Abstract
Variations in percent mammographic density (PMD) reflect variations in the amounts of collagen and number of epithelial and non-epithelial cells in the breast. Extensive PMD is associated with a markedly increased risk of invasive breast cancer. The PMD phenotype is important in the context of breast cancer prevention because extensive PMD is common in the population, is strongly associated with risk of the disease, and, unlike most breast cancer risk factors, can be changed. Work now in progress makes it likely that measurement of PMD will be improved in the near future and that understanding of the genetics and biological basis of the association of PMD with breast cancer risk will also improve. Future prospects for the application of PMD include mammographic screening, risk prediction in individuals, breast cancer prevention research, and clinical decision making.
Introduction
Breast density, assessed by mammography and expressed as a percentage of the mammogram occupied by radiologically dense tissue (percent mammographic density, or PMD), reflects variations in breast tissue composition and is strongly associated with breast cancer risk [1]. Here, we review the evidence that PMD is a risk factor for breast cancer, histological and other factors associated with variations in PMD, and the biological plausibility of the associations with risk of breast cancer. We discuss the potential clinical applications of this risk factor in screening, in research on breast cancer prevention, and in risk prediction in individuals. Mammographic density has been the subject of a meta-analysis (see next section) [1] and a recent review [2] and readers are referred to these sources for additional information.
Mammographic density and risk of breast cancer
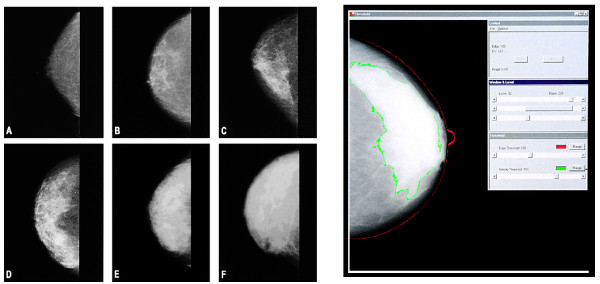
The radiographic appearance of the breast on mammography varies among women, as illustrated in Figure 1, and reflects variations in breast tissue composition and the different x-ray attenuation characteristics of these tissues [3]. Fat is radiologically lucent and appears dark on a mammogram. Connective and epithelial tissues are radiologically dense and appear light. This appearance is usually expressed as a percentage of the breast area, or (as referred to here) as percent mammographic density (PMD).
Figure 1.
Examples of mammographic density. (a) 0% mammographic density, (b) less than 10%, (c) less than 25%, (d) less than 50%, (e) less than 75%, and (f) greater than 75%. On the right is an illustration of Cumulus in the measurement of mammographic density. The red line outlines the breast, and the green line outlines the area of density. Republished with permission from [2].
In a systematic meta-analysis of data for more than 14,000 cases and 226,000 non-cases from 42 studies, McCormack and dos Santos Silva [1] reviewed the data on the association of PMD with risk of breast cancer. The authors found that PMD was consistently associated with risk of breast cancer. Associations were stronger in studies in the general population rather than symptomatic women, in studies of incident rather than prevalent cancer, and for percent density rather than Wolfe's classification. Wolfe was the first to describe differences in breast cancer risk associated with variations in the mammographic appearance of the breast [4,5]; he used four categories: N1 (predominately fat), P1 and P2 (ductal prominence in less than 25% or more than 25% of the breast, respectively), and DY (extensive 'dysplasia'). A quantitative method of measuring breast density, Cumulus, is illustrated in Figure 1. Thresholds placed at the edge of the breast (red line) and the edge of density (green line) are used to calculate PMD [6].
Table 1 summarizes selected features of the cohort studies, or studies nested within cohorts, that used quantitative methods to classify PMD [7-15]. The 10 studies shown were carried out in the US, Europe, or Canada and all found a statistically significant increase in risk associated with more extensive PMD after adjustment for other risk factors, and the increase in risk persisted for at least 8 to 10 years from the date of the mammogram used to classify PMD [9,15]. There is also evidence of a dose-response relationship (that is, of risk increasing with increasing PMD).
Table 1.
Selected characteristics of cohort studies with quantitative classification of percent mammographic density
| Authors/study, region | Subject age, years | Sample sizea | Measurementb | Partitionc | OR (95% CI) | Follow-up, years | Adjustmentsd |
|---|---|---|---|---|---|---|---|
| Kato et al. [7], USA | 35-65 | 197/521 | Planimetry | Upper versus lower tertile | 3.6 (1.4 to 9.1) | 5.5 | BMI, parity, and menopause |
| Saftlas et al. [8], USA | 35-74 | 266/301 | Planimetry | <5% versus ≥65% | 4.3 (2.1 to 8.8) | 5 | Age, weight, and parity |
| Byrne et al. [9], USA | 35-74 | 1,880/2,152 | Planimetry | 0% versus ≥75% | 4.3 (3.1 to 6.1) | 10 | Weight, age at first birth, family history, years of education, alcohol use, previous benign biopsies, and reproductive years |
| Torres-Mejia et al. [10], Europe | 40-80 | 111/3,100 | Computer-assisted | 0.5% versus >46% | 3.5 (1.4 to 5.2) | 14 | Age, education, parity, height, and BMI |
| van Gils et al. [11], Europe | >45 | 129/517 | Automated | <5% versus >25% | 2.9 (1.6 to 5.6) | 10 | Age and parity |
| Thomas et al. [12], USA | <50 | 547/472 | Estimation | Upper versus lower quartiles | 4.4 (3.0 to 6.7) | >6 | Age and study |
| Maskarinec et al. [13], USA | 60e | 607/667 | Computer-assisted | <10% versus >50% | 3.6 (2.3 to 5.6) | 7 | Ethnicity, age, BMI, age at first birth, number of births, age at menarche, age at menopause, HRT, and family history of breast cancer |
| Boyd et al./NBSS [14], Canada | 40-59 | 330 | a. Estimation b. Computer-assisted |
0% versus ≥75% | a. 6.0 (2.8 to 13.0) b. 4.0 (2.1 to 7.7) | 7 | Age, parity, age at first birth, weight, height, number of births, age at menarche, and family history |
| Boyd et al./SMPBC [15], Canada | 40-70 | 398 | a. Estimation b. Computer-assisted |
<10% versus ≥75% | a. 4.5 (1.9 to 11.0) b. 4.4 (2.1 to 5.0) | 6 | Age, parity, age at first birth, weight, height, number of births, age at menarche, and family history |
| Boyd et al./OBSP [15], Canada | 50-69 | 386 | a. Estimation b. Computer-assisted |
<10% versus ≥75% | a. 3.4 (1.1 to 10.3) b. 4.1 (2.0 to 8.6) |
8 | Age, parity, age at first birth, weight, height, number of births, age at menarche, and family history |
| Boyd et al./Combined [15], Canada | 40-70 | 1,114 | a. Estimation b. Computer-assisted |
<10% versus ≥75% | a. 4.7 (3.0 to 7.4) b. 4.4 (2.9 to 6.7) | 6-8 | Age, parity, age at first birth, weight, height, number of births, age at menarche, and family history |
aReported as the number of case subjects/number of control subjects or as the number of pairs of case and control subjects. bEstimation means visual estimation by an observer (radiologist). cThe most and least extensive categories of density from which odds ratios (ORs) were calculated. dFactors included in the analysis of risk associated with mammographic density. Factors controlled for by matching are also included. eAverage age. Table reproduced from [2]. BMI, body mass index; CI, confidence interval; HRT, hormone replacement therapy; NBSS, National Breast Screening Study; OBSP, Ontario Breast Screening Program; SMPBC, Screening Mammography Program of British Columbia. Republished with permission from [2].
Other qualitative classifications, such as the four-category system developed by the American College of Radiology (Breast Imaging-Reporting and Data System, or BI- RADS), also create groups with different risks of breast cancer [16,17]. The BI-RADS classification of mammographic density has four categories: (1) almost entirely fatty, (2) scattered fibroglandular densities, (3) heterogeneously dense, and (4) extremely dense. BI-RADS is the only classification of mammographic density currently in clinical use in the US but, of the available methods, appears to be the least reliable. Reliability between readers is modest (kappa statistic = 0.56) [18], whereas the interclass correlation coefficient for trained readers using Cumulus is more than 0.9 [15]. Nonetheless, the BI-RADS classification does distinguish women at different risks for the development of breast cancer, and a summary by Cummings and colleagues [17] estimated a fourfold gradient in risk between BI-RADS categories 1 and 4.
