Abstract
Human Vγ2Vδ2 T cells monitor isoprenoid metabolism by recognizing (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP), an intermediate in the 2-C-methyl-D-erythritol-4-phosphate pathway used by microbes, and isopentenyl pyrophosphate (IPP), an intermediate in the mevalonate pathway used by humans. Aminobisphosphonates and alkylamines indirectly stimulate Vγ2Vδ2 cells by inhibiting farnesyl diphosphate synthase (FDPS) in the mevalonate pathway, thereby increasing IPP/ApppI that directly stimulate. In this study, we further characterize stimulation by these compounds, and define pathways used by new classes of compounds. Consistent with FDPS inhibition, stimulation of Vγ2Vδ2 cells by aminobisphosphonates and alkylamines was much more sensitive to statin inhibition than stimulation by prenyl pyrophosphates. However, the continuous presence of aminobisphosphonates was toxic for T cells, and blocked their proliferation. Aminobisphosphonate stimulation was rapid and prolonged, independent of known antigen presenting molecules, and resistant to fixation. New classes of stimulatory compounds–mevalonate, the alcohol of HMBPP, and alkenyl phosphonates–likely stimulate differently. Mevalonate, a rate-limiting metabolite, appears to enter cells to increase IPP levels whereas the alcohol of HMBPP and alkenyl phosphonates are directly recognized. The critical chemical feature of bisphosphonates is the amino moiety, because its loss switched aminobisphosphonates to direct antigens. Transfection of APC with siRNA downregulating FDPS rendered them stimulatory for Vγ2Vδ2 cells, and increased cellular IPP. siRNAs for isopentenyl diphosphate isomerase functioned similarly. Our results show that a variety of manipulations affecting isoprenoid metabolism lead to stimulation of Vγ2Vδ2 T cells and that pulsing aminobisphosphonates would be more effective for the ex vivo expansion of Vγ2Vδ2 T cells for adoptive cancer immunotherapy.
Keywords: gamma delta T cell, Vgamma2Vdelta2 T cells, human, bisphosphonate, antigen presentation, prenyl pyrophosphates, isopentenyl pyrophosphate, isoprenoid metabolism, farnesyl diphosphate synthase, siRNA
Introduction
Human γδ T cells expressing the Vγ2Vδ2 TCR (also termed Vγ9Vδ2) recognize both exogenous prenyl pyrophosphates (also termed prenyl diphosphates) from bacteria and parasitic protozoa, as well as endogenous prenyl pyrophosphates from the mevalonate pathway (1). This recognition is important for the control of infections (2, 3) and for tumor immunotherapy (4-8). In this sense, γδ T cells function as a bridge between the innate and adaptive immune systems, by monitoring intermediates in isoprenoid metabolism (9).
There have been three major classes of nonpeptide compounds described that stimulate Vγ2Vδ2 T cells: prenyl pyrophosphates (10, 11), aminobisphosphonates (12, 13), and alkylamines (14). Prenyl pyrophosphates and alkylamines are natural antigens that can be produced by bacteria and other human pathogens during infections. Aminobisphosphonates are synthetic compounds that mimic prenyl pyrophosphates and that are used to treat bone diseases such as osteoporosis (15), Paget’s disease (16), and metastatic tumors in bone (17, 18). Like prenyl pyrophosphates and alkylamines, aminobisphosphonates recognition is mediated by the Vγ2Vδ2 TCR (19), requires antigen presentation by species-specific APCs (20), and is enhanced by costimulatory molecules (20, 21). Once stimulated, Vγ2Vδ2 T cells secrete high levels of inflammatory cytokines and chemokines such as IFN-γ and TNF-α (22), and kill tumor cells (4, 23).
Although aminobisphosphonates are structural analogs of prenyl pyrophosphates, the mechanism by which aminobisphosphonates stimulate γδ T cells differs from that of prenyl pyrophosphates. Although the exact molecular mechanisms are unclear, prenyl pyrophosphates are directly presented by APC for Vγ2Vδ2 TCR recognition primarily by germline-encoded regions (24) leading to T cell activation (25). In contrast, aminobisphosphonates appear to stimulate Vγ2Vδ2 T cells through an indirect mechanism by inhibiting farnesyl diphosphate synthase (FDPS) thereby increasing the level of the upstream metabolite, isopentenyl pyrophosphate (IPP) (Fig. 1). In support of this mechanism, increases in HMG-CoA reductase (HMGCR) activity, the rate limiting step in IPP synthesis, increase the stimulatory ability of tumor cells in a manner similar to treatment with aminobisphosphonates (26). Moreover, statins that inhibit the HMGCR enzyme also inhibit aminobisphosphonate and alkylamine stimulation of Vγ2Vδ2 T cells (26-28).
FIGURE 1. Mevalonate pathway and key downstream branches in isoprenoid biosynthesis.
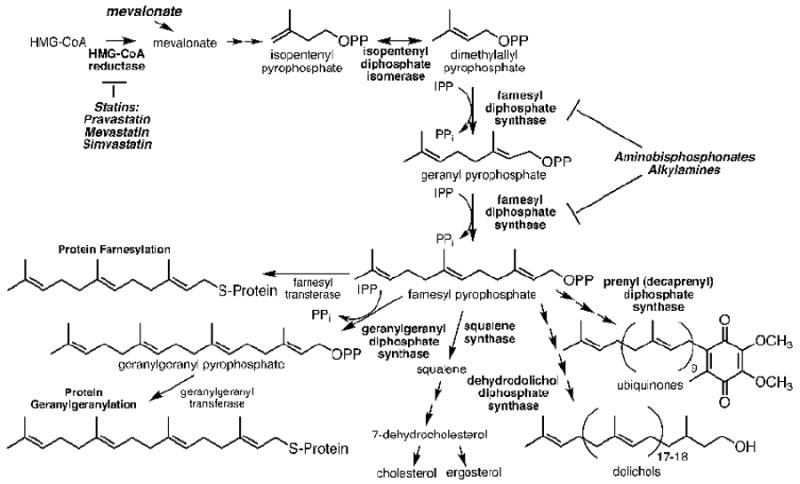
HMG-CoA reductase is the rate-controlling enzyme in the mevalonate pathway and is subject to feedback regulation by downstream products. It is also inhibited by statins. FPP synthase converts IPP and DMAPP to GPP and FPP intermediates and is inhibited by aminobisphosphonates and alkylamines. Loss of FPP and GGPP leads to the loss of membrane anchoring of signaling proteins causing signaling defects and, in some cases, apoptosis.
Although the basic mechanisms for indirect stimulation of Vγ2Vδ2 T cells by aminobisphosphonates and alkylamines have been established, many questions remain. For example: Why are they unable to stimulate proliferative response by Vγ2Vδ2 T cell clones (21)? How quickly do bisphosphonates render APC stimulatory? How specific is statin inhibition of aminobisphosphonate responses, given reports that statins also alter the responses of αβ T cells and other cells of the immune system (29-31)? Moreover, besides direct stimulators such as prenyl pyrophosphates and other ester linked carbon-phosphate analogs (32, 33), how do other novel compounds, such as mevalonate (27), the alcohol of HMBPP (34), alkenyl-pyrophosphonates, and alkyl-bisphosphonates, stimulate Vγ2Vδ2 T cells? And finally, are there enzymes other than FDPS whose inhibition will stimulate Vγ2Vδ2 T cells?
To address these questions, we examined in detail how bisphosphonates and alkylamines, as well as other novel classes of compounds, stimulate Vγ2Vδ2 T cells. We find that stimulation of Vγ2Vδ2 T cells by aminobisphosphonates and alkylamines is more sensitive to statin inhibition than other compounds that either activate directly, or use other indirect pathways, and that there are additional pathways for the stimulation of human Vγ2Vδ2 T cells, through alterations in isoprenoid metabolism.
Materials and Methods
Compounds
Synthesis of bisphosphonates was as described (13, 35, 36). Prior to use, 20 mM stock solutions were prepared by dissolving the compounds in ultra-purified distilled H2O, adjusting the pH to 7.0 as required, and filtering through a 0.22 μm Spin-X mini-filter (Corning Incorporated, Lowell, MA). HMBPP was prepared as described (37). BrHPP was prepared as described (38). Crude mono-ethyl-pyrophosphate was prepared as described (10). ApppI was prepared as described (11). Tetanus toxoid was from the University of Massachusetts Biologic Laboratories, Jamaica Plain, MA.
Derivation and culture of T cell clones
T cell lines and clones were maintained by periodic restimulation with PHA as previously described (39). The derivation of the CD8αα+ T cell clone, 12G12, and the weakly cytotoxic CD4+ γδ T cell clones, HF.2, JN.23, and JN.24, have been described (10, 40, 41). 10G4 is a randomly derived Vγ1Vδ1 clone (42). SP-F3 is a CD4+ αβ T cell clone that recognizes tetanus toxoid C fragment (residues 947-961) presented by HLA-DR MHC class II molecules (43).
Maintenance, treatment, and pulsing of APCs
APCs were maintained as previously described (44). For proliferation assays, APCs were treated either with mitomycin C (mit. C) or fixed with glutaraldehyde. For mit. C treatment, APCs (1-3×107 cells/ml) in Dulbecco’s PBS without calcium or magnesium were incubated with fresh mit. C (Sigma-Aldrich, St. Louis, MO) at 100 μg/ml for 1 h at 37°C in a 5% CO2 incubator, washed three times in PBS, and resuspended in either supplemented RPMI 1640 media (termed P-media, 44) or PBS for use. For glutaraldehyde fixation, APCs were adjusted to 1-3×107 cells/ml in PBS and reacted with 0.05% glutaraldehyde (EM grade; Sigma-Aldrich) for 15 s at room temperature while gently vortexing. The reaction was stopped by adding an equal volume of 0.2 M L-lysine (in H2O at pH 7.4) and incubating for 2 min. The fixed cells were then washed three times in PBS and resuspended in either P-media or PBS for use. For antigen pulsing, mit. C-treated or glutaraldehyde-fixed APCs were plated in round bottom 96-well plates (Corning Incorporated) at 1×105 cells per well in PBS and incubated with the compound indicated at 37°C for 1 h. The cells were then washed three times with PBS and resuspended in P-media for mixing with T cells. For statin inhibition experiments, Vγ2Vδ2 T cell responses were generally adjusted such that they were at least 45% of the maximum response. Pravastatin stock solution was made directly with water, mevastatin and simvastatin were first dissolved in 100% ethanol and then an equal volume of water was added to make stock solutions. All statins were from Calbiochem and were the (active) sodium carboxylate salts. For statin inhibition, APC were preincubated with the statin for 30 min. For pulsing, APC were cultured with stimulatory compounds in the presence of the statin and then washed. T cells were then added with the statin so that the statin was present during for the entire duration of culture. Similarly, APC were preincubated with cell transport inhibitors (chloroquine, ammonium chloride, bafilomycin A1, brefeldin A, monensin, wortmannin, cytochalasin D, and nocodazole (Sigma-Aldrich)) for 30 minutes, followed by either pulsing with stimulatory compounds for 60 min or continuously culturing with stimulatory compounds, in the presence of the inhibitors. T cells were then added in the presence of the inhibitors.
