Abstract
Inhibition of sirtuin 2 (SIRT2) is known to be protective against the toxicity of disease proteins in Parkinson’s and Huntington’s models of neurodegeneration. Previously, we developed SIRT2 inhibitors based on the 3-(N-arylsulfamoyl)benzamide scaffold, including 3-(N-(4-bromophenyl)sulfamoyl)-N-(4-bromophenyl)benzamide (C2-8, 1a), which demonstrated neuroprotective effects in a Huntington’s mouse model, but had low potency of SIRT2 inhibition. Here we report that N-methylation of 1a greatly increases its potency and results in excellent selectivity for SIRT2 over SIRT1 and SIRT3 isoforms. Structure-activity relationships observed for 1a analogs and docking simulation data suggest that the para-substituted amido moiety of these compounds could occupy two potential hydrophobic binding pockets in SIRT2. These results provide a direction for the design of potent drug-like SIRT2 inhibitors.
Keywords: Sirtuin 2, neurodegenerative disease, 3-(N-arylsulfamoyl)benzamides, sirtuin selectivity, hydrophobic pocket
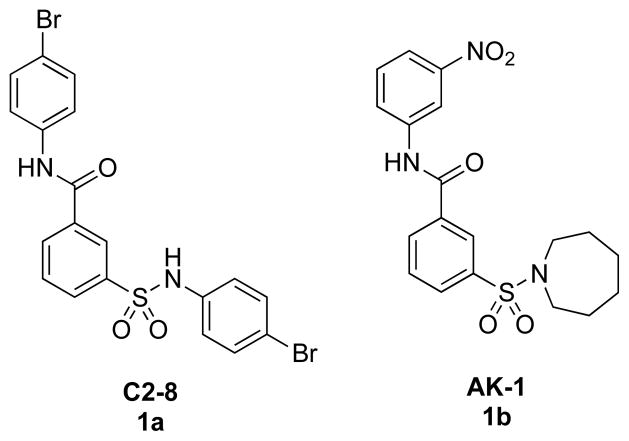
Sirtuin 2 (SIRT2),§ one of seven known human sirtuins, is a NAD+-dependent enzyme that catalyzes the deacetylation of histone Nε-acetyllysines with concomitant formation of nicotinamide and 2′-O-acetyl-ADP-ribose.1 Previous studies showed that inhibition of SIRT2 mediated neuroprotective effects in Parkinson’s disease (PD) and Huntington’s disease (HD) models.2,3,4 In in vitro models of PD and HD, neuroprotective effects of SIRT2 inhibition have been associated with changes in aggregation of the α-synuclein and huntingtin proteins, respectively. A previously identified inhibitor of polyglutamine aggregation, a hallmark of many neurodegenerative diseases,5 namely C2-8 (1a, Figure 1), has potential as a therapeutic candidate based on its neuroprotective effects achieved in transgenic HD models and, apparently, good drug-like properties.6 AK-1 (1b), which has a common 3-sulfobenzamide scaffold to that of 1a and is neuroprotective against α-synuclein toxicity,2 is a SIRT2 inhibitor that was reported to have good selectivity for SIRT2 over SIRT1 and SIRT3.2 Most known SIRT2 inhibitors show low selectivities for SIRT1 and SIRT3, even though their potencies are better than that of 1b.7,8,9,10,11 Compound 1a, however, displayed low potency as a SIRT2 inhibitor. Here we test the feasibility of enhancing SIRT2 inhibition and selectivity of the therapeutically promising structural scaffold 1a. We report the discovery of more potent and highly selective SIRT2 inhibitors and describe related structure-activity relationship (SAR) studies.
Figure 1.