As shown in Table 2, PMD is associated with risk of breast cancer both at screening and between screening examinations. In the three Canadian studies shown in Table 1[15], the method of breast cancer detection was recorded by each of the programs. We used those classifications to subdivide the breast cancers into those detected at screening, those detected within 12 months of a negative screen, and those detected more than 12 months after a negative screening examination. In a comparison of those with less than 10% density and those with more than 75% density, the odds ratio was 4.74 (95% confidence interval (CI) 3.0, 7.4) for all cancers. In the 717 cases of breast cancer detected at screening, the odds ratio was 3.52 (95% CI 2.0, 6.2). In the 124 cases of breast cancer detected within 12 months of the last screening examination, the odds ratio for risk of breast cancer in those with more than 75% density was 17.81 (95% CI 4.8, 65.9). For cancers detected more than 12 months after the last screen, the odds ratio for those with more than 75% density was 5.68 (95% CI 2.1, 15.5). Within each category of detection, there was a monotonic increase in risk with each category of density, and the trend tests were all highly significant. Similar results were seen in each of the three screening programs.
Table 2.
Mammographic density and risk of breast cancer according to method of detection: unmatched analysis and radiologists' classification of density
| Categories of percent density, percentage | ||||||||
|---|---|---|---|---|---|---|---|---|
| Number of pairsa | <10 | 10 to <25 | 25 to <50 | 50 to <75 | >75 | P valueb | ||
| All | Case | 1,112 | 230 | 272 | 336 | 178 | 96 | |
| Control | 1,112 | 362 | 270 | 290 | 144 | 46 | ||
| ORc | 1 | 1.75 | 2.06 | 2.43 | 4.74 | <0.0001 | ||
| (95% CI) | (1.4, 2.2) | (1.6, 2.6) | (1.8, 3.3) | (3.0, 7.4) | ||||
| Screen-detected | Case | 717 | 173 | 171 | 219 | 102 | 52 | |
| Control | 717 | 242 | 162 | 196 | 88 | 29 | ||
| ORc | 1 | 1.65 | 1.77 | 1.98 | 3.52 | <0.0001 | ||
| (95% CI) | (1.2, 2.2) | (1.3, 2.4) | (1.3, 2.9) | (2.0, 6.2) | ||||
| Non-screen-detected <12 monthsd | Case | 124 | 12 | 22 | 33 | 32 | 25 | |
| Control | 124 | 35 | 29 | 29 | 23 | 8 | ||
| ORc | 1 | 2.11 | 3.61 | 5.65 | 17.81 | <0.0001 | ||
| (95% CI) | (0.9, 5.2) | (1.5, 8.7) | (2.1, 15.3) | (4.8, 65.9) | ||||
| Non-screen-detected >12 monthse | Case | 262 | 43 | 79 | 80 | 42 | 18 | |
| Control | 262 | 82 | 79 | 62 | 30 | 9 | ||
| ORc | 1 | 2.00 | 2.64 | 3.13 | 5.68 | <0.0001 | ||
| (95% CI) | (1.2, 3.4) | (1.5, 4.6) | (1.6, 6.2) | (2.1, 15.5) | ||||
aNine pairs were excluded from the screen or non-screen group analysis because of missing information on detection (n = 1) or the last mammogram date (n = 8). bP value for the Cochran-Armitage trend test. cAdjusted for age, body mass index, age at menarche, parity, number of live births, age at first birth, menopausal status, age at menopause, hormone replacement therapy (ever/never), breast cancer in first-degree relatives (0, 1, and 2+), study (National Breast Screening Study, Ontario Breast Screening Program, and Screening Mammography Program of British Columbia), and observation time (2 years, 2 to 4 years, and greater than 4 years). dCancers detected within 12 months of the last screening date. eCancers detected 12 months or more after the last screening date. Table reproduced from [15]. CI, confidence interval; OR, odds ratio. Republished with permission from [15].
More extensive PMD was thus associated with an increased risk of breast cancer at screening, in the presence of potential masking by density. The marked elevation in risk associated with PMD in the 12 months after a negative screening examination does, however, probably reflect the masking of tumors by density. The annual incidence of breast cancer associated with di erent degrees of density may be best estimated by combining the incident cancers detected at screening with those found by other methods in the 12 months following screening [15].
Comparison with other risk factors
Relative risk
Among other menstrual, reproductive, and familial risks of breast cancer, only age and BRCA carrier status are associated with larger relative risks of breast cancer than PMD (for example, [19]). The relative risk associated with density is substantially larger than the relative risk of breast cancer associated with a family history of the disease or any of the menstrual and reproductive risk factors.
Attributable risk
Because extensive PMD is common in the population and associated with a large relative risk, if the association with breast cancer risk is causal, the proportion of the disease attributable to this risk factor is expected to be substantial. According to data from three Canadian screening programs [15], the risks of breast cancer attributable to density of 50% or more were 16% for all cancers, 12% for screen-detected cancers, 40% for cancers detected within 12 months of a negative screen, and 16% for cancers detected more than 12 months after a screening examination.
For women below the median age of 56 years, the prevalence of density of 50% or more was about three times greater than in older women, in each category of detection, and the attributable risks of breast cancer were 26% for all cancers, 21% for screen-detected cancers, 50% for cancers detected within 12 months of a negative screen, and 28% for cancers detected more than 12 months after a screening examination. Similar estimates of attributable risk have been calculated for PMD in the Breast Cancer Detection and Demonstration project [9]. These estimates of attributable risk are larger than for any other risk factor for breast cancer, including BRCA carrier status, which is estimated to be responsible for 5% or less of all breast cancer [20,21].
Biological plausibility of the association of mammographic density and breast cancer risk
Hypotheses concerned with the biological basis of the association of PMD with risk of breast cancer have been reviewed elsewhere [22] and will be discussed only briefly here. The change in PMD with age reflects the reduction in glandular tissue and accompanying increase in fat which occur with increasing age. This decline in the risk factor of density with age may seem paradoxical, as breast cancer incidence increases with age. However, cumulative exposure to PMD reflects cumulative exposure of breast stroma and epithelium to hormonal and growth factor stimuli to cell division. Cumulative exposure to PMD increases with age and may be related to the age-specific incidence of breast cancer [23].
As reviewed in [22], PMD is also less extensive in women who are parous and in those with a larger number of live births and is reduced by menopause. After adjustment for age and other potential influences, a family history of breast cancer is associated with a more extensive PMD [24]. PMD has consistently been found to be inversely associated with body weight. Greater birth weight and adult height have been shown to be positively associated with PMD [25,26] and with an increased risk of breast cancer [27]. With the exception of weight, PMD may be on the causal pathway for breast cancer for some or all of these other risk factors.
Many of the factors that are associated with PMD are also associated with alterations in exposure to hormones that may influence the number and proliferative state of epithelial and stromal cells in the breast. To date, most studies of blood levels of ovarian hormones have found either no association or an inverse association with PMD in premenopausal or postmenopausal women (reviewed in [22]). Positive associations with PMD have been found between serum levels of growth hormone and breast water (a surrogate for PMD) in young women from 15 to 30 years old [28], and serum insulin-like growth factor I (IGF-I) levels in premenopausal women and postmenopausal women, and with serum levels of prolactin in postmenopausal women (reviewed in detail in [22]).
Radiologically dense breast tissue - in addition to greater amounts of collagen and cells and greater stained area (on immunohistochemistry) of IGF-I - also have greater amounts of the tissue inhibitor metalloprotease 3 (TIMP-3) [29]. Aromatase immunoreactivity is also associated with dense breast tissue [30]. The proteoglycans lumican and decorin have been associated with breast cancer and have also been found to be present in greater amounts in women with extensive PMD [31].
Mammographic density and risk of histological precursors to breast cancer
Mammographic density reflects the proportions of fat, stromal, and epithelial tissue in the breast and does not denote any histological abnormality [32,33]. Extensive mammographic density is, however, associated with increased risks for the development of most of the histological abnormalities that are non-obligate precursors of breast cancer. The breast lesions of ductal carcinoma in situ (DCIS), atypical hyperplasia, hyperplasia without atypia, and columnar cell lesions (CLLs) are, to different degrees, associated with an increased risk of breast cancer, and, as discussed below, risk of each type of lesion is also increased by extensive PMD.
