at E13.5 (Fig. S8 M and N). These findings indicate that expression of PTHrP is not only induced by Eda (Fig. 5A), but also requires NF-κB. Somewhat surprisingly, there was no obvious difference in Wnt/β-catenin activity, as measured with TOP-gal and BAT-gal reporters, or in Lef1 expression between Eda−/− and control embryos at E14.5 or E15.5 (Fig. S9). Intriguingly, two kinds of changes were observed in K14-Eda embryos; epithelial activity, particularly Lef1 staining, was reduced, whereas the number of mesenchymal BAT-gal–expressing cells was increased, especially at later stages when the mesenchymal condensate appeared to be more expanded (Fig. S9 D–F and J–O). In line with qRT-PCR data, both areg and epgn were barely detectable by in situ hybridization, indicating that their mRNA levels were low in vivo.
Egf Ligands, PTHrP, and Wnt3A Accelerate Embryonic Ductal Branching ex Vivo.
To assess the functional relevance of the potential Eda target genes on embryonic branching morphogenesis, we established an ex vivo mammary bud culture system that entailed slight modifications to previously described methods (7, 25). Under this setup, a remarkable proportion of WT mammary buds gave rise to successful branching outgrowths, with success rates of 90% for NMRI (29 of 32) and B6CBA (70 of 79) and 70% for C57BL/6 (23 of 29) (Fig. 5 B, D, and G). Mammary buds isolated from K14-Eda embryos (in C57BL/6) were readily cultured ex vivo and recapitulated the in vivo phenotype (Fig. 5C). Only 15% (19 of 144) of buds dissected from Eda−/− embryos (in B6CBA) gave rise to successful outgrowths (Fig. 5 E and G), and the rudiments typically regressed before sprouting. However, application of Fc-Eda had a prominent restorative effect on Eda−/− mammary buds, and approximately half of the buds (74 of 156) sprouted and branched (Fig. 5 E–G).
We first tested the effect of recombinant PTHrP, areg, epgn, and Wnt3A (a representative of canonical Wnt ligands) on WT buds, which formed an average of three branches after 5 d of culture (Fig. 5 H and M). Application of PTHrP or of either Egfr agonist doubled the number of end buds (Fig. 5 H–K and M). Wnt3A had a positive effect of the same range (Fig. 5 L and M). However, none of these factors given alone or in combination had any discernible impact on the success rate of Eda−/− bud outgrowths (Fig. 5 N–S).
Eda Enhances Cell Proliferation but Has No Effect on Apoptosis in Vivo.
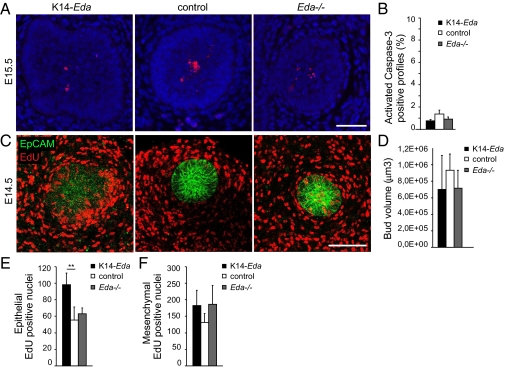
The significantly increased ductal growth of K14-Eda mammary glands and the reduced growth of Eda−/− glands suggests that Eda can promote epithelial cell proliferation, protect these cells from apoptosis, or both. No significant difference in activated caspase-3 staining was seen in epithelial cells of E15.5 Eda−/−, WT, and K14-Eda buds (Fig. 6 A and B).
Fig. 6.
Eda induces cell proliferation in the mammary bud epithelium, but has no effect on apoptosis. (A) Activated caspase-3 staining of E15.5 K14-Eda, control, and Eda−/− mammary buds. (Scale bar: 50 μm.) (B) Percentage of active caspase-3–positive cells ± SD in each genotype (n = 8–11 buds from three or four embryos of each genotype). (C) Eda−/− (n = 6), control (n = 4), and K14-Eda (n = 5) E14.5 embryos were killed at 2 h after EdU injection, followed by whole-mount analysis of EdU and the epithelial marker EpCAM in mammary bud 4 by confocal microscopy. (Scale bar: 100 μm.) (D) Quantification of the volume of mammary bud 4 at E14.5. (E) Quantification of the number of EdU-positive nuclei in the epithelium of mammary bud 4 (normalized to EpCAM+ cells). (F) Quantification of EdU-positive nuclei in the mesenchyme. **P < 0.01.
