Abstract
Previous studies showed that angiotensin-(1-7) [Ang-(1-7)] attenuates cardiac remodeling by reducing both interstitial and perivascular fibrosis. Although a high affinity binding site for Ang-(1-7) was identified on cardiac fibroblasts, the molecular mechanisms activated by the heptapeptide hormone were not identified. We isolated cardiac fibroblasts from neonatal rat hearts to investigate signaling pathways activated by Ang-(1-7) that participate in fibroblast proliferation. Ang-(1-7) reduced 3H-thymidine, -leucine and –proline incorporation into cardiac fibroblasts stimulated with serum or the mitogen endothelin-1 (ET-1), demonstrating that the heptapeptide hormone decreases DNA, protein and collagen synthesis. The reduction in DNA synthesis by Ang-(1-7) was blocked by the AT(1-7) receptor antagonist [D-Ala7]-Ang-(1-7), showing specificity of the response. Treatment of cardiac fibroblasts with Ang-(1-7) reduced the Ang II- or ET-1-stimulated increase in phospho-ERK1 and –ERK2. In contrast, Ang-(1-7) increased dual-specificity phosphatase DUSP1 immunoreactivity and mRNA, suggesting that the heptapeptide hormone increases DUSP1 to reduce MAP kinase phosphorylation and activity. Incubation of cardiac fibroblasts with ET-1 increased cyclooxygenase 2 (COX-2) and prostaglandin synthase (PGES) mRNAs, while Ang-(1-7) blocked the increase in both enzymes, suggesting that the heptapeptide hormone alters the concentration and the balance between the proliferative and anti-proliferative prostaglandins. Collectively, these results indicate that Ang-(1-7) participates in maintaining cardiac homeostasis by reducing proliferation and collagen production by cardiac fibroblasts in association with up-regulation of DUSP1 to reduce MAP kinase activities and attenuation of the synthesis of mitogenic prostaglandins. Increased Ang-(1-7) or agents that enhance production of the heptapeptide hormone may prevent abnormal fibrosis that occurs during cardiac pathologies.
Keywords: angiotensin-(1-7), cardiac fibrosis, mitogen-activated protein kinases, dual specificity phosphatase, cyclooxygenase, prostaglandin E synthase
1. INTRODUCTION
Cardiac fibroblasts constitute the major type of cell in the heart, accounting for 60 to 70% of the total number of cells in the heart and over 90% of the non-myocytes [2,4,41,49]. In contrast, the majority of the volume of the heart is composed of cardiac myocytes, providing the contractile force for cardiac function. Cardiac fibroblasts are located in sheets, in parallel with myocytes, providing the structure for cardiac contraction by synthesizing and secreting extracellular matrix proteins including fibronectin and collagens as well as matrix metalloproteases (MMPs) and MMP inhibitors, to regulate collagen degradation. Cardiac fibroblasts are also a source of cytokines and growth factors which function in both the autocrine and paracrine regulation of cardiac cell proliferation.
Under pathological conditions, such as after a myocardial infarction, cardiac fibroblasts differentiate into myofibroblasts [4,41]. Myofibroblasts are characterized by the expression of α-smooth muscle actin and conversion to a secretory phenotype, exhibiting increased production and secretion of both collagens and fibronectin. Although the production of extracellular matrix proteins is important in reparative remodeling, excess production of collagens and fibronectin by activated myofibroblasts leads to loss of cardiac function, cardiac fibrosis and heart failure. Chronic hypertension, due to increased hemodynamic forces or humoral factors, such as endothelin 1 (ET-1), angiotensin II (Ang II) and transforming growth factor-β1 (TGF-β1), also results in myofibroblast differentiation and adverse cardiac remodeling [3,17]. Cardiac fibrosis poses an increased risk for adverse cardiovascular events including ventricular dysfunction and arrhythmias, ultimately culminating in heart failure [57].
Angiotensin-(1-7) [Ang-(1-7)] is a seven amino acid peptide of the renin-angiotensin system that often opposes the physiological effects elicited by bioactive hormones such as Ang II and ET-1. Ang-(1-7), which is present in the heart [1], improves contractility [11,15,46], reduces cardiac hypertrophy and attenuates cardiac myocyte cell growth [31,52]. We recently showed that infusion of Ang-(1-7) into Ang II-treated rats reduces cardiac hypertrophy and fibrosis, independent of a reduction in blood pressure [33] in agreement with other studies showing that increased Ang-(1-7), by either direct infusion or using an Ang-(1-7)-generating fusion protein, prevented cardiac remodeling in deoxycorticosterone (DOCA) salt-induced hypertension, in Ang II-mediated models of hypertension, and in a model of diabetes induced by fructose-feeding [20,23,24,36,45]. The discovery of angiotensin converting enzyme 2 or ACE2, which converts Ang II to Ang-(1-7) [13,54,56], and its localization in the heart provide additional evidence of a role for Ang-(1-7) in cardiac function. Genetic deletion of cardiac ACE2, while decreasing Ang-(1-7) and increasing Ang II, is associated with enhanced cardiac hypertrophy and reduced pumping ability [10]. Although Donoghue et al. [14] originally reported that over-expression of ACE2 was detrimental to the heart, subsequent studies using human recombinant ACE2, lentiviral-mediated over-expression of ACE2, an ACE2 agonist, or an ACE2 inhibitor demonstrate that ACE2, through either a reduction in Ang II or other ACE2 substrates (angiotensin I, des-Arginine9-bradykinin, apelin-13 or opioids), an increase in Ang-(1-7), or a combination of these alterations in peptide concentrations improves cardiac function and reduces left ventricular fibrosis and remodeling [12,16,22,28,37,42,53,55,60,61]. Treatment of cardiac fibroblasts with recombinant human ACE2 reduced collagen production and the Ang II-mediated increase in phospho-ERK1/2, effects blocked by an Ang-(1-7) receptor antagonist [60], the ACE2 activator XNT reduced phospho-MAP kinases in cardiac fibroblasts from hypertensive rats [16], and transfection of cardiac fibroblasts with lenti-ACE2 reduced hypoxia-induced collagen production [22], suggesting that Ang-(1-7) reduces collagen production and MAP kinase activities in cardiac fibroblasts. Collectively, these studies provide support for a cardioprotective role for the heptapeptide hormone.
Iwata et al. [29] showed that cardiac fibroblasts contain a high affinity 125I-Ang-(1-7) binding site and that treatment of cardiac fibroblasts with Ang-(1-7) reduced the production of various growth factors and cytokines including ET-1 and leukemia inhibitory factor. However, the signaling mechanisms activated by Ang-(1-7) to inhibit the growth of cardiac fibroblasts and the production of collagen have not been examined. In this study, we investigated the effect of Ang-(1-7) on cardiac fibroblasts, to identify signaling pathways associated with the reduction in cell proliferation and cytokine production.
2. MATERIALS AND METHODS
2.1 Materials
Ang-(1-7), D-Alanine7-angiotensin-(1-7) [D-Ala7]-Ang-(1-7) and ET-1 were obtained from Bachem California (Torrance, CA). For Western blot hybridization, a phospho-specific antibody against ERK1/ERK2 was obtained from Cell Signaling Technologies (Cambridge, MA) and an antibody against DUSP1 (MKP-1) was purchased from Upstate (Lake Placid, NY). All other reagents were purchased from Sigma Chemical (St. Louis, MO) unless otherwise noted.
2.2 Isolation of neonatal cardiac fibroblasts
Cardiac fibroblasts were isolated from the ventricles of neonatal Sprague-Dawley rats by proteolytic digestion and differential plating, as previously described [18]. All procedures complied with the policies of the Wake Forest University Animal Care and Use Committee. Cardiac fibroblasts were collected and maintained in DMEM-F12 with 10% fetal bovine serum (FBS) and the antibiotics ampicillin and streptomycin. Cardiac fibroblasts were passaged one time, using trypsin/EDTA, to eliminate any residual myocytes. Cells isolated by this protocol were fixed in 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, incubated with antibodies to fibronectin, vimentin, sarcomeric myosin and α-smooth muscle-specific actin (1:1,000 dilutions, Sigma, St. Louis, MO), and visualized with FITC-conjugated secondary antibodies using a fluorescence microscope.