In the Multiethnic cohort, women with more than 50% PMD had, compared with those with less than 10% PMD, an increased risk of both invasive breast cancer (OR = 3.58; 95% CI 2.3, 5.7) and DCIS (OR = 2.9; 95% CI 1.4, 5.9) [26]. A case control study in the Canadian National Breast Screening Study showed that, in women with more than 75% density, compared with those with no density, risk of in situ breast cancer and atypical hyperplasia combined was greater (OR = 9.7; 95% CI 1.7, 53.9), as was risk of hyperplasia without atypia (OR = 12.2; 95% CI 2.9, 50.1) [34]. Additional studies have also shown PMD to be associated with risk of DCIS [35,36].
CLL, thought to be the earliest recognizable histological feature that is a non-obligate precursor to breast cancer, has been found to be more frequent (OR = 2.2; 95% CI 1.03, 4.8) in biopsies from breasts with more than the median density of 30%. CLLs were also strongly positively associated with the percentage of the biopsy occupied by collagen (P = 9.2 × 10-5) and glandular area (P = 2 × 10-5) [37]. Age-related atrophy of breast lobules (lobular involution) has been found to be inversely associated with risk of breast cancer [38], and it appears that PMD and lobular involution are independently associated with risk of breast cancer [39].
Future prospects
Potential improvements in measuring breast tissue composition
All of the methods currently used to assess breast density by mammography have limitations. None takes into account the thickness of the breast, and all are based on the projected area, rather than the volume, of breast tissue. All current methods depend upon a trained observer and thus are subjective. These potential sources of error in measurement are likely to attenuate the observed associations between percent PMD and other risk factors for breast cancer and risk of the disease itself.
To date, three published case control studies have examined the association between percent PMD and risk of breast cancer by measuring breast tissue volumes. One used standard mammography form (SMF) software that uses information about the non-fat tissue in the breast, in conjunction with the thickness of the compressed breast and the breast imaging variables of tube voltage and exposure time, to generate estimates of breast tissue volumes [40]. In an alternative approach to the measurement of tissue volumes, we acquired images prospectively from mammography machines calibrated to allow examination of the relationship between the image signal in each pixel (that is, optical density or blackness of the processed film value), the exposure factors (that is, kilovoltage, milliampere-seconds, tube target, and beam filter), and the amount of radiation transmitted by the breast. Corrected breast tissue thickness and breast tissue volumes were calculated [41].
In two of these studies, the volume-based measures of percent density were associated with breast cancer risk, though less strongly than the area-based measures of percent density. It is not yet clear whether these results reflect as-yet-uncorrected errors in the measurement of breast tissue volumes or the failure to capture additional breast risk information that is present in the area-based measures. An alternative method of measuring percent fibroglandular tissue volumes by using single x-ray absorptiometry has been shown to more accurately predict breast cancer risk than percent dense area [42] but has not yet been replicated or applied to digital mammograms. Other methods of measuring tissue volumes are under development [43,44].
Potential alternatives to the assessment of breast tissue composition by mammography include measurement of the breast water (reflecting the stromal and epithelial tissue) and fat content by magnetic resonance (MR) and ultrasound tomography (UST). Both have been discussed elsewhere as alternatives to mammography in measuring density [2]. Percent PMD in the mammogram is strongly correlated both with percent water by MR (Spearman r = 0.85; P < 0.001) [45] and average sound speed by UST (Spearman r = 0.77; P < 0.001) [46].
Etiology of mammographic density
Because PMD is strongly associated with risk of breast cancer, factors that influence PMD may also contribute to the causes of breast cancer, and the identification of factors that change PMD may lead to the identification of factors that can reduce the incidence of breast cancer. Age, parity, and menopausal status (see 'Biological plausibility of the association of mammographic density and breast cancer risk' section above) account for only 20% to 30% of the PMD variation observed in the population [47], and genetic factors might explain a proportion of variation (that is, the heritability) of PMD. Two large, twin studies have added to the evidence that PMD is a heritable quantitative trait. In one, 951 twin pairs (age range of 40 to 70 years) in Australia and North America were recruited, and mammograms and information on the factors associated with variations in PMD were collected. After adjustment for age and other covariates, the proportion of the residual variation in PMD accounted for by additive genetic factors (heritability) was estimated to be 63% (95% CI 59% to 67%) in the combined studies [48]. In a second study, with 553 twin pairs, the proportion of the residual variation in PMD heritability was estimated to be 53% [49]. Research now in progress seeks to identify genetic variants associated with PMD, and, of the 12 single-nucleotide polymorphisms reproducibly associated with risk of breast cancer, at least 3 have been found to be also associated with PMD [50,51].
Understanding of biological mechanisms
Epithelial and stromal cells, collagen, and fat are the tissue components that contribute to variations in PMD. The twin studies described in the previous section indicate that the quantities of these tissue components in the breast are determined largely by heritable factors. Furthermore, each component has properties that may influence the risk and progression of breast cancer.
Breast cancer arises from epithelial cells and the number and proliferative state of these cells may influence both the radiological density of the breast and the probability of genetic damage that can give rise to cancer. In addition, collagen and the stromal matrix are products of stromal cells, which may, through mechanical and other properties, facilitate tumor invasion. Interactions between stroma and epithelium are known to influence breast development and the changes in breast structure that take place during pregnancy, lactation, and involution and during tumorigenesis. The extracellular matrix, which comprises collagens, fibronectin, laminins, polysaccharides, and proteoglycans, plays a key role in these processes, and there is a large and rapidly growing body of literature on the molecules that mediate how the extracellular matrix influences the epithelium (see [52-55] for reviews). Proteoglycans (see 'Biological plausibility of the association of mammographic density and breast cancer risk' section above) bind growth factors, contribute to the mechanical integrity of tissues, may reflect the stiffness of breast tissue, and can modify tissue behavior [55]. To date, there has been limited application of these basic science findings to understanding the association between PMD and risk of breast cancer. Animal models now being developed may clarify the biological mechanisms that underlie the association of PMD with breast cancer risk.
Potential clinical applications of mammographic density
Mammographic screening
The evidence given above shows that women undergoing screening for breast cancer with mammography are heterogeneous with respect to cancer risk and the ease with which breast cancer can be detected by mammography. Women with extensive PMD are doubly disadvantaged as they are both at higher risk of developing breast cancer and at greater risk that cancer will not be detected by mammography, because of 'masking' by density of the radiological signs of cancer. In the presence of this underlying heterogeneity in the population undergoing screening, it does not seem likely that screening with a single modality and a single screening frequency will be optimal. It seems possible that, for women with extensive PMD, screening more often than once every 2 to 3 years and with modalities such as MR or UST in addition to mammography would improve cancer detection rates at screening and reduce the frequency of interval cancers. For women with radio-lucent breast tissue and a negative screening mammogram, in whom risk is lower and detection easier, re-screening less frequently than every 2 to 3 years might be safe. Research is required into optimizing screening frequency and modality according to the breast tissue characteristics of women. An approach to mammographic screening that starts at age 40 and that bases the frequency of screening on a women's age, breast density (by BI-RADS score), and other risk factors was recently advocated and shown to be cost-effective [56]. However, in an editorial accompanying that paper, a number of potential limitations of this approach were raised [57]. These limitations include lack of knowledge of the biological basis of the risk associated with mammographic density and of the effects of density on the risk and detection of breast cancer subtypes (see 'Breast cancer characteristics and clinical outcomes' section below).
Individual risk prediction
Currently, the most widely used method of predicting risk of breast cancer in individuals is the Gail model [58], which takes into account a woman's age, age at menarche, age at first live birth, number of previous benign breast biopsies, and number of first-degree relatives with breast cancer. Breast density is more strongly associated with breast cancer risk than the other variables included in the Gail model, and the addition of breast density, measured by a manual method tracing, to the Gail model increased predictive accuracy, as shown by the concordance statistic, from 0.607 to 0.642 [59]. Tice and colleagues [60] developed a predictive model for breast cancer by using the BI-RADS classification; the model had a concordance statistic of 0.66. The Gail and Tice models have only moderate levels of risk prediction that might be improved by the improvements in measuring breast density described above.
Breast cancer prevention trials
In contrast to most other risk factors for breast cancer, mammographic density can be changed (as described below), suggesting that MD might be used as a surrogate marker in clinical trials of potential approaches to breast cancer prevention. Clinical trials of breast cancer prevention require large numbers of subjects and long periods of observation and thus are expensive. The number of subjects required in a breast cancer prevention trial can, however, be reduced by the selection of subjects at increased risk of breast cancer. We have carried out a long-term dietary intervention study in 4,690 women selected because they had mammographic density in 50% or more of the breast. During an average follow-up of 10 years (range of 7 to 17 years), invasive breast cancer was detected in 220 women, an observed age-specific incidence twice that of women of the same age in the Canadian population followed for the same length of time. However, a potential limitation of the selection of a high-risk group is that the results of such a trial may not be applicable to women who are not at increased risk [61].
