Abstract
Fully functional CD8+ T cell memory is highly dependent upon CD4+ T cell support. CD4+ T cells play a critical role in inducing the expression of CD70, the ligand for CD27, on dendritic cells. Here we demonstrate that CD27 stimulation during primary CD8+ T cell responses regulates the ability to mount secondary CD8+ T cell responses. CD27 stimulation during vaccinia and dendritic cell immunization controls the expression of the IL-7 receptor (CD127), which has been shown to be necessary for memory CD8+ T cell survival. Further, CD27 stimulation during primary CD8+ T cell responses to vaccinia virus restrained the late expression on memory precursor cells of cytokine receptors that support terminal differentiation. The formation of CD8+ T cell memory precursors and secondary CD8+ T cell responses were restored in the absence of CD27 costimulation when endogenous IL-12 was not available. Similarly, the lesion in CD8+ T cell memory that occurs in the absence ofCD4+ T cells did not occur in mice lacking IL-12. These data indicate that CD4+ T cell help and, by extension, CD27 stimulation supports CD8+ T cell memory by modulating the expression of cytokine receptors that influence the differentiation and survival of memory CD8+ T cells.
Keywords: CD8+ T cell memory, CD27, CD70, IL-12, IL-7R
Introduction
In the search for more effective vaccine regimens, there is a continuing need to understand the basis by which CD8+ T cell memory develops and is sustained. Two competing hypotheses account for CD8+ T cell memory: first, that a subset of less differentiated primary CD8+ T cells survives at the end of the response (1); second, that memory precursors split from primary effectors at early stages of the primary response and develop as parallel population (2). Recent data indicate that naïve CD8+ T cells have the capacity to form either effector or memory CD8+ T cells (3), and that at least some memory cells show evidence of previous effector activity(4), supporting a linear differentiation model. From the pool of CD8+ T cells that expand in response to immunization, those with a greater capacity for survival (termed Memory Precursors Effector Cells (MPECs)) are enriched within a population of cells that reexpress the IL-7 receptor (IL-7R) (5,6), while terminally differentiated effector cells with little capacity to survive long term (termed Short Lived Effector Cells (SLECs)) frequently express KLRG1 (7). Loss ofIL-7 receptor expression has been shown to be influenced by T cell receptor engagement and the binding of IL-7, but the factors that influence it’s re-expression on MPECs are not known (8).
The factors that influence the fate-decisions of primary CD8+ T cells are therefore of considerable interest. Recent studies have elucidated that the extent of inflammation that accompanies exposure to antigen is a critical determinant in the differentiation of primary CD8+ T cells into SLECs. CD8+ T cell responses to dendritic cell immunization are dominated by cells with MPEC phenotype, and the addition of pro-inflammatory TLR-agonists increases the proportion of KLRG1-expressing SLECs in the response (9). Differentiation into KLRG1-expressing SLECs is strongly enhanced by IL-12-driven induction of T-bet and BLIMP-1 (7,10-12). Genetically limiting T-bet expression enhances CD8+ T cell memory in some but not all cases(7,13), suggesting that SLECs arise from the same common precursor as MPECs, and that inflammation-driven differentiation might come at the expense of MPECs and memory CD8+ T cells.
This leads to the hypothesis that differentiation into memory precursors is the default pathway for activated CD8+ T cells that have not received effector cell differentiation signals. However, CD4+ T cells have also been shown to provide important contributions to memory CD8+ T cell development and function in many (14-16) but not all responses to pathogens(17). Our understanding of the mechanistic basis behind CD4+ T cell-mediated promotion of CD8+ T cell memory is incomplete. In some studies, expression of IL-2 or IL-21 receptor is necessary for CD8+ T cell memory (18-20), suggesting that CD4+ T cells support CD8+ T cell memory via the provision of paracrine cytokines. Alternatively, direct stimulation of CD40 on CD8+ T cells by CD4+ T cells can enhance CD8+ T cell activation (21) and survival (22). CD4+ T cell-mediated stimulation of CD40also play an important role in up-regulating the activation state of dendritic cells (DC) to support CD8+ T cell responses. Direct stimulation of CD40 on DC has been shown to overcome the necessity of CD4+ T cells for the generation of primary CD8+ T cell responses (23-25), and subsequent development into fully functioning memory CD8+ T cells (25), indicating that paracrine cytokines provided by CD4+ T cells may support but are not required for CD8+ T cell memory. These studies indicate that DC that have been activated by CD4+ T cells induce a program of proliferation and differentiation in CD8+ T cells that is sufficient for long term survival and homeostatic proliferation. However, our understanding of the mechanistic basis by which CD4+ T cell-stimulated DC regulate CD8+ T cell memory programming is limited.
CD40-stimulated DC upregulate the expression of CD70, the ligand for CD27, and blockade of CD70 potently reduces primary CD8+T cell responses (25-27), demonstrating a prominent role for CD70 expression in a “licensed” DC. Importantly, memory CD8+ T cell responses to influenza infection and LCMV infection have been reported to be curtailed in CD27-knockout mice(28), and blocking CD70-CD27 interactions results in diminished CD8+ T cell memory (29,30). Therefore we hypothesized that the defects in the quantity and quality of CD8+ T cell memory that occur in the absence of CD4+ T cell help are a consequence of inadequate CD27 stimulation. To test this hypothesis, we utilized combinations of blocking antibodies to CD70 and agonistic antibodies to CD27, either in the context of highly inflammatory infections with recombinant vaccinia virus or weakly inflammatory DC-based immunizations, to determine the extent to which CD27 stimulation during primary CD8+ T cells responses influences the fate-decisions made by primary CD8+ T cells. Our results indicate that CD27-mediated stimulation strongly supports CD8+ T cell differentiation to MPECs, and protects against IL-12 mediated terminal differentiation.
Materials and Methods
Animals
C57BL/6Y (B6) mice were obtained from NCI (Frederick, MD). IL-12 p35 (B6.129S1-Il12atm1Jm/J stock # 002692) and IL-12p40 knockout mice (B6.129S1-Il12btm1Jm/J stock # 002693) were purchased from The Jackson Laboratory (Bar Habor, Maine). OT-I transgenic mice, expressing T cell receptors specific for OVA257 peptide in complex with H-2Kb, were purchased from The Jackson Laboratory (C57BL/6-Tg(TcraTcrb)1100Mjb/J, stock # 003831) and crossed onto Thy1.1+(B6.PL-Thy1a/CyJ stock # 000406) mice obtained from The Jackson Laboratory. CD27-knockout mice (31) were provided by Dr Stephen Schoenberger (La Jolla Institute for Allergy and Immunology), with the permission of DrJannieBorst (Netherlands Cancer Institute).Mice were maintained in specific pathogen-free facilities and were treated in accordance with the guidelines established by the Animal Care and Use Committee at the University of Virginia.
Cell lines and viruses
Recombinant vaccinia expressing ovalbumin (OVA) was provided by Dr Jon Yewdell (NIAID), and was propagated on HuTK− cells. Recombinant adenovirus expressing OVA was kindly provided by Dr Young Hahn, University of Virginia, and was propagated on 293A fibroblasts. LB15.13 hybridoma was obtained from ATCC (Frederick, MD) and maintained in RPMI with 5% FBS (Hyclone; Logan, UT)).
Antibodies
Agonistic AT124.1 anti-mouse CD27 has been described (32). FR70 blocking anti-mouse CD70 has been described(33). Control immunoglobulin was purchased from Sigma (St Louis, MO.).
BMDC generation
BMDC were expanded from mouse bone marrow in the presence of GM-CSF and IL-4 as previously described(34). D7 BMDC were isolated by negative selection on magnetic columns (Stemcell, Vancouver, BC), incubated overnight in culture with CD40L-expressing 3T3 cells and media containing 10μg/ml OVA257 peptide.
Peptides and protein
Synthetic peptides were purchased from Genscript (Piscataway, NJ) OVA was purchased from Sigma. Endotoxin was removed by Detoxi-Gel endotoxin-removal kit (Pierce, Rockford, IL).