2.3 Measurement of thymidine, leucine and proline incorporation
Tritiated thymidine, leucine and proline incorporation into cardiac fibroblasts was measured in cells growing in 24-well culture plates. Cells were incubated in serum-free media for 24 h prior to experimentation and were treated for 48 h in the presence and absence of 1% FBS, 100 nM Ang II, 10 nM ET-1, and the Ang-(1-7) receptor antagonist [D-Ala7]-Ang-(1-7), as indicated in individual experiments. One microcurie of 3H-thymidine, 3H-leucine, or 3H-proline per milliliter of culture medium was added to each well, during the last 24 h of the treatment period. Little cell death was observed during the serum-deprivation or treatment period. The incorporation of 3H-thymidine into newly synthesized DNA, 3H-leucine into newly synthesized protein or 3H-proline into collagen was determined after precipitation of acid-insoluble material with ice-cold 10% trichloroacetic acid. The acid-insoluble material was dissolved in 0.25 N NaOH-0.1% sodium dodecyl sulfate and quantified by liquid scintillation spectrometry.
2.4 Preparation of cell lysates and Western blot hybridization
Cells were pre-incubated with 100 nM Ang-(1-7) for 6 h followed by a 10-min treatment with 10 nM ET-1 or 100 nM Ang II, to measure phospho-ERK1/2 or for 6 h to measure DUSP1. Cell lysates were prepared by washing the cells with phosphate-buffered saline (PBS, 50 mmol/L NaHPO4 and 0.15 mmol/L NaCl, pH 7.2) containing 0.01 mmol/L NaVO4 to prevent dephosphorylation of activated, phosphorylated proteins. Cellular protein was solubilized in lysis buffer (100 mmol/L NaCl, 50 mmol/L NaF, 5 mmol/L EDTA, 1% Triton X-100, and 50 mmol/L Tris-HCl, pH 7.4) containing 0.01 mmol/L NaVO4, 0.1 mmol/L PMSF, and 0.6 μM leupeptin for 30 min on ice. The supernatant was clarified by centrifugation (12,000 ×g for 10 min at 4°C), and the protein concentration was measured by the Lowry method [32].
For Western blot hybridization, solubilized proteins (20 μg/well) were separated on 10% polyacrylamide gels using the buffer system of Laemmli and then transferred to polyvinylidene difluoride membranes (Amersham Pharmacia, Pisctaway, NJ) by electrophoresis. Nonspecific binding to the membranes was blocked by incubation in 5% Blotto (5% evaporated milk, 0.1% Tween 20 in 50 mmol/L Tris-HCl, pH 7.4; and 50 mmol/L NaCl). Membranes were subsequently probed with a specific antibody to phosphorylated ERK1/ERK2 MAP kinases (1:1,000) or DUSP1 (1:1,000), followed by incubation with goat anti-rabbit antibody (1:1,000 dilution) coupled to horseradish peroxidase. Actin (β-actin; Sigma, St. Louis, MO) immunostaining was used as a loading control. Immunoreactive bands were visualized with the use of enhanced chemiluminescence reagents and quantified by densitometry.
2.5 RNA isolation and RT real-time PCR
Cardiac fibroblasts were incubated with Ang-(1-7) or ET-1 or the combination for increasing periods of time and RNA was isolated using the TRIzol reagent (GIBCO Invitrogen, Carlsbad, CA) as directed by the manufacturer. The RNA concentration and integrity were assessed with an Agilent 2100 Bioanalyzer with an RNA 6000 Nano LabChip (Agilent Technologies, Palo Alto, CA). Approximately 1 μg of total RNA was reverse transcribed using AMV reverse transcriptase in a 20 μL reaction mixture containing deoxyribonucleotides, random hexamers, and RNase inhibitor in reverse transcriptase buffer. Heating the reverse transcriptase reaction product at 95°C terminated the reaction. For RT-PCR, 2 μL of the resultant cDNA was added to TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA) with either a DUSP1, cyclooxygenase 2 (COX-2) or prostaglandin E synthase 1 (PGES-1) primer/probe set (Applied Biosystems), and amplification was performed on an ABI 7000 Sequence Detection System. The mixtures were heated at 50°C for 2 min, at 95°C for 10 min followed by 40 cycles at 95°C for 15 s, and 60°C for 1 min. All reactions were performed in triplicate and 18S ribosomal RNA, amplified using TaqMan Ribosomal RNA control kit (Applied Biosystems), served as an internal control. The results were quantified as Ct values, where Ct is defined as the threshold cycle of PCR at which the amplified product is first detected, and expressed as the ratio of target/control.
2.6 Statistics
All data are presented as means ± SEM. Statistical differences were evaluated by Student’s t-tests or ANOVA followed by Dunnett’s post hoc test. The criterion for statistical significance was set at p < 0.05.
3. RESULTS
3.1 Ang-(1-7) reduces DNA, protein and collagen synthesis in cardiac fibroblasts
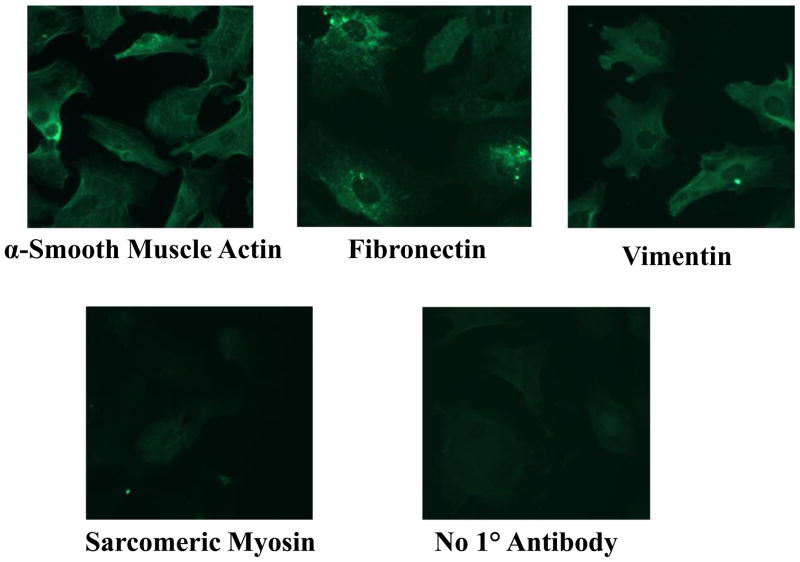
Cardiac fibroblasts were isolated from the hearts of 1 to 2 day old Sprague-Dawley rats by proteolytic digestion and differential plating, to demonstrate the effect of Ang-(1-7) on cell proliferation, protein and collagen production, and signaling pathways associated with cell growth. The isolated cells were identified as activated myofibroblasts by immunocytochemistry, as shown in Figure 1: essentially all of the isolated cells showed positive immunoreactivity with antibodies to α-smooth muscle actin, fibronectin, and vimentin but were not reactive when incubated with an antibody to sarcomeric myosin.
Figure 1. Immunocytochemical identification of cardiac fibroblasts.
Isolated cardiac cells were incubated with antibodies to α-smooth muscle actin, fibronectin, vimentin or sarcomeric myosin and visualized with FITC-coupled secondary antibodies. The control was incubated without a primary antibody, as indicated.