It would make possible smaller, shorter, and less expensive trials of breast cancer prevention strategies if there were a breast cancer surrogate that after a short period of observation would allow the identification of interventions that would reduce breast cancer incidence. To be used as a surrogate for breast cancer, a biomarker such as PMD should meet the criteria proposed by Prentice [62] and further by Schatzkin and Gail [63]. These are that (a) the marker should be associated with risk of breast cancer, (b) the marker should be changed by the intervention, and (c) the change in the marker should mediate the effect of the intervention on breast cancer risk.
In a case control study nested within the first International Breast Cancer Intervention Study (IBIS), a randomized prevention trial of tamoxifen versus placebo, Cuzick and colleagues [64] showed that, compared with all women in the placebo group, those in the tamoxifen group who experienced a 10% or greater reduction in breast density had a 63% reduction in breast cancer risk, whereas those who took tamoxifen but experienced a reduction in PMD of less than 10% had no risk reduction. In the placebo arm, breast cancer risk was similar in subjects who experienced less than a 10% reduction in PMD and those who experienced a greater reduction. The authors conclude that the change in PMD 12 to 18 months after starting treatment is an excellent predictor of response to tamoxifen in the preventive setting [64].
These results (and others) show that PMD is associated with risk of breast cancer and is changed by intervention with tamoxifen. However, although the change in PMD was associated with the effect of tamoxifen on breast cancer risk, no evidence is given that the change in PMD mediated the effect of tamoxifen on breast cancer risk.
Even if it were convincingly shown that change in PMD did mediate the effects of tamoxifen on breast cancer risk, it should not be concluded that all other causes of a reduction in PMD will reduce risk of breast cancer. For example, as discussed above, average PMD decreases with increasing age whereas breast cancer incidence increases with age. A randomized controlled trial of physical activity for 1 year in postmenopausal women, which may reduce breast cancer risk, showed that PMD was increased as a result of the weight loss associated with the intervention [65].
Other interventions that are known to influence PMD and breast cancer risk include combined hormone therapy (but not estrogen alone), which increases PMD and risk of breast cancer [66-68], and a gonadotrophinreleasing hormone agonist reduces PMD in premenopausal women [69]. It is not yet known whether PMD can be used as a surrogate for breast cancer in any of these settings. In the IBIS trial, the association observed between change in PMD and reduction in breast cancer incidence with tamoxifen suggests that change in PMD after the initiation of hormone therapy might be useful in the prediction of effect in therapeutic settings.
Breast cancer characteristics and clinical outcomes
Tables 3 and 4 show, respectively, summaries of published studies that have examined the associations of breast density with tumor characteristics and the clinical course of breast cancer. To date, most studies examining the association of breast density with tumor characteristics have used a qualitative measure of density (for example, BI-RADS), lacked information on covariates, and differed in whether and how the cancer was detected (by screening or other means).
Table 3.
Summary of studies of the association of mammographic density and tumor characteristics
| Association with | |||||||
|---|---|---|---|---|---|---|---|
| Authors, region (year) |
Design | Sample size | Measurement of MD |
ER status/phenotypea | Sizea, b | Nodal statusa, b |
Adjustmentsc |
| Yaghjyan et al. [70], USA (2011) | Nested case control | 1,042 cases 1,794 controls | Computer-assisted | Case control: Increased risk of ER+ and ER- tumors (greater for ER-) Increased risk of PR+ and PR- and HER2- and HER2+ tumors | Increased risk for tumors >2 cm but not for tumors <2 cm | Increased risk with node+ and node- disease | Age, BMI, age at menarche, age at first birth, parity, age at menopause, HRT use, family history, history of benign breast disease, alcohol intake, and smoking |
| Conroy et al. [71], USA (2011) | Nested case control | 607 cases 667 controls | Computer- assisted | Case control: Increased risk of ER+ tumors only Case only: ER+ > PMD than ER- cases |
n/a | n/a | Age, ethnicity, BMI, parity, age at first birth, age at menarche, menopausal status, HRT use, and family history |
| Ding et al. [72], Europe (2010) |
Nested case control | 370 cases 1,904 controls | Computer- assisted | Case control: Increased risk of ER+ tumors only | Increased risk for tumors of all sizes | Increased risk with node+ and node- disease | Age |
| Case only: ER+ > PMD than ER- cases |
No association | No association | |||||
| Olsen et al. [73], Europe (2009) | Cohort | 694 cases 48,052 total | Mixed/dense versus fatty | Increased risk of ER+ and ER- tumors (greater for ER+) | n/a | n/a | Age |
| Ziv et al. [74], USA (2004) | Cohort | 701 cases 44,811 total | BI-RADS | Increased risk of ER+ and ER- tumors | n/a | n/a | Age, HRT use, BMI, parity, family history, menopause, and race |
| Ma et al. [75], USA (2009) | Case control | 479 cases 376 controls | Computer-assisted | Case control: Increased risk of ER+/PR+, ER-/PR-, HER2-, luminal A, and triple-negative tumorsd Case analysis: Molecular subtyped: no association | n/a | n/a | Age, family history, BMI, age at menarche, parity, age at first birth, menopause, and HRT use |
| Gierach et al. [76], Europe (2010 abstract) | Case only | 227 cases | Computer- assisted | No significant difference in PMD between luminal A, luminal B, HER2+, basal-like, or unclassified tumorsd | n/a | n/a | Not available (abstract only) |
| Arora et al. [77], USA (2010) | Case only | 1,323 cases | BI-RADS | Molecular subtype: no association | No association | No association | Age |
| Yang et al. [78], USA (2008) | Case only | 198 cases | BI-RADS | Molecular subtyped: no association | n/a | n/a | None |
| Cil et al. [79], Canada (2009) | Case only | 335 cases | Wolfe score | No association | No association | No association | None |
| Nickson and Kavanagh [86], Australia (2009) | Case only | 1,348 cases | Semi- automated | n/a | No association | n/a | Age, HRT use, and family history |
| Ghosh et al. [80], USA (2008) | Case only | 286 cases | Computer- assisted | No association | No association | n/a | Age, parity, BMI, family history, and HRT use |
| Porter et al. [87], Europe (2007) | Case only | 759 cases | BI-RADS | n/a | Positive (screen- detected) | No association | None |
| Fasching et al. [81], Europe (2006) | Case only | 434 cases | BI-RADS | No association | Negative | No association | None |
| Aiello et al. [82], USA (2005) | Case only | 546 cases | BI-RADS | No association | Positive | Positive | Age, BMI, menopause, and age at first birth |
| Morishita et al. [83], Japan (2005) | Case only | 163 cases | BI-RADS | No association | No association | n/a | None |
| Roubidoux et al. [84], USA (2004) | Case only | 121 cases | BI-RADS | No association | Positive | No association | Age |
| Sala et al. [88], Europe (2000) | Nested case control | 875 cases | Wolfe | n/a | Positive | Positive | None |
| Hinton et al. [85], Europe (1985) | Case only | 337 cases | Wolfe | DY pattern associated with greater frequency of ER+ versus ER- tumors | n/a | n/a | None |
| Boyd et al. [89], Canada (1982) | Case only | 183 cases | Wolfe | n/a | No association | No association | None |
aNo association: association is not statistically significant. bPositive: higher percent mammographic density (PMD) associated with higher tumor size or higher frequency of positive nodal status (node+); negative (inverse) association: higher PMD associated with smaller tumor size or lower frequency of positive nodal status (node+). cFactors included in the analysis of risk associated with mammographic density or of the association of mammographic density with tumor characteristics. dMolecular subtypes determined by immunohistochemistry. BI-RADS, Breast Imaging-Reporting and Data System; BMI, body mass index; DY, dysplastic; ER, estrogen receptor; HRT, hormone replacement therapy; MD, mammographic density; n/a: not assessed; PR, progesterone receptor.
Table 4.