Immunization
For the generation of chimeric mice, 1000-5000 Thy1.1+CD45.2+ OT-I cells were transferred into recipient mice. Depletion of CD4+ T cells was achieved by i.p. injection of 200 μg GK1.5 (ATCC) 7d and 3d prior to generation of chimeric mice, and confirmed by tail vein bleed. CD27 stimulation was performed by injecting 50 μg AT124-1 i.p. on d0, d3 and d6. CD70 blockade was performed by injecting 500 μg FR70 i.p. on d0, d2, d4 and d6. IL-12 blockade was performed by injecting 500 μg C17.8 (Bioxcell, Hanover, NH) on d0, d2, d4 and d6 after immunization. rIL-12 (eBioscience) was delivered by i.p. injection of 500μg 24h and 48h after immunization. Primary CD8+ T cell responses were generated by injecting mice i.v. with 107 p.f.u. OVA-vac, or 105 CD40L-activated OVA257-pulsed BMDC. Secondary responses were initiated in primed mice by i.p. challenge with 2×108 p.f.u. recombinant OVA-adeno.
Viral titers
Virus titers from infected mice were determined 4d after i.p. challenge with 1×108pfu of naïve or previously immunized mice. Ovaries were excised and digested with collagenase/DNAse/hyaluronidase then homogenized. Homogenate was subject to 3 cycles of freezing and thawing then sonicated. Sonicate was cleared of particulate matter by a centrifugation, and the supernatant used to infect HuTK-cells. Virus plaques were revealed 48h later by crystal violet staining.
Tetramer staining
H2-Kb-tetramers that had been folded around OVA257 were provided by Dr. Vic Engelhard, University of Virginia. Lymphocytes were isolated from blood or homogenized spleens and were co-incubated for 30 min at 4°C with tetramer-APC. Antibodies described were purchased from eBioscience, with the exception of anti-CD44-Pacific Blue, anti-IL-21R-PE and anti-IFNαβR-PE which were purchased from Biolegend. Anti-CD212 (IL-12Rβ1)-PE and anti-CD107a-PECy7 were purchased from Becton Dickenson (Franklin Lakes, NJ). Anti-KLRG1-PE was purchased from Abcam (Cambridge, MA). Staining was assessed by flow cytometry on a FACS Canto II (Becton Dickinson) and analyzed using FlowJo Software (Treestar, OR).
Memory adoptive transfers
CD8+ T cells were enriched from CD45.1+ mice by magnetic bead-based negative selection (Stemcell) from the spleens and lymph nodes of d90 mice. OT-1 cells were enumerated by staining for Thy1.1, and ~1000 OT-1 cells were transferred into recipient CD45+.2 mice.
Statistics
Statistical significance of differences between comparison groups was determined by performing unpaired two-tailed Student T tests for 95% confidence limits using GraphPad Prism software (San Diego, CA).
Results
Stimulation of CD27 on CD8+ T cells promotes CD8+ T cell memory in the absence of CD4+ T cell help
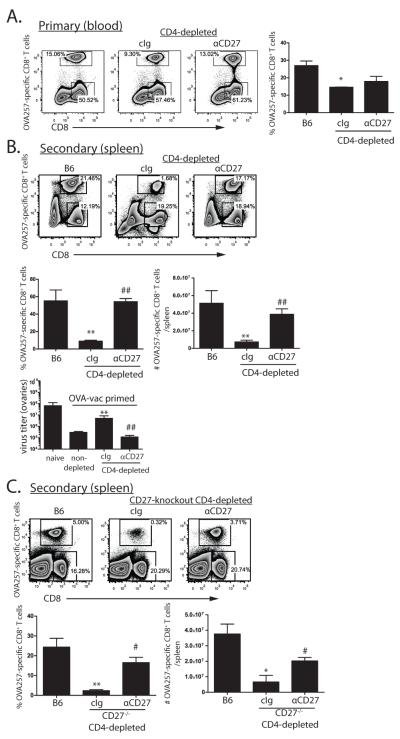
To begin to test whether the defect in CD8+ T cell memory that occurs in the absence of CD4+ T cells is a consequence of failed induction of CD27 co-stimulation, we first asked whether direct stimulation of CD27 promotes CD8+ T cell memory in the absence of CD4+ T cells. To facilitate tracking CD8+ T cell responses, and to allow genetic manipulation of responding CD8+ T cell populations, we generated chimeric mice by transferring ~1000 Thy1.1+ OT-I TCR transgenic cells into Thy1.2+ recipient mice. At this frequency, approximately 70% of the OVA257-specific response is composed of OT-I cells, while 30% is derived from endogenous sources. This relationship is maintained through memory and subsequent secondary expansion (Supplemental Figure 1).CD4+ T cell-depleted OT-1 chimeric mice were immunized with recombinant vaccinia expressing ovalbumin (OVA-vac), and treated with an agonistic antibody to CD27 or control immunoglobulin (cIg) during the primary response. Compared to non-depleted control mice, the frequency of the OVA257-specific primary CD8+ T cells in CD4-depleted mice was approximately 2-fold lower. CD27-stimulation modestly increased the magnitude of the OVA257-specific primary response in CD4-depleted mice (Figure 1A). After 90d rest we assessed the ability of these mice to mount secondary CD8+ T cell responses to a heterologous challenge with recombinant adenovirus expressing OVA (OVA-adeno). As anticipated, secondary OVA257-specific CD8+ T cell responses were highly compromised in cIg-treated, CD4+ T cell-depleted mice as compared to non-depleted mice. In contrast, CD4+ T cell depleted mice treated with anti-CD27 mounted secondary OVA257-specific CD8+ T cell responses that were equivalent in magnitude to those found in non-depleted mice (Figure 1B). Similar results were achieved using MHC II-deficient mice (data not shown). The ability of anti-CD27 treatment to rescue secondary CD8+ T cell responses corresponded to a significant reduction in the amount of virus found in the ovaries of infected mice compared to control treated counterparts (Figure 1C). CD27 stimulation worked directly on CD8+ T cells as anti-CD27 treatment restored CD8+ T cell memory in CD4-depleted, CD27−/− mice containing wild-type OT-1 (Supplemental Figure 2). Therefore, the deficiency in CD8+ T cell memory that develops in the absence of CD4+ T cells can be overcome by direct stimulation of CD27 on primary CD8+ T cells.
Figure 1. CD27 stimulation promotes CD8+ T cell memory in the absence of CD4+ T cells.
OT-1 chimeric mice (n=3 mice per cohort) were depleted of CD4+ T cells then immunized with OVA-vac and treated with cIg or anti-CD27. A. Primary OT-1 responses in blood 7d after OVA-vac immunization. B. Secondary OT-1 responses in spleens 5d after OVA-adeno challenge of mice from A. rested for >35d. C. Vaccinia titers from the ovaries of naïve mice or mice primed 60d previously with OVA-vac under the indicated conditions. Plots are derived from representative individual mice within an experimental cohort. Histograms contain compiled cohort data, showing median responses +/− SEM. *p<0.05; ** p<0.01 compared to B6. #p<0.05; ## p<0.01 compared to cIg-treated mice. Data from one of 5 similar experiments.
CD70 blockade during the primary CD8+ T cell response abrogates CD8+ T cell memory
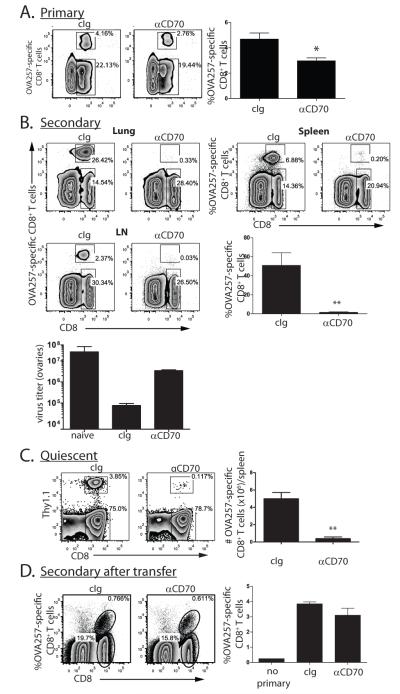
We reasoned that if insufficient CD27 stimulation was responsible for the defect in CD8+ T cell memory in the absence of CD4+ T cell help, then blocking CD27 stimulation in mice replete for CD4+ T cells should abrogate memory. We prevented CD27 stimulation by infusing a CD70-blocking antibody after OVA-vac infection of OT-1 chimeric mice, and the magnitude of the primary CD8+ T cell response was determined in the blood or the spleen. We found statistically significant yet small reduction in the magnitude of the OVA257-specific response compared to control treated mice (Figure 2A). In contrast to the primary CD8+ T cell response, the secondary response from mice treated with anti-CD70 was almost completely absent from both lymphoid (spleen, lymph nodes) and peripheral tissues (lungs) (Figure 2B). Thus, as with CD8+ T cell responses to vaccinia virus in the absence of CD4+ T cells (15,35,36), the primary CD8+ T cell responses to recombinant vaccinia virus is not strongly dependent upon CD70 stimulation. However, the secondary CD8+ T cell response after re-exposure to antigen was highly compromised, indicating a critical role for CD70-mediated stimulation in promoting CD8+memory T cell formation or function. This fact is further emphasized by the significantly higher vaccinia virus titer found in the ovaries of αCD70 treated mice rechallenged with vaccinia compared to control treated mice (Figure 2C).