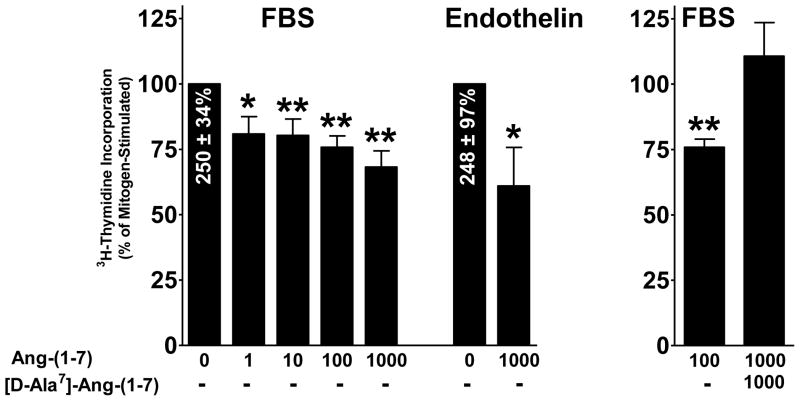
Cardiac fibroblasts were incubated with 3H-thymidine, in the presence and absence of Ang-(1-7), to determine whether the heptapeptide hormone inhibits DNA synthesis. Semi-confluent monolayers of cardiac fibroblasts were incubated in media depleted of serum for 48 h and then treated with 1% FBS or 10 nM ET-1, in the presence and absence of Ang-(1-7); 3H-thymidine was added to measure DNA synthesis. Both FBS and ET-1 stimulated 3H-thymidine incorporation into DNA; FBS increased 3H-thymidine incorporation by 250 ± 34% above control, and ET-1 caused a 248 ± 97% increase above control (Figure 2). Treatment with Ang-(1-7) caused a dose-dependent reduction in serum-stimulated 3H-thymidine incorporation, with a maximum reduction by 100 nM Ang-(1-7) (a reduction of 24.2 %, p < 0.01, n = 3–6). Ang-(1-7) also caused a pronounced reduction in ET-1-stimulated 3H-thymidine incorporation (a reduction of 39.0%, p < 0.01, n=3). [D-Ala7]-Ang-(1-7), the selective AT(1-7) receptor antagonist, blocked the inhibitory effects Ang-(1-7) in serum-stimulated cardiac fibroblasts. These results show that Ang-(1-7) inhibits DNA synthesis in cardiac fibroblasts through activation of a [D-Ala7]-Ang-(1-7)-sensitive receptor.
Figure 2. Inhibition of DNA synthesis by Ang-(1-7) in cardiac fibroblasts.
Cardiac fibroblasts were serum-deprived for 24 h and subsequently treated for 48 h with increasing concentrations of Ang-(1-7), in the presence of 1% FBS, 10 nM endothelin (ET-1), or 1 μM [D-Ala7]-Ang-(1-7), as indicated. DNA synthesis was detected by 3H-thymidine incorporation, which was added during the last 24 h of treatment. Data are presented as the percentage of mitogen-stimulated growth, in the absence of Ang-(1-7). n = 4-6, in triplicate. *denotes p < 0.05 and **denotes p < 0.01 compared to mitogen alone.
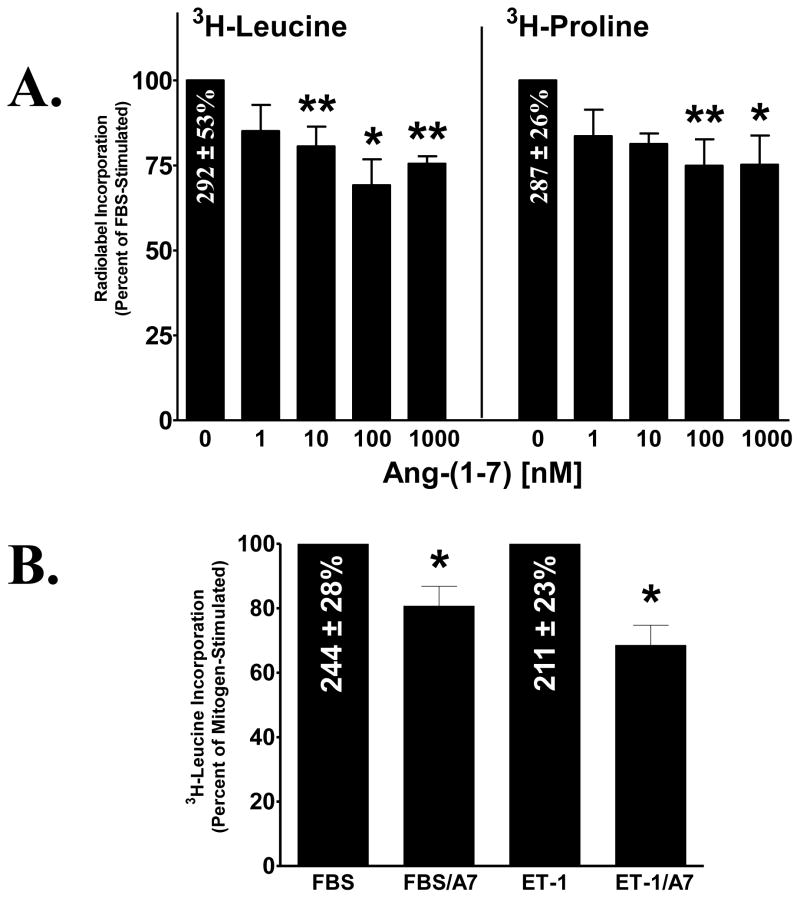
The effect of Ang-(1-7) on mitogen-stimulated protein and collagen synthesis in cardiac fibroblasts was also determined, by measuring the incorporation of 3H-leucine into newly synthesized protein and 3H-proline into newly synthesized collagen. 3H-leucine or 3H-proline was added to isolated cardiac fibroblasts pretreated for 48 h in media deplete of serum and treated with Ang-(1-7) and either 1% FBS or 10 nM ET-1. FBS stimulated both 3H-leucine and 3H-proline incorporation into cardiac fibroblasts; serum increased 3H-leucine incorporation by 292 ± 53% above control and increased 3H-proline incorporation by 287± 26% above control, while Ang-(1-7) caused a dose-dependent reduction in both 3H-leucine and 3H-proline incorporation, as shown in Figure 3A. A similar stimulation of 3H-leucine incorporation was observed with ET-1 (211 ± 23% increase above control) and co-treatment with Ang-(1-7) significantly reduced the ET-1-mediated increase in 3H-leucine incorporation (Figure 3B). These results demonstrate that the heptapeptide hormone inhibits mitogen-stimulated protein and collagen synthesis in cardiac fibroblasts, in agreement with the Ang-(1-7)-mediated reduction in fibrosis in vivo.
Figure 3. Inhibition of protein and collagen synthesis by Ang-(1-7) in cardiac fibroblasts.
Cardiac fibroblasts were deprived of serum for 24 h and subsequently treated for 48 h with various concentrations of Ang-(1-7), in the presence of 1% FBS or 10 nM ET-1. Protein and collagen was detected by 3H-leucine and 3H-proline incorporation, respectively, which were added during the last 24 h of the treatment period. Data are presented as the percentage of mitogen-stimulated growth, in the absence of Ang-(1-7). n = 4 – 6, in triplicate. *denotes p < 0.05 and **denotes p < 0.01 compared to mitogen alone.