T cell proliferation and cytokine release
T cell proliferation assays were performed as previously described (45). Assays were in duplicate or triplicate in round-bottom 96-well plates using 1×105 T cells per well in the presence of non-fixed (mit. C-treated) or fixed APC at 1×105 cells per well for antigen and PHA stimulation, or in the absence of APC for IL-2 stimulation. Stimulating compounds and inhibitors were used as indicated in the figure legends. The cultures were pulsed with 1 μCi of 3H-thymidine (2 Ci/mmol) on day 1 and harvested 16-18 h later. Mean proliferation and SEM of duplicate or triplicate cultures is shown. For digestion of phosphorylated compounds, shrimp alkaline phosphatase was added (Fermentas, Thermo Fisher Scientific). For cytokine release, culture supernatants were harvested after 16 h and assayed for TNF-α or IFN-γ levels by DuoSet sandwich ELISA (R&D Systems, Minneapolis, MN). A standard curve was derived from serial dilution of each cytokine standard, and used to calculate the cytokine concentration in pg/ml.
In vitro expansion of Vγ2Vδ2 T cells
For in vitro expansion of blood Vγ2Vδ2 T cells by bisphosphonates, peripheral blood mononuclear cells (PBMC) were prepared from the blood or leukopacs of normal donors by Ficoll-Hypaque density centrifugation. 1×105 PBMC in 0.2 ml media in 96-well round bottom wells were pulsed with the compounds for 2-6 hours, washed twice, or cultured continuously with the compounds. IL-2 containing media was added on day 3. The cells were harvested on day 9, stained with the HIT3a FITC-anti-CD3 (eBioscience) and B6 PE-anti-Vδ2 (BD Pharmingen) monoclonal antibodies, and analyzed using flow cytometry.
Measurement of calcium flux by flow cytometry
Calcium flux was measured using a flow cytometric assay with indo-1 (Invitrogen, Molecular Probes, Eugene, OR) as described previously (25). Indo-1-loaded T cells (without APC) were incubated at 37°C for 2 min, analyzed for 30 s to establish baseline levels, then antigen was added. For “not spun” samples, cells were analyzed for an additional 3 min. For “spun” samples, cells were analyzed for an additional 30 s to establish baseline calcium levels, after antigen addition. The T cells were then centrifuged for 20 s in a micro-centrifuge to initiate cell-cell contact, then incubated for a further 50 s at 37°C. The cells were resuspended, introduced into the flow cytometer, and analyzed for an additional 2-3 min. The mean ratios of indo-1 fluorescence at 405/485 nm are shown.
Measurement of intracellular IPP levels
Cells were treated with various compounds or siRNA, harvested from culture, washed twice with PBS, counted, and spun down. 300 μl of ice-cold acetonitrile (ACN) was then added to the cell pellet to precipitate macromolecules, followed by the addition of 200 μl of water. The precipitate was removed by centrifugation (13,000×g for 3 min) and the supernatant immediately transferred to a new tube. The cell extracts were then evaporated in vacuo and stored at -80°C until use. For LC/MS determination of IPP levels of siRNA treated APC, samples were re-dissolved in 50 μl of 12 mM ammonium formate, metabolites separated by reverse phase HPLC using a ZORBAX Eclipse XDB-C8 column (Agilent Technologies), and analyzed by positive ion electrospray mass spectrometry using an MSD Trap XCT Plus spectrometer (Agilent Technologies) as described (36). For LC/MS determination of IPP and ApppI in APCs incubated with different compounds, MCF-7 cells were incubated with the various compounds and cell extracts prepared as above. Levels of IPP and ApppI were determined by separation of metabolites on high-performance ion-pairing reverse phase liquid chromatography using a Gemini C18 column (Phenomenex) with N,N-dimethylhexylamine formate as the ion pairing agent and analysis by negative ion electrospray ionization mass spectrometry as described (46).
siRNA transfection and real-time PCR
For each enzyme, three different siRNAs were purchased from Invitrogen. Enzymes targeted were isopentenyl diphosphate isomerase (IDI), farnesyl diphosphate synthase (FDPS), squalene synthase (SQS) (also termed farnesyl-diphosphate farnesyltransferase 1), dehydrodolichol diphosphate synthase (DHDDS), and prenyl (decaprenyl) diphosphate synthase subunit 2 (PDSS2). Note that diphosphate is also termed pyrophosphate. For transfection, HeLa cells were plated at 2×105 cells per well in 6-well plates one day before use. For transfection, 12 μl of HiPerFect transfection reagent (Qiagen, Germantown, MD) was added to 150 ng siRNA diluted in 100 μl of serum-free OPTI-MEM I (Invitrogen). After votexing for 10 s and incubating at RT for 5-10 min, the transfection complexes were added drop-wise onto the cells in each well. The transfected cells were then incubated at 37°C and 10% CO2 for 24 to 96 h before harvesting for future use. For mRNA detection, siRNA transfected HeLa cells were harvested at 72 h post-transfection. RNA was extracted from 1×106 cells using the RNeasy Mini Kit (Qiagen). 4.0 μg of RNA were reverse-transcribed to cDNA using the SuperScript First-Strand Kit (Invitrogen). 1 μg of the synthesized cDNA was then added as template into PCR Master Mix, and the gene of interest amplified using probes validated for real time PCR from Invitrogen (except for DHDDS) according to the protocol for TaqMan Gene Expression Assays (Applied Biosystems). mRNA expression by each gene was assessed by real-time quantitative PCR using the ABI PRISM 7700 Sequence Detection System. Target gene mRNA levels were calculated by using the comparative CT method, and compared with control siRNA transfectants.
Stimulation of DBS43 Vγ2Vδ2 TCR transfectant
Derivation of the DBS43 Vγ2Vδ2 TCR transfectant is described (47). Stimulation of TCR transfectants for IL-2 release was performed as described (47, 48). Briefly, 1×105 transfectants or the parent J.RT3-T3.5 cells were cultured with anti-TCRδ1 mAb concentrated culture supernatant, HMBPP, ionomycin, or siRNA-treated HeLa tumor cells in the presence of 1×105 glutaraldehyde-fixed Va-2 cells (except for tumor cells) and 10 ng/ml PMA. After 24 h, supernatants were harvested and frozen at -20°C. For IL-2 assays, the supernatants were thawed and used at a 1/8 dilution to stimulate the proliferation of the IL-2-dependent cell line, HT-2. The cultures were pulsed with 1 μCi of 3H-thymidine (2 Ci/mmol) at 18 h and harvested 6 h later.
Results
Aminobisphosphonates can be pulsed into APC to reduce their nonspecific inhibition of T cell proliferation
Aminobisphosphonates stimulate Vγ2Vδ2 T cells by inhibiting FDPS, leading to the accumulation of IPP (Fig.1). Previous experiments on aminobisphosphonate stimulation of Vγ2Vδ2 T cell clones and lines have focused on cytokine release (26), because the cells did not proliferate (21). Confirming these studies, the JN.23 Vγ2Vδ2 T cell clone released TNF-α in response to the aminobisphosphonate, risedronate (Fig. 2A), and to the prenyl pyrophosphate analog, mono-ethyl-pyrophosphate (Supplemental Fig. 1A). However, whereas Vγ2Vδ2 T cells proliferated with exposure to monoethyl pyrophosphate (with a slight response even in the absence of APC), there was little proliferation with exposure to risedronate, either in the absence or presence of APC (Fig. 2A).
FIGURE 2. Aminobisphosphonate toxicity for Vγ2Vδ2 T cell can be avoided by pulsing.
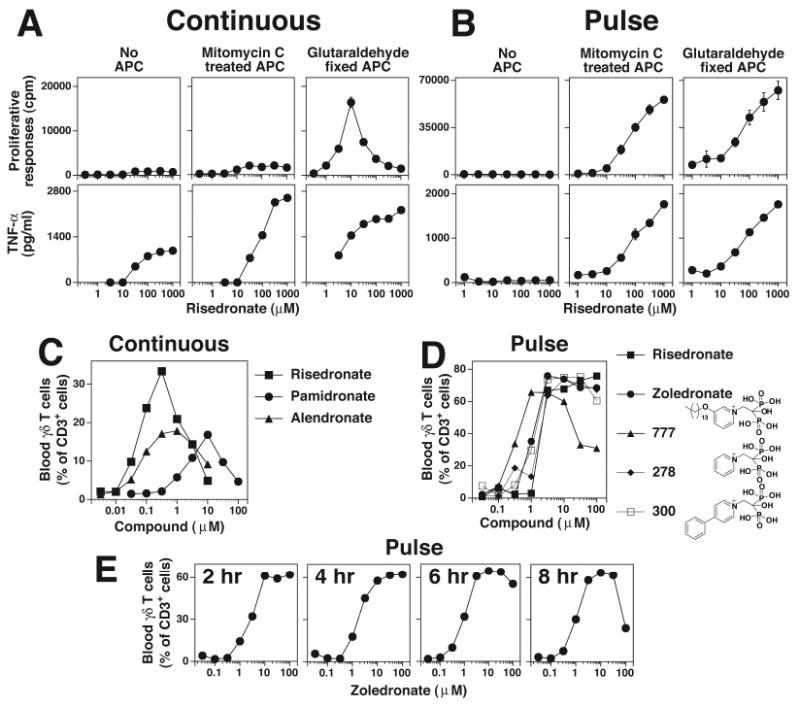
A, Continuous culture of Vγ2Vδ2 T cells with aminobisphosphonates inhibits their proliferation but not TNF-α release. Mit. C treated or glutaraldehyde fixed CP.EBV cells were continuously cultured with risedronate and the CD4+ Vγ2Vδ2 T cell clone, JN.23. Supernatants were collected at 16 h for the measurement of TNF-α. The cells were pulsed with 3H-thymidine and harvested 18 h later. B, APC pulsed with risedronate stimulate both proliferation and TNF-α release. Risedronate was pulsed into APC, washed, and then mixed with the CD4+ Vγ2Vδ2 T cell clone, JN.23. TNF-α and cell proliferation were measured as in A. C, Variable expansion of blood Vγ2Vδ2 T cells with continuous exposure to aminobisphosphonates. PBMC were cultured with various aminobisphosphonates for 10 days and Vγ2Vδ2 T cells and CD3+ T cells determined by flow cytometry. D, Consistent blood Vγ2Vδ2 T cell responses to aminobisphosphonates pulsed into monocytes. PBMC were pulsed for 4 hours with the various aminobisphosphonates. The PBMC were then washed and cultured in the presence of IL-2. After 9 days, Vγ2Vδ2 T cells and CD3+ T cells were determined by flow cytometry. E, Expansion of blood Vγ2Vδ2 T cells in PBMC pulsed with zoledronate. PBMC were pulsed for the indicated time with zoledronate, washed, and cultured in the presence of IL-2. After 9 days, Vγ2Vδ2 T cells and CD3+ T cells were determined by flow cytometry.
We showed previously that glutaraldehyde fixation increases costimulatory/accessory functions of APC for Vγ2Vδ2 T cells (45). When glutaraldehyde-fixed APC were used, Vγ2Vδ2 T cell proliferation was observed with risedronate, but only in a narrow dose range, with responses observed at 10-fold lower concentrations than TNF-α release in the presence of non-fixed APC (Fig. 2A). This lack of Vγ2Vδ2 T cell proliferation was not observed when APC were pulsed with risedronate, because both non-fixed and fixed APC pulsed with risedronate induced strong proliferative responses (Fig. 2B). Similar results were noted with other aminobisphosphonates (Supplemental Fig. 2). These results suggest that continuous exposure to risedronate blocks Vγ2Vδ2 T cell proliferation unless highly effective APC are used.