Structures of polyglutamine aggregation inhibitor C2-8 (1a) and SIRT2 inhibitor AK-1 (1b)
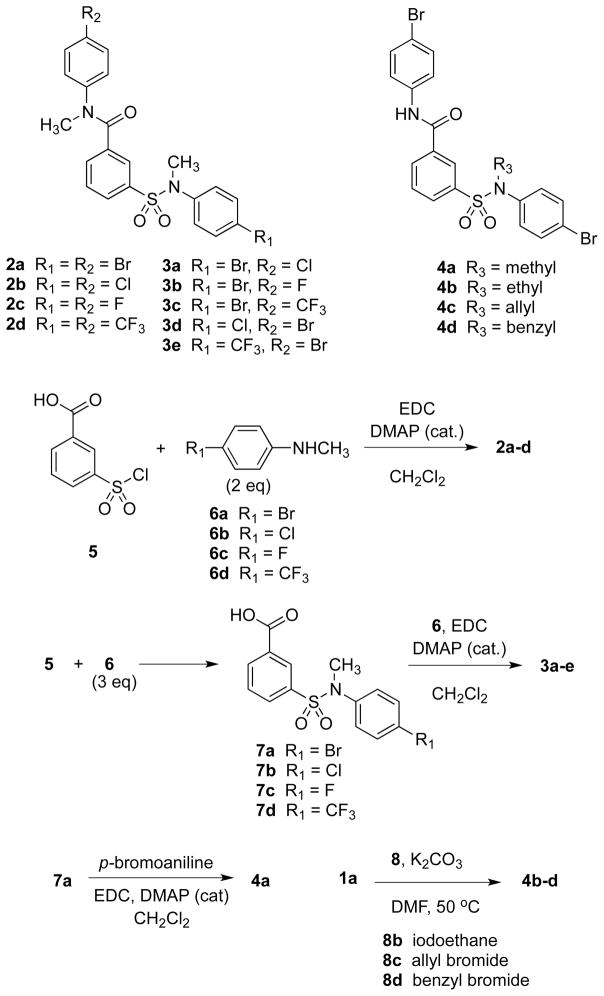
Scheme 1 shows the structures of and related synthetic routes to analogs of 1a. Compounds 2a–2d were prepared from 3-(chlorosulfonyl)benzoic acid (5) and the corresponding para-substituted anilines 6 in one step. Compounds 3a–e and 4a were prepared from 5 and 6 in two steps by consecutive amide coupling reactions. Compounds 4b–4d were synthesized from 1a in one step by selective N-alkylation using potassium carbonate and the corresponding alkyl halide at 50 °C.
Scheme 1.
Syntheses of 2–4
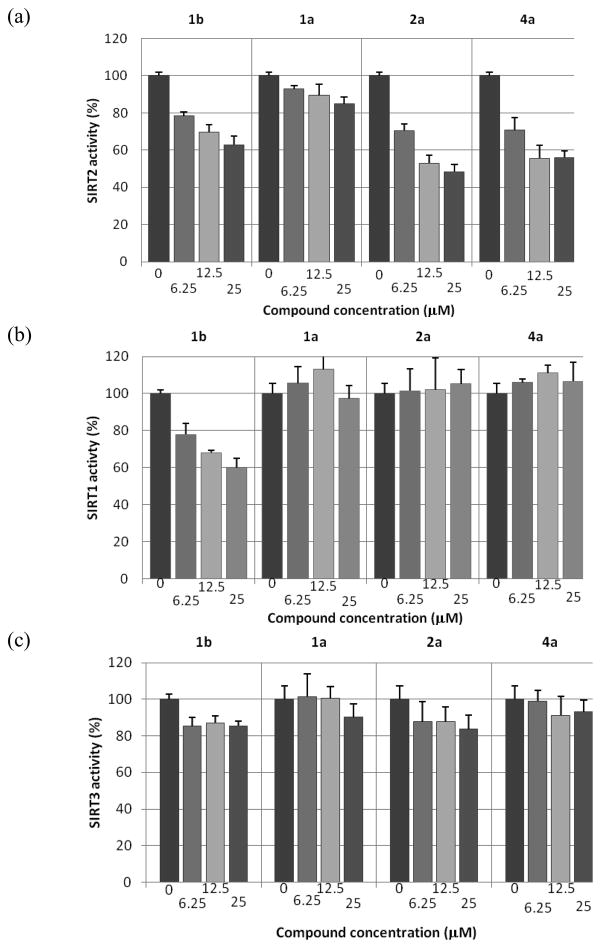
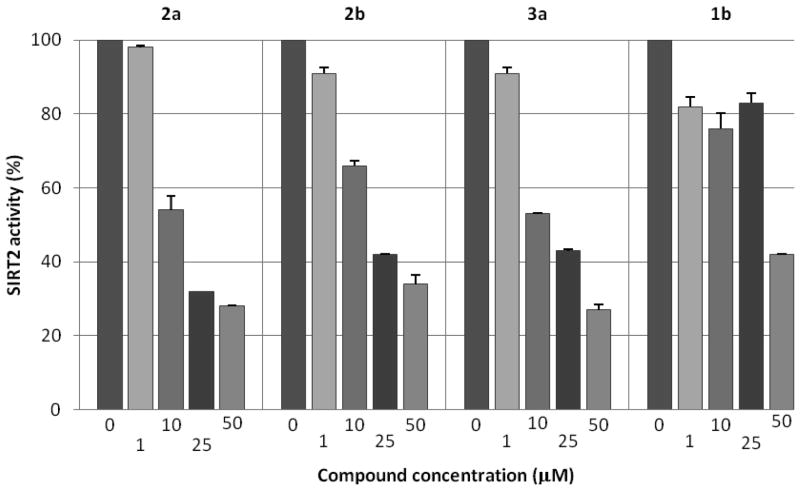
Figure 2 shows in vitro SIRT1, SIRT2, and SIRT3 inhibition assay results for 1a, 1b, and two N- methylsufonamide analogs, 2a and 4a. Compound 1a is a weaker SIRT2 inhibitor than 1b, as reported previously.4 The potencies of 2a and 4a with SIRT2, however, are very similar and are slightly better than that of 1b. In addition, 2a and 4a are more selective SIRT2 inhibitors than 1b; 2a and 4a are virtually inactive with SIRT1 and SIRT3 up to 50 μM, while 1b shows some inhibitory activity with SIRT1. These results suggest that simple modifications of 1a (methylation) can enhance both potency and selectivity toward SIRT2. A subsequent SAR study, changing the para substituents (2) or the N-alkyl substituent (4), identified 2b as a more