Figure 2. CD70 stimulation during the primary CD8+ T cell response to OVA vac is required for secondary CD8+ T cell expansion.
OT-I chimeric mice (n=3 mice per cohort) were challenged with OVA-vac and were treated with anti-CD70 or cIg during the expansion of the primary response. A. Magnitude of the primary OT-1 response in spleens on d7 in cIg or anti-CD70 treated mice. B. Magnitude of secondary responses in lung, spleen and lymph nodes (LN) 5d after challenge with OVA-adeno, >35d after initial priming with OVA-vac. Dot plots are from individual mice within representative experiments. Histograms contain data from spleens of each cohort. C. Vaccinia titers from the ovaries of naïve or mice primed 60d previously with OVA-vac under the indicated conditions. D. Frequency and number of quiescent memory CD8+ T cells enriched from the spleens of OVA-vac immunized OT-1 chimeric mice >90d after priming. E. Magnitude of secondary OT-1 response from 1000 transferred quiescent memory OT-1 5d after OVA-adeno challenge. Numbers in plots indicate percentage of cells within plot within the indicated region. Each histogram shows the median value of the cohort +/− SEM. *p<0.05; ** p<0.01 compared to cIg-treated mice. Data from experiments performed 3-5 times.
Reduction in the frequency of memory CD8+ T cells in the absence of CD70 co-stimulation
The critical role of CD70 in establishing the ability to mount a secondary CD8+ T cell response implicated that CD70 stimulation might be needed in the proper formation or survival of memory CD8+ T cells, or their ability to expand upon challenge. To address this question, we next determined whether CD27 co-stimulation during the primary response altered the number of quiescent memory CD8+ T cells. At 90d after infection, very few OVA257-specific memory cells were found in mice that were blocked from CD70 costimulation during the primary response (Figure 2D). Those that remained expressed similar surface molecules as the CD8+ T cells found in control treated mice. The majority (55-60%) expressedIL-7R and CD27, but only 10-20% expressed CD62L and CCR7 (not shown). Therefore, effector memory phenotype cells dominated in the spleen of both cIg and anti-CD70-treated mice that have been infected with OVA-vac, but no significant differences were apparent between control and anti-CD70 treated mice. Thus, CD70 co-stimulation during the primary CD8+ T cell response enhances the number of memory CD8+ T cells.
We next determined whether memory CD8+ T cells that developed in the absence of CD70 costimulation have defects in their ability to proliferate in response to antigen challenge(14). ~1000 memory OT-1 CD45.2+ cells were transferred into recipient CD45.1 mice and challenged with OVA-adeno. Memory CD8+ T cells from either control or CD70-blocked mice were able to expand equivalently (Figure 2E) and the subsequent secondary effectors had the same ability to expose CD107a (a marker of cellular degranulation) and produce IFNγ(not shown). Therefore the absence of CD70 stimulation during priming with OVA-vac abrogates the ability of mice to mount secondary CD8+ T cell responses, and this is predominantly a consequence of diminished memory CD8+ T cell numbers, rather than the type or function of the memory CD8+ T cells that do form.
CD70 stimulation during primary OVA-vac infection supports IL-7 receptor-expressing memory precursor CD8+ T cells
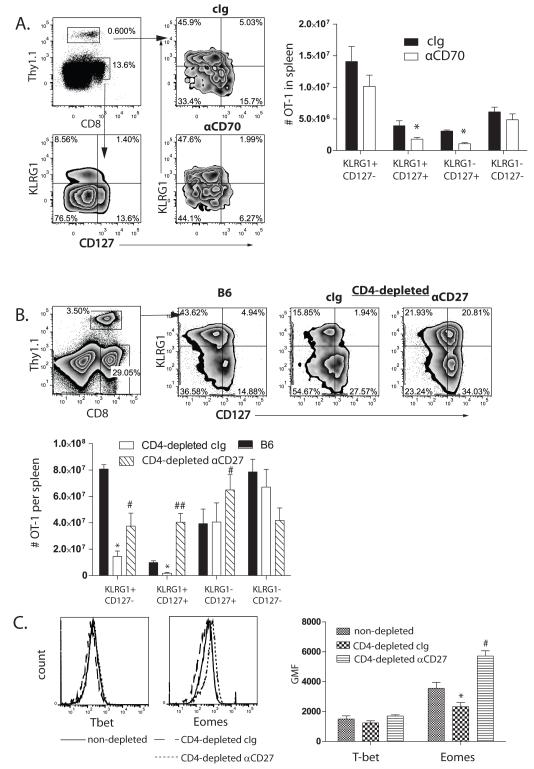
To establish how CD70 stimulation supported memory CD8+ T cells, we next asked whether it played a role in the formation of CD8+ T cell memory precursors, which are identified by the expression of the IL-7 receptor (CD127) and the absence of KLRG1 expression (5,7). First, we determined the frequency and number of MPECs in the spleens of control or CD70-blocked mice 7d after priming, and found a 55% reduction in the number of primary OT-1 cells that express IL-7R (Figure 3A). A similar outcome was found for the endogenous component of the primary CD8+ T cell response (Supplemental Figure 3).We found no significant increase in KLRG1-expression by OT-1 cells, indicating that CD70-blockade does not enhance terminal differentiation of effector CD8+ T cells. Rather, an increase in the frequency of OT-1 cells that express neither IL-7R nor KLRG1 (termed early effector cells; EEC (37)) was noted (Figure 3A), suggesting that CD27 stimulation promotes either the formation or persistence of IL-7R-expressing CD8+ T cells.
Figure 3. CD70-CD27 stimulation modulates the frequency CD127-expressing primary effector CD8+ T cells.
A. KLRG1 and CD127 expression by d7 OT-1 (Top left plot; Thy1.1+) cells from chimeric mice challenged with OVA-vac and treated with cIg (top plots) or anti-CD70 (bottom plots). cIg and anti-CD70 plots are gated on Thy1.1, while bottom left hand plot shows KLRG1 and CD127 expression on total CD8 for comparative purposes. B. Expression of CD127 and KLRG1 on d7 OT-1 cells from spleens of non-depleted or CD4-depleted mice, treated with cIg or anti-CD27, that were primed with OVA-vac. C. Intracellular expression of T-bet and Eomes in d7 OT-1 MPEC that expanded in non-depleted (solid lines) or CD4-depleted mice treated with cIg (dashed lines) or anti-CD27 (doted lines), gated on Thy1.1+ CD127+ CD8+ T cells. Dot plots and overlays are from representative mice. Numbers in dot plots indicated the percentage of cells within the indicated regions or quadrants. Histograms show the number of OT-1 expressing KLRG1 and/or CD127 in the spleens of B6 or CD4-depleted mice treated with cIg or anti-CD27 treated mice on d7 and the GMF expression of T-bet and Eomes. Each histogram shows the median value of the cohort (n=3) +/− SEM. *p<0.05; compared to cIg-treated or B6 mice. #p<0.05; ## p<0.01 compared to cIg-treated CD4-depleted mice. Data represent experiments repeated at least twice.
We next assessed whether augmented CD27 stimulation enhanced the frequency and number of IL-7R expressing memory precursors. Somewhat unexpectedly (38), we found that CD4-depleted mice had a dramatically reduced frequency and number of KLRG1hi SLECS, yet did not have a lower frequency of IL-7R+MPEC CD8+ T cells (Figure 3B). However, CD4+ T cell-depleted mice treated with anti-CD27 had a significantly greater frequency of IL-7R-expressing cells than either non-depleted mice, or CD4+ T cell-depleted mice treated with cIg (Figure 3B). Notably, we found increases in both the frequency of KLRG1−IL-7R+ and in IL-7R+KLRG1+ CD8+ T cells (Figure 3B). Again, these data were recapitulated in the smaller endogenous response to OVA-vac (Supplemental Figure 4A and 4B).Thus, stimulation of CD27 during primary CD8+ T cell responses to OVA-vac results in a far greater proportion of CD8+ T cells with characteristics of cells with potential to survive into memory. Together, these data indicate that CD70/CD27 costimulation regulates either the expression of IL-7R by primary effector cells, or is integral to either the proliferation or survival ofIL-7R-expressing MPECs.