Toxicity was also noted when various aminobisphosphonates were used continuously to expand Vγ2Vδ2 T cells from PBMC, with variable maximal expansions and narrow dose response ranges (Fig. 2C). In contrast, when aminobisphosphonate exposure was limited to 4 h, blood Vγ2Vδ2 T cells expanded to similar maximal levels for both conventional (zolendronate), pyridinium (BPH-278 and BPH-300), and lipophilic (BPH-777) aminobisphosphonates with broad peak responses over a 10 to 30-fold range (Fig. 2D). Exposure of Vγ2Vδ2 T cells to 100 μM zoledronate for 8 h reduced expansion by >50% whereas exposure for 6 h or less had minimal effect (Fig. 2E).
To determine if this loss of proliferation was specific for Vγ2Vδ2 T cells, the effect of aminobisphosphonates on IL-2- and mitogen-induced proliferation of Vγ1Vδ1, Vγ2Vδ1, and αβ T cell clones was tested. All T cell proliferative responses were inhibited by risedronate (inhibitory concentration reducing responses by 50% (IC50) ranging from 50-1000 μM) (Supplemental Fig. 1B). Thus, aminobisphosphonate inhibition of FDPS within APC blocks isoprenoid metabolism resulting in IPP accumulation. This block also can cause non-specific inhibition of T cell proliferation at higher concentrations.
Aminobisphosphonate rapidly stimulate Vγ2Vδ2 T cells in an MHC-independent manner
To determine how rapidly aminobisphosphonates make APC stimulatory for Vγ2Vδ2 T cells, calcium flux responses of Vγ2Vδ2 T cells to risedronate were compared with responses to HMBPP. When in cell-cell contact, aminobisphosphonates stimulated calcium flux in Vγ2Vδ2 T cells within 2 min, with similar kinetics as HMBPP (Fig. 3A). Risedronate had no effect on Vγ1Vδ1 T cells (Fig. 3A, right bottom panel) and without cell-cell contact, no responses were observed (Fig. 3A, left panel). Consistent with the rapid calcium flux, APC exposure to risedronate for as short as 5 min, rendered the APC stimulatory for Vγ2Vδ2 T cells similar to pulsing with HMBPP (Supplemental Fig. 3A). Prolonged risedronate exposure for 120 min only increased EC50% 3-fold compared with APC pulsed for 5 min. Thus, risedronate stimulation is rapid and dependent on cell-cell contact.
FIGURE 3. Aminobisphosphonate stimulation of Vγ2Vδ2 T cells is a rapid, cell-cell contact-dependent process that is persistent, not inhibited by glutaraldehyde fixation of APC, and does not require known antigen presenting molecules.
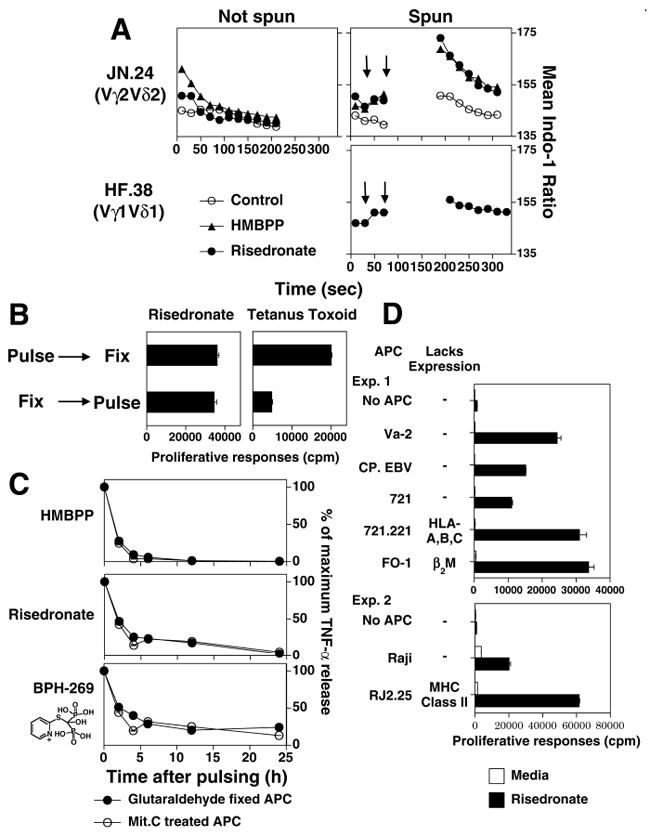
A, Calcium flux of Vγ2Vδ2 T cells in response to risedronate or HMBPP. The Vγ2Vδ2 T cell clone, JN.24 (top), and the Vγ1Vδ1 T cell clone, HF.38 (bottom), were loaded with indo-1 and calcium flux assessed by flow cytometry. PBS (opened circle), HMBPP (2.5 μM, closed triangle), or risedronate (300 μM, closed circle) was added at the time indicated by the first arrow. Then the cells were either not centrifuged (left) or centrifuged for 50 sec to initiate cell-cell contact (second arrow), incubated for 1 min, and gently resuspended for analysis (right). The mean indo-1 ratio is plotted. B, Glutaraldehyde fixation does not inhibit bisphosphonate stimulation of Vγ2Vδ2 T cells. CP.EBV cells were fixed before or after pulsing with a mix of tetanus toxoid (5 μg/ml) and risedronate (1 mM). The JN.24 Vγ2Vδ2 T cell clone and the SP.F3 αβ T cell clone, that is specific for tetanus toxoid, were then added and cell proliferation measured. C, Time kinetics for the loss of stimulation by pulsed APC. CP.EBV APC were pulsed with HMBPP (3.16 μM), risedronate (316 μM), or the bisphosphonate, BPH-269 (1 mM) followed by the addition of JN.24 T cells at different times. Culture supernatants were harvested 16 hours after T cell addition and TNF-α production assessed by ELISA. Responses at each time point are shown as a percentage of the maximum response. D, Vγ2Vδ2 T cells respond to pulsed risedronate in the absence of classical MHC class I molecules (HLA-A, HLA-B, or HLA-C), MHC class II molecules (HLA-DR, HLA-DQ, HLA-DP, HLA-DMA, or HLA-DMB), CD1 (CD1a, -b, -c, and –d), and β2M-dependent molecules. The JN.24 Vγ2Vδ2 T cell clone was stimulated with APC pulsed with the bisphosphonate, risedronate (1 mM). APC included (Exp. 1) the human fibrosarcoma cell line, Va-2, the EBV B cell lines, CP.EBV and 721 (lacking CD1a, -b, -c, and –d), the mutant EBV line, 721.221 (lacking HLA-A, HLA-B and HLA-C), and the melanoma cell line, FO-1 (lacking β2M) or (Exp. 2) the Burkitt lymphoma, Raji, and its class II-deficient mutant, RJ.2.2.5.
Because aminobisphosphonates are proposed to function intracellularly, fixation of APC could disrupt uptake. However, when APC were fixed and then pulsed with risedronate, no significant reduction in Vγ2Vδ2 T cell stimulation was observed (Fig. 3B). APC fixation was judged adequate because APC fixation before pulsing, but not after, inhibited the response of the SP-F3 CD4 αβ T cell clone to tetanus toxoid, indicating sufficient APC fixation to abolish the presentation of a protein antigen by MHC class II HLA-DR. In contrast, APC fixation before or after risedronate pulsing had no effect on Vγ2Vδ2 T cell responses (Fig. 3B) demonstrating that aminobisphosphonate stimulation is resistant to glutaraldehyde fixation.
We next determined how long APC pulsed with aminobisphosphonates remain stimulatory for Vγ2Vδ2 T cells. APC pulsed with aminobisphosphonates stimulated Vγ2Vδ2 T cells for up to 24 h whereas APC pulsed with HMBPP lost their ability to stimulate by 4 h (Fig. 3C). APC fixation did not affect the retention of aminobisphosphonate activity since both nonfixed and fixed APC lost their ability to stimulate with the same kinetics (Fig. 3C).
The ability of aminobisphosphonates to pulse into APC allowed us to determine the requirement for known antigen presenting molecules under conditions where self-presentation of antigens was not possible. Expression of MHC class I (HLA-A, -B, and –C), MHC class II, β2M, and CD1a, CD1b, CD1c, and CD1d (absent on CP.EBV, 721, and 721.221) was not required because APCs lacking these molecules stimulated Vγ2Vδ2 T cells when pulsed with risedronate (Fig. 3D). In addition, like prenyl pyrophosphates and contrary to a report using the stimulatory Daudi cell line as the APC (26), stimulation by aminobisphosphonates using a conventional B cell line was not greatly affected by low temperature or monensin (Supplemental Fig. 3B,C). However, monensin treatment did abolish the intrinsic stimulatory activity of Daudi (Supplemental Fig. 3B). There was moderate inhibition by other cellular inhibitors (chloroquine, ammonium chloride, bafilomycin A1, brefeldin A, wortmannin, cytochalasinD, and nocodazole), but none blocked completely (data not shown).
Aminobisphosphonate-stimulated Vγ2Vδ2 T cell responses are more sensitive to statin inhibition than responses induced by prenyl pyrophosphates and superantigens
Statins inhibit HMGCR, the rate-limiting enzyme in the mevalonate pathway that is upstream from FDPS (Fig. 1). Statins are reported to specifically inhibit Vγ2Vδ2 T cells responses to aminobisphosphonates (26, 27) and alkylamines (28). However, they are also reported to alter αβ T cell responses as well as the functions of other cells of the immune system (29-31). To reconcile these apparent differences, we investigated the effect of statins on Vγ2Vδ2 T cell responses in more detail.
We first determined the relationship between the magnitude of the Vγ2Vδ2 T cell response to risedronate and its sensitivity to statin inhibition. Risedronate responses between 50 to 100% of the maximum response were inhibited by mevastatin at concentration varying only between 1 to 3 μM (Supplemental Fig. 4A). In contrast, when risedronate responses were weaker (<50% of maximum), sensitivity to statin inhibition increased 10 to 100-fold (IC50 values between 0.013 to 0.01 μM) (Supplemental Fig. 4A,B). Much of the aminobisphosphonate response could be restored by mevalonate (the product of the HMGCR enzyme) (Supplemental Fig. 4C). Thus, to accurately assess statin sensitivity, the magnitude of the Vγ2Vδ2 T cell response must be considered because sensitivity to statin inhibition increases greatly for responses less than 45% of maximum.
Taking this into consideration, we assessed the sensitivity of different Vγ2Vδ2 T cell stimulators to statin inhibition. Staphylococcal enterotoxin A (SEA) is a superantigen that activates Vγ2+ T cells through direct presentation by MHC class II (45). Despite a weak response to SEA (3% of the HMBPP maximum), the response to SEA was relatively resistant to mevastatin inhibition (IC50 = 100 μM), requiring a concentration similar to that needed to inhibit the response to HMBPP (IC50 = 63 μM) (Fig. 4A). In contrast, risedronate was 333-fold more sensitive to mevastatin inhibition (IC50 = 0.3 μM) (Fig. 4A). Two additional statins, pravastatin (lower potency) and simvastatin (higher potency), also preferentially inhibited risedronate responses compared with HMBPP and PHA responses (Fig. 4B). The differences in concentration were ~10-fold for pravastatin, 30-48-fold for mevastatin, and 76-154-fold for simvastatin (Fig. 4B). Finally, statin treatment of APC pulsed with both risedronate and tetanus toxoid showed that risedronate-induced Vγ2Vδ2 T cell responses were 38-fold more sensitive to mevastatin inhibition than tetanus toxoid-induced αβ T cell responses presented by the same APC (Fig. 4C).