3.2 Ang-(1-7) reduces ERK1/ERK2 activity and up-regulates DUSP1 in cardiac fibroblasts
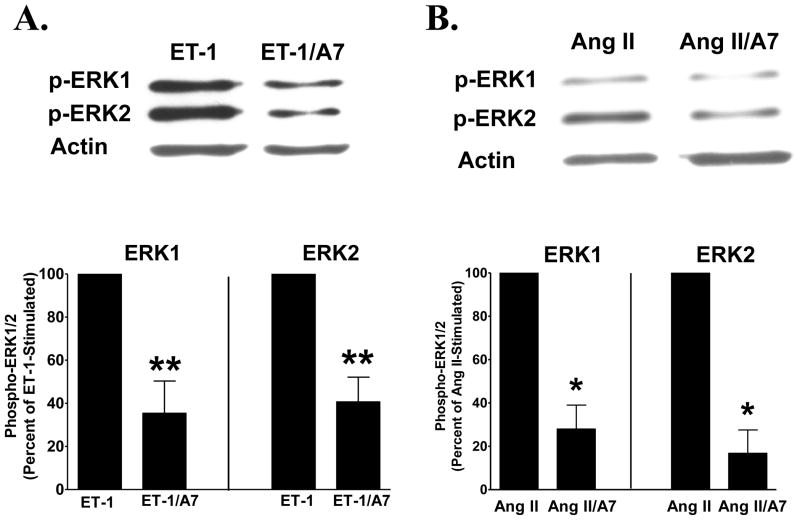
Phospho-ERK1 and -ERK2 were quantified following the addition of 10 nM ET-1 or 100 nM Ang II in the presence or absence of 100 nM Ang-(1-7), to determine the effect of the heptapeptide hormone on activation of the MAP kinases in isolated cardiac fibroblasts. ERK1 and ERK2 were measured using phospho-specific antibodies to the enzymes, as markers of MAP kinase activities. Neither phospho-ERK1 nor phospho-ERK2 was detected in unstimulated cardiac fibroblasts, indicating an absence of active MAP kinases. Immunoreactive phospho-ERK1 and -ERK2 were visualized in lysates from cardiac fibroblasts stimulated with either ET-1 or Ang II. Pre-incubation with 100 nM Ang-(1-7) reduced phospho-ERK1 and phospho-ERK2, by 64% and 59%, respectively, following ET-1 stimulation, or by 72% and 83%, respectively, by incubation with Ang II, shown in representative blots or graphically in Figure 4. These findings demonstrate that the heptapeptide hormone attenuates mitogen-stimulated ERK1/ERK2 MAP kinase activities in cardiac fibroblasts.
Figure 4. Ang-(1-7) reduces phospho-ERK1/ERK2.
Cardiac fibroblasts pre-treated with 100 nM Ang-(1-7) [A7] were stimulated with 10 nM ET-1 (Panel A) or 100 nM Ang II (Panel B) and phospho-ERK1/ERK2 were determined using a phospho-specific antibody. Representative blots show ET-1 or Ang II-stimulated phospho-ERK1/ERK2 and actin immunoreactivity in the presence or absence of Ang-(1-7). Graphs show percent control of ET-1 or Ang II phospho-ERK1 and ERK2. n = cells isolated from 2 to 4 litters of neonatal pups, in duplicate or triplicate. * p <0.05 compared with Ang II alone; **denotes p < 0.01 compared with ET-1 treatment alone.
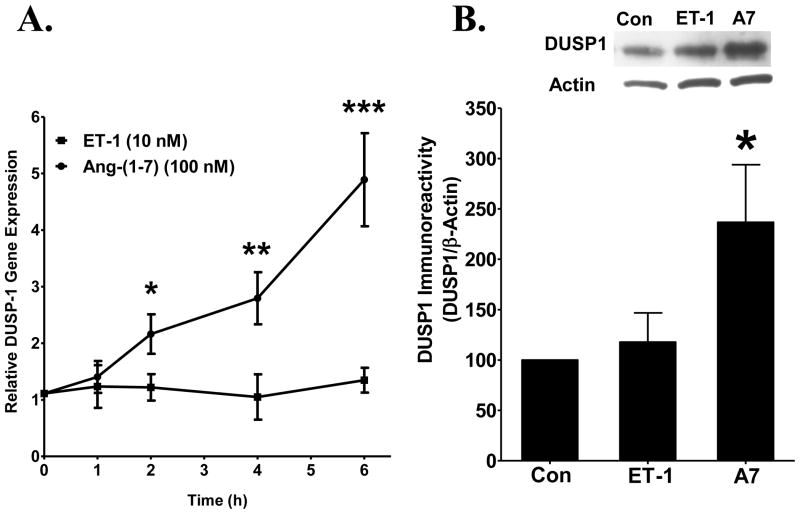
DUSP1 was quantified in lysates from cardiac fibroblasts treated with Ang-(1-7), since this MAP kinase phosphatase inactivates phospho-ERK1/2 [5]. Cardiac fibroblasts were treated with 10 nM ET-1, in the presence or absence of 100 nM Ang-(1-7), and total RNA and protein were isolated to determine whether the heptapeptide hormone regulates DUSP1. Ang-(1-7) caused a time-dependent increase in DUSP1 mRNA during a 6 h treatment period, with a maximal 4-fold increase observed at 6 h (Figure 5A). ET-1 did not alter the expression of DUSP1 mRNA throughout the 6 h treatment period. In addition, Ang-(1-7) significantly increased immunoreactive DUSP1 in isolated cardiac fibroblasts treated with the heptapeptide hormone alone which was not observed when cells were stimulated with ET-1 alone (Figure 5B). These data demonstrate that Ang-(1-7) up-regulates DUSP1 and suggest that the increase in DUSP1 may reduce the phosphorylation of ERK1/ERK2, to attenuate this major growth promoting pathway and decrease the proliferation of cardiac fibroblasts.
Figure 5. Ang-(1-7) up-regulates DUSP-1 in neonatal cardiac fibroblasts.
Cardiac fibroblasts in serum-free media were treated with either 100 nM Ang-(1-7) or ET-1 (10 nM) and incubated for 6 h. A) DUSP1 mRNA isolated from cardiac fibroblasts was analyzed by RT real-time PCR. n= cells isolated from 4 litters of neonatal rat pups. * denotes p < 0.05, ** denotes p < 0.005, *** denotes p < 0.001 compared to treatment with ET-1. B) A 40-kDa immunoreactive protein was visualized by Western blot hybridization in cultured cardiac fibroblasts using a specific antibody to DUSP1 and quantified by densitometry, with β-actin as a loading control. n = cells isolated from 3 litters of neonatal rat pups. * denotes p < 0.05 compared to control (Con), in serum-free media alone.
3.3 Ang-(1-7) Attenuates COX-2 and PGES Expression in Cardiac Fibroblasts
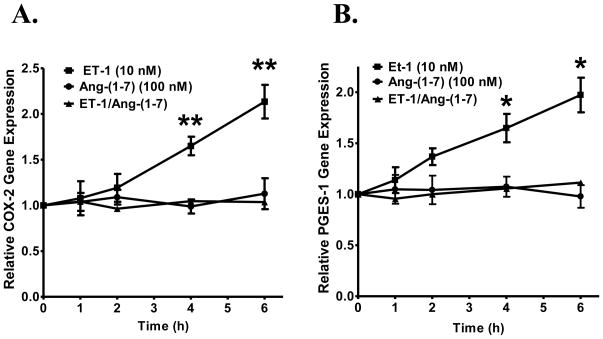
Increases in humoral peptides and hormones such as ET-1, Ang II and TGF-β1 are implicated in the inflammatory process that accompanies remodeling of the heart [43]. COX-2 is a key factor in the progression of inflammation and is expressed following myocardial infarction [47]. COX-2 was quantified in cardiac fibroblasts treated with ET-1 and Ang-(1-7) to evaluate the effects of Ang-(1-7) on mitogen-stimulated changes in inflammatory mediators. Cardiac fibroblasts were pre-incubated with 100 nM Ang-(1-7) followed by stimulation with 10 nM ET-1 and time-dependent changes in COX-2 mRNA were measured. ET-1 alone resulted in an increase in relative COX-2 expression that was significantly elevated at 4 and 6 h, as shown in Figure 6A. However, co-administration of Ang-(1-7) prevented the ET-1-stimulated increase in COX-2 gene expression while Ang-(1-7) treatment alone had no effect on COX-2 mRNA. These findings suggest that Ang-(1-7) attenuates mitogen-stimulated induction of COX-2 in cardiac fibroblasts and thus may play an important role in modulating the inflammatory and proliferative effects observed with induction of this pathway.
Figure 6. Ang-(1-7) reduces COX-2 and PGES gene expression in cardiac fibroblasts.