Summary of studies of mammographic density and risk of second breast cancers
| Results | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Authors, region (year) |
Study population |
Median follow-up |
Measurement of MD |
Eventsb | Number | HR (95% CI) |
Adjustmentsa | Comments |
| Habel et al. [91], USA (2010) | 935 patients with DCIS | 8 years | Planimeter Highest versus lowest quintile of dense area |
All Ips. |
228 164 |
1.8 (1.2 to 2.9) 1.7 (1.0 to 2.9) | Age, BMI, treatment, and diagnosis year | Similar HR in subgroups of age, BMI, treatment, and menopausal status |
| Cont. | 59 | 3.0 (1.3 to 6.9) | ||||||
| Hwang et al. [93], USA (2007) | 3,274 patients with DCIS | 39 months | BI-RADS High (3 or 4) versus low (1 or 2) | All inv. | 133 | 1.4 (0.9 to 2.1) | Age and radiation treatment | No interaction of density with radiation treatment |
| Ips. inv. | 83 | 1.0 (0.6 to 1.6) | ||||||
| Cont. inv. | 52 | 3.1 (1.6 to 6.1) | ||||||
| Habel et al. [90], USA (2004) | 334 patients with DCIS | 11 years | Planimetry >75% versus <25% PMD | All | 112 | 2.8 (1.3 to 6.1) | Age, BMI, and radiation treatment | No interaction with radiation treatment or menopausal Status |
| Ips. | 80 | 3.0 (1.2 to 7.5) | ||||||
| Cont. | 28 | 3.4 (0.7 to 16.2) | ||||||
| Cil et al. [79], Canada (2009) | 335 patients with invasive breast cancer | 8 years | Wolfe score High versus low Wolfe score | Ips. inv. | 34 | 5.7 (1.6 to 20.0) | Age, menopause, and radiation treatment | Association stronger in those who did not receive radiation treatment |
| Dist. inv. | 31 | No association (HR not given) | ||||||
| Park et al. [92], USA (2008) | 136 patients with invasive breast cancer | 7.7 years | Computer- assisted >75% versus <25% PMD | Ips. inv. | 19 | 3.4 (1.6 to 7.5) | BMI | |
| Cont./Dist. inv. | 25 | No association (HR not given) | ||||||
aFactors included in the analysis of mammographic density and risk of second breast cancer. bEvents include in situ and invasive cancer unless specified as invasive (inv.). All, all second breast cancers; BI-RADS, Breast Imaging-Reporting and Data System; BMI, body mass index; CI, confidence interval; Cont., second cancer in contralateral breast; DCIS, ductal carcinoma in situ; Dist, distant metastasis; HR, hazard ratio; Ips, second cancer in ipsilateral breast; MD, mammographic density; PMD, percent mammographic density.
Tumor characteristics
Studies that have examined the association of breast density with tumor characteristics of estrogen receptor status, tumor size, and nodal status are summarized in Table 3. These studies vary in size, design, methods used to classify mammographic density, and factors adjusted for in analysis. Differences in these factors may contribute to the inconsistency of the results of the association of breast density with tumor characteristics.
Of 16 studies that examined the association of breast density with hormone receptor status or molecular phenotype [70-85], most found no associations. More extensive density was found to be associated with risk of ER+ tumors in 6 studies [70-75] and of ER- tumors in 4 studies [70,73-75]. Of 12 studies that examined tumor size in relation to breast density [70,72,77,79-84,86-89], 4 found larger tumors [82,84,87,88] and 1 found smaller tumors [81] associated with more extensive density. The remainder found no association. Ten studies examined nodal status [70,72,77,79,81,82,84,87-89], and 2 found nodal involvement to be more frequent in those with extensive density [82,88] and the remainder found no association. In addition, Yaghjyan and colleagues [70] found that the associations between breast density and breast cancer were stronger for in situ than for invasive tumors and for high-grade than for low-grade tumors.
Risk of second breast cancer
Studies that have examined risk of a second invasive or in situ breast cancer are summarized in Table 4. Four [79,90-92] of the five [79,90-93] studies show an increased risk of a second cancer in the ipsilateral breast, and three [90,91,93] of the five show an increased risk in the contralateral breast. Only one [79] of the three [79,91,93] studies to examine the potential modifying role of radiation therapy found evidence that risk of a second breast cancer was higher in those who did not receive radiation.
Women with higher density have been shown to have a higher risk of dying from breast cancer compared with those with lower density, but this is due largely to the increased breast cancer incidence associated with density [73,94]. In terms of survival after a breast cancer diagnosis, one study reported a non-significant trend to better survival in women with dense breasts [68], and another reported that women with mixed/dense breasts had a significantly lower risk of death from any cause or from breast cancer specifically (case fatality rates of 60% and 53%, respectively) compared with women with fatty breasts [73].
Summary
There is now extensive evidence that extensive PMD is a strong risk factor for breast cancer and is associated with large relative and attributable risks for the disease. As discussed above (in the 'Breast cancer prevention trials' section), unlike most breast cancer risk factors, PMD can be changed. Work now in progress is likely to improve measurement of PMD, understanding of the genetics and biological basis of the association of PMD with breast cancer risk, and the clinical significance of change in PMD. Future prospects for the application of PMD include improvements in mammographic screening, risk prediction in individuals, breast cancer prevention research, and clinical decision making.
Abbreviations
BI-RADS: Breast Imaging-Reporting and Data System; CI: confidence interval; CLL: columnar cell lesion; DCIS: ductal carcinoma in situ; ER: estrogen receptor; IBIS: International Breast Cancer Intervention Study; IGF-I: insulin-like growth factor I; MR: magnetic resonance; OR: odds ratio; PMD: percent mammographic density; UST: ultrasound tomography.
Competing interests
MJY is a founding partner of, and holds shares in, Matakina Technology (Wellington, New Zealand), a company that develops software for measurement of breast density. The other authors declare that they have no competing interests.
Contributor Information
Norman F Boyd, Email: boyd@uhnres.utoronto.ca.
Lisa J Martin, Email: lmartin@uhnres.utoronto.ca.
Martin J Yaffe, Email: martin.yaffe@swri.ca.
Salomon Minkin, Email: minkin@uhnres.utoronto.ca.
Acknowledgements
The work described here was supported in part by grants from the Canadian Breast Cancer Research Alliance and the National Cancer Institute of Canada and by the Ontario Ministry of Health and Long-Term Care.