FIGURE 4. Indirect stimulation of Vγ2Vδ2 T cells by aminobisphosphonates is more sensitive to statin inhibition than direct stimulation by prenyl pyrophosphates or superantigens.
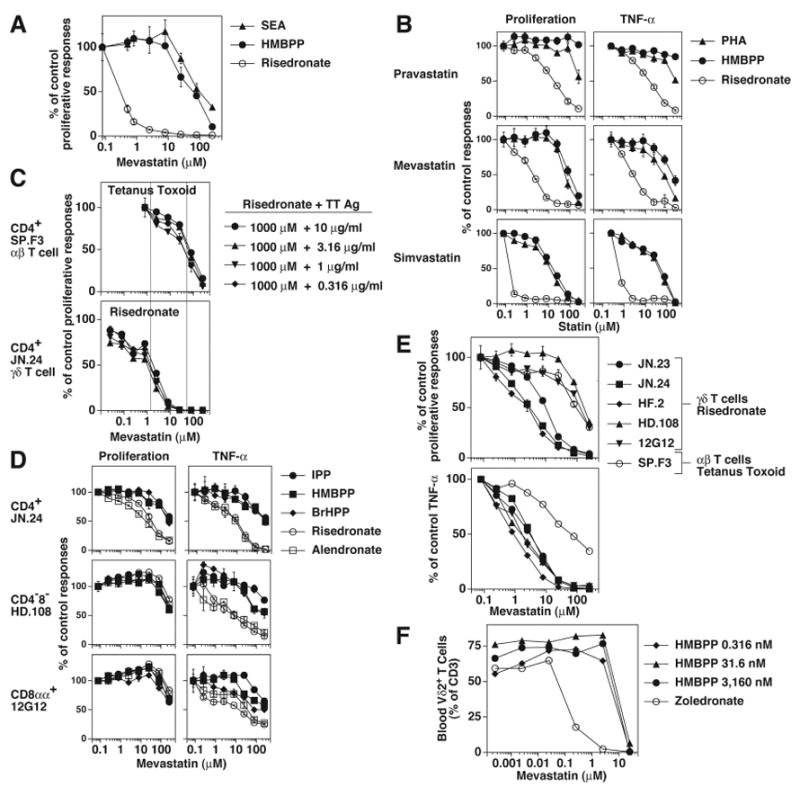
A, Mevastatin inhibition of Vγ2Vδ2 T cell proliferation to staphylococcal enterotoxin A (1 μg/ml), HMBPP (1 μM), or risedronate (10 μM). Mit. C treated CP.EBV cells were pulsed with the above compounds for 1 h, and then cultured with JN.24 T cells. B, Different statins inhibit Vγ2Vδ2 T cell responses. Inhibition by pravastatin, mevastatin, and simvastatin of Vγ2Vδ2 T cell responses to the mitogen, PHA (1:1000), HMBPP (1 μM), or risedronate (1 mM). CP.EBV were preincubated with the indicated statin for 1 h, pulsed with the compounds in the presence of the statin, and then cultured with JN.23 T cells in the presence of the statin. TNF-α and cell proliferation were measured as in Fig. 2A. C, Risedronate-induced Vγ2Vδ2 T cell response is more sensitive to mevastatin inhibition than a tetanus toxoid-induced αβ T cell response presented by the same APC. CP.EBV cells were treated with varying concentrations of mevastatin for 1 h and then pulsed with the mixture of 1 mM risedronate and indicated concentrations of tetanus toxoid. T cells were added to the culture in the presence of mevastatin. After 18 h, the cells were pulsed and harvested 1 day later. D, Mevastatin inhibition of Vγ2Vδ2 T cell responses. The effect of mevastatin on the proliferative and TNF-α responses of the CD4+ γδ T cell clone, JN.24, and the CD4- γδ clones, HD.108 and 12G12 by three prenyl pyrophosphates (100 μM IPP, 1 μM HMBPP, and 10 μM BrHPP) and two bisphosphonates (1mM risedronate and 1 mM alendronate) was determined. E, Mevastatin inhibition of the proliferative and TNF-α responses of five Vγ2Vδ2 T cell clones to risedronate (1 mM) and an αβ T cell clone to tetanus toxoid (10 μg/ml). F, Mevastatin inhibition of blood Vγ2Vδ2 T cell expansion in response to HMBPP or zoledronate. PBMC were incubated with varying concentrations of mevastatin and either HMBPP (3,160-0.316 nM) or zoledronate (3.16 μM) for 6 h, washed, and then cultured with mevastatin. IL-2 was added on day 3. After 8 days, Vγ2Vδ2 T cells and CD3+ T cells were determined by flow cytometry.
Because these experiments used a CD4+ Vγ2Vδ2 T cell clone, we evaluated two non-CD4 Vγ2Vδ2 T cell clones to determine whether sensitivity to statin inhibition varied. Mevastatin inhibition was determined for proliferative and TNF-α responses to prenyl pyrophosphates (IPP, HMBPP, and BrHPP) and to aminobisphosphonates (risedronate and alendronate) (Fig. 4D). Aminobisphosphonate-induced TNF-α responses were more sensitive to mevastatin inhibition than prenyl pyrophosphate-induced TNF-α responses for all three clones. In contrast, for proliferative responses only the CD4+ T cell clone exhibited this increased sensitivity to statin inhibition of aminobisphosphonate responses. No difference in inhibition sensitivity was noted for the CD8αα+ and CD4-8- T cell clones. This pattern was further confirmed using additional CD4+ Vγ2Vδ2 T cell clones (Fig. 4E). We next stimulated freshly isolated blood Vγ2Vδ2 T cells to determine their sensitivity to statin inhibition (Fig. 4F). Zoledronate-induced expansion of blood Vγ2Vδ2 T cells was 80-fold more sensitive to mevastatin inhibition than HMBPP-induced expansion (IC50 = 0.1 μM versus 8 μM). Thus, the pattern of statin inhibition of blood Vγ2Vδ2 T cells (largely CD8αα+ or CD4-8-) was similar to that of CD4+ Vγ2Vδ2 T cell clones.
High statin concentrations (similar to those inhibiting prenyl pyrophosphate and SEA responses) also inhibited Vγ2Vδ2, non-Vγ2Vδ2 γδ, and αβ T cell proliferative responses to IL-2 (Supplemental Fig. 4D) and both proliferative and TNF-α responses to the mitogen, PHA (Supplemental Fig. 4E). Statin effects were not due to reductions in APC numbers since APC numbers did not vary with treatment (data not shown). In summary, statins preferentially inhibit the aminobisphosphonate-stimulated proliferation of blood Vγ2Vδ2 T cells and CD4+ Vγ2Vδ2 T cell clones, but not CD8αα+/CD4-8- Vγ2Vδ2 T cell clones. Statins also preferentially inhibit TNF-α release to aminobisphosphonates for all Vγ2Vδ2 T cells. At high doses, statins non-specifically inhibit T cell responses. Therefore, the sensitivity to statin inhibition can distinguish indirect stimulation of Vγ2Vδ2 T cells from direct recognition of antigens by Vγ2Vδ2 T cells.
Statin inhibition distinguishes indirect stimulation because of FDPS inhibition from other pathways for stimulation of Vγ2Vδ2 T cells
Because sensitivity to statin inhibition distinguishes indirect stimulation by aminobisphosphonates from direct recognition of prenyl pyrophosphates and superantigens, statin inhibition can help distinguish between different pathways for stimulation of Vγ2Vδ2 T cells. Additional classes of compounds have been shown to stimulate Vγ2Vδ2 T cells. Alkylamines are natural products present in some foods, and produced by certain bacteria, that stimulate Vγ2Vδ2 T cells in vitro (14) and prime Vγ2Vδ2 T cells in vivo for increased responsiveness to prenyl pyrophosphates (49). The alcohol of HMBPP, (E)-2-methyl-but-2-ene-1,4-diol (HMB-OH), stimulates the expansion of Vγ2Vδ2 T cells (34), despite lacking the phosphate groups that are normally essential for the activity of prenyl pyrophosphates. Finally, mevalonate by itself also stimulates the expansion of Vγ2Vδ2 T cells (27).
Consistent with these reports, these compounds stimulated both proliferation and TNF-α release by Vγ2Vδ2 T cells. Whereas HMBPP and risedronate rendered APC strongly stimulatory after pulsing, HMB-OH and mevalonate rendered APC only weakly stimulatory (Fig. 5A). The alkylamine, sec-butylamine, had no effect with pulsing of either nonfixed or fixed APC (data not shown) but could stimulate Vγ2Vδ2 T cells when present continuously (Fig. 5B). Vγ2Vδ2 T cell responses to these compounds were then tested for their sensitivity to statin inhibition when the compounds were either pulsed with the APC, or continuously cultured with the APC and T cells. Whereas Vγ2Vδ2 T cell responses to PHA and HMBPP were relatively resistant to inhibition by mevastatin (Fig. 6A, top 2 rows), and completely resistant to inhibition by pravastatin (data not shown), responses to risedronate was highly sensitive to statin inhibition when the compounds were either pulsed, or continuously present (Fig. 6A, bottom row). Similarly, Vγ2Vδ2 T cell responses to sec-butylamine was highly sensitive to statin inhibition, consistent with a report that alkylamines inhibit FDPS activity in cells (28). Finally, like HMBPP and PHA, Vγ2Vδ2 T cell responses to HMB-OH and mevalonate were relatively resistant to statin inhibition when the compounds were either continuously present or pulsed (for HMB-OH) (Fig. 6A, third and fourth rows). Thus, the activity of HMB-OH and mevalonate do not appear dependent on FDPS inhibition. To confirm this finding, the levels of intracellular IPP and its metabolite, ApppI, were measured after incubation of MCF-7 cells with the various stimulators. While zoledronate treatment greatly increased IPP and ApppI levels, IPP was still undetectable after HMBPP, HMB-OH, or mevalonate treatment (Fig. 6B).
FIGURE 5. Multiple compounds stimulate Vγ2Vδ2 T cells.