A deficiency in CD4+ T cells during LCMV infection has been previously shown to result in excessive expression of the transcription factor T-bet and reduced expression of Eomesodermin (Eomes), resulting in repression of the IL-7R and a corresponding loss of central memory CD8+ T cells (13). Therefore we determined whether CD27 stimulation modulated the expression of T-bet and Eomes. While both transcription factors were induced in effector CD8+ T cells compared to naïve CD8+ T cells (not shown), we found little difference in T-bet expression in CD8+ T cells that expand in response to OVA-vac non-depleted and CD4-depleted mice (Figure 3C). In contrast, the expression of Eomes was significantly reduced in CD8+ T cells that expanded in CD4-depleted mice compared to non-depleted. Treatment with anti-CD27 restored Eomes expression to a level even higher than found in non-depleted mice (Figure 3C). Thus, ability of CD27 stimulation to promote secondary CD8+ T cell responses after vaccinia immunization closely correlates with the induction of Eomes expression, but not T-bet expression.
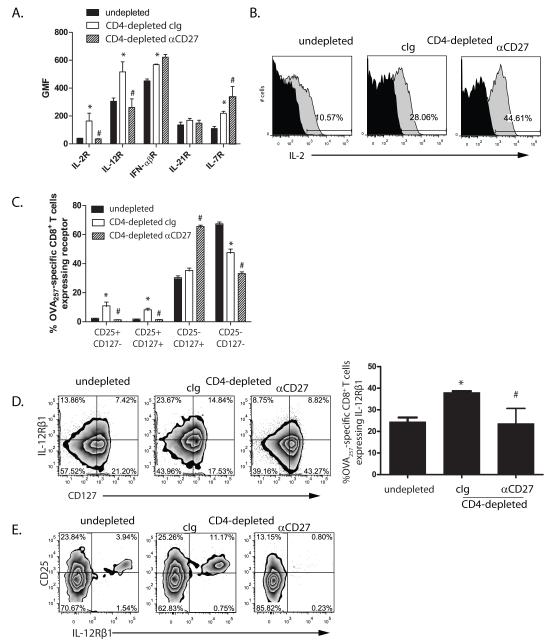
Promotion of CD8+ T cell memory by CD27 co-stimulation correlates with decreased expression of IL-12 and IL-2 receptors
The preceding data indicates that CD27 co-stimulation strongly influences CD8+ T cell memory potential. However, IL-7R expression is not sufficient for memory CD8+ T cell function(39,40). Further, while CD27 stimulation augmented the frequency of IL-7R expressing cells, the absence of CD4+ T cells did not substantially reduce the frequency of IL-7R-expressing cells compared to control mice. These data suggested that CD27 stimulation modulates additional factors that influence memory CD8+ T cell differentiation or survival. Neither the expression of IL-21R or IFNαβR (Figure 4A), nor autocrine IL-2(41)(which was strongly induced by CD27stimulation; Figure 4B), correlated with the ability to generate CD8+ T cell memory in CD4-depleted mice. In contrast, we found that in CD4+ T cell depleted mice IL-7R+OT-1 retained expression of CD25, the high affinity IL-2 receptor which marks terminally differentiated cells (42,43). Stimulation with CD27 significantly reduced the frequency of IL-7R+ cells that expressedCD25 (Figure 4C, and Supplemental Figure 4C). This suggested that in the absence of CD4+ T cells MPECs retain the expression of cytokine receptors that could lead to terminal differentiation. Supporting this, the expression of IL-12Rβ1 on IL-7R+ OT-1 inversely correlated with the capacity to form CD8+ T cell memory (Figure 4D). Of particular interest, IL-7R-expressing OT-1 that co-expressed CD25 also expressed IL-12Rβ1, and this subpopulation was 3 times more prevalent in CD4-depleted mice compared to non-depleted, yet was absent from CD4-depleted mice treated with anti-CD27 (Figure 4E). Therefore, the absence of CD4+ T cells during primary CD8+ T cell responses is associated with increased expression of receptors for cytokines associated with the differentiation and survival of effector cells on IL-7R-expressing MPECs. Stimulation of CD27 suppresses the generation of cells with this phenotype.
Figure 4. The ability of CD27 stimulation to promote CD8+ T cell memory inversely correlates with CD25 and IL-12R expression.
Thy1.1+ OT-1 were transferred into non-depleted or CD4-depleted mice that were infected with OVA-vac and treated with cIg or anti-CD27. 7d after OVA-vac infection spleens and/or LN were excised and stained for the presence of CD44hi CD8+ Thy1.1+ OT-1 cells. A. Expression of cytokine receptors on Thy1.1+ OT-1 gated cells. Histogram shows the geometric mean fluorescence (GMF) or the respective cytokine receptors. B. Expression of IL-2 by d7 OT-1 cells after short term in vitro stimulation of splenocytes (gray plots). Black areas are from non-stimulated controls. Numbers in plots show percent OT-1 expressing IL-2. C. Co-expression of CD127 and CD25 on d7 OT-1 cells. D. Co-expression of IL-12Rβ1 and CD127 on OT-1. Dot plots show expression in individual mice. Histogram shows percent OT-1 expressing IL-12Rβ1 in each cohort. E. Co-expression of CD25 and IL-12Rβ1 gated on CD127-expressing OT-1 cells. *p<0.05 compared to non-depleted. Each histogram shows the median value of the cohort (n=3) +/− SEM. # p<0.05 compared to cIg-treated. Data represent one of three similar experiments.
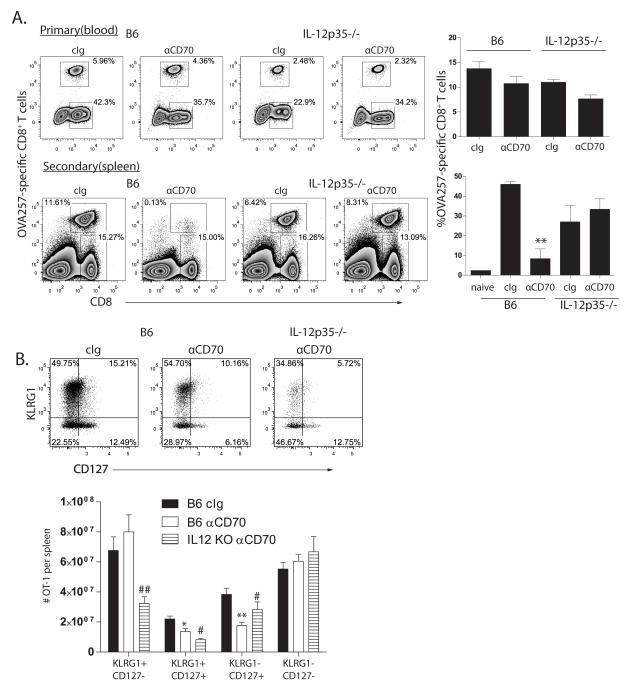
CD70 co-stimulation moderates the influence of IL-12 on MPEC formation and CD8+ T cell memory
Recent studies have implicated IL-12 as a critical mediator of CD8+ T cell fate decisions (7,10,44,45), and as CD27 co-stimulation modulated the expression of the IL-12Rβ1 chain, we assessed whether IL-12 is responsible for the loss of memory cell formation in the absence of CD27 co-stimulation. IL-12 only moderately contributed to the development of OT-1 primary effector CD8+ T cell responses to recombinant vaccinia, but concomitantly blocking CD70 had an additive effect, leading to a significant reduction in the primary OT-1 CD8+ T cell response (Figure 5A). In contrast, we found that the magnitude of the OT-1 secondary CD8+ T cell response IL-12-deficient mice treated with anti-CD70 was equivalent to that of either IL-12-deficient or wild-type mice treated with cIg (Figure 5A). Similar results were found with IL-12p40-knockout mice (data not shown). To define the basis for this difference, we examined the impact of CD70-blockade on memory precursors. We found that in the absence of IL-12, the proportion of the responding CD8+ T cells that expressed KLRG1 was reduced. Further, in the absence of IL-12, CD70 blockade did not significantly reduce the frequency and number of IL-7R-expressing memory precursors (Figure 5B). Therefore, we conclude that CD27 stimulation plays a critical role in controlling the influence of IL-12 on the differentiation and survival of MPECs.