Cardiac fibroblasts were pre-treated with 100 nM Ang-(1-7) for 4 h and incubated with or without 10 nM ET-1 for increasing periods of time, as indicated. COX-2 mRNA (in Panel A) or PGES mRNA (in Panel B) were measured by RT real-time PCR. n = 5. For COX-2 mRNA in Panel A, ** denotes p < 0.005 compared to Ang-(1-7) or Ang-(1-7) and ET-1 treatment; for PGES mRNA in Panel B, * denotes p < 0.05 compared to Ang-(1-7) or Ang-(1-7) and ET-1 treatment.
Prostaglandins are important in normal cellular conditions as well as pathophysiological conditions such as inflammation [59]. The synthesis of prostaglandin E2 (PGE2) following COX-2 induction involves activation of microsomal glutathione-dependent PGES [21]. Cardiac fibroblasts pre-incubated with 100 nM Ang-(1-7) were stimulated with 10 nM ET-1 to detect changes in the relative gene expression of PGES. ET-1 stimulation alone caused an increase in relative PGES expression that was markedly elevated at 4 and 6 h, as shown in Figure 6B. Combination treatment with Ang-(1-7) significantly prevented the ET-1 stimulated increase in PGES gene expression, while Ang-(1-7) treatment alone had no effect. These results suggest that Ang-(1-7) may play a role in reducing cardiac fibroblast cell growth associated with COX-2 induction and PGE2 synthesis.
4 DISCUSSION
Ang-(1-7) treatment of Ang II-infused hypertensive rats, DOCA-salt hypertensive rats or spontaneously hypertensive rats decreases cardiac fibrosis, suggesting that the heptapeptide hormone reduces the proliferation and production of extracellular matrix proteins and cytokines by cardiac fibroblasts [20,23,24,33,36,40,45]. Although a high affinity binding site for Ang-(1-7) was identified on cardiac fibroblasts [29], few studies have investigated the signaling mechanisms activated by Ang-(1-7) in cardiac fibroblasts. We now show that Ang-(1-7) regulates two primary growth-promoting pathways, suggesting that alterations in cellular mechanisms that enhance production of the heptapeptide hormone or interventions that increase circulating concentrations of Ang-(1-7) may have pleiotrophic effects on fibroblast proliferation and function in the heart.
Ang-(1-7) inhibited serum- and ET-1-stimulated DNA, protein, and collagen synthesis in cardiac fibroblasts, suggesting that the heptapeptide hormone reduces the proliferation and pro-fibrotic protein production by cardiac fibroblasts. This is in agreement with previous studies showing that Ang-(1-7) reduces 3H-proline incorporation into collagen in isolated rat cardiac fibroblasts [29]. The Ang-(1-7)-mediated reduction in 3H-thymidine incorporation was blocked by the selective Ang-(1-7) receptor antagonist [D-Ala7]-Ang-(1-7), indicating that the heptapeptide hormone mediates anti-proliferative effects in cardiac fibroblasts through activation of a distinct [D-Ala7]-Ang-(1-7) sensitive receptor. These results suggest that Ang-(1-7) activation of the AT(1-7) on cardiac fibroblasts not only inhibits proliferation but also the production of extracellular matrix proteins and are in agreement with the in vivo inhibition of fibrosis in hypertensive rats by the heptapeptide hormone [20,23,24,33,36,40,45]. We previously showed that Ang-(1-7) reduced the growth of cancer-associated fibroblasts and reduced fibrosis in human estrogen-receptor positive breast tumor xenografts in immunocompromised mice [9]. The heptapeptide hormone concomitantly reduced interstitial fibrosis in association with a significant decrease in collagen I deposition. A similar decrease in perivascular fibrosis was observed. The pro-fibrotic protein fibronectin was reduced markedly in cancer-associated fibroblasts isolated from orthotopic breast tumors, again demonstrating the suppression of extracellular matrix protein production by Ang-(1-7).
The heptapeptide hormone also plays a regulatory role in collagen degradation. The reduction in collagen production by chronic treatment of spontaneously hypertensive rats with Ang-(1-7) was associated with abrogation of the decrease in MMP-2 and of the increase in tissue inhibitor of matrix metalloprotease (TIMP)-1, to alter the MMP/TIMP ratio; MMP-2 and -9 were increased and TIMP-1 and -2 were decreased in isolated rat cardiac fibroblasts treated with Ang-(1-7) [40]. Pan et al. [39] also noted a decrease in TIMP-2 in human fibroblasts treated with Ang-(1-7) and the heptapeptide hormone reversed the increase in MMP-9 and the decrease in TIMP-2 in fibroblasts treated with Ang II, to alter the MMP/TIMP ratios. Collectively, these results indicate that Ang-(1-7) reduces fibrosis in the heart by decreasing collagen production and altering the ratio of MMPs to TIMPs to regulate collagen degradation. Decades of study have elucidated the pro-fibrotic signaling molecules, such as ET-1 and Ang II, which promote the synthesis of extracellular matrix proteins by cardiac fibroblasts. Taken together, our results as well as published studies by others suggest that Ang-(1-7) may play a prominent role in maintaining homeostasis of the extracellular matrix in the heart by counter-regulating the actions of pro-fibrotic signaling molecules.
Ang II activates AT1 receptors to initiate a cascade of signaling events leading to the activation of MAP kinases that are important in the growth of cardiac cells [34]. In addition, Ang II stimulation of AT1 receptors on cardiac fibroblasts increases endothelin mRNA, demonstrating that Ang II up-regulates endothelin production [7]. Moreover, Ang II stimulation of 3H-thymidine incorporation into cardiac fibroblasts was blocked by either an AT1 receptor antagonist or an endothelin receptor antagonist, suggesting that Ang II increases proliferation through the increase in endothelin and subsequent endothelin-mediated effects on growth [6,7]. Stimulation of cardiac fibroblasts with either ET-1 or Ang II increased phospho-ERK1/ERK2, in agreement with previous studies [6,7]. Conversely, pretreatment with Ang-(1-7) significantly attenuated the ET-1 or Ang II-mediated phosphorylation of ERK1 and ERK2. Previous reports show that Ang-(1-7) attenuates the mitogen-stimulated increase in phosphorylation and activation of ERK1 and ERK2 in rat aortic VSMC [51], rat cardiac myocytes [52], human endothelial cells [44] and human lung cancer cells [19], suggesting that inactivation of ERK1/ERK2 MAP kinase is a global mechanism whereby Ang-(1-7) regulates cell proliferation. In addition, we showed that the Ang-(1-7)-mediated inhibition of ERK1/ERK2 activation in isolated neonatal cardiac myocytes was blocked by antisense oligonucleotides to the Ang-(1-7) receptor mas [52], demonstrating that the anti-proliferative responses to Ang-(1-7) are mediated by a specific AT(1-7) receptor. Together, these studies suggest that a primary function of the heptapeptide hormone is to attenuate the phosphorylation of ERK1/ERK2 to prevent aberrant cell growth in the heart. The increased mitogenic factors produced during pathological processes, including hypertension, heart failure or myocardial infarction, perturb this delicate balance, such that sufficient concentrations of endogenous Ang-(1-7) are not produced to block growth promoting pathways which lead to enhanced cardiac fibrosis and remodeling.
MAP kinases are activated by phosphorylation on both threonine and tyrosine residues by MAP kinase kinases (MEKs) and are inactivated through dephosphorylation by either serine/threonine and tyrosine protein phosphatases or dual specificity phosphatases. Members of a family of dual specificity phosphatases (DUSPs) dephosphorylate and inactivate MAP kinase isoforms in mammalian cells [25,38]. DUSP1 (also known as MAP kinase phosphatase 1 or MKP-1) is a critical counteracting phosphatase that directly regulates the magnitude and duration of p38, JNK and ERK auto-phosphorylation and activation [38]. Ang-(1-7) increased DUSP1 in cultured cardiac fibroblasts, with a time-dependent up-regulation of DUSP1 mRNA and an increase in DUSP immunoreactivity. These results suggest that Ang-(1-7) maintains the balance of fibroblast proliferation in the heart by regulating the mitogenic activation of MAP kinase signaling through the phosphatase DUSP1. The endotoxin lipopolysaccharide (LPS) also up-regulated DUSP1 in cardiac fibroblasts and reduced the Ang II-mediated increase in phospho-ERK1/2 while silencing DUSP1 prevented the LPS-mediated reduction in MAP kinase activity and cell proliferation [50]. In addition, Choudhary and colleagues demonstrated that pressure overload-induced cardiac fibrosis and phosphorylation of ERK1/ERK2, JNK and p38 MAP kinase was inhibited by retinoic acid, through up-regulation of both DUSP1 and DUSP2 [8]. These results suggest that Ang-(1-7) as well as other agents up-regulate cardiac DUSP1 to reduce MAP kinase signaling.