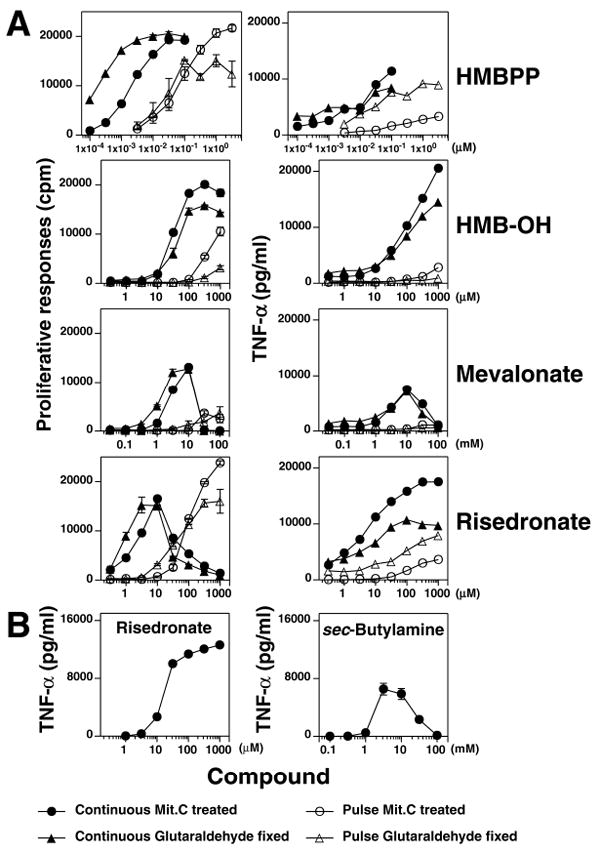
A, Stimulation of Vγ2Vδ2 T cells by HMBPP, HMB-OH, mevalonate, and risedronate. The JN.24 Vγ2Vδ2 T cell clone was cultured with non-fixed (mit. C-treated) or fixed Va2 APC that had been pulsed with the indicated compounds or the compounds were added continuously. T cell proliferation and TNF-α release were measured as described in 2A. B, Stimulation of Vγ2Vδ2 T cells by sec-butyamine and risedronate. Untreated CP.EBV and the HF.2 Vγ2Vδ2 T cell clone were cultured continuously with either risedronate or sec-butylamine. Supernatants were collected 16 h later for determination of TNF-α.
FIGURE 6. Mevalonate and HMB-OH are relatively resistant to statin inhibition, do not greatly increase intracellular IPP or ApppI levels, and are resistant to alkaline phosphatase.
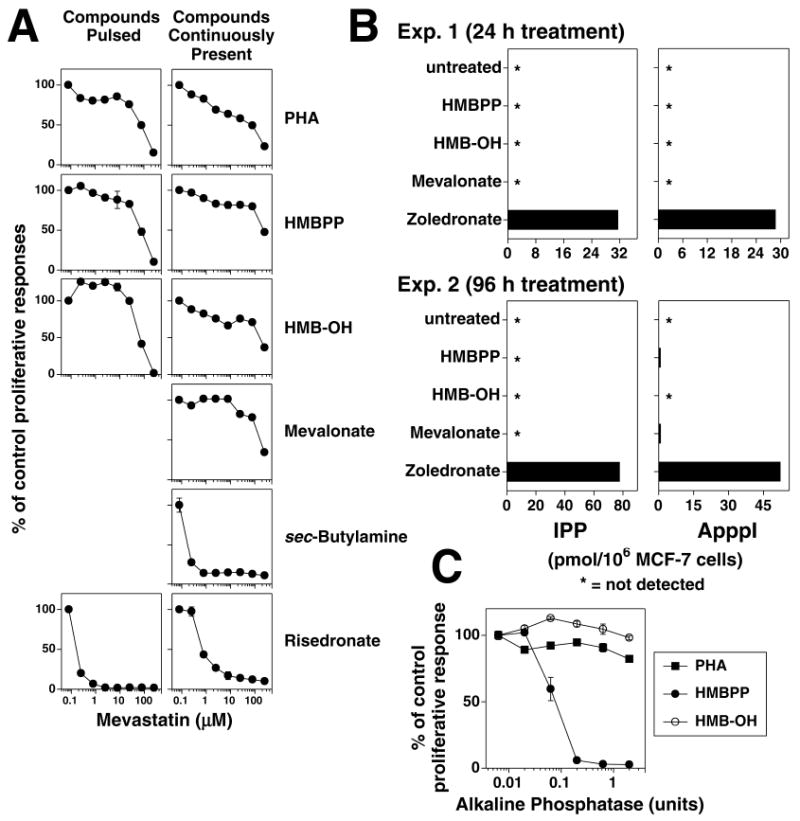
A, Mevastatin inhibition of the response of the CD4+ HF.2 Vγ2Vδ2 T cell clone stimulated (left panels) by APC pulsed with PHA (1:1000), HMBPP (1 μM), HMB-OH (1 mM), or risedronate (1 mM), or (right panels) by APC continuously cultured with PHA (1:1000), HMBPP (1 μM), HMB-OH (1 mM), mevalonate (25 mM), sec-butylamine (5 mM), or risedronate (31.6 μM) continuously present in culture. B, HMB-OH and mevalonate do not greatly increase IPP or ApppI levels. MCF-7 cells were untreated or incubated with HMBPP (10 nM for 24 h or 100 nM for 96 h), HMB-OH (100 μM), or mevalonate (10 mM) for 24 h (Exp. 1, top panels) or 96 h (Exp. 2, bottom panels). For a control, MCF-7 cells were treated for zoledronate (25 μM) for 24 h for both experiments. Cells were then harvested, washed, and lysed with acetonitrile for determination of IPP and ApppI levels by LC/MS (46). C, HMB-OH stimulation is not affected by alkaline phosphatase. Mit. C-treated CP.EBV were cultured in the presence of shrimp alkaline phosphatase continuously with 0.1 μM HMBPP, 1 mM HMB-OH, or 1:4000 diluted PHA or after pulsing with 1 mM risedronate. HF.2 T cells were added and cell proliferation assessed on day 2.
A recent report detailed extracellular IPP produced by cells treated with zoledronate (50). HMB-OH could similarly enter cells, become phosphorylated to HMBPP, and then secreted for presentation to Vγ2Vδ2 T cells. To rule out this mechanism of action, alkaline phosphatase was added to the cultures to hydrolyze extracellular HMBPP. Addition of alkaline phosphatase totally abrogated stimulation by HMBPP but had no effect on HMB-OH stimulation of Vγ2Vδ2 T cells (Fig. 6C) showing that extracellular HMBPP was not responsible for stimulation by HMB-OH. Also, lysates from HMB-OH-treated cells did not contain HMBPP bioactivity upon HPLC separation (data not shown).
One property of directly presented prenyl pyrophosphates is their ability to stimulate Vγ2Vδ2 T cell responses in the absence of APC due to daughter-daughter T cell presentation (25). In contrast, stimulation by ApppI is minimal in the absence of APC since APC are required to provide nucleotide phosphorylase to release IPP (51). Because our findings suggested that HMB-OH might be directly presented, the requirement for APC was tested. Whereas ApppI stimulation was suboptimal in the absence of APC (Fig. 7A, B), HMB-OH stimulated Vγ2Vδ2 T cells in the absence of APC with kinetics identical to HMBPP and IPP; this was especially evident for TNF-α release (Fig. 7B). Finally, despite lacking phosphates, HMB-OH (EC50% of 3.2 μM) stimulates Vγ2Vδ2 T cells at similar concentrations as the HMB phosphonate analogs, HMB-CPCP and HMB-OPCP (EC50% of 4.6 μM and 5.5 μM (34), respectively). Taken together, HMB-OH does not inhibit FDPS but appears to stimulate Vγ2Vδ2 T cells directly.
FIGURE 7. HMB-OH stimulation of Vγ2Vδ2 T cells in the absence of APC is similar to stimulation by HMBPP and IPP.
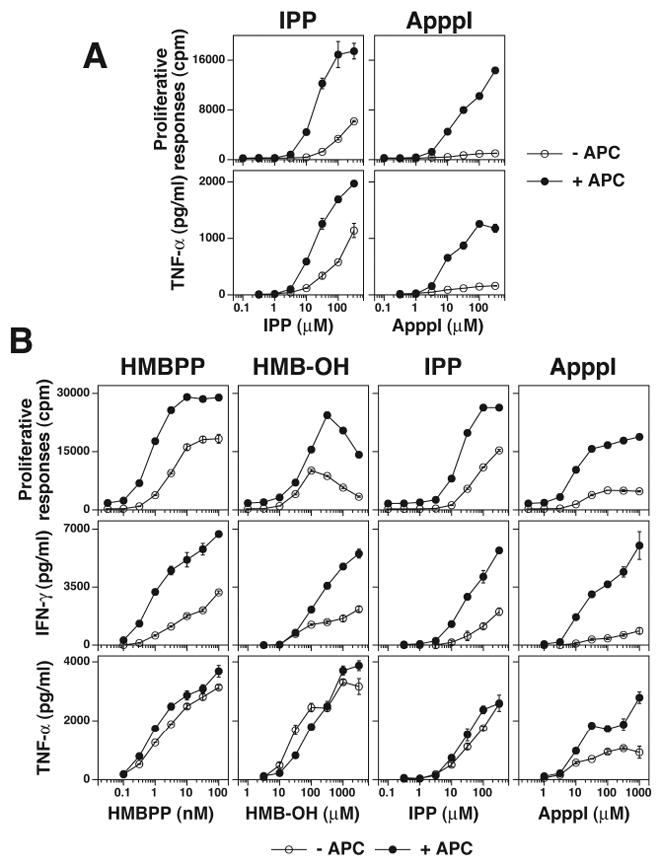
A, ApppI stimulation is relatively APC dependent. The HF.2 Vγ2Vδ2 T cell clone was cultured with either IPP or ApppI in the presence or absence of mitomycin C-treated CP.EBV. TNF-α and cell proliferation were measured as in Fig. 2A. B, HMB-OH stimulation in the absence of APC is similar to stimulation by HMBPP and IPP. The HF.2 Vγ2Vδ2 T cell clone was cultured with HMBPP, HMB-OH, IPP, or ApppI in the presence or absence of mitomycin C-treated CP.EBV. TNF-α and cell proliferation were measured as in Fig. 2A.
Alkenyl-pyrophosphonates and alkyl-bisphosphonates directly stimulate Vγ2Vδ2 T cells
Other classes of phosphonate compounds that stimulate Vγ2Vδ2 T cells include alkenyl pyrophosphonates containing -CPOP moieties (52, 53), alkenyl-methylene diphosphonates (-OPCP), and alkenyl-phosphorylmethylphosphonates (-CPCP). These compounds can either stimulate Vγ2Vδ2 T cells (for example HMB-OPCP, 34, 54) or antagonize prenyl pyrophosphate responses (for example BrH-OPCP, 44, 55). To assess the mechanism of action of these compounds, we compared statin inhibition of the response to HMB-CPCP with that of HMBPP and risedronate. As expected, HMBPP was relatively resistant to statin inhibition while risedronate was highly sensitive. Consistent with direct recognition, HMB-CPCP required high statin concentrations for inhibition that were identical to those required by HMBPP (Fig. 8A). Thus, given the structural similarities, alkenyl-pyrophosphonates, alkenyl-methylene diphosphonates, and alkenyl-phosphorylmethylphosphonates directly stimulate Vγ2Vδ2 T cells.
FIGURE 8. Linear pyrophosphonates and alkyl-bisphosphonates directly stimulate Vγ2Vδ2 T cells.