Figure 5. IL-12 prevents secondary CD8+ T cell responses in the absence of CD70 stimulation.
A. OT-1 chimeric B6 or IL-12p35-knockout mice (n=3) were primed with OVA-vac in the presence of cIg or anti-CD70. Primary OT-1 responses (top plots) were determined by Thy1.1-staining in blood 7d after infection. Secondary OT-1 responses (bottom plots) were initiated by challenge with OVA-adeno 35d after initial priming with OVA-vac, and determined by staining spleens 5d later for Thy1.1-expressing CD8+ T cells. Naïve indicates the primary response generated 5d after OVA-adeno. B. Expression of KLRG1 and CD127 (dot plots) by d7, gated on Thy1.1+ OT-1, in the spleens of mice described in A, and enumeration of the number of OT-1 with each phenotype per spleen (histogram). Numbers in dot plots indicate the percentage of cells within each region. Plots are derived from representative individual mice within an experimental cohort. Histograms contain compiled cohort data, showing median responses +/− SEM. *p<0.05; ** p<0.01 compared to cIg-treated B6. # p<0.05; ##p<0.01 compared to anti-CD70-treated mice. Data from one of 5 similar experiments.
CD70-blockade does not abrogate CD8+ T cell memory in BMDC-primed mice
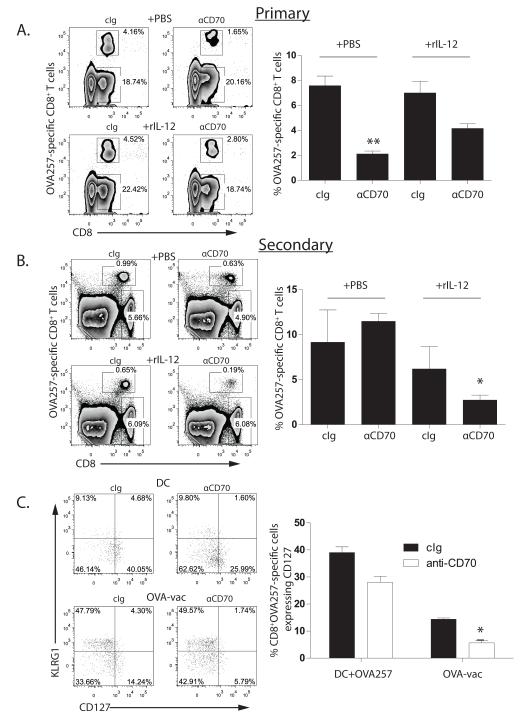
We next investigated whether CD70 co-stimulation is generally necessary for the development of IL-7R-expressing MPECs, even under non-inflammatory conditions. Cohorts of mice were immunized with OVA257-pulsed, CD40L-activated BMDC and treated with anti-CD70 or cIg. Analysis of the primary CD8+ T cell response revealed a significant reduction (~75%) in mice treated with anti-CD70, consistent with our previous results using BMDC pre-incubated with anti-CD70 (25)(Figure 6A). Strikingly, the magnitude of the secondary CD8+ T cell response elicited by OVA-adeno challenge was equivalent between the two cohorts (Figure 6B). Therefore, under immunization conditions that induce weak inflammation, CD70-blockade had a large impact on the development of the primary CD8+ T cell response but no apparent impact on the survival or differentiation of CD8+ T cell memory.
Figure 6. IL-12 induces memory CD8+ T cell susceptibility to CD70 blockade after immunization with BMDC.
Cohorts (n=3) of OT-1 chimeric mice were primed with OVA257-pulsed, CD40L-activated BMDC in the presence of cIg (left panels) or anti-CD70 (right panels). Half the cohorts received PBS (top panels) and the other half rIL-12 (bottom panels) on d0 and d2 after BMDC. A. Primary OT-1 responses and B. secondary OT-1 responses were assessed 7d and 5d after immunization with BMDC or challenge with OVA-vac, respectively. Histograms contain compiled cohort data, showing median responses +/− SEM. C. SLEC/MPEC phenotype of d7 primary OT-1 cells in spleens elicited by either BMDC (top panels) or OVA-vac (bottom plots) in mice treated with cIg or anti-CD70. Plots are derived from representative individual mice within an experimental cohort. Histograms contain compiled cohort data, showing median responses +/− SEM. *p<0.05; ** p<0.01 compared to cIg-treated. Data are from one of 2 similar experiments.
We reasoned that CD70 blockade during BMDC immunization had little impact on CD8+ T cell memory due to the minimal IL-12 produced by this immunization system. We therefore immunized cohorts of OT-1 chimeras and treated with anti-CD70 or cIg with supplemental IL-12 or PBS. Inclusion of IL-12 had little impact on the overall magnitude of the OT-1 response generated in cIg-treated mice. However, IL-12 significantly increased the size of the primary CD8+ T cell response in anti-CD70-treated mice (Figure 6A), indicating that IL-12 and CD70 stimulation non-redundantly support the expansion of primary effector CD8+ T cells. However, mice that had received IL-12 and anti-CD70 during the primary response to BMDC immunization made very poor secondary CD8+ T cell responses (Figure 6B). Therefore, CD8+ T cells that respond to BMDC immunization require stimulation by CD70 to form functional CD8+ T cell memory in the presence of IL-12.
To understand the basis of the difference between the effect of CD70 blockade on the development of CD8+ T cell memory after OVA-vac and BMDC immunization, we examined the impact of CD70 blockade on MPEC generation in response to BMDC. Notably, considerably fewer OT-1 that responded to BMDC immunization expressed KLRG1, while the proportion that expressed IL-7R was significantly higher (Figure 6C). Surprisingly, we found that blocking CD70 during BMDC immunization resulted in a substantial reduction in IL-7R-expressing OT-1; nevertheless, a considerably larger frequency of IL-7R-expressing cells remained after CD70-blockade of BMDC immunization compared to OVA-vac immunization. Blockade of CD70 during BMDC immunization resulted in an increase in the proportion of EEC OT-1 that express neither KLRG1 nor IL-7R (Figure 6C), indicating that CD70 blockade does not enhance terminal differentiation in OT-1 responding to BMDC immunization. Thus, CD70 blockade reduces the proportion of IL-7R-expressing MPEC primary CD8+ T cells that respond to BMDC, but their frequency remains relatively high.
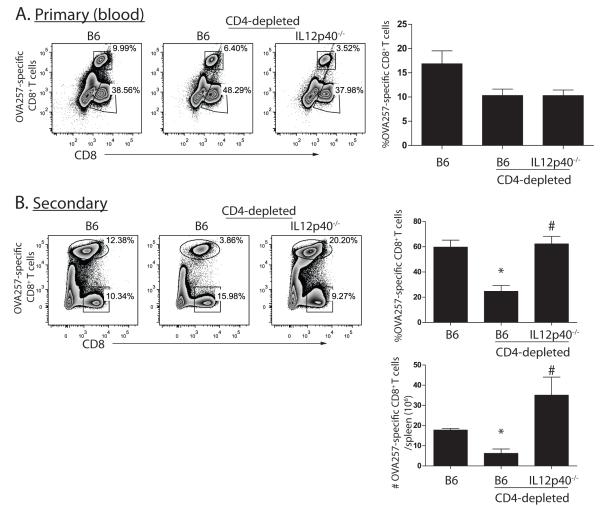
Loss of CD8+ T cell memory in the absence of CD4+ T cells is caused by IL-12
As the defect in CD8+ T cell memory that is found in CD4+ T cell-deficient mice could be overcome by stimulation of CD27, and closely correlated with the expression of the IL-12 receptor, we hypothesized that the loss of CD8+ T cell memory in the absence of CD4+ T cells might be a consequence of weak CD70-mediated protection against terminal differentiation induced by IL-12. We depleted wild-type or IL-12-deficient OT-I chimeric mice of CD4+ T cells, and challenged with OVA-vac. As previously observed, the OVA257-specific primary CD8+ T cell response was ~2-fold lower in CD4-depleted mice (Figure 7A). However, compared to CD4-depleted wild-type mice, secondary CD8+ T cell responses elicited by OVA-adeno in CD4-depleted IL-12-deficient mice were not compromised (Figure 7B). Thus we conclude that the inability to mount secondary CD8+ T cell responses from memory CD8+ T cells primed in the absence of helper CD4+ T cells can be attributed to IL-12 and can be overcome by direct stimulation of CD27 on CD8+ T cells.