Cell proliferation was assessed at 2 h after injection of the nucleoside analog 5-ethynyl-2′-deoxyuridine (EdU) at E14.5 by whole-mount staining for EdU and EpCAM, a marker of epithelial cells (Fig. 6C). The number of epithelial (EpCAM+) EdU-positive cells, normalized to bud volume (Fig. 6D), was significantly higher in K14-Eda buds compared with controls (Fig. 6E). No differences in mesenchymal cell proliferation were detected at this stage (Fig. 6F). Similar findings were observed when ex vivo cultured mammary buds were analyzed, except that notable changes were seen in mesenchymal cell proliferation as well (Fig. S10).
Discussion
The embryonic development of the mammary gland is regulated by crosstalk between the ectodermal epithelium and two types of mesenchymal tissues: the primary condensed mesenchyme, which has the capability to induce mammogenesis even when combined with nonmammary epithelium, and the prospective fat pad tissue, which supports ductal outgrowth and dictates organ-specific branching patterns (4, 26). Pioneering tissue recombination experiments have indicated that mammary branching morphogenesis is regulated largely by mesenchymal signals (5, 25, 26), which might act as either survival/proliferative cues or guidance factors. With the exception of Fgf10 (27), the identity of these mesenchymal signals has remained elusive. Here we identify Eda as a mesenchymal factor regulating embryonic morphogenesis via activation of Edar in epithelial basal cells. However, modulation of the Eda pathway activity did not seem to affect branching pattern per se, but rather affected cell proliferation (see below). Likewise, we recently discovered that the extent of submandibular salivary gland branching is correlated with Eda/NF-κB activity, yet Eda−/− and K14-Eda glands retain the typical salivary gland-type branching pattern (28).
Our data reveal a previously unrecognized function for NF-κB in embryonic mammogenesis. A role for NF-κB in pubertal development has been inferred from studies showing that transplantation of IκΒα-deficient epithelium into cleared fat pads of WT mice resulted in a substantial increase in branching (29) and that mouse mammary tumor virus-driven expression of a nondegradable I-κBα implicated NF-κB in pregnancy-associated side branching (30). Thus, tight control of NF-κB activity is necessary at several stages to ensure correct mammary gland development.
It is well established that NF-κB can promote cell proliferation and/or suppress apoptosis (16). Based on EdU incorporation and cleaved caspase-3 staining, we find it likely that the Eda/NF-κB pathway exerts its function during embryonic ductal morphogenesis via its ability to enhance proliferation, similar to findings in IκBα-deficient mammary epithelia during puberty (29). The ability of Eda/NF-κB to enhance cell proliferation may result from a direct impact on signal-receiving cells and/or indirect impacts via autocrine or paracrine effects. At E14.5, just when the differences between K14-Eda and control buds are emerging, K14-Eda buds exhibited highly increased epithelial cell proliferation. Ex vivo studies have suggested that mesenchymal cell proliferation also may be affected, but perhaps moreso at later time points.
We identified several secreted factors—Egfr ligands, Wnt10a and Wnt10b, and PTHrP—as putative target genes of Eda. These factors accelerated branching of embryonic mammary glands ex vivo, suggesting that they may contribute to the observed Eda loss-of-function and gain-of-function phenotypes, although additional Eda target genes are bound to be involved. The relative significance of distinct Eda target genes may differ in Eda−/− and K14-Eda mammary glands, however. Studies in other ectodermal organs have revealed a tight but complex relationship between Eda/NF-κB and Wnt/β-catenin pathways (11, 24, 28). Our data suggest that Eda overexpression may lead to both decreased (epithelium) and increased (mesenchyme) changes in Wnt/β-catenin signaling. We speculate that these dual effects might be due to up-regulation of two kinds of paracrine Wnt factors: Wnt agonists (Wnt10a and Wnt10b) and Wnt inhibitor Dkk4. These findings raise the intriguing possibility that enhancement of mesenchymal Wnt signaling, suppression of epithelial Wnt signaling, or both are linked to accelerated branching morphogenesis. The elucidation of the functional importance of epithelial and mesenchymal Wnt/β-catenin signaling must await the generation of inducible, tissue-specific β-catenin–deficient mice.