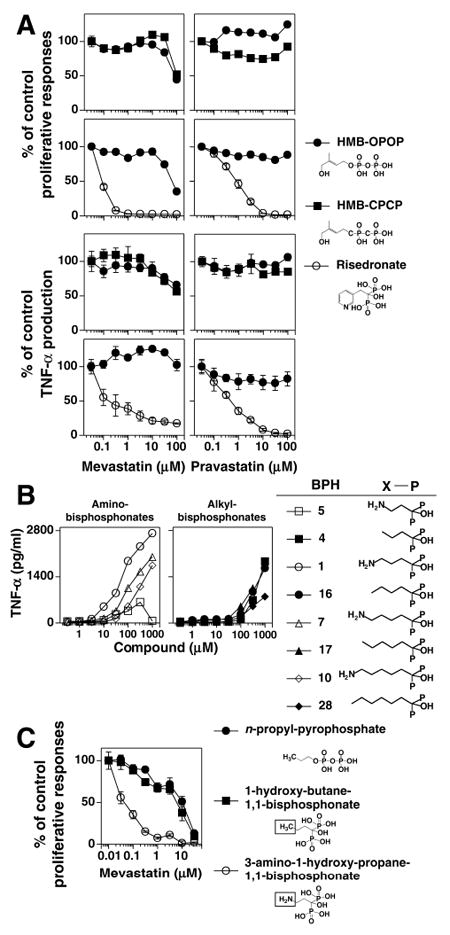
A, Direct recognition of HMBPP and HMB-CPCP by Vγ2Vδ2 T cells. Mevastatin and pravastatin inhibition of proliferative and TNF-α responses by the HF.2 Vγ2Vδ2 T cell clone stimulated by 0.1 μM of HMB-OPOP or 316 μM of HMB-CPCP continuously present (top panels) or by 1 μM of HMB-OPOP or 1 mM of risedronate that were pulsed with APC (bottom panels). B, Amino- and alkyl-bisphosphonates stimulate Vγ2Vδ2 T cells. Bisphosphonates were tested for their ability to stimulate TNF-α release by the CD4+ JN.23 Vγ2Vδ2 T cell clone. C, Substitution of an amino moiety for carbon 4 in 1-hydroxy-butane-1,1 bisphosphonate switches direct to indirect stimulation. The CD4+ HF.2 Vγ2Vδ2 T cell clone was continuously stimulated by either n-propyl pyrophosphate (20 μM), 1-hydroxy-butane-1,1 bisphosphonate (20 μM), or 3 amino-1-hydroxy-propane-1,1 bisphosphonate (400 μM) in the presence of mevastatin.
In our testing of different bisphosphonates, we found a new class of compounds, alkyl-bisphosphonates, that stimulate Vγ2Vδ2 T cells (Fig. 8B). These compounds have identical alkyl-1,1-bisphosphonate structures as aminobisphosphonates but lack amino moieties and thus have similarities also to alkyl-pyrophosphates (10, 32). Alkyl-bisphosphonates stimulate Vγ2Vδ2 T cells with EC50 of ~300-700 μM (Fig. 8B). To determine the effect of the loss of the amino moiety on the mechanism of action for Vγ2Vδ2 T cell stimulation, the sensitivity to statin inhibition of the Vγ2Vδ2 T cell response to an alkyl-bisphosphonate (1-hydroxy-butane-1,1-bisphosphonate) was compared with an alkyl-pyrophosphate (n-propyl-pyrophosphate) and the aminobisphosphonate, pamidronate (3-amino-1-hydroxy-propane-1,1-bisphosphonate). As expected, the Vγ2Vδ2 T cell response to the aminobisphosphonate pamidronate was highly sensitive to mevastatin inhibition (IC50 = 0.04 μM) (Fig. 8C). Surprisingly, the loss of the amino group in 1-hydroxy-butane-1,1-bisphosphonate increased the resistance to statin inhibition 175-fold (IC50 = 7 μM) to concentrations similar to those required to inhibit n-propyl-pyrophosphate responses (IC50 = 10 μM) (Fig. 8C). We hypothesize that the loss of the amino moiety switches aminobisphosphonates from indirect stimulators to direct stimulators. Thus, the amino moiety of bisphosphonates plays a key role in determining their mechanism of action for stimulating Vγ2Vδ2 T cells.
Small interfering RNA (siRNA) treatment of APC identifies FDPS and IDI as enzyme targets for the development of Vγ2Vδ2 T cell stimulators
Given that aminobisphosphonates and alkylamines inhibit FDPS to stimulate Vγ2Vδ2 T cells, we sought to determine whether the inhibition of other enzymes involved in isoprenoid biosynthesis might stimulate Vγ2Vδ2 T cells. IPP is required for many isoprenoid biosynthetic reactions such as the synthesis of GGPP and CoQ10. HeLa cells were therefore transfected with siRNAs specific for key downstream enzymes in the isoprenoid pathway including IDI, FDPS, GGPS, SQS, PDSS2, and DHDDS (Fig. 1). Transfection of siRNAs greatly decreased mRNA levels (most >90%) in all cases tested (Fig. 9A). Consistent with the proposed mechanism of action of aminobisphosphonates and alkylamines and with experiments using short hairpin RNA for FDPS (56), HeLa cells transfected with siRNA targeting FDPS stimulated Vγ2Vδ2 T cells (Fig. 9B). HeLa cells transfected with FDPS siRNA began to stimulate Vγ2Vδ2 T cells by 72 h and peaked at 96 h. The ability of FDPS siRNA transfectants to stimulate Vγ2Vδ2 T cells correlated with intracellular IPP levels. At 72 h post-transfection, when FDPS siRNA transfected-HeLa cells begin to show weak stimulatory activity, their intracellular IPP levels were slightly increased. At 96 h post-transfection, when the ability of transfectants to stimulate Vγ2Vδ2 T cells peaked, IPP level were dramatically elevated (Fig. 9C). Mevastatin preferentially inhibited the Vγ2Vδ2 T cell response to APC transfected with siRNA for FDPS with an identical dose response to that of risedronate (Fig. 9D). Finally, recognition of FDPS siRNA-treated cells was mediated by the Vγ2Vδ2 TCR because β- Jurkat cells transfected with Vγ2Vδ2 TCRs (DBS43) released IL-2 in response to FDPS siRNA transfected HeLa cells whereas these APC had no effect on the parent cell line (J.RT3-T3.5) (Fig. 9E).
FIGURE 9. siRNA downregulation of either FDPS mRNA or isopentenyl diphosphate isomerase mRNA in APC results in indirect stimulation of Vγ2Vδ2 T cells with elevations in intracellular IPP levels in APC.
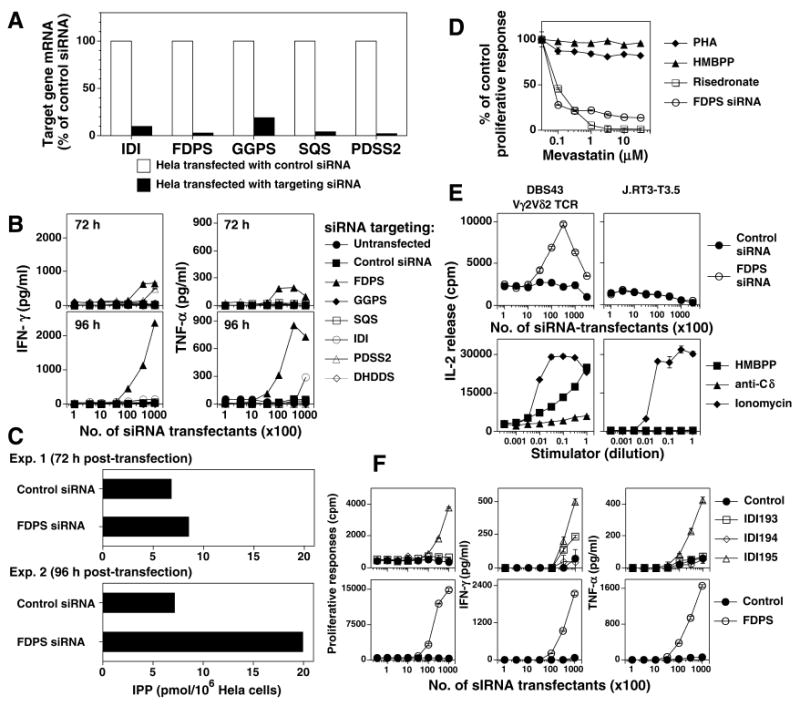
A, siRNA treatment greatly decreases mRNA levels of most enzymes in isoprenoid biosynthesis. mRNA levels of enzymes targeted by siRNA were measured in comparison to control siRNA using real-time PCR as detailed in the Materials and Methods. B, Downregulation of FDPS results in APC that stimulate Vγ2Vδ2 T cells. HeLa cells were either untransfected or transfected with control siRNA or siRNA targeting mRNAs for enzymes required for the synthesis of isoprenoid compounds. After 72 h and 96 h, transfected HeLa cells were mixed with HF.2 Vγ2Vδ2 T cells. Supernatants were harvested 16 hours later, and the levels of IFN-γ (left panels) and TNF-α (right panels) determined by ELISA. For each enzyme, 3 siRNAs were tested with the best siRNA shown. Results are representative of 3 experiments. C, Increased intracellular IPP levels in HeLa cells after transfection with siRNA to FDPS. HeLa cells were transfected with either a control siRNA or a siRNA to FDPS. After 72 h or 96 h, the cells were harvested and intracellular IPP level measured. D, Stimulation by APC treated with siRNA to FDPS is sensitive to statin inhibition. HeLa cells were transfected with siRNA to FDPS and after 72 h cultured with HF.2 Vγ2Vδ2 T cells in the presence of mevastatin. For comparison, untransfected HeLa cells were either continuously cultured with 0.1 μM HMBPP or 1:4000 PHA, or pulsed with 1 mM risedronate with HF.2 T cells in the presence of mevastatin. Cultures were pulsed with 1mCi of H-thymidine on day 1 and harvested 16-18 h later. E, Recognition of FDPS siRNA-treated APC is mediated by the Vγ2Vδ2 TCR. The DBS43 Vγ2Vδ2 TCR transfectant or the parent mutant Jurkat cell line, J.RT3-T3.5, was cultured with HeLa cells treated with either a control siRNA or siRNA to FDPS and PMA or with anti-TCRδ1, ionomycin (1 μg/ml), or HMBPP (1 μM) in the presence of Va2 cells and PMA. The supernatants were harvested and IL-2 levels assessed by proliferation of the IL-2-dependent HT-2 cell line. F, Downregulation of isopentenyl diphosphate isomerase (IDI) renders APC stimulatory for Vγ2Vδ2 T cells. Mit. C-treated HeLa cells were transfected with either a control siRNA, 3 different siRNAs targeting IDI, or with a siRNA targeting FDPS. After 96 h, transfected HeLa cells were mixed with HF.2 Vγ2Vδ2 T cells. Culture supernatants were harvested 16 hours later and IFN-γ (middle panels) and TNF-α (right panels) determined by ELISA. Proliferation was assessed on day 2 (left panels).
siRNA specific for IDI also stimulated Vγ2Vδ2 T cells. HeLa cells transfected with an siRNA specific for IDI (IDI 195) stimulated moderate levels of Vγ2Vδ2 T cell proliferation, IFN-γ release, and TNF-α release (Fig. 9F, top panels) that were ~25% of the FDPS stimulation levels (Fig. 9F, bottom panels). Thus, downregulation of either FDPS or IDI renders APC stimulatory for Vγ2Vδ2 T cells whereas no stimulation of Vγ2Vδ2 T cells was found with the downregulation of other enzymes.