Figure 7. IL-12 is responsible for the loss of CD8+ T cell memory to OVA-vac in absence of CD4+ T cells.
Cohorts of non-depleted, or CD4-depleted B6 or IL-12p40−/− OT-1 chimeric mice (n=3) were primed with OVA-vac. A. Frequency of primary OT-1 response in blood on 7d after priming. B. Frequency and total number of secondary OT-1 responses in spleen 5d after challenging mice from A. with OVA-adeno. Histograms contain compiled cohort data, showing median responses +/− SEM. n.s.=not significant. *p<0.05 compared to non-depleted; # p<0.05 compared to CD4-depleted B6. Data from one of 2 similar experiments.
Discussion
The studies presented herein demonstrate that CD70-mediated co-stimulation has a major influence on CD8+ T cell memory, particularly in situations where IL-12 expression is strongly induced. Our data indicate that CD70-CD27 interaction plays a significant role in either the expansion/survival of IL-7R expressing cells, or directly modulates the expression of the IL-7R. We find that CD70 stimulation dictates the frequency and number of IL-7R expressing putative memory precursor CD8+T cells found at the peak of the primary response, which is significant as little is known about the stimuli that promote MPECs. Together these data illuminate an unappreciated role for CD27-mediated costimulation in the promoting the frequency of IL-7R expressing cells and constraining the influence of IL-12 on CD8+ T cell differentiation, and mechanistically link the induction of CD70 expression on DC by CD4+ T cells with the programming of IL-12 resistance in memory CD8+ T cell differentiation
As the expression of CD70 on DC generally requires ligation of CD40, we hypothesizedthat the dysfunctional nature of memory CD8+ T cells that develops in the absence of CD4+ T cell help can be attributed to a failure to induce CD70 expression. Supporting the hypothesis, direct stimulation of CD27 bypassed the requirement for CD4+ T cell help in the promotion of CD8+ T cell memory, while CD70 blockade abrogated CD8+ T cell memory; in both cases, loss of CD8+ T cell memory was dependent upon IL-12. However, there is some divergence in the impact of CD70 blockade and CD4+ T cell depletion on the subsets of CD8+ T cells at the peak of the primary response. The absence of CD4+ T cells had a more profound effect on KLRG1+ SLEC numbers than on either the frequency or number of IL-7R-expressing MPECs, highlighting the role for CD4+ T cells in the support of SLEC survival. However, autocrine IL-2 expression in primary effector CD8+ T cells, which has recently been implicated in dictating the ability of CD8+ T cells to become memory cells (46), was not reduced in the absence of CD4+ T cell help. Conversely, CD27 stimulation strongly induced both IL-7R and autocrine IL-2 expression. These data indicate that CD27 stimulation and CD4+ T cell help have some overlapping effects on CD8+ T cell phenotype and function; however, they are not surprisingly incompletely synonymous. Alternatively, other mechanisms for inducing low levels of CD70expression independent of CD4+ T cells, perhaps NK or NKT cells, may be at play during viral infections. Thus, some limited CD70-mediated stimulation available in the absence of CD4+ T cells may allow the development of cells with MPEC characteristics but not the ability to fully differentiate into long-lived memory CD8+ T cells.
The contribution of CD27 stimulation to CD8+ T cell fate-decisions appears to be highly associated with the extent of inflammation associated with the type of immunization. We identified IL-12 as the critical mediator that regulates memory CD8+ T cell loss in the absence of CD27 stimulation or CD4+ T cell help during the primary CD8+ T cell response. IL-12 has been described as a strong promoter of CD8+ T cell expansion, and promotes differentiation into KLRG1-expressing effector cells after LCMV (7,47), Listeria monocytogenes(48) and Toxoplasma gondii infection (10). Further, it has been suggested that IL-12 may have a detrimental outcome on memory CD8+ T cell differentiation (10,45), although it is unclear whether this is due to forced differentiation into KLRG1-expressing terminally differentiated cells, or activation-induced death of memory precursors. The studies presented here demonstrate that in the absence of balancing stimulation by CD27, IL-12 can result in the failure to generate long-lived CD8+ T cell memory. We found that CD8+ T cells that expand in the absence of helper CD4+ T cells over-express IL-12Rβ1 compared to controls, while those that received supplemental CD27 stimulation had normal levels of IL-12Rβ1 expression. This suggests that CD27 stimulation and CD4+ T cell help regulates the extent to which expanding CD8+ T cells are responsive to IL-12. IL-12Rβ expression on CD4+ T cells is in part controlled by a positive feedback loop activated by IFNγ mediated induction of T-bet (49) and IL-2(50), but less is known about its regulation on CD8+ T cells. The reduced expression of IL-12R on CD8+ T cells after CD27 stimulation is an unexpected result given that stimulation of CD27 has been reported to enhance IL-12Rβ expression on human CD4+ T cells(51), but provides a rationale for how CD27 engagement regulates the sensitivity of CD8+ T cells to IL-12 mediated differentiation. Pertaining to this, we found that Eomesexpression, but not T-bet expression, correlated with CD4+ T cell help and CD27 stimulation. This is consistent with the notion that T-bet is initially induced by IFNγ, rather than IL-12, and suggests that Eomes is either a novel downstream target of CD27 signaling, or that the reduction in IL-12R expression abrogates IL-12-mediated suppression of Eomes(52). In either case, the data presented here argue that the importance of CD27 stimulation in promoting CD8+ T cell memory can be amplified by the extent of accompanying inflammatory cytokines. Interestingly, the contribution of CD27 stimulation for CD8+ T cell memory was first noted in studies using influenza virus (30,31), yet a recent study utilizing a different strain of influenza found no role for CD27 stimulation in CD8+ T cell memory (53). This, together with DC immunization data presented here, indicates that the requirement for CD27 and potentially other TNF-superfamily members such as 4-1BB (CD137) (54) for CD8+ T cell memory development may be exacerbated by the inflammatory context present during the initial expansion of the primary CD8+ T cell response.
We found that CD27 stimulation strongly influenced the frequency and number of IL-7R-expressing primary CD8+ T cells, which are putative memory precursors. To date, the control of IL-7R expression has generally been attributed to either TCR engagement or IL-7 binding. Currently we do not know whether the change in frequency of IL-7R-expressing cells induced by CD27 stimulation is a consequence of augmentedproliferation, differentiation or survival, and/or by direct regulation of IL-7R expression. However, CD27-stimulation profoundly up-regulated the expression of IL-7R expression in differentiated KLRG1-expressing cells. Further, the increase in IL-7R expressing OT-1 obtained after CD27 stimulation occurred without a concomitant increase in total OT-1 numbers. Together, these dataargue that CD27 stimulation controls IL-7R expression, rather than supporting the survival of IL-7R-expressing cells. IL-7R expression is controlled by the opposing actions of the transcription factors GABPα1 and Gfi-1 (55), and Foxo1 and Foxp1 (56,57), and potentially ETS-1(58), suggesting a role for CD27-signaling in the mobilization of these factors. Future studies will elucidate whether there is a transcriptional cassette that is elicited by CD27-stimulation that directly accounts for IL-7R expression, or whether CD27-stimulation impacts on the ability of other cytokines (such as IL-12) to induce epigenetic silencing or transcriptional repression of IL-7R. Further, as both IL-7R-stimulation and CD27-stimulation induce anti-apoptotic molecules, it is interesting to speculate whether CD27-stimulation operates through IL-7(59), and whether IL-7R-expressing KLRG1+ cells can survive to become memory CD8+ T cells.