References
- McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159–1169. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- Boyd NF, Martin LJ, Bronskill MJ, Yaffe MJ, Duric N, Minkin S. Breast tissue composition and susceptibility to breast cancer. J Natl Cancer Inst. 2010;102:1224–1237. doi: 10.1093/jnci/djq239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns PC, Yaffe MJ. X-ray characterisation of normal and neoplastic breast tissues. Phys Med Biol. 1987;32:675–695. doi: 10.1088/0031-9155/32/6/002. [DOI] [PubMed] [Google Scholar]
- Wolfe JN. Risk for breast cancer development determined by mammographic parenchymal pattern. Cancer. 1976;37:2486–2492. doi: 10.1002/1097-0142(197605)37:5<2486::aid-cncr2820370542>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Wolfe JN. Breast patterns as an index of risk for developing breast cancer. AJR Am J Roentgenol. 1976;126:1130–1139. doi: 10.2214/ajr.126.6.1130. [DOI] [PubMed] [Google Scholar]
- Byng JW, Boyd NF, Fishell E, Jong RA, Yaffe MJ. The quantitative analysis of mammographic densities. Phys Med Biol. 1994;39:1629–1638. doi: 10.1088/0031-9155/39/10/008. [DOI] [PubMed] [Google Scholar]
- Kato I, Beinart C, Bleich A, Su S, Kim M, Toniolo PG. A nested case-control study of mammographic patterns, breast volume, and breast cancer (New York City, NY, United States) Cancer Causes Control. 1995;6:431–438. doi: 10.1007/BF00052183. [DOI] [PubMed] [Google Scholar]
- Saftlas AF, Hoover RN, Brinton LA, Szklo M, Olson DR, Salane M, Wolfe JN. Mammographic densities and risk of breast cancer. Cancer. 1991;67:2833–2838. doi: 10.1002/1097-0142(19910601)67:11<2833::aid-cncr2820671121>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Byrne C, Schairer C, Wolfe J, Parekh N, Salane M, Brinton LA, Hoover R, Haile R. Mammographic features and breast cancer risk: Effects with time, age, and menopause status. J Natl Cancer Inst. 1995;87:1622–1629. doi: 10.1093/jnci/87.21.1622. [DOI] [PubMed] [Google Scholar]
- Torres-Mejía G, De Stavola B, Allen DS, Pérez-Gavilán JJ, Ferreira JM, Fentiman IS, Dos Santos Silva I. Mammographic features and subsequent risk of breast cancer: A comparison of qualitative and quantitative evaluations in the Guernsey prospective studies. Cancer Epidemiol Biomarkers Prev. 2005;14:1052–1059. doi: 10.1158/1055-9965.EPI-04-0717. [DOI] [PubMed] [Google Scholar]
- van Gils CH, Hendriks JH, Otten JD, Holland R, Verbeek AL. Parity and mammographic breast density in relation to breast cancer risk: indication of interaction. Eur J Cancer Prev. 2000;9:105–111. doi: 10.1097/00008469-200004000-00006. [DOI] [PubMed] [Google Scholar]
- Thomas DB, Carter RA, Bush WH Jr, Ray RM, Stanford JL, Lehman CD, Daling JR, Malone K, Davis S. Risk of subsequent breast cancer in relation to characteristics of screening mammograms from women less than 50 years of age. Cancer Epidemiol Biomarkers Prev. 2002;11:565–571. [PubMed] [Google Scholar]
- Maskarinec G, Pagano I, Lurie G, Kolonel LN. A longitudinal investigation of mammographic density: the multiethnic cohort. Cancer Epidemiol Biomarkers Prev. 2006;15:732–739. doi: 10.1158/1055-9965.EPI-05-0798. [DOI] [PubMed] [Google Scholar]
- Boyd NF, Byng JW, Jong RA, Fishell EK, Little LE, Miller AB, Lockwood GA, Tritchler DL, Yaffe MJ. Quantitative classification of mammographic densities and breast cancer risk: results from the Canadian National Breast Screening Study. J Natl Cancer Inst. 1995;87:670–675. doi: 10.1093/jnci/87.9.670. [DOI] [PubMed] [Google Scholar]
- Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, Jong RA, Hislop G, Chiarelli A, Minkin S, Yaffe MJ. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- American College of Radiology. Breast Imaging Reporting and Data System Atlas. 4. Reston, VA: American College of Radiology; 2003. [Google Scholar]
- Cummings SR, Tice JA, Bauer S, Browner WS, Cuzick J, Ziv E, Vogel V, Shepherd J, Vachon C, Smith-Bindman R, Kerlikowske K. Prevention of breast cancer in postmenopausal women: approaches to estimating and reducing risk. J Natl Cancer Inst. 2009;101:384–398. doi: 10.1093/jnci/djp018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerlikowske K, Grady D, Barclay J, Frankel SD, Ominsky SH, Sickles EA, Ernster V. Variability and accuracy in mammographic interpretation using the American college of radiology breast imaging reporting and data system. J Natl Cancer Inst. 1998;90:1801–1809. doi: 10.1093/jnci/90.23.1801. [DOI] [PubMed] [Google Scholar]
- Veronesi U, Boyle P, Goldhirsch A, Orecchia R, Viale G. Breast cancer. Lancet. 2005;365:1727–1741. doi: 10.1016/S0140-6736(05)66546-4. [DOI] [PubMed] [Google Scholar]
- Newman B, Mu H, Butler LM, Millikan R, Moorman PG, King M-C. Frequency of breast cancer attributable to BRCA1 in a population-based series of American women. JAMA. 1998;279:915–921. doi: 10.1001/jama.279.12.915. [DOI] [PubMed] [Google Scholar]
- Peto J, Collins N, Barfoot R, Seal S, Warren W, Rahman N, Easton DF, Evans C, Deacon J, Stratton MR. Prevalence of BRCA1 and BRCA2 gene mutations in patients with early-onset breast cancer. J Natl Cancer Inst. 1999;91(11):943–949. doi: 10.1093/jnci/91.11.943. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Boyd N. Potential mechanisms of breast cancer risk associated with mammographic density: hypotheses based on epidemiological evidence. Breast Cancer Res. 2008;10:201. doi: 10.1186/bcr1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd NF, Rommens JM, Vogt K, Lee V, Hopper JL, Yaffe MJ, Paterson AD. Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol. 2005;6:798–808. doi: 10.1016/S1470-2045(05)70390-9. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Melnichouk O, Guo H, Chiarelli AM, Hislop TG, Yaffe MJ, Minkin S, Hopper JL, Boyd NF. Family history, mammographic density, and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:456–463. doi: 10.1158/1055-9965.EPI-09-0881. [DOI] [PubMed] [Google Scholar]
- Sellers TA, Vachon CM, Pankratz VS, Janney CA, Fredericksen Z, Brandt KR, Huang Y, Couch FJ, Kushi LH, Cerhan JR. Association of childhood and adolescent anthropometric factors, physical activity, and diet with adult mammographic breast density. Am J Epidemiol. 2007;166:456–464. doi: 10.1093/aje/kwm112. [DOI] [PubMed] [Google Scholar]
- Brisson J, Morrison AS, Kopans DB. Height and weight, mammographic features of breast tissue, and breast cancer risk. Am J Epidemiol. 1984;119:371–381. doi: 10.1093/oxfordjournals.aje.a113755. [DOI] [PubMed] [Google Scholar]
- Hunter DJ, Willett WC. Diet, body size, and breast cancer. Epidemiol Rev. 1993;15:110–132. doi: 10.1093/oxfordjournals.epirev.a036096. [DOI] [PubMed] [Google Scholar]
- Boyd N, Martin L, Chavez S, Gunasekara A, Salleh A, Melnichouk O, Yaffe M, Friedenreich C, Minkin S, Bronskill M. Breast-tissue composition and other risk factors for breast cancer in young women: a cross-sectional study. Lancet Oncol. 2009;10:569–580. doi: 10.1016/S1470-2045(09)70078-6. [DOI] [PubMed] [Google Scholar]
- Guo YP, Martin LJ, Hanna W, Banerjee D, Miller N, Fishell E, Khokha R, Boyd NF. Growth factors and stromal matrix proteins associated with mammographic densities. Cancer Epidemiol Biomarkers Prev. 2001;10:243–248. [PubMed] [Google Scholar]
- Vachon CM, Sasano H, Ghosh K, Brandt KR, Watson DA, Reynolds C, Lingle WL, Goss PE, Li R, Aiyar SE, Scott CG, Pankratz VS, Santen RJ, Ingle JN. Aromatase immunoreactivity is increased in mammographically dense regions of the breast. Breast Cancer Res Treat. 2011;125:243–252. doi: 10.1007/s10549-010-0944-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alowami S, Troup S, Al-Haddad S, Kirkpatrick I, Watson PH. Mammographic density is related to stroma and stromal proteoglycan expression. Breast Cancer Res. 2003;5:R129–R135. doi: 10.1186/bcr622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartow SA, Mettler FA Jr, Black WC III. Correlations between radiographic patterns and morphology of the female breast. Rad Patterns Morph. 1997;13:263–275. [Google Scholar]
- Li T, Sun L, Miller N, Nicklee T, Woo J, Hulse-Smith L, Tsao MS, Khokha R, Martin L, Boyd N. The association of measured breast tissue characteristics with mammographic density and other risk factors for breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:343–349. doi: 10.1158/1055-9965.EPI-04-0490. [DOI] [PubMed] [Google Scholar]
- Boyd NF, Jensen H, Cooke G, Lee Han HW. Relationship between mammographic and histological risk factors for breast cancer. J Natl Cancer Inst. 1992;84:1170–1179. doi: 10.1093/jnci/84.15.1170. [DOI] [PubMed] [Google Scholar]
- Reinier KS, Vacek PM, Geller BM. Risk factors for breast carcinoma in situ versus invasive breast cancer in a prospective study of pre- and postmenopausal women. Breast Cancer Res Treat. 2006;103:343–348. doi: 10.1007/s10549-006-9375-9. [DOI] [PubMed] [Google Scholar]
- MacKenzie TA, Vacek PM, Geller B, Weiss JE, Goodrich ME, Carney PA. Breast density in relation to risk of ductal carcinoma in situ of the breast in women undergoing screening mammography. Cancer Causes Control. 2007;18:939–945. doi: 10.1007/s10552-007-9035-3. [DOI] [PubMed] [Google Scholar]
- Turashvili G, McKinney S, Martin L, Gelmon KA, Watson P, Boyd N, Aparicio S. Columnar cell lesions, mammographic density and breast cancer risk. Breast Cancer Res Treat. 2009;115:561–571. doi: 10.1007/s10549-008-0099-x. [DOI] [PubMed] [Google Scholar]