Mitogens such as ET-1 and Ang II which stimulate the proliferation of cardiac fibroblasts and increase the synthesis of extracellular matrix proteins also promote the production of pro-inflammatory mediators such as COX-2 and the release of prostaglandins [48]. We observed up-regulation of both COX-2 and PGES following mitogen stimulation of isolated cardiac fibroblasts in agreement with previous studies showing that Ang II induced COX-2 and stimulated PGE2 production in cardiac fibroblasts [47] and PGES was up-regulated by IL1-beta in isolated cardiac fibroblasts [21]. Conversely, pretreatment with Ang-(1-7) abrogated both the increase in COX-2 and PGES, suggesting that Ang-(1-7) reduces the production of prostaglandins. COX-2 is increased after myocardial infarction and treatment with a COX-2 inhibitor improved cardiac parameters, attenuated inflammatory cell infiltration and reduced fibrosis [30]. Although COX-2 plays an important role in the physiological and pathophysiological conditions in response to stress signals, over-production of COX-2 is a key mediator not only of inflammation and tissue injury but also of cell proliferation. COX-2 and PGE2 production were observed in patients with heart failure [58] as well as animal models of myocardial damage [30] and increased PGE2 promotes hypertrophic growth of cardiac myocytes [35]. PGE2 also increases the proliferation of cardiac fibroblasts, by activation of the EP1 receptor and activation of both ERK1/2 and Akt signaling [26]; treatment of cardiac fibroblasts with PGE2 reduced the number of cells in S phase by 24.9%, in agreement with the 30 to 40% decrease in mitogen-stimulated DNA and protein synthesis that we observed in response to Ang-(1-7). Ang II treatment of mice with ablated PGES resulted in a further reduction in cardiac function and an increase in apoptosis, demonstrating important roles for PGE2 in the heart following Ang II infusion [27]. In previous studies in vascular smooth muscle cells, we showed that the Ang-(1-7)-mediated reduction in proliferation was blocked by a COX-2 inhibitor, suggesting that the anti-proliferation response to the heptapeptide hormone was a result of prostaglandin production. Collectively, these results suggest that the Ang-(1-7)-mediated reduction in COX-2 and PGES reduces the proliferation of cardiac fibroblasts and may also regulate additional processes in fibroblasts, associated with the regulation of cardiac parameters, inflammation and fibrosis.
In conclusion, our results demonstrate that Ang-(1-7) reduces the proliferation of cardiac fibroblasts and collagen production. These anti-fibrotic effects of Ang-(1-7) were associated with increased expression of DUSP1 and decreased activation of ERK1/ERK2 MAP kinases. Additionally Ang-(1-7) appears to play a prominent role in attenuating inflammatory and proliferative signals produced by the COX-2/PGES pathway. The anti-proliferative, anti-fibrotic and anti-inflammatory actions of Ang-(1-7) in cardiac fibroblasts contribute to maintaining normal cardiac function by regulating fibroblast proliferation and extracellular matrix homeostasis in the heart. Alterations in the ratio of the mitogenic signaling molecules to negative regulators, such as Ang-(1-7), disrupt the balance and lead to cardiac remodeling. This suggests that increasing the endogenous concentration of Ang-(1-7) or other proliferation suppressors may provide a therapeutic agent to prevent the cardiac pathologies associated with hypertensive disease and heart failure.
Highlights.
Angiotensin-(1-7) [Ang-(1-7)] inhibited DNA, protein, and collagen synthesis in cardiac fibroblasts.
Ang-(1-7) increased the dual specificity phosphatase DUSP1 in cardiac fibroblasts, in association with a reduction in phospho-ERK1/ERK2 MAP kinases.
Ang-(1-7) attenuated the mitogen-stimulated increase in cyclooxygenase 2 (COX-2) and prostaglandin E synthase (PGES) in cardiac fibroblasts to reduce the production of proliferative prostaglandins.
Acknowledgments
We acknowledge the technical assistance of L. Tenille Shields, Mark Landrum, and Robert Lanning. Present address of LaTronya T. McCollum is: Mount Sinai School of Medicine, One Gustave L. Levy Place, New York, NY 10029.
GRANTS
This work was supported by Grant HL-51952 from the National Institutes of Health. In addition, the studies described were supported, in part, by Unifi, Inc., Greensboro, NC, and the Farley-Hudson Foundation, Jacksonville, NC.
Footnotes
DISCLOSURES
NA
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Averill DB, Ishiyama Y, Chappell MC, Ferrario CM. Cardiac angiotensin-(1-7) in ischemic cardiomyopathy. Circulation. 2003 Oct;108(17):2141–2146. doi: 10.1161/01.CIR.0000092888.63239.54. [DOI] [PubMed] [Google Scholar]
- 2.Baum J, Duffy HS. Fibroblasts and myofibroblasts: what are we talking about? J Cardiovasc Pharmacol. 2011 Apr;57(4):376–379. doi: 10.1097/FJC.0b013e3182116e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther. 2010 Oct;128(1):191–227. doi: 10.1016/j.pharmthera.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Brown RD, Ambler SK, Mitchell MD, Long CS. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol. 2005;45:657–687. doi: 10.1146/annurev.pharmtox.45.120403.095802. [DOI] [PubMed] [Google Scholar]
- 5.Bueno OF, Molkentin JD. Involvement of extracellular signal-regulated kinases 1/2 in cardiac hypertrophy and cell death. Circ Res. 2002 Nov;91(9):776–781. doi: 10.1161/01.res.0000038488.38975.1a. [DOI] [PubMed] [Google Scholar]
- 6.Cheng CM, Hong HJ, Liu JC, Shih NL, Juan SH, Loh SH, Chan P, Chen JJ, Cheng TH. Crucial role of extracellular signal-regulated kinase pathway in reactive oxygen species-mediated endothelin-1 gene expression induced by endothelin-1 in rat cardiac fibroblasts. Mol Pharmacol. 2003 May;63(5):1002–1011. doi: 10.1124/mol.63.5.1002. [DOI] [PubMed] [Google Scholar]
- 7.Cheng TH, Cheng PY, Shih NL, Chen IB, Wang DL, Chen JJ. Involvement of reactive oxygen species in angiotensin II-induced endothelin-1 gene expression in rat cardiac fibroblasts. J Am Coll Cardiol. 2003 Nov;42(10):1845–1854. doi: 10.1016/j.jacc.2003.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Choudhary R, Palm-Leis A, Scott RC, III, Guleria RS, Rachut E, Baker KM, Pan J. All-trans retinoic acid prevents development of cardiac remodeling in aortic banded rats by inhibiting the renin-angiotensin system. Am J Physiol Heart Circ Physiol. 2008 Feb;294(2):H633–H644. doi: 10.1152/ajpheart.01301.2007. [DOI] [PubMed] [Google Scholar]