Together the data present here define a mechanism by which CD70-CD27 stimulation regulates CD8+ T cell memory, and provide a link between CD4+ T cell-mediated licensing of DC and CD8+ T cell memory. Importantly, these data argue that targeting CD70-expression, or CD27-stimulation, will provide a mechanism for generating long-term CD8+ T cell memory in the absence of CD4+ T cell help. This has significant ramifications in the design of vaccines individuals with degraded CD4+T cell populations. Further, the capability of CD27 stimulation to induce IL-7R expression may provide opportunities to augment IL-7-based immunotherapies for cancer and chronic viral infections (60,61).. Further, these data indicate that vaccine adjuvants, such as TLR agonists, that elicit high or sustained levels of IL-12 in the absence of concomitant induction of CD70 expression will possibly lead to poor memory populations. Thus, for vaccines based upon minimal MHC class I restricted peptides, combination of TLR agonists with CD40 stimulation to induce CD70 expression, or the inclusion of peptide epitopes that elicit CD4+ T cell responses are likely to be significantly more effective at generating CD8+ T cell memory.
Supplementary Material
Acknowledgements
We would like to thank Ms Alison Robbins for her technical assistance; the Beirne B. Carter Center for Immunology Research for use of flow cytometry equipment; Drs Steven Schoenberger and JannieBorst for providing CD27−/− mice; Dr Vic Engelhard for MHC tetramers; Dr Mike Brown for critical feedback on the manuscript.
These studies were supported by National Cancer Institute (CA115882), the Cancer Research Institute/Libby Bartnick Memorial Investigator Award and the University of Virginia Cancer Center Support Grant P30 CA44579 (all to TNJB).
Abbreviations used
- BMDC
bone marrow dendritic cells
- MPEC
memory precursor effector cells
- SLEC
short lived effector cells
- cIg
control immunoglobulin
- LN
lymph node
- OVA
chicken egg ovalbumin
- OVA-vac
recombinant vaccinia expressing OVA
- OVA-adeno
recombinant adenovirus expressing OVA
Footnotes
Author contributions: H.D. designed experiments, performed experiments, and analyzed data. N.F. and D.R. performed experiments and analyzed data. H.Y. and M.G. provided critical reagents. T.N.J.B. designed experiments and wrote the manuscript.
Reference List
- 1.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 2.Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, Longworth SA, Vinup KE, Mrass P, Oliaro J, Killeen N, Orange JS, Russell SM, Weninger W, Reiner SL. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 3.Stemberger C, Huster KM, Koffler M, Anderl F, Schiemann M, Wagner H, Busch DH. A single naive CD8+ T cell precursor can develop into diverse effector and memory subsets. Immunity. 2007;27:985–997. doi: 10.1016/j.immuni.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Bannard O, Kraman M, Fearon DT. Secondary replicative function of CD8+ T cells that had developed an effector phenotype. Science. 2009;323:505–509. doi: 10.1126/science.1166831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huster KM, Busch V, Schiemann M, Linkemann K, Kerksiek KM, Wagner H, Busch DH. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc. Natl. Acad. Sci. U. S. A. 2004;101:5610–5615. doi: 10.1073/pnas.0308054101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 7.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: intelligent design. Nat. Rev. Immunol. 2007;7:144–154. doi: 10.1038/nri2023. [DOI] [PubMed] [Google Scholar]
- 9.Pham NL, Badovinac VP, Harty JT. A default pathway of memory CD8 T cell differentiation after dendritic cell immunization is deflected by encounter with inflammatory cytokines during antigen-driven proliferation. J. Immunol. 2009;183:2337–2348. doi: 10.4049/jimmunol.0901203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson DC, Matthews S, Yap GS. IL-12 signaling drives CD8+ T cell IFN-gamma production and differentiation of KLRG1+ effector subpopulations during Toxoplasma gondii Infection. J. Immunol. 2008;180:5935–5945. doi: 10.4049/jimmunol.180.9.5935. [DOI] [PubMed] [Google Scholar]
- 11.Rutishauser RL, Martins GA, Kalachikov S, Chandele A, Parish IA, Meffre E, Jacob J, Calame K, Kaech SM. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kallies A, Xin A, Belz GT, Nutt SL. Blimp-1 transcription factor is required for the differentiation of effector CD8(+) T cells and memory responses. Immunity. 2009;31:283–295. doi: 10.1016/j.immuni.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 13.Intlekofer AM, Takemoto N, Kao C, Banerjee A, Schambach F, Northrop JK, Shen H, Wherry EJ, Reiner SL. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J. Exp. Med. 2007;204:2015–2021. doi: 10.1084/jem.20070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 16.Janssen EM, Lemmens EE, Wolfe T, Christen U, Von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 17.Khanolkar A, Badovinac VP, Harty JT. CD8 T cell memory development: CD4 T cell help is appreciated. Immunol. Res. 2007;39:94–104. doi: 10.1007/s12026-007-0081-4. [DOI] [PubMed] [Google Scholar]
- 18.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yi JS, Ingram JT, Zajac AJ. IL-21 deficiency influences CD8 T cell quality and recall responses following an acute viral infection. J. Immunol. 2010;185:4835–4845. doi: 10.4049/jimmunol.1001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Novy P, Huang X, Leonard WJ, Yang Y. Intrinsic IL-21 signaling is critical for CD8 T cell survival and memory formation in response to vaccinia viral infection. J. Immunol. 2011;186:2729–2738. doi: 10.4049/jimmunol.1003009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002;297:2060–2063. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy R, Celis E. T helper lymphocytes rescue CTL from activation-induced cell death. J. Immunol. 2006;177:2862–2872. doi: 10.4049/jimmunol.177.5.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schoenberger SP. T cell memory. Semin. Immunol. 2009;21:51–52. doi: 10.1016/j.smim.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahonen CL, Doxsee CL, McGurran SM, Riter TR, Wade WF, Barth RJ, Vasilakos JP, Noelle RJ, Kedl RM. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J. Exp. Med. 2004;199:775–784. doi: 10.1084/jem.20031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bullock TN, Yagita H. Induction of CD70 on dendritic cells through CD40 or TLR stimulation contributes to the development of CD8+ T cell responses in the absence of CD4+ T cells. J. Immunol. 2005;174:710–717. doi: 10.4049/jimmunol.174.2.710. [DOI] [PubMed] [Google Scholar]
- 26.Taraban VY, Rowley TF, Al Shamkhani A. Cutting edge: a critical role for CD70 in CD8 T cell priming by CD40-licensed APCs. J. Immunol. 2004;173:6542–6546. doi: 10.4049/jimmunol.173.11.6542. [DOI] [PubMed] [Google Scholar]
- 27.Sanchez PJ, McWilliams JA, Haluszczak C, Yagita H, Kedl RM. Combined TLR/CD40 stimulation mediates potent cellular immunity by regulating dendritic cell expression of CD70 in vivo. J. Immunol. 2007;178:1564–1572. doi: 10.4049/jimmunol.178.3.1564. [DOI] [PubMed] [Google Scholar]
- 28.Nolte MA, van Olffen RW, van Gisbergen KP, Van Lier RA. Timing and tuning of CD27-CD70 interactions: the impact of signal strength in setting the balance between adaptive responses and immunopathology. Immunol. Rev. 2009;229:216–231. doi: 10.1111/j.1600-065X.2009.00774.x. [DOI] [PubMed] [Google Scholar]
- 29.Taraban VY, Rowley TF, Tough DF, Al Shamkhani A. Requirement for CD70 in CD4+ Th Cell-Dependent and Innate Receptor-Mediated CD8+ T Cell Priming. J. Immunol. 2006;177:2969–2975. doi: 10.4049/jimmunol.177.5.2969. [DOI] [PubMed] [Google Scholar]