Discussion
This study shows that there are other indirect pathways leading to the stimulation of Vγ2Vδ2 T cells besides the inhibition of FDPS. Downregulation of IDI stimulates Vγ2Vδ2 T cells as does exposure to high concentrations of mevalonate (Fig. 10). All are related by the fact that they alter isoprenoid metabolism leading to the increased production of prenyl pyrophosphates that directly activate Vγ2Vδ2 T cells. These findings suggest that Vγ2Vδ2 T cells may be involved in surveillance for cancer cells because relatively small increases in IPP levels are recognized. Moreover, prolonged exposure of Vγ2Vδ2 T cells to higher doses of aminobisphosphonates has the paradoxical effect of inhibiting their ability to proliferate. This is due to blocking isoprenoid metabolism in the T cells. These findings suggest that aminobisphosphonates should be pulsed to limit toxicity when used for ex vivo expansion of Vγ2Vδ2 T cells for cancer immunotherapy.
FIGURE 10. Proposed mechanisms for stimulation of Vγ2Vδ2 T cells.
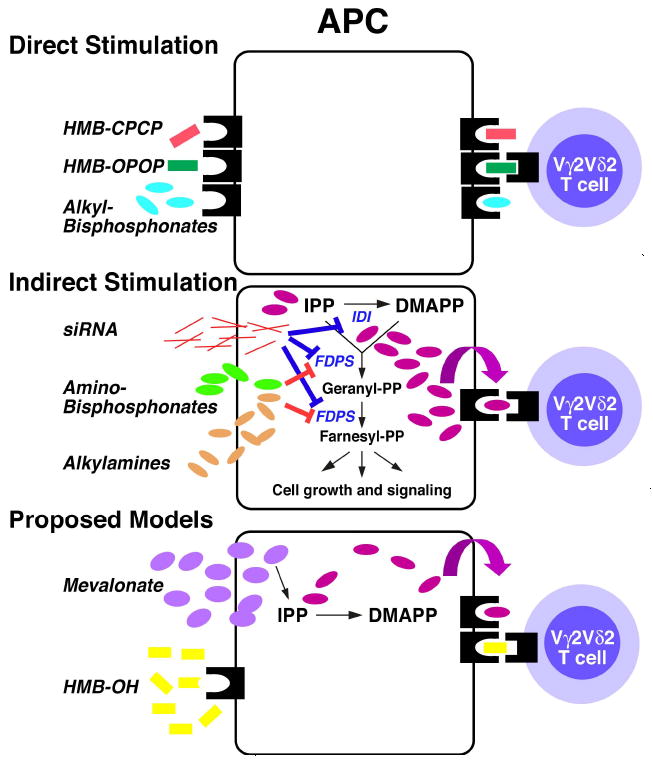
Top, Vγ2Vδ2 T cells recognize HMBPP, HMB-CPCP, and alkyl-bisphosphonates presented directly without internalization by an antigen presenting molecule on APC. Middle, aminobisphosphonates and alkylamines indirectly stimulate Vγ2Vδ2 T cells by inhibiting FDPS leading to the accumulation of IPP that can then be presented by the antigen presenting molecule. Transfection of FDPS and IDI siRNA also cause IPP to accumulate and stimulate Vγ2Vδ2 T cells. Bottom, proposed models for mevalonate and HMB-OH. Exogenous mevalonate, a rate limiting intermediate, increases IPP levels modestly that then stimulate Vγ2Vδ2 T cells. In contrast, HMB-OH is likely directly presented as it is relatively APC independent and there is no evidence for IPP accumulation.
Because IPP is used for the synthesis of many isoprenoid compounds, inhibition of other enzymes besides FDPS might also increase IPP levels sufficiently to stimulate Vγ2Vδ2 T cells. One candidate enzyme, IDI, is upstream of FDPS and its inhibition would be predicted to cause IPP (its substrate) to accumulate (Fig. 1). Consistent with this prediction, we found that treatment of APC with siRNA targeting IDI made them stimulatory for Vγ2Vδ2 T cells (Fig. 10, middle). This suggests that inhibitors of IDI would also stimulate Vγ2Vδ2 T cells. Because an aminobisphosphonate exists that inhibits both IDI and FDPS (57) and stimulates Vγ2Vδ2 T cells (27), it may be possible to design specific bisphosphonate inhibitors of IDI. Given that DMAPP exhibits 3-30-fold lower bioactivity than IPP (32), such compounds could have increased potency for stimulating Vγ2Vδ2 T cells or different biological effects compared with FDPS inhibitors because only IPP will accumulate rather than both IPP and DMAPP that accumulate with FDPS inhibitors. Besides IDI, no effects were seen upon GPPS inhibition using aminobisphosphonates specific for this enzyme (36) or upon APC transfection with siRNAs specific for GGPS, SQS, PDSS2, or DHDDS, suggesting that blocking only one branch of downstream isoprenoid biosynthesis is not sufficient for IPP accumulation and Vγ2Vδ2 stimulation.
Mevalonate also stimulated Vγ2Vδ2 T cells. Because mevalonate is the product of HMGCR, a rate limiting enzyme subject to tight regulation (58-61), high exogenous mevalonate concentrations would bypass normal regulation and increase the levels of downstream products including IPP and DMAPP (Fig. 1). Statins would be unable to block this stimulation as was observed in this study (Fig. 6A). Because intracellular IPP levels were below detection in both normal and mevalonate-treated cells (Fig. 6B), the degree of IPP increase is unclear, but certainly less than those observed with aminobisphosphonate treatment. Because relatively small increases in IPP levels (25% for FDPS siRNA treated cells) stimulated Vγ2Vδ2 T cells (Fig. 9C), there easily could have been sufficient increases in IPP to stimulate γδ T cells. Based on our findings, we propose that mevalonate acts indirectly—stimulating Vγ2Vδ2 T cells by increasing endogenous IPP levels in APC (Fig. 10, bottom).
Although aminobisphosphonates stimulate Vγ2Vδ2 T cells to release TNF-α, we found that they could also inhibit Vγ2Vδ2 T cell proliferation upon continuous exposure. The blocking of FDPS by aminobisphosphonates results in decreased levels of farnesyl pyrophosphate and geranylgeranyl pyrophosphate. Upon activation of T cells, farnesyl and geranylgeranyl moieties are transferred to the C termini of GTPases allowing them to anchor in the inner leaflet of the plasma membrane and function in signal transduction. Prolonged exposure to aminobisphosphonates, therefore, would block signal transduction required for T cell proliferation and survival. Statin inhibition of HMGCR activity also works similarly (62). Besides blocking GTPases, statins reduce the association of Lck and Linker of Activation of T cells with membrane rafts in T cells (63). In our experiments, both aminobisphosphonates and statins blocked γδ and αβ T cell proliferation in response to a variety of different stimuli if sufficiently high doses were used. These results are consistent with reports of the broad immunological effects of statin treatment in immune and autoimmune responses that do not involve γδ T cells (29-31, 64).
Ex vivo expansion of blood Vγ2Vδ2 T cells was also inhibited by continuous exposure to aminobisphosphonates. We observed highly variable levels of Vγ2Vδ2 T cell expansion (ranging from 17-33% of CD3 T cells (Fig. 2C)) with narrow dose responses that were similar to the results of other studies (25-85% (23), and 8-49% (65)). Aminobisphosphonate toxicity occurred with exposure periods as short as 6 hours. However, pulsing aminobisphosphonates to limit T cell exposure resulted in uniform expansions of Vγ2Vδ2 T cells over a 10-30-fold dose range (Fig. 2D) rather than the 3-5-fold dose range commonly observed with continuous culture (66). During the pulsing period, monocytes take up zoledronate through fluid phase endocytosis more efficiently than Vγ2Vδ2 T cells (67), thereby reducing T cell toxicity. Pulsing aminobisphosphonate replicates in vivo exposure because aminobisphosphonates are rapidly cleared through renal excretion (they are not metabolized) and by binding to bone such that they have a half-life of ~1-2 h and less than 1% remains 24 h after infusion (68, 69). Aminobisphosphonates are being commonly used in clinical studies to expand Vγ2Vδ2 T cells ex vivo for adoptive transfer into cancer patients for immunotherapy (70-75). Our results suggest that pulsing of PBMC for 4-6 hours with higher aminobisphosphonate doses would give more consistent ex vivo expansions and, potentially, more vigorous Vγ2Vδ2 T cells for adoptive transfer.
Besides pharmacological inhibitors like aminobisphosphonates, we found that downregulation of FDPS mRNA by siRNA makes tumor cells stimulatory for Vγ2Vδ2 T cells and that this stimulation is highly sensitive to statin inhibition. Our findings confirm a study reporting that short hairpin RNA for FDPS stably expressed by tumor cells makes the tumor cells stimulatory for Vγ2Vδ2 T cells (56). Moreover, we now show that reductions in FDPS activity increase cellular IPP levels and that recognition of treated cells, like recognition of the Daudi and RPMI 8226 cell lines (47), is mediated by the Vγ2Vδ2 TCR.
Differences in the sensitivity to statin inhibition can help distinguish between different pathways of stimulation of Vγ2Vδ2 T cells(26, 27). Indirect stimulation of Vγ2Vδ2 T cells by aminobisphosphonates, alkylamines, or siRNAs inhibiting FDPS, was more sensitive to statin inhibition than direct stimulation. However, the difference in statin sensitivity varied depending on the statin used (from 10- to 154-fold difference) and on the strength of stimulation. Statin inhibition of aminobisphosphonate responses was increasingly efficient when the Vγ2Vδ2 responses were less than 45% of the maximum response (Supplemental Fig. 4). In contrast, stimulation by prenyl pyrophosphates or the SEA superantigen was relatively resistant to statin inhibition over a broad response range, requiring concentrations similar to those required to inhibit γδ responses to IL-2 and PHA and αβ T cell responses to tetanus toxoid and IL-2. Therefore, because statins inhibit both indirect and direct Vγ2Vδ2 T cell responses, it is important to measure statin inhibition over a wide statin dose range in comparison to known Vγ2Vδ2 stimulators. When performed in this manner, sensitivity to statin inhibition distinguishes between indirect stimulation by FDPS inhibition and direct stimulation of Vγ2Vδ2 T cells.
Using statin inhibition, we studied alkyl-bisphosphonates, a new class of bisphosphonates that lack amino moieties. The amino moiety in aminobisphosphonates is critically important for FDPS inhibition (76) and for inhibiting bone resorption (77). However, we found that aminobisphosphonate analogs lacking this amino moiety stimulated Vγ2Vδ2 T cells, although requiring somewhat higher concentrations (EC50 of ~300-600 μM). Vγ2Vδ2 stimulation by an alkyl-bisphosphonate was highly resistant to statin inhibition with a dose response curve identical to the directly stimulating alkyl pyrophosphate analog, n-propyl-pyrophosphate. In contrast, the similar aminobisphosphonate, pamidronate, was highly sensitive to statin inhibition (Fig. 8C). Given the differences in statin inhibition, we propose that the loss of the amino moiety switches aminobisphosphonates from indirectly stimulating through FDPS inhibition to directly stimulating Vγ2Vδ2 T cells (Fig. 10, top).