- 9.Cook KL, Metheny-Barlow LJ, Tallant EA, Gallagher PE. Angiotensin-(1-7) reduces fibrosis in orthotopic breast tumors. Cancer Research. 2010;70(21):8319–8328. doi: 10.1158/0008-5472.CAN-10-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira-dos-Santos AJ, da Costa J, Zhang L, Pei Y, Scholey J, Ferrario CM, Manoukian AS, Chappell MC, Backx PH, Yagil Y, Penninger JM. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002 Jun;417(6891):822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 11.De Mello WC. Angiotensin (1-7) re-establishes impulse conduction in cardiac muscle during ischaemia-reperfusion. The role of the sodium pump. J Renin Angiotensin Aldosterone Syst. 2004 Dec;5(4):203–208. doi: 10.3317/jraas.2004.041. [DOI] [PubMed] [Google Scholar]
- 12.Der Sarkissian S, Grobe JL, Yuan L, Narielwala DR, Walter GA, Katovich MJ, Raizada MK. Cardic overexpression of angiotensin converting enzyme 2 protects the heart from ischemia-induced pathophysiology. Hypertension. 2008;51:712–718. doi: 10.1161/HYPERTENSIONAHA.107.100693. [DOI] [PubMed] [Google Scholar]
- 13.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000 Sep;87(5):E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 14.Donoghue M, Wakimoto H, Maguire CT, Acton S, Hales P, Stagliano N, Fairchild-Huntress V, Xu J, Lorenz JN, Kadambi V, Berul CI, Breitbart RE. Heart block, ventricular tachycardia, and sudden death in ACE2 transgenic mice with downregulated connexins. J Mol Cell Cardiol. 2003 Sep;35(9):1043–1053. doi: 10.1016/s0022-2828(03)00177-9. [DOI] [PubMed] [Google Scholar]
- 15.Ferreira AJ, Santos RA, Almeida AP. Angiotensin-(1-7): cardioprotective effect in myocardial ischemia/reperfusion. Hypertension. 2001 Sep;38(3 Pt 2):665–668. doi: 10.1161/01.hyp.38.3.665. [DOI] [PubMed] [Google Scholar]
- 16.Ferreira AJ, Shenoy V, Qi Y, Fraga-Silva RA, Santos RA, Katovich MJ, Raizada MK. Angiotensin-converting enzyme 2 activation protects against hypertension-induced cardiac fibrosis involving extracellular signal-regulated kinases. Exp Physiol. 2011 Mar;96(3):287–294. doi: 10.1113/expphysiol.2010.055277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frohlich ED, Gonzalez A, Diez J. Hypertensive left ventricular hypertrophy risk: beyond adaptive cardiomyocytic hypertrophy. J Hypertens. 2011 Jan;29(1):17–26. doi: 10.1097/HJH.0b013e328340d787. [DOI] [PubMed] [Google Scholar]
- 18.Gallagher PE, Ferrario CM, Tallant EA. Regulation of ACE2 in cardiac myocytes and fibroblasts. Am J Physiol Heart Circ Physiol. 2008 Dec;295(6):H2373–H2379. doi: 10.1152/ajpheart.00426.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallagher PE, Tallant EA. Inhibition of human lung cancer cell growth by angiotensin-(1-7) Carcinogenesis. 2004 Nov;25(11):2045–2052. doi: 10.1093/carcin/bgh236. [DOI] [PubMed] [Google Scholar]
- 20.Giani JF, Munoz MC, Mayer MA, Veiras LC, Arranz C, Taira CA, Turyn D, Toblli JE, Dominici FP. Angiotensin-(1-7) improves cardiac remodeling and inhibits growth-promoting pathways in the heart of fructose-fed rats. Am J Physiol Heart Circ Physiol. 2010 Mar;298(3):H1003–H1013. doi: 10.1152/ajpheart.00803.2009. [DOI] [PubMed] [Google Scholar]
- 21.Giannico G, Mendez M, LaPointe MC. Regulation of the membrane-localized prostaglandin E synthases mPGES-1 and mPGES-2 in cardiac myocytes and fibroblasts. Am J Physiol Heart Circ Physiol. 2005 Jan;288(1):H165–H174. doi: 10.1152/ajpheart.00726.2004. [DOI] [PubMed] [Google Scholar]
- 22.Grobe JL, Der Sarkissian S, Stewart JM, Meszaros JG, Raizada MK, Kotovich MJ. ACE2 overexpression inhibits hypoxia-induced collagen production by cardiac fibroblasts. Clin Sci (Lond) 2007;113:357–364. doi: 10.1042/CS20070160. [DOI] [PubMed] [Google Scholar]
- 23.Grobe JL, Mecca AP, Lingis M, Shenoy V, Bolton TA, Machado JM, Speth RC, Raizada MK, Katovich MJ. Prevention of angiotensin II-induced cardiac remodeling by angiotensin-(1-7) Am J Physiol Heart Circ Physiol. 2007 Feb;292(2):H736–H742. doi: 10.1152/ajpheart.00937.2006. [DOI] [PubMed] [Google Scholar]
- 24.Grobe JL, Mecca AP, Mao H, Katovich MJ. Chronic angiotensin-(1-7) prevents cardiac fibrosis in DOCA-salt model of hypertension. Am J Physiol Heart Circ Physiol. 2006 Jun;290(6):H2417–H2423. doi: 10.1152/ajpheart.01170.2005. [DOI] [PubMed] [Google Scholar]
- 25.Haneda M, Sugimoto T, Kikkawa R. Mitogen-activated protein kinase phosphatase: a negative regulator of the mitogen-activated protein kinase cascade. Eur J Pharmacol. 1999 Jan;365(1):1–7. doi: 10.1016/s0014-2999(98)00857-7. [DOI] [PubMed] [Google Scholar]
- 26.Harding P, LaPointe MC. Prostaglandin E2 increases cardiac fibroblast proliferation and increases cyclin D expression via EP1 receptor. Prostaglandins Leukot Essent Fatty Acids. 2011 May;84(5-6):147–152. doi: 10.1016/j.plefa.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harding P, Yang XP, He Q, LaPointe MC. Lack of microsomal prostaglandin E synthase-1 reduces cardiac function following angiotensin II infusion. Am J Physiol Heart Circ Physiol. 2011 Mar;300(3):H1053–H1061. doi: 10.1152/ajpheart.00772.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huentelman MJ, Grobe JL, Vazquez J, Steward JM, Mecca AP, Katovich MJ, Ferrario CM, Raizada MK. Protection from angiotensin II-induced cardiac hypertrophy and fibrosis by systemic lentiviral delivery of ACE2 in rats. Exp Physiol. 2005;90.5:783. doi: 10.1113/expphysiol.2005.031096. [DOI] [PubMed] [Google Scholar]
- 29.Iwata M, Cowling RT, Gurantz D, Moore C, Zhang S, Yuan XJ, Greenberg BH. Angiotensin-(1-7) binds to specific receptors on cardiac fibroblasts to initiate antifibrotic and antitrophic effects. Am J Physiol Heart Circ Physiol. 2005;289:2356–2363. doi: 10.1152/ajpheart.00317.2005. [DOI] [PubMed] [Google Scholar]
- 30.LaPointe MC, Mendez M, Leung A, Tao Z, Yang XP. Inhibition of cyclooxygenase-2 improves cardiac function after myocardial infarction in the mouse. Am J Physiol Heart Circ Physiol. 2004;286:H1416–H1424. doi: 10.1152/ajpheart.00136.2003. [DOI] [PubMed] [Google Scholar]
- 31.Loot AE, Roks AJ, Henning RH, Tio RA, Suurmeijer AJ, Boomsma F, van Gilst WH. Angiotensin-(1-7) attenuates the development of heart failure after myocardial infarction in rats. Circulation. 2002 Apr;105(13):1548–1550. doi: 10.1161/01.cir.0000013847.07035.b9. [DOI] [PubMed] [Google Scholar]
- 32.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- 33.McCollum LT, Gallagher PE, Tallant EA. Angiotensin-(1-7) attenuates angiotensin II-induced cardiac remodeling associated with up-regulation of dual specificity phosphatase 1. Am J Physiol Heart Circ Physiol. 2011 Dec; doi: 10.1152/ajpheart.00908.2011. [epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 35.Mendez M, LaPointe MC. Trophic effects of the cyclooxygenase-2 product prostaglandin E(2) in cardiac myocytes. Hypertension. 2002 Feb;39(2 Pt 2):382–388. doi: 10.1161/hy02t2.102808. [DOI] [PubMed] [Google Scholar]