potent SIRT2 inhibitor (see Supporting Information Figure S1). Compound 2b was selective for SIRT2; at 10 μM concentration 2b did not inhibit SIRT1 and inhibited SIRT3 by only 5% (see Supporting Information Figure S2).
Figure 2.
Compound inhibition activities in in vitro sirtuin-catalyzed lysine deacetylation assays. Potency and selectivity of 1b, 1a, 2a, and 4a have been evaluated in dose-response assays against deacetylase activities of SIRT2, SIRT1, and SIRT3 at indicated concentrations. Each dose has been tested in triplicate. Compound 1b was included as a reference compound.
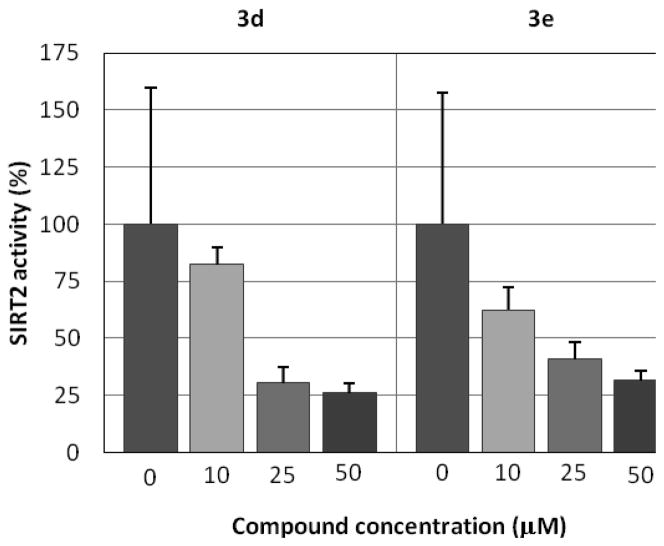
Further modification of the para-substituents (3a–e, Table 1) shows that two compounds, 3a and 3e, inhibit the SIRT2 activity by about half at 10 μM concentration. Compounds 3a and 3e were highly selective; there was no inhibition of SIRT1 or SIRT3 at 10 μM concentration. However, a comparison of the dose-response activities of 3d and 3e (Figure 3) indicated that 3e was not as potent as initially observed. On the basis of these preliminary assay results, three compounds, 2a, 2b, and 3a, were retested in comparison with 1b. Figure 4 shows that all of these analogs of 1a are more potent than 1b. It should be noted that experiments represented by Figures 2 and 4 were carried out months apart, and the values differ somewhat. Compound 1b was routinely included in assays for normalization of results.
Table 1.