Sexual dimorphism of embryonic mammogenesis, observed in mice and some other rodents, depends on AR expression downstream of PTHrP (2, 4, 21). We detected all of the hallmarks of AR activation in the K14-Eda males. Accordingly, the connection between the prospective nipple and the ductal bulb was severed in most cases. However, the entire anlage was not destroyed, most likely because of the precocious growth of the ductal tip(s) into the underlying secondary, AR negative stroma. Interestingly, Wnt1 and Wnt10b transgenes also can overcome the androgen-dependent developmental block and display extensive ductal hyperplasia (31, 32).
In conclusion, this study has identified the role of a unique TNF pathway in mammary gland morphogenesis. Thus, two different TNF pathways are now emerging as important mitogenic regulators of distinct stages of mammary gland development: the Eda/Edar/NF-κB pathway as a driver of hormone-independent ductal growth and branching, and the RANK pathway as the essential mediator of progesterone-induced proliferation and side branching during pregnancy (33). Reports on breast defects of patients with HED are scattered (34, 35) but, taken together with our present findings, suggest that Eda is an important regulator of human breast development as well. Elevated levels of NF-κB activity have been associated with breast cancer, particularly with hormone-independent tumors with poor prognosis, but the molecular mechanisms leading to constitutive NF-κB activation remain incompletely understood (36, 37). Future studies should examine whether Eda plays any role in breast tumorigenesis.
Materials and Methods
Embryonic mammary bud cultures were prepared according to a protocol modified from the techniques described by Kratochwil (25) and Hens et al. (7). The ventral skin encompassing the mammary line area was dissected from E13.5 embryos, explants were treated for 4–8 min with 0.75% pancreatin and 0.25% trypsin (Sigma-Aldrich) at room temperature. After a 1-h incubation on ice in DMEM supplemented with 10% (vol/vol) FCS, 2 mM l-glutamine, and antibiotics, excess epithelium surrounding the mammary buds was microsurgically peeled off. A typical explant consisted of an ample amount of mesenchyme with mammary buds 2, 3, and 4 attached. Explants were cultured for 5–6 d in a Trowell-type culture setting (14, 22) in 1:1 F12/DMEM with the same supplements as above and ascorbic acid (75 mg/L). The medium was changed every other day.
Details on animals, recombinant proteins, qRT-PCR, in situ hybridization, EdU labeling, immunofluorescence, and confocal microscopy are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Riikka Santalahti, Raija Savolainen, and Merja Mäkinen for excellent technical assistance, Julie Hens and Hannu Sariola for advice on mammary bud cultures and testis histology, respectively, and Irma Thesleff for a critical reading of the manuscript. This study was supported by the Academy of Finland, the Sigrid Jusélius Foundation, European Union Marie Curie training programs, the Finnish Cultural Foundation, the Helsinki Graduate Program for Biotechnology and Molecular Biology, and the Viikki Graduate School for Molecular Biosciences. P.S. is supported by the Swiss National Science Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1110627109/-/DCSupplemental.