- 36.Mercure C, Yogi A, Callera GE, Aranha AB, Bader M, Ferreira AJ, Santos RA, Walther T, Touyz RM, Reudelhuber TL. Angiotensin(1-7) blunts hypertensive cardiac remodeling by a direct effect on the heart. Circ Res. 2008 Nov;103(11):1319–1326. doi: 10.1161/CIRCRESAHA.108.184911. [DOI] [PubMed] [Google Scholar]
- 37.Oudit GY, Penninger JM. Recombinant human angiotensin-converting enzyme 2 as a new renin-angiotensin system peptidase for heart failure therapy. Curr Heart Fail Rep. 2011 Sep;8(3):176–183. doi: 10.1007/s11897-011-0063-7. [DOI] [PubMed] [Google Scholar]
- 38.Owens DM, Keyse SM. Differential regulation of MAP kinase signaling by dual-specificity phosphatases. Oncogene. 2007;26:3203–3213. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- 39.Pan CH, Wen CH, Lin CS. Interplay of angiotensin II and angiotensin(1-7) in the regulation of matrix metalloproteinases of human cardiocytes. Exp Physiol. 2008;93:599–612. doi: 10.1113/expphysiol.2007.041830. [DOI] [PubMed] [Google Scholar]
- 40.Pei Z, Meng R, Li G, Yan G, Xu C, Zhuang Z, Ren J, Wu Z. Angiotensin-(1-7) ameliorates myocardial remodeling and interstitial fibrosis in spontaneous hypertension: Role of MMPs/TIMPs. Toxicol Lett. 2010 Sep; doi: 10.1016/j.toxlet.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 41.Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther. 2009 Aug;123(2):255–278. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 42.Qi Y, Shenoy V, Wong F, Li H, Afzal A, Mocco J, Sumners C, Raizada MK, Katovich MJ. Lentiviral mediated overexpression of Angiotensin-(1-7) attenuated ischemia-induced cardiac pathophysiology. Exp Physiol. 2011 Jun; doi: 10.1113/expphysiol.2011.056994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sadoshima J, Xu Y, Slayter HS, Izumo S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell. 1993 Dec;75(5):977–984. doi: 10.1016/0092-8674(93)90541-w. [DOI] [PubMed] [Google Scholar]
- 44.Sampaio WO, Henrique dC, Santos RA, Schiffrin EL, Touyz RM. Angiotensin-(1-7) counterregulates angiotensin II signaling in human endothelial cells. Hypertension. 2007 Dec;50(6):1093–1098. doi: 10.1161/HYPERTENSIONAHA.106.084848. [DOI] [PubMed] [Google Scholar]
- 45.Santiago NM, Guimaraes PS, Sirvente RA, Oliveira LA, Irigoyen MC, Santos RA, Campagnole-Santos MJ. Lifetime overproduction of circulating Angiotensin-(1-7) attenuates deoxycorticosterone acetate-salt hypertension-induced cardiac dysfunction and remodeling. Hypertension. 2010 Apr;55(4):889–896. doi: 10.1161/HYPERTENSIONAHA.110.149815. [DOI] [PubMed] [Google Scholar]
- 46.Santos RA, Ferreira AJ, Nadu AP, Braga AN, de Almeida AP, Campagnole-Santos MJ, Baltatu O, Iliescu R, Reudelhuber TL, Bader M. Expression of an angiotensin-(1-7)-producing fusion protein produces cardioprotective effects in rats. Physiol Genomics. 2004 May;17(3):292–299. doi: 10.1152/physiolgenomics.00227.2003. [DOI] [PubMed] [Google Scholar]
- 47.Scheuren N, Jacobs M, Ertl G, Schorb W. Cyclooxygenase-2 in myocardium stimulation by angiotensin-II in cultured cardiac fibroblasts and role at acute myocardial infarction. J Mol Cell Cardiol. 2002;34:29–37. doi: 10.1006/jmcc.2001.1484. [DOI] [PubMed] [Google Scholar]
- 48.Sigusch HH, Campbell SE, Weber KT. Angiotensin II-induced myocardial fibrosis in rats: role of nitric oxide, prostaglandins and bradykinin. Cardiovasc Res. 1996 Apr;31(4):546–554. [PubMed] [Google Scholar]
- 49.Souders CA, Bowers SL, Baudino TA. Cardiac fibroblast: the renaissance cell. Circ Res. 2009 Dec;105(12):1164–1176. doi: 10.1161/CIRCRESAHA.109.209809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stawowy P, Goetze S, Margeta C, Fleck E, Graf K. LPS regulate ERK1/2-dependent signaling in cardiac fibroblasts via PKC-mediated MKP-1 induction. Biochem Biophys Res Commun. 2003 Mar;303(1):74–80. doi: 10.1016/s0006-291x(03)00301-2. [DOI] [PubMed] [Google Scholar]
- 51.Tallant EA, Clark MA. Molecular mechanisms of inhibition of vascular growth by angiotensin-(1-7) Hypertension. 2003 Oct;42(4):574–579. doi: 10.1161/01.HYP.0000090322.55782.30. [DOI] [PubMed] [Google Scholar]
- 52.Tallant EA, Ferrario CM, Gallagher PE. Angiotensin-(1-7) inhibits growth of cardiac myocytes through activation of the mas receptor. Am J Physiol Heart Circ Physiol. 2005 Oct;289(4):H1560–H1566. doi: 10.1152/ajpheart.00941.2004. [DOI] [PubMed] [Google Scholar]
- 53.Tikellis C, Bernardi S, Burns WC. Angiotensin-converting enzyme 2 is a key modulator of the renin-angiotensin system in cardiovascular and renal disease. Curr Opin Nephrol Hypertens. 2011 Jan;20(1):62–68. doi: 10.1097/MNH.0b013e328341164a. [DOI] [PubMed] [Google Scholar]
- 54.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000 Oct;275(43):33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 55.Trask AJ, Groban L, Westwood BM, Varagic J, Ganten D, Gallagher PE, Chappell MC, Ferrario CM. Inhibition of angiotensin-converting enzyme 2 exacerbates cardiac hypertrophy and fibrosis in Ren-2 hypertensive rats. Am J Hypertens. 2010 Jun;23(6):687–693. doi: 10.1038/ajh.2010.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, Acton S, Patane M, Nichols A, Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 2002 Apr;277(17):14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- 57.Weber KT. Fibrosis in hypertensive heart disease: focus on cardiac fibroblasts. J Hypertens. 2004 Jan;22(1):47–50. doi: 10.1097/00004872-200401000-00011. [DOI] [PubMed] [Google Scholar]
- 58.Wong SC, Fukuchi M, Melnyk P, Rodger I, Giaid A. Induction of cyclooxygenase-2 and activation of nuclear factor-kappaB in myocardium of patients with congestive heart failure. Circulation. 1998 Jul;98(2):100–103. doi: 10.1161/01.cir.98.2.100. [DOI] [PubMed] [Google Scholar]
- 59.Wu KK, Liou JY. Cellular and molecular biology of prostacyclin synthase. Biochem Biophys Res Commun. 2005 Dec;338(1):45–52. doi: 10.1016/j.bbrc.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 60.Zhong J, Basu R, Guo D, Chow FL, Byrns S, Schuster M, Loibner H, Wang XH, Penninger JM, Kassiri Z, Oudit GY. Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation. 2010 Aug;122(7):717–28. 18. doi: 10.1161/CIRCULATIONAHA.110.955369. [DOI] [PubMed] [Google Scholar]
- 61.Zhong J, Guo D, Chen CB, Wang W, Schuster M, Loibner H, Penninger JM, Scholey JW, Kassiri Z, Oudit GY. Prevention of angiotensin II-mediated renal oxidative stress, inflammation, and fibrosis by angiotensin-converting enzyme 2. Hypertension. 2011 Feb;57(2):314–322. doi: 10.1161/HYPERTENSIONAHA.110.164244. [DOI] [PubMed] [Google Scholar]