In vitro SIRT2 inhibition assay results for 3a–3e
| Compound | Relative SIRT2 activity (%)a | Concentration of compounds (μM) |
|---|---|---|
| 3a | 54 | 10 |
| 3b | 57 | 50 |
| 3c | 76 | 50 |
| 3d | 72 | 10 |
| 3e | 55 | 10 |
Measured by the relative fluorescence observed from the SIRT2 assay
Figure 3.
Comparison of SIRT2 dose-response activities for 3d and 3e
Figure 4.
SIRT2 inhibition by three analogs of 1a compared with that of 1b
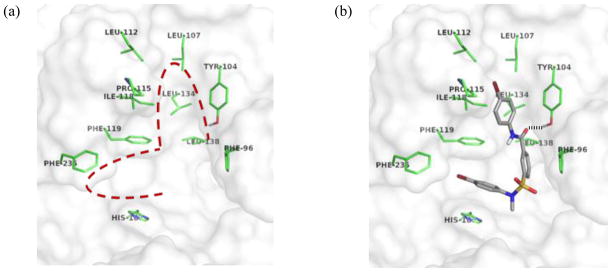
To date, the only available crystal structure of SIRT2 does not contain any ligand molecule bound;1 it is likely that the uncomplexed SIRT2 structure is different from that of a ligand-bound conformation. A few computational methods have been reported to find the active conformation of SIRT2, including those that use energy minimization and/or molecular dynamics simulations9,12,13,14 and a homology model constructed from the SIRT2 structure and other homolog-ligand complex structures.2,15 The SIRT2 structure without any modification has been used in a few cases.10,16 We carried out docking simulations with the original crystal structure of SIRT2.1 Although quantitative assessment of binding interactions using a binding score would not be reliable, we assumed that at least a qualitative evaluation of binding conformations of analogs of 1a with SIRT2 could be garnered. Figure 5(a) shows a putative ligand-binding site in SIRT2, proposed previously by a comparison with the crystal structures of other sirtuin homolog-ligand complexes. 17, 18, 19 There are two potential hydrophobic binding pockets in the active site. A small cleft between Phe119 and His187 would be a good hydrophobic binding pocket for an aromatic ring, which could be stabilized by π-π interactions with the phenyl group of Phe119 and the imidazole ring of His187. This channel has been proposed as the binding site for the side chain of the acetylated lysine residue of a substrate. There is another potential hydrophobic binding pocket surrounded by residues with hydrophobic side chains Phe96, Tyr104, Leu107, Leu112, Pro115, Ile118, Phe119, Leu134, and Leu138. Docking simulation results predict that the two para-susbstituted anilino moieties of analogs of 1a occupy the two potential hydrophobic pockets. Figure 5(b) shows a potential binding conformation for 4a. The two p-bromophenyl groups are bound into the two hydrophobic pockets of SIRT2. Additionally, there is a hydrogen bond between the carbonyl group of 4a and the hydroxyl group of Tyr104. Other active analogs of 1a adopted very similar conformations in docking simulations.
Figure 5.
(a) Putative binding site of SIRT2; hydrophobic pockets are surrounded by a red dotted line. (b) Binding conformation of 4a predicted by a docking simulation with a potential H-bond shown
The inhibitory assay data for 2a–2d and 3a–3e suggested that the potency might be correlated with the size of the two para-substituents, R1 and R2 (Scheme 1), both of which contribute to the hydrophobic interactions in the purported hydrophobic pockets. It is reasonable that there would be an optimal size for R1 or R2 that is dependent on the size of a hydrophobic binding pocket to maximize hydrophobic contact. Among the four compounds with the same R1 group (R1 = Br), 2a (R2 = Br), and 3a (R2 = Cl) showed comparable activities that were much higher than those of 3b (R2 = F) and 3c (R2 = CF3). The order of van der Waals volumes for the four R1 substituents is CF3 > Br > Cl > F. It is therefore likely that the maximum hydrophobic contact might be achieved with an R2 group having a van der Waals volume between Cl and Br. By the same analogy, the activities of the three compounds with the same R2 group (R2 = Br) can be compared to derive the optimal size for the R1 group. The activity of 2a (R1 = Br) is greater than those of 3d (R1 = Cl) and 3e (R1 = CF3), suggesting that the size of the hydrophobic binding pocket for the R1 group might be similar to that of the R2-binding pocket.