- Ghosh K, Hartmann LC, Reynolds C, Visscher DW, Brandt KR, Vierkant RA, Scott CG, Radisky DC, Sellers TA, Pankratz VS, Vachon CM. Association between mammographic density and age-related lobular involution of the breast. J Clin Oncol. 2010;28:2207–2212. doi: 10.1200/JCO.2009.23.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh K, Vachon CM, Pankratz VS, Vierkant RA, Anderson SS, Brandt KR, Visscher DW, Reynolds C, Frost MH, Hartmann LC. Independent association of lobular involution and mammographic breast density with breast cancer risk. J Natl Cancer Inst. 2010;102:1716–1723. doi: 10.1093/jnci/djq414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken Z, McCormack VA, Highnam RP, Martin L, Gunasekara A, Melnichouk O, Mawdsley G, Peressotti C, Yaffe M, Boyd NF, dos Santos Silva I. Screen-film mammographic density and breast cancer risk: a comparison of the volumetric standard mammogram form and the interactive threshold measurement methods. Cancer Epidemiol Biomarkers Prev. 2010;19:418–428. doi: 10.1158/1055-9965.EPI-09-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd N, Martin L, Gunasekara A, Melnichouk O, Maudsley G, Peressotti C, Yaffe M, Minkin S. Mammographic density and breast cancer risk: evaluation of a novel method of measuring breast tissue volumes. Cancer Epidemiol Biomarkers Prev. 2009;18:1754–1762. doi: 10.1158/1055-9965.EPI-09-0107. [DOI] [PubMed] [Google Scholar]
- Shepherd JA, Kerlikowske K, Ma L, Duewer F, Fan B, Wang J, Malkov S, Vittinghoff E, Cummings SR. Volume of dense breast tissue and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:1473–1482. doi: 10.1158/1055-9965.EPI-10-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine JJ, Cao K, Rollison DE. Calibrated measures for breast density estimation. Acad Radiol. 2011;18:547–555. doi: 10.1016/j.acra.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine JJ, Cao K, Rollison DE, Tiffenberg G, Thomas JA. A quantitative description of the percentage of breast density measurement using fullfield digital mammography. Acad Radiol. 2011;18:556–564. doi: 10.1016/j.acra.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham SJ, Bronskill MJ, Byng JW, Yaffe MJ, Boyd NF. Quantitative correlation of breast tissue parameters using magnetic resonance and X-ray mammography. Br J Cancer. 1996;73:162–168. doi: 10.1038/bjc.1996.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glide-Hurst CK, Duric N, Littrup P. Volumetric breast density evaluation from ultrasound tomography images. Med Phys. 2008;35:3988–3997. doi: 10.1118/1.2964092. [DOI] [PubMed] [Google Scholar]
- Vachon CM, Kuni CC, Anderson K. Association of mammographically defined percent breast density with epidemiologic risk factors for breast cancer (United States) Cancer Causes Control. 2000;11:653–662. doi: 10.1023/a:1008926607428. [DOI] [PubMed] [Google Scholar]
- Boyd NF, Dite GS, Stone J, Gunasekara A, English DR, McCredie MR, Giles GG, Tritchler D, Chiarelli A, Yaffe MJ, Hopper JL. Heritability of mammographic density, a risk factor for breast cancer. N Engl J Med. 2002;347:886–894. doi: 10.1056/NEJMoa013390. [DOI] [PubMed] [Google Scholar]
- Ursin G, Lillie EO, Lee E, Cockburn M, Schork NJ, Cozen W, Parisky YR, Hamilton AS, Astrahan MA, Mack T. The relative importance of genetics and environment on mammographic density. Cancer Epidemiol Biomarkers Prev. 2009;18:102–112. doi: 10.1158/1055-9965.EPI-07-2857. [DOI] [PubMed] [Google Scholar]
- Lindström S, Vachon CM, Li J, Varghese J, Thompson D, Warren R, Brown J, Leyland J, Audley T, Wareham NJ, Loos RJ, Paterson AD, Rommens J, Waggott D, Martin LJ, Scott CG, Pankratz VS, Hankinson SE, Hazra A, Hunter DJ, Hopper JL, Southey MC, Chanock SJ, Silva Idos S, Liu J, Eriksson L, Couch FJ, Stone J, Apicella C, Czene K. et al. Common variants in ZNF365 are associated with both mammographic density and breast cancer risk. Nat Genet. 2011;43:185–187. doi: 10.1038/ng.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odefrey F, Stone J, Gurrin LC, Byrnes GB, Apicella C, Dite GS, Cawson JN, Giles GG, Treloar SA, English DR, Hopper JL, Southey MC. Australian Twins and Sisters Mammographic Density Study. Common genetic variants associated with breast cancer and mammographic density measures that predict disease. Cancer Res. 2010;70:1449–1458. doi: 10.1158/0008-5472.CAN-09-3495. [DOI] [PubMed] [Google Scholar]
- Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass L, Erler JT, Dembo M, Weaver VM. Mammary epithelial cell: Influence of extracellular matrix composition and organization during development and tumorigensis. Int J Biochem Cell Biol. 2007;39:1987–1994. doi: 10.1016/j.biocel.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schousboe JT, Kerlikowske K, Loh A, Cummings SR. Personalizing mammography by breast density and other risk factors for breast cancer: analysis of health benefits and cost-effectiveness. Ann Intern Med. 2011;155:10–20. doi: 10.7326/0003-4819-155-1-201107050-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelblatt JS, Stout N, Trentham-Dietz A. To screen or not to screen women in their 40s for breast cancer: is personalized risk-based screening the answer? Ann Intern Med. 2011;155:58–60. doi: 10.7326/0003-4819-155-1-201107050-00008. [DOI] [PubMed] [Google Scholar]
- Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, Mulvihill JJ. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- Chen J, Pee D, Ayyagari R, Graubard B, Schairer C, Byrne C, Benichou J, Gail MH. Projecting absolute invasive breast cancer risk in white women with a model that includes mammographic density. J Natl Cancer Inst. 2006;98:1215–1226. doi: 10.1093/jnci/djj332. [DOI] [PubMed] [Google Scholar]
- Tice JA, Cummings SR, Smith-Bindman R, Ichikawa L, Barlow WE, Kerlikowske K. Using clinical risk factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model. Ann Intern Med. 2008;148:337–347. doi: 10.7326/0003-4819-148-5-200803040-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Li Q, Melnichouk O, Greenberg C, Minkin S, Hislop G, Boyd NF. A randomized trial of dietary intervention for breast cancer prevention. Cancer Res. 2011;71:123–133. doi: 10.1158/0008-5472.CAN-10-1436. [DOI] [PubMed] [Google Scholar]
- Prentice RL. Surrogate endpoints in clinical trials: Definition and operational criteria. Stat Med. 1988;8:431–440. doi: 10.1002/sim.4780080407. [DOI] [PubMed] [Google Scholar]
- Schatzkin A, Gail M. The promise and peril of surrogate end points in cancer research. Nat Rev Cancer. 2002;2:19–27. doi: 10.1038/nrc702. [DOI] [PubMed] [Google Scholar]
- Cuzick J, Warwick J, Pinney E, Duffy SW, Cawthorn S, Howell A, Forbes JF, Warren RM. Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case-control study. J Natl Cancer Inst. 2011;103:744–752. doi: 10.1093/jnci/djr079. [DOI] [PubMed] [Google Scholar]
- Woolcott CG, Courneya KS, Boyd NF, Yaffe MJ, Terry T, McTiernan A, Brant R, Ballard-Barbash R, Irwin ML, Jones CA, Brar S, Campbell KL, McNeely ML, Karvinen KH, Friedenreich CM. Mammographic density change with 1 year of aerobic exercise among postmenopausal women: a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2010;19:1112–1121. doi: 10.1158/1055-9965.EPI-09-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerlikowske K, Cook AJ, Buist DS, Cummings SR, Vachon C, Vacek P, Miglioretti DL. Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J Clin Oncol. 2010;28:3830–3837. doi: 10.1200/JCO.2009.26.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greendale GA, Reboussin BA, Slone S, Wasilauskas C, Pike MC, Ursin G. Postmenopausal hormone therapy and change in mammographic density. J Natl Cancer Inst. 2003;95:30–37. doi: 10.1093/jnci/95.1.30. [DOI] [PubMed] [Google Scholar]
- Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D, Rodabough RJ, Gilligan MA, Cyr MG, Thomson CA, Khandekar J, Petrovitch H, McTiernan A. WHI Investigators. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women. The Women's Health Initiative Randomized Trial. JAMA. 1989;289(24):3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- Spicer DV, Ursin G, Parisky YR, Pearce JG, Shoupe D, Pike A, Pike MC. Changes in mammographic densities induced by a hormonal contraceptive designed to reduce breast cancer risk. J Natl Cancer Inst. 1994;86:431–436. doi: 10.1093/jnci/86.6.431. [DOI] [PubMed] [Google Scholar]
- Yaghjyan L, Colditz GA, Collins LC, Schnitt SJ, Rosner B, Vachon C, Tamimi RM. Mammographic breast density and subsequent risk of breast cancer in postmenopausal women according to tumor characteristics. J Natl Cancer Inst. 2011;103:1179–1189. doi: 10.1093/jnci/djr225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy SM, Pagano I, Kolonel LN, Maskarinec G. Mammographic density and hormone receptor expression in breast cancer: The Multiethnic Cohort Study. Cancer Epidemiol. 2011;35:448–52. doi: 10.1016/j.canep.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Warren R, Girling A, Thompson D, Easton D. Mamographic density, estrogen receptor status and other breast cancer tumor characteristics. Breast J. 2010;16:279–289. doi: 10.1111/j.1524-4741.2010.00907.x. [DOI] [PubMed] [Google Scholar]
- Olsen AH, Bihrmann K, Jensen MB, Vejborg I, Lynge E. Breast density and outcome of mammography screening: a cohort study. Br J Cancer. 2009;100:1205–1208. doi: 10.1038/sj.bjc.6604989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv E, Tice J, Smith-Bindman R, Shepherd J, Cummings S, Kerlikowske K. Mammographic density and estrogen receptor status of breast cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:2090–2095. [PubMed] [Google Scholar]
- Ma H, Luo J, Press MF, Wang Y, Berstein L, Ursin G. Is there a difference in the association between percent mammographic density and subtypes of breast cancer? Luminal A and triple-negative breast cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:479–485. doi: 10.1158/1055-9965.EPI-08-0805. [DOI] [PubMed] [Google Scholar]
- Gierach GL, Lissowska J, Garcia-Closas M, Yang XR, Figueroa JD, Anzick S, Wesolowska E, Brinton LA, Meltzer PS, Boyd NF, Sherman ME. Relationship of mammographic density with breast cancer subtypes. Paper presented at: American Association for Cancer Research 101st Annual Meeting; 17-21 April 2010; Washington, DC. p. Abstract 2779.