References
- 1.Mikkola ML, Millar SE. The mammary bud as a skin appendage: Unique and shared aspects of development. J Mammary Gland Biol Neoplasia. 2006;11:187–203. doi: 10.1007/s10911-006-9029-x. [DOI] [PubMed] [Google Scholar]
- 2.Cowin P, Wysolmerski J. Molecular mechanisms guiding embryonic mammary gland development. Cold Spring Harb Perspect Biol. 2010;2:a003251. doi: 10.1101/cshperspect.a003251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brisken C, O'Malley B. Hormone action in the mammary gland. Cold Spring Harb Perspect Biol. 2010;2:a003178. doi: 10.1101/cshperspect.a003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veltmaat JM, Mailleux AA, Thiery JP, Bellusci S. Mouse embryonic mammogenesis as a model for the molecular regulation of pattern formation. Differentiation. 2003;71:1–17. doi: 10.1046/j.1432-0436.2003.700601.x. [DOI] [PubMed] [Google Scholar]
- 5.Sakakura T, Sakagami Y, Nishizuka Y. Dual origin of mesenchymal tissues participating in mouse mammary gland embryogenesis. Dev Biol. 1982;91:202–207. doi: 10.1016/0012-1606(82)90024-0. [DOI] [PubMed] [Google Scholar]
- 6.Wysolmerski JJ, et al. Rescue of the parathyroid hormone-related protein knockout mouse demonstrates that parathyroid hormone-related protein is essential for mammary gland development. Development. 1998;125:1285–1294. doi: 10.1242/dev.125.7.1285. [DOI] [PubMed] [Google Scholar]
- 7.Hens JR, et al. BMP4 and PTHrP interact to stimulate ductal outgrowth during embryonic mammary development and to inhibit hair follicle induction. Development. 2007;134:1221–1230. doi: 10.1242/dev.000182. [DOI] [PubMed] [Google Scholar]
- 8.Lindvall C, et al. The Wnt co-receptor Lrp6 is required for normal mouse mammary gland development. PLoS ONE. 2009;4:e5813. doi: 10.1371/journal.pone.0005813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sternlicht MD, et al. Mammary ductal morphogenesis requires paracrine activation of stromal EGFR via ADAM17-dependent shedding of epithelial amphiregulin. Development. 2005;132:3923–3933. doi: 10.1242/dev.01966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciarloni L, Mallepell S, Brisken C. Amphiregulin is an essential mediator of estrogen receptor α function in mammary gland development. Proc Natl Acad Sci USA. 2007;104:5455–5460. doi: 10.1073/pnas.0611647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mikkola ML. TNF superfamily in skin appendage development. Cytokine Growth Factor Rev. 2008;19:219–230. doi: 10.1016/j.cytogfr.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Mikkola ML. The Edar subfamily in hair and exocrine gland development. Adv Exp Med Biol. 2011;691:23–33. doi: 10.1007/978-1-4419-6612-4_3. [DOI] [PubMed] [Google Scholar]
- 13.Mustonen T, et al. Stimulation of ectodermal organ development by Ectodysplasin-A1. Dev Biol. 2003;259:123–136. doi: 10.1016/s0012-1606(03)00157-x. [DOI] [PubMed] [Google Scholar]
- 14.Mustonen T, et al. Ectodysplasin A1 promotes placodal cell fate during early morphogenesis of ectodermal appendages. Development. 2004;131:4907–4919. doi: 10.1242/dev.01377. [DOI] [PubMed] [Google Scholar]
- 15.Chang SH, Jobling S, Brennan K, Headon DJ. Enhanced Edar signalling has pleiotropic effects on craniofacial and cutaneous glands. PLoS ONE. 2009;4:e7591. doi: 10.1371/journal.pone.0007591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baud V, Karin M. Is NF-κB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009;8:33–40. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt-Ullrich R, et al. Requirement of NF-κB/Rel for the development of hair follicles and other epidermal appendices. Development. 2001;128:3843–3853. doi: 10.1242/dev.128.19.3843. [DOI] [PubMed] [Google Scholar]
- 18.Pispa J, Pummila M, Barker PA, Thesleff I, Mikkola ML. Edar and Troy signalling pathways act redundantly to regulate initiation of hair follicle development. Hum Mol Genet. 2008;17:3380–3391. doi: 10.1093/hmg/ddn232. [DOI] [PubMed] [Google Scholar]
- 19.Pispa J, Mikkola ML, Mustonen T, Thesleff I. Ectodysplasin, Edar and TNFRSF19 are expressed in complementary and overlapping patterns during mouse embryogenesis. Gene Expr Patterns. 2003;3:675–679. doi: 10.1016/s1567-133x(03)00092-9. [DOI] [PubMed] [Google Scholar]