- 30.Dolfi DV, Boesteanu AC, Petrovas C, Xia D, Butz EA, Katsikis PD. Late signals from CD27 prevent Fas-dependent apoptosis of primary CD8+ T cells. J. Immunol. 2008;180:2912–2921. doi: 10.4049/jimmunol.180.5.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hendriks J, Gravestein LA, Tesselaar K, Van Lier RA, Schumacher TN, Borst J. CD27 is required for generation and long-term maintenance of T cell immunity. Nat. Immunol. 2000;1:433–440. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 32.Roberts DJ, Franklin NA, Kingeter LM, Yagita H, Tutt AL, Glennie MJ, Bullock TN. Control of established melanoma by CD27 stimulation is associated with enhanced effector function and persistence, and reduced PD-1 expression of tumor infiltrating CD8(+) T cells. J. Immunother. 2010;33:769–779. doi: 10.1097/CJI.0b013e3181ee238f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oshima H, Nakano H, Nohara C, Kobata T, Nakajima A, Jenkins NA, Gilbert DJ, Copeland NG, Muto T, Yagita H, Okumura K. Characterization of murine CD70 by molecular cloning and mAb. Int. Immunol. 1998;10:517–526. doi: 10.1093/intimm/10.4.517. [DOI] [PubMed] [Google Scholar]
- 34.Bullock TNJ, Colella TA, Engelhard VH. The density of peptides displayed by dendritic cells affects immune responses to human tyrosinase and gp100 in HLA-A2 transgenic mice. J. Immunol. 2000;164:2354–2361. doi: 10.4049/jimmunol.164.5.2354. [DOI] [PubMed] [Google Scholar]
- 35.Novy P, Quigley M, Huang X, Yang Y. CD4 T cells are required for CD8 T cell survival during both primary and memory recall responses. J. Immunol. 2007;179:8243–8251. doi: 10.4049/jimmunol.179.12.8243. [DOI] [PubMed] [Google Scholar]
- 36.Fuse S, Tsai CY, Molloy MJ, Allie SR, Zhang W, Yagita H, Usherwood EJ. Recall responses by helpless memory CD8+ T cells are restricted by the up-regulation of PD-1. J. Immunol. 2009;182:4244–4254. doi: 10.4049/jimmunol.0802041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Obar JJ, Lefrancois L. Memory CD8+ T cell differentiation. Ann. NY Acad. Sci. 2010;1183:251–266. doi: 10.1111/j.1749-6632.2009.05126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitchell DM, Ravkov EV, Williams MA. Distinct roles for IL-2 and IL-15 in the differentiation and survival of CD8+ effector and memory T cells. J. Immunol. 2010;184:6719–6730. doi: 10.4049/jimmunol.0904089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hand TW, Morre M, Kaech SM. Expression of IL-7 receptor alpha is necessary but not sufficient for the formation of memory CD8 T cells during viral infection. Proc. Natl. Acad. Sci. U. S. A. 2007;104:11730–11735. doi: 10.1073/pnas.0705007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun JC, Lehar SM, Bevan MJ. Augmented IL-7 signaling during viral infection drives greater expansion of effector T cells but does not enhance memory. J. Immunol. 2006;177:4458–4463. doi: 10.4049/jimmunol.177.7.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peperzak V, Xiao Y, Veraar EA, Borst J. CD27 sustains survival of CTLs in virus-infected nonlymphoid tissue in mice by inducing autocrine IL-2 production. J. Clin. Invest. 2010;120:168–178. doi: 10.1172/JCI40178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity. 2010;32:91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 43.Obar JJ, Molloy MJ, Jellison ER, Stoklasek TA, Zhang W, Usherwood EJ, Lefrancois L. CD4+ T cell regulation of CD25 expression controls development of short-lived effector CD8+ T cells in primary and secondary responses. Proc. Natl. Acad. Sci. U. S. A. 2010;107:193–198. doi: 10.1073/pnas.0909945107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valenzuela J, Schmidt C, Mescher M. The roles of IL-12 in providing a third signal for clonal expansion of naive CD8 T cells. J. Immunol. 2002;169:6842–6849. doi: 10.4049/jimmunol.169.12.6842. [DOI] [PubMed] [Google Scholar]
- 45.Pearce EL, Shen H. Generation of CD8 T cell memory is regulated by IL-12. J. Immunol. 2007;179:2074–2081. doi: 10.4049/jimmunol.179.4.2074. [DOI] [PubMed] [Google Scholar]
- 46.Feau S, Arens R, Togher S, Schoenberger SP. Autocrine IL-2 is required for secondary population expansion of CD8(+) memory T cells. Nat. Immunol. 2011;12:908–913. doi: 10.1038/ni.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cui W, Joshi NS, Jiang A, Kaech SM. Effects of Signal 3 during CD8 T cell priming: Bystander production of IL-12 enhances effector T cell expansion but promotes terminal differentiation. Vaccine. 2009;27:2177–2187. doi: 10.1016/j.vaccine.2009.01.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keppler SJ, Theil K, Vucikuja S, Aichele P. Effector T-cell differentiation during viral and bacterial infections: Role of direct IL-12 signals for cell fate decision of CD8(+) T cells. Eur. J. Immunol. 2009;39:1774–1783. doi: 10.1002/eji.200839093. [DOI] [PubMed] [Google Scholar]
- 49.Afkarian M, Sedy JR, Yang J, Jacobson NG, Cereb N, Yang SY, Murphy TL, Murphy KM. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat. Immunol. 2002;3:549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 50.Liao W, Lin JX, Leonard WJ. IL-2 family cytokines: new insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr. Opin. Immunol. 2011;23:598–604. doi: 10.1016/j.coi.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Oosterwijk MF, Juwana H, Arens R, Tesselaar K, van Oers MH, Eldering E, Van Lier RA. CD27-CD70 interactions sensitise naive CD4+ T cells for IL-12-induced Th1 cell development. Int. Immunol. 2007;19:713–718. doi: 10.1093/intimm/dxm033. [DOI] [PubMed] [Google Scholar]
- 52.Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, Reiner SL. Cutting Edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J. Immunol. 2006;177:7515–7519. doi: 10.4049/jimmunol.177.11.7515. [DOI] [PubMed] [Google Scholar]
- 53.van Gisbergen KP, Klarenbeek PL, Kragten NA, Unger PP, Nieuwenhuis MB, Wensveen FM, ten BA, Tak PP, Eldering E, Nolte MA, Van Lier RA. The costimulatory molecule CD27 maintains clonally diverse CD8(+) T cell responses of low antigen affinity to protect against viral variants. Immunity. 2011;35:97–108. doi: 10.1016/j.immuni.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 54.Fuse S, Bellfy S, Yagita H, Usherwood EJ. CD8+ T cell dysfunction and increase in murine gammaherpesvirus latent viral burden in the absence of 4-1BB ligand. J. Immunol. 2007;178:5227–5236. doi: 10.4049/jimmunol.178.8.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chandele A, Joshi NS, Zhu J, Paul WE, Leonard WJ, Kaech SM. Formation of IL-7 Ralphahigh and IL-7Ralphalow CD8 T cells during infection is regulated by the opposing functions of GABPalpha and Gfi-1. J. Immunol. 2008;180:5309–5319. doi: 10.4049/jimmunol.180.8.5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kerdiles YM, Beisner DR, Tinoco R, Dejean AS, Castrillon DH, DePinho RA, Hedrick SM. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat. Immunol. 2009;10:176–184. doi: 10.1038/ni.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feng X, Wang H, Takata H, Day TJ, Willen J, Hu H. Transcription factor Foxp1 exerts essential cell-intrinsic regulation of the quiescence of naive T cells. Nat. Immunol. 2011;12:544–550. doi: 10.1038/ni.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grenningloh R, Tai TS, Frahm N, Hongo TC, Chicoine AT, Brander C, Kaufmann DE, Ho IC. Ets-1 maintains IL-7 receptor expression in peripheral T cells. J. Immunol. 2011;186:969–976. doi: 10.4049/jimmunol.1002099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carr JM, Carrasco MJ, Thaventhiran JE, Bambrough PJ, Kraman M, Edwards AD, Al-Shamkhani A, Fearon DT. CD27 mediates interleukin-2-independent clonal expansion of the CD8+ T cell without effector differentiation. Proc. Natl. Acad. Sci. U. S. A. 2006;103:19454–19459. doi: 10.1073/pnas.0609706104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pellegrini M, Calzascia T, Elford AR, Shahinian A, Lin AE, Dissanayake D, Dhanji S, Nguyen LT, Gronski MA, Morre M, Assouline B, Lahl K, Sparwasser T, Ohashi PS, Mak TW. Adjuvant IL-7 antagonizes multiple cellular and molecular inhibitory networks to enhance immunotherapies. Nat. Med. 2009;15:528–536. doi: 10.1038/nm.1953. [DOI] [PubMed] [Google Scholar]
- 61.Pellegrini M, Calzascia T, Toe JG, Preston SP, Lin AE, Elford AR, Shahinian A, Lang PA, Lang KS, Morre M, Assouline B, Lahl K, Sparwasser T, Tedder TF, Paik JH, DePinho RA, Basta S, Ohashi PS, Mak TW. IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell. 2011;144:601–613. doi: 10.1016/j.cell.2011.01.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.