- Arora N, King TA, Jacks LM, Stempel MM, Patil S, Morris E, Morrow M. Impact of breast density on the presenting features of malignancy. Ann Surg Oncol. 2010;17:211–218. doi: 10.1245/s10434-010-1237-3. [DOI] [PubMed] [Google Scholar]
- Yang WT, Dryden M, Broglio K, Gilcrease M, Dawood S, Dempsey PJ, Valero V, Hortobagyi G, Atchley D, Arun B. Mammographic features of triple receptor-negative primary breast cancers in young premenopausal women. Breast Cancer Res Treat. 2008;111:405–410. doi: 10.1007/s10549-007-9810-6. [DOI] [PubMed] [Google Scholar]
- Cil T, Fishell E, Hanna W, Sun P, Rawlinson E, Narod SA, McCready DR. Mammographic density and the risk of breast cancer recurrence after breast-conserving surgery. Cancer. 2009;115:5780–5787. doi: 10.1002/cncr.24638. [DOI] [PubMed] [Google Scholar]
- Ghosh K, Brandt KR, Sellers TA, Reynolds C, Scott CG, Maloney SD, Carston MJ, Pankratz VS, Vachon CM. Association of mammographic density with the pathology of subsequent breast cancer among postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2008;17:872–879. doi: 10.1158/1055-9965.EPI-07-0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasching PA, Heusinger K, Loehberg CR, Wenkel E, Lux MP, Schrauder M, Koscheck T, Bautz W, Schulz-Wendtland R, Beckmann MW, Bani MR. Influence of mammographic density on the diagnostic accuracy of tumor size assessment and association with breast cancer tumor characteristics. Eur J Radiol. 2006;60:398–404. doi: 10.1016/j.ejrad.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Aiello EJ, Buist DSM, White E, Porter PL. Association between mammographic breast density and breast cancer tumor characteristics. Cancer Epidemiol Biomarkers Prev. 2005;14:662–668. doi: 10.1158/1055-9965.EPI-04-0327. [DOI] [PubMed] [Google Scholar]
- Morishita M, Ohtsuru A, Hayashi T, Isomoto I, Itoyanagi N, Maeda S, Honda S, Yano H, Uga T, Nagayasu T, Kanematsu T, Yamashita S. Clinical significance of categorisation of mammographic density for breast cancer prognosis. Int J Oncol. 2005;26:1307–1312. [PubMed] [Google Scholar]
- Roubidoux MA, Bailey JE, Wray LA, Helvie MA. Invasive cancers detected after breast cancer screening yielded a negative result: relationship of mammographic density to tumor prognostic factors. Radiology. 2004;230:42–48. doi: 10.1148/radiol.2301020589. [DOI] [PubMed] [Google Scholar]
- Hinton CP, Roebuck EJ, Williams MR, Blamey RW, Glaves J, Nicholson RI, Griffiths K. Mammographic parenchymal patterns: value as a predictor of hormone dependency and survival in breast cancer. AJR Am J Roentgenol. 1985;144:1103–1107. doi: 10.2214/ajr.144.6.1103. [DOI] [PubMed] [Google Scholar]
- Nickson C, Kavanagh AM. Tumour size at detection according to different measures of mammographic breast density. J Med Screen. 2009;16:140–146. doi: 10.1258/jms.2009.009054. [DOI] [PubMed] [Google Scholar]
- Porter GJ, Evans AJ, Cornford EJ, Burrell HC, James JJ, Lee AH, Chakrabarti J. Influence of mammographic parenchymal pattern in screening-detected and interval invasive breast cancers on pathologic features, mammographic features, and patient survival. AJR Am J Roentgenol. 2007;188:676–683. doi: 10.2214/AJR.05.1950. [DOI] [PubMed] [Google Scholar]
- Sala E, Solomon L, Warren R, McCann J, Duffy S, Luben R, Day N. Size, node status and grade of breast tumors: association with mammographic parenchymal patterns. Eur Radiol. 2000;10:157–162. doi: 10.1007/s003300050025. [DOI] [PubMed] [Google Scholar]
- Boyd NF, O'Sullivan B, Campbell JE, Fishell E, Simor I, Cooke G, Germanson T. Mammographic patterns and bias in breast cancer detection. Radiology. 1982;143:671–674. doi: 10.1148/radiology.143.3.7079494. [DOI] [PubMed] [Google Scholar]
- Habel LA, Dignam JJ, Land SR, Salane M, Capra AM, Juliano RL. Mammographic density and breast cancer after ductal carcinoma in situ. J Natl Cancer Inst. 2004;96:1467–1472. doi: 10.1093/jnci/djh260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habel LA, Capra AM, Achacoso NS, Janga A, Acton L, Puligandla B, Quesenberry CP Jr. Mammographic density and risk of second breast cancer after ductal carcinoma in situ. Cancer Epidemiol Biomarkers Prev. 2010;19:2488–2495. doi: 10.1158/1055-9965.EPI-10-0769. [DOI] [PubMed] [Google Scholar]
- Park CC, Rembert J, Chew K, Moore D, Kerlikowske K. High mammographic breast density is independent predictor of local but not distant recurrence after lumpectomy and radiotherapy for invasive breast cancer. Int J Radiat Oncol Biol Phys. 2009;73:75–79. doi: 10.1016/j.ijrobp.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Hwang ES, Miglioretti DL, Ballard-Barbash R, Weaver DL, Kerlikowske K. National Cancer Institute Breast Cancer Surveillance Consortium. Association between breast density and subsequent breast cancer following treatment for ductal carcinoma in situ. Cancer Epidemiol Biomark Prev. 2007;16(12):2587–2593. doi: 10.1158/1055-9965.EPI-07-0458. [DOI] [PubMed] [Google Scholar]
- Chiu SY, Duffy S, Yen AM, Tabár L, Smith RA, Chen HH. Effect of baseline breast density on breast cancer incidence, stage, mortality, and screening parameters: 25-year follow-up of a Swedish mammographic screening. Cancer Epidemiol Biomarkers Prev. 2010;19:1219–1228. doi: 10.1158/1055-9965.EPI-09-1028. [DOI] [PubMed] [Google Scholar]