- 20.Kratochwil K. In vitro analysis of the hormonal basis for the sexual dimorphism in the embryonic development of the mouse mammary gland. J Embryol Exp Morphol. 1971;25:141–153. [PubMed] [Google Scholar]
- 21.Dunbar ME, et al. Parathyroid hormone-related protein signaling is necessary for sexual dimorphism during embryonic mammary development. Development. 1999;126:3485–3493. doi: 10.1242/dev.126.16.3485. [DOI] [PubMed] [Google Scholar]
- 22.Fliniaux I, Mikkola ML, Lefebvre S, Thesleff I. Identification of dkk4 as a target of Eda-A1/Edar pathway reveals an unexpected role of ectodysplasin as inhibitor of Wnt signalling in ectodermal placodes. Dev Biol. 2008;320:60–71. doi: 10.1016/j.ydbio.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 23.Lefebvre S, Fliniaux I, Schneider P, Mikkola ML. Identification of ectodysplasin target genes reveals involvement of chemokines in hair development. J Invest Dermatol. 2012;132:1094–1102. doi: 10.1038/jid.2011.453. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, et al. Reciprocal requirements for EDA/EDAR/NF-κB and Wnt/β-catenin signaling pathways in hair follicle induction. Dev Cell. 2009;17:49–61. doi: 10.1016/j.devcel.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kratochwil K. Organ specificity in mesenchymal induction demonstrated in the embryonic development of the mammary gland of the mouse. Dev Biol. 1969;20:46–71. doi: 10.1016/0012-1606(69)90004-9. [DOI] [PubMed] [Google Scholar]
- 26.Sakakura T. Mammary embryogenesis. In: Neville MC, Daniel CW, editors. The Mammary Gland: Development, Regulation, and Function. New York: Plenum; 1987. pp. 37–66. [Google Scholar]
- 27.Mailleux AA, et al. Role of FGF10/FGFR2b signaling during mammary gland development in the mouse embryo. Development. 2002;129:53–60. doi: 10.1242/dev.129.1.53. [DOI] [PubMed] [Google Scholar]
- 28.Häärä O, et al. Ectodysplasin and Wnt pathways are required for salivary gland branching morphogenesis. Development. 2011;138:2681–2691. doi: 10.1242/dev.057711. [DOI] [PubMed] [Google Scholar]
- 29.Brantley DM, et al. Nuclear factor-κB (NF-κB) regulates proliferation and branching in mouse mammary epithelium. Mol Biol Cell. 2001;12:1445–1455. doi: 10.1091/mbc.12.5.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demicco EG, et al. RelB/p52 NF-κB complexes rescue an early delay in mammary gland development in transgenic mice with targeted superrepressor IκB-α expression and promote carcinogenesis of the mammary gland. Mol Cell Biol. 2005;25:10136–10147. doi: 10.1128/MCB.25.22.10136-10147.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsukamoto AS, Grosschedl R, Guzman RC, Parslow T, Varmus HE. Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell. 1988;55:619–625. doi: 10.1016/0092-8674(88)90220-6. [DOI] [PubMed] [Google Scholar]
- 32.Lane TF, Leder P. Wnt-10b directs hypermorphic development and transformation in mammary glands of male and female mice. Oncogene. 1997;15:2133–2144. doi: 10.1038/sj.onc.1201593. [DOI] [PubMed] [Google Scholar]
- 33.Beleut M, et al. Two distinct mechanisms underlie progesterone-induced proliferation in the mammary gland. Proc Natl Acad Sci USA. 2010;107:2989–2994. doi: 10.1073/pnas.0915148107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clarke A, Phillips DIM, Brown R, Harper PS. Clinical aspects of X-linked hypohidrotic ectodermal dysplasia. Arch Dis Child. 1987;62:989–996. doi: 10.1136/adc.62.10.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mégarbané H, et al. Unusual presentation of a severe autosomal recessive anhydrotic ectodermal dysplasia with a novel mutation in the EDAR gene. Am J Med Genet A. 2008;146A:2657–2662. doi: 10.1002/ajmg.a.32509. [DOI] [PubMed] [Google Scholar]
- 36.Sovak MA, et al. Aberrant nuclear factor-κB/Rel expression and the pathogenesis of breast cancer. J Clin Invest. 1997;100:2952–2960. doi: 10.1172/JCI119848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biswas DK, et al. NF-κ B activation in human breast cancer specimens and its role in cell proliferation and apoptosis. Proc Natl Acad Sci USA. 2004;101:10137–10142. doi: 10.1073/pnas.0403621101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.