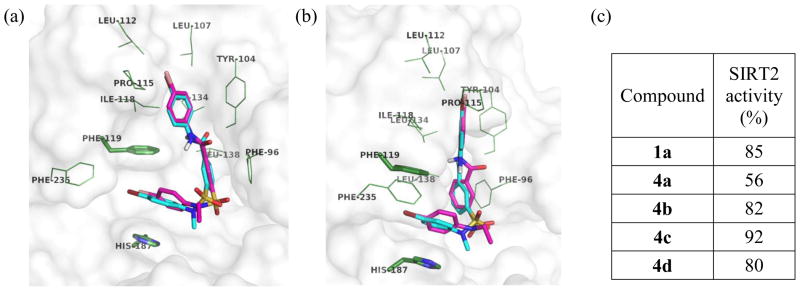
Five analogs of 1a, including 1a and 4a–4d, contain the same R1 and R2 groups (R1 = R2 = Br) and are structurally different only by the R3 substituent. Among these five compounds, only 4a (R3 = Me) showed significant activity against SIRT2, indicating that the N-methylsulfonamide moiety is crucial to the SIRT2 activity. Considering that the docked conformation of 1a is very similar to that of 4a, the increased potency of 4a over 1a could be attributed to the additional van der Waals contact between the N-methylsulfonamide moiety of 4a and SIRT2. However, this one additional hydrophobic interaction should not be sufficient to explain the much greater potency of 4a. One possible explanation is that the N-methyl substituent behaves as an anchor to direct the adjacent para-bromoanilino group close to the channel between Phe119 and His187, resulting in more favorable hydrophobic interactions.
Figure 6 shows two views of an overlay of the binding conformations of 4a and 4b. Although 4b differs from 4a by only one methylene unit, the N-ethylsulfonamide moiety of 4b causes steric hindrance with SIRT2 and distorts the orientation of the adjacent p-bromoanilino group. The view in Figure 6(b) clearly shows that the p-bromophenyl ring at the sulfonamide moiety of 4b is twisted out of plane for optimal π-π interactions with Phe119 and His187, while the corresponding p-bromophenyl ring of 4a is aligned parallel to Phe119 and His187. The R3 groups of 4c and 4d would cause even larger steric hindrance with SIRT2, rationalizing their lower potencies. In contrast to the N-substituent of the sulfonamide moiety, the N-substituent of the amide moiety does not seem to affect the SIRT2 activity significantly; 2a and 4a had comparable activities. The binding conformation of 4a in Figure 6(b) shows that the proton of the amide moiety is exposed to solvent, suggesting that no significant binding interaction is contributed by the N-methylamide moiety of 2a.
Figure 6.
(a, b) Overlay of binding conformations of 4a (cyan) and 4b (magenta) from different views (c) Relative SIRT2 activity from treatment with 1a and 4a–4d at 25 μM
We have demonstrated that 1a could serve as a lead scaffold for inhibitors of SIRT2. The N-methylsulfonamide moiety of analogs of 1a increases both SIRT2 activity and selectivity, both of which are higher than the known SIRT2 inhibitor 1b. The observed structure-activity relationships with various R1 and R2 groups are consistent with the binding conformation of analogs of 1a predicted by docking simulations. Both terminal aniline moieties might occupy the two potential hydrophobic binding pockets having strict size requirements. These observed SARs should be valuable for structure-based design of more potent SIRT2 inhibitors.
Supplementary Material
Acknowledgments
The authors are grateful to the National Institutes of Health (5 U01 NS066912) for financial support of this research.
Footnotes
Abbreviations: SIRT, sirtuin; PD, Parkinson’s disease; HD, Huntington’s disease; SAR, structure-activity relationship
Experimental procedures, in vitro SIRT2 inhibition data, NMR spectra, and HRMS data.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Finnin MS, Donigian JR, Pavletich NP. Nat Struct Biol. 2001;8:621. doi: 10.1038/89668. [DOI] [PubMed] [Google Scholar]
- 2.Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, Amore AM, Volk CB, Maxwell MM, Rochet JC, McLean PJ, Young AB, Abagyan R, Feany MB, Hyman BT, Kazantsev AG. Science. 2007;317:516. doi: 10.1126/science.1143780. [DOI] [PubMed] [Google Scholar]
- 3.Luthi-Carter R, Taylor DM, Pallos J, Lambert E, Amore A, Parker A, Moffitt H, Smith DL, Runne H, Gokce O, Kuhn A, Xiang Z, Maxwell MM, Reeves SA, Bates GP, Neri C, Thompson LM, Marsh JL, Kazantsev AG. Proc Nat Acad Sci USA. 2010;107:7927. doi: 10.1073/pnas.1002924107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor DM, Balabadra U, Xiang Z, Woodman B, Meade S, Amore A, Maxwell MM, Reeves S, Bates GP, Luthi-Carter R, Lowden PA, Kazantsev AG. ACS Chem Biol. 2011;6:540. doi: 10.1021/cb100376q. [DOI] [PubMed] [Google Scholar]
- 5.Riley BE, Orr HT. Genes Dev. 2006;20(16):2183. doi: 10.1101/gad.1436506. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, Smith DL, Meriin AB, Engemann S, Russel DE, Roark M, Washington SL, Maxwell MM, Marsh JL, Thompson LM, Wanker EE, Young AB, Housman DE, Bates GP, Sherman MY, Kazantsev AG. Proc Natl Acad Sci USA. 2005;102:892. doi: 10.1073/pnas.0408936102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rotili D, Carafa V, Tarantino D, Botta G, Nebbioso A, Altucci L, Mai A. Bioorg Med Chem. 2011;19:3659. doi: 10.1016/j.bmc.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 8.Huhtiniemi T, Salo HS, Suuronen T, Poso A, Salminen A, Leppänen J, Jarho E, Lahtela-Kakkonen M. J Med Chem. 2011;54:6456. doi: 10.1021/jm200590k. [DOI] [PubMed] [Google Scholar]
- 9.Huber K, Schemies J, Uciechowska U, Wagner JM, Rumpf T, Lewrick F, Süss R, Sippl W, Jung M, Bracher F. J Med Chem. 2010;53:1383. doi: 10.1021/jm901055u. [DOI] [PubMed] [Google Scholar]
- 10.Medda F, Russell RJ, Higgins M, McCarthy AR, Campbell J, Slawin AM, Lane DP, Lain S, Westwood NJ. J Med Chem. 2009;52:2673. doi: 10.1021/jm8014298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trapp J, Jochum A, Meier R, Saunders L, Marshall B, Kunick C, Verdin E, Goekjian P, Sippl W, Jung M. J Med Chem. 2006;49:7307. doi: 10.1021/jm060118b. [DOI] [PubMed] [Google Scholar]
- 12.Neugebauer RC, Uchiechowska U, Meier R, Hruby H, Valkov V, Verdin E, Sippl W, Jung M. J Med Chem. 2008;51:1203. doi: 10.1021/jm700972e. [DOI] [PubMed] [Google Scholar]
- 13.Kiviranta PH, Salo HS, Leppanen J, Rinne VM, Kyrylenko S, Kuusisto E, Suuronen T, Salminen A, Poso A, Lahtela-Kakkonen M, Wallen EA. Bioorg Med Chem. 2008;16:8054. doi: 10.1016/j.bmc.2008.07.059. [DOI] [PubMed] [Google Scholar]
- 14.Tervo AJ, Kyrylenko S, Niskanen P, Salminen A, Leppanen J, Nyronen TH, Jarvinen T, Poso A. J Med Chem. 2004;47:6292. doi: 10.1021/jm049933m. [DOI] [PubMed] [Google Scholar]
- 15.Trapp J, Meier R, Hongwiset D, Kassack MU, Sippl W, Jung M. Chem Med Chem. 2007;2:1419. doi: 10.1002/cmdc.200700003. [DOI] [PubMed] [Google Scholar]
- 16.Kiviranta PH, Leppanen J, Kyrylenko S, Salo HS, Lahtela-Kakkonen M, Tervo AJ, Wittekindt C, Suuronen T, Kuusisto E, Jarvinen T, Salminen A, Poso A, Wallen EA. J Med Chem. 2006;49:7907. doi: 10.1021/jm060566j. [DOI] [PubMed] [Google Scholar]
- 17.Hawse WF, Hoff KG, Fatkins DG, Daines A, Zubkova OV, Schramm VL, Zheng W, Wolberger C. Structure. 2008;16:1368. doi: 10.1016/j.str.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoff KG, Avalos JL, Sens K, Wolberger C. Structure. 2006;14:1231. doi: 10.1016/j.str.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Avalos JL, Bever KM, Wolberger C. Mol Cell. 2005;17:855. doi: 10.1016/j.molcel.2005.02.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.