Like alkyl-bisphosphonates, HMB-CPCP is a phosphonate compound that is an analog of HMBPP. Prenyl-pyrophosphonates (–CPOP), -methylene diphosphonates (-OPCP), and -phosphorylmethylphosphonates (-CPCP) have identical carbon chains as natural prenyl pyrophosphates but have phosphonate linkages. The change in linkages affects their ability to stimulate Vγ2Vδ2 T cells. Changing both ester linkages in HMBPP to phosphonate linkages (HMB-CPCP) reduces activity by 5.8 logs (681,000-fold). Much of this decrease can be attributed to the change of the pyrophosphate to a methylene diphosphonate, since HMB-CPOP is only 2.3-fold less active than HMB-OPOP (53) whereas HMB-OPCP is ~70,800-fold less active (34, 54). Despite the differences in activity, HMB-CPCP had similar low sensitivity to statin inhibition like HMBPP. Thus, phosphonate analogs of prenyl pyrophosphates also appear to directly stimulate Vγ2Vδ2 T cells (Fig. 10, top).
HMB-OH is another analog of HMBPP that stimulates Vγ2Vδ2 T cells although it totally lacks phosphate groups (34) that are generally required for stimulation (32). To determine its mechanism of stimulation, we first assessed HMB-OH responses for their sensitivity to statin inhibition. Like HMBPP, HMB-OH was relatively resistant to inhibition suggesting that HMB-OH does not inhibit FDPS to stimulate Vγ2Vδ2 T cells. Further confirming this hypothesis, there were no increases in cellular IPP after treatment with HMB-OH. Another possibility is that HMB-OH enters cells, becomes phosphorylated to HMBPP, and then is secreted for stimulation. Some isoprenoid alcohols likely rescue aminobisphosphonate-blocked cells in this way, presumably due to a two-step salvage pathway (78-82). However, there was no evidence of extracellular HMBPP during HMB-OH stimulation given that the addition of alkaline phosphatase had no effect (Fig. 6C) but totally abrogated stimulation by HMBPP. Phosphorylation of HMB-OH might also be expected to be at least partially dependent on APC. However, HMB-OH stimulated Vγ2Vδ2 T cells with kinetics identical to HMBPP and IPP, unlike ApppI that was partially dependent on APC as reported earlier (51). Moreover, HMB-OH stimulates Vγ2Vδ2 T cells at concentrations similar to those required by HMB-CPCP and HMB-OPCP that are directly presented. Therefore, we propose that HMB-OH is directly presented (Fig. 10, bottom) and that phosphate groups are not absolutely required for stimulation of Vγ2Vδ2 T cells.
In conclusion, stimulation of Vγ2Vδ2 T cells can be classified as either direct or indirect. For direct stimulation, compounds such as prenyl pyrophosphates, prenyl pyrophosphonates, and alkyl-bisphosphonates associate with an unidentified protein at the cell surface for direct presentation to the Vγ2Vδ2 TCR (Fig. 10, top). In contrast, aminobisphosphonates and alkylamines use an indirect pathway to stimulate Vγ2Vδ2 T cells (Fig. 10, middle). These compounds enter APC and block the FDPS enzyme, leading to the accumulation of IPP that is then transported to the cell surface through an unknown process where it stimulates Vγ2Vδ2 T cells. siRNAs for FDPS and IDI decrease enzyme levels thus diminishing their action, resulting in IPP accumulation that directly stimulates Vγ2Vδ2 T cells. Indirect stimulation due to blocking FDPS (and likely IDI) function is highly sensitive to statin inhibition of the upstream HMGCR enzyme since the accumulation of IPP is dependent on metabolite flow down the pathway. Exogenous mevalonate, the rate limiting metabolite, will increase intracellular IPP levels if present at high concentration (Fig. 10, bottom). However, in this situation, statins will not easily block this indirect stimulation. Finally, HMB-OH is likely directly presented to Vγ2Vδ2 T cells since its stimulation is statin and alkaline phosphatase resistant, does not increase IPP levels, is active at similar concentrations as HMB-CPCP, and is independent of APC like HMBPP (Fig. 10, bottom). Our results demonstrate that there are multiple ways to stimulate Vγ2Vδ2 T cells. Further characterization of these indirect and direct pathways will deepen our understanding of the roles that γδ T cells play in human immunity and may improve current approaches to cancer immunotherapy using Vγ2Vδ2 T cells.
Supplementary Material
Acknowledgments
We thank Kristin J. Ness-Schwickerath and Grefachew Workalemahu for critical review of this manuscript. We thank Markku Taskinen, Amy M. Raker, Ian Kenning, and Zhimei Fang for technical assistance.
Abbreviations used in this paper
- ApppI
triphosphoric acid 1-adenosin-5′-yl ester 3-(3-methylbut-3-enyl) ester
- β2M
β2-microglobulin
- BPH
bisphosphonate
- BrH-PCP
([[(4-bromo-3-hydroxy-3-methylbutoxy)hydroxyphosphinyl]methyl]-phosphonic acid)
- BrHPP
bromohydrin pyrophosphate (3-bromo-3-hydroxybutyl pyrophosphate)
- DHDDS
dehydrodolichol diphosphate synthase
- EPP
ethyl-pyrophosphate
- FPP
farnesyl pyrophosphate
- FDPS
farnesyl disphosphate synthase
- GGPP
geranylgeranyl pyrophosphate
- GGPS
geranylgeranyl disphosphate synthase
- GPP
geranyl pyrophosphate
- HMBCPCP
(E)-(hydroxy(5-hydroxy-4-methylpent-3-enyl)phosphoryl)methylphosphonic acid
- HMBCPOP
(E)-1-hydroxy-2-methyl-pent-2-enyl pyrophosphonate
- HMB-OH
(E)-4-hydroxy-3-methyl-but-2-enol
- HMBOPCP
(E)-1-hydroxy-2-methyl-but-2-enyl4-(methylene-diphosphonate)
- HMBOPOP
(E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate
- HMBPP
(E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate
- HMGCR
3-hydroxy-3-methylglutaryl-CoA reductase
- IDI
isopentenyl diphosphate isomerase
- IPP
isopentenyl pyrophosphate
- LC/MS
liquid chromatography/mass spectrometry
- MEP
2-C-methyl-d-erythritol-4-phosphate
- mit. C
mitomycin C
- OPP
pyrophosphate (diphosphate)
- PHA
phytohemagglutinin
- PDSS2
prenyl (decaprenyl) diphosphate synthase subunit 2
- SEA
staphylococcal enterotoxin A
- siRNA
small interferring RNA
- SQS
squalene synthase
Footnotes
This work was supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health (AR045504), National Cancer Institute, National Institutes of Health (CA113874), and the Department of Veterans Affairs (BX000972) to C.T.M., by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (AI074233) to E.O., and by the Academy of Finland to J.M.
Disclosures: C.T.M. is a coinventor of U.S. Patent 8,012,466 on the development of live bacterial vaccines for activating γδ T cells. E.O. is a coinventor of U.S. Patents 7,358,361; 7,687, 482; 7,745,422; and 8,012,949 on bisphosphonates for cancer therapy.
The online version of this article contains supplemental material.
References
- 1.Morita CT, Jin C, Sarikonda G, Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vγ2Vδ2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol Rev. 2007;215:59–76. doi: 10.1111/j.1600-065X.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- 2.Shen Y, Zhou D, Qiu L, Lai X, Simon M, Shen L, Kou Z, Wang Q, Jiang L, Estep J, Hunt R, Clagett M, Sehgal PK, Li Y, Zeng X, Morita CT, Brenner MB, Letvin NL, Chen ZW. Adaptive immune response of Vγ2Vδ2+ T cells during mycobacterial infections. Science. 2002;295:2255–2258. doi: 10.1126/science.1068819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang L, Kamath A, Das H, Li L, Bukowski JF. Antibacterial effect of human Vγ2Vδ2 T cells in vivo. J Clin Invest. 2001;108:1349–1357. doi: 10.1172/JCI13584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilhelm M, Kunzmann V, Eckstein S, Reimer P, Weissinger F, Ruediger T, Tony H-P. γδ T cells for immune therapy of patients with lymphoid malignancies. Blood. 2003;102:200–206. doi: 10.1182/blood-2002-12-3665. [DOI] [PubMed] [Google Scholar]
- 5.Dieli F, Vermijlen D, Fulfaro F, Caccamo N, Meraviglia S, Cicero G, Roberts A, Buccheri S, D’Asaro M, Gebbia N, Salerno A, Eberl M, Hayday AC. Targeting human γδ T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 2007;67:7450–7457. doi: 10.1158/0008-5472.CAN-07-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobayashi H, Tanaka Y, Shimmura H, Minato N, Tanabe K. Complete remission of lung metastasis following adoptive immunotherapy using activated autologous γδ T-cells in a patient with renal cell carcinoma. Anticancer Res. 2010;30:575–579. [PubMed] [Google Scholar]
- 7.Kobayashi H, Tanaka Y, Yagi J, Minato N, Tanabe K. Phase I/II study of adoptive transfer of γδ T cells in combination with zoledronic acid and IL-2 to patients with advanced renal cell carcinoma. Cancer Immunol Immunother. 2011;60:1075–1084. doi: 10.1007/s00262-011-1021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meraviglia S, Eberl M, Vermijlen D, Todaro M, Buccheri S, Cicero G, La Mendola C, Guggino G, D’Asaro M, Orlando V, Scarpa F, Roberts A, Caccamo N, Stassi G, Dieli F, Hayday AC. In vivo manipulation of Vγ9Vδ2 T cells with zoledronate and low-dose interleukin-2 for immunotherapy of advanced breast cancer patients. Clin Exp Immunol. 2010;161:290–297. doi: 10.1111/j.1365-2249.2010.04167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morita CT, Mariuzza RA, Brenner MB. Antigen recognition by human γδ T cells: pattern recognition by the adaptive immune system. Springer Semin Immunopathol. 2000;22:191–217. doi: 10.1007/s002810000042. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka Y, Sano S, Nieves E, De Libero G, Roca D, Modlin RL, Brenner MB, Bloom BR, Morita CT. Nonpeptide ligands for human γδ T cells. Proc Natl Acad Sci USA. 1994;91:8175–8179. doi: 10.1073/pnas.91.17.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka Y, Morita CT, Tanaka Y, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature. 1995;375:155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 12.Kunzmann V, Bauer E, Wilhelm M. γ/δ T-cell stimulation by pamidronate. N Engl J Med. 1999;340:737–738. doi: 10.1056/NEJM199903043400914. [DOI] [PubMed] [Google Scholar]
- 13.Sanders JM, Ghosh S, Chan JMW, Meints G, Wang H, Raker AM, Song Y, Colantino A, Burzynska A, Kafarski P, Morita CT, Oldfield E. Quantitative structure-activity relationships for γδ T cell activation by bisphosphonates. J Med Chem. 2004;47:375–384. doi: 10.1021/jm0303709. [DOI] [PubMed] [Google Scholar]
- 14.Bukowski JF, Morita CT, Brenner MB. Human γδ T cells recognize alkylamines derived from microbes, edible plants, and tea: implications for innate immunity. Immunity. 1999;11:57–65. doi: 10.1016/s1074-7613(00)80081-3. [DOI] [PubMed] [Google Scholar]
- 15.Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP, Monkkonen J, Frith JC. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000;88(Suppl. 12):2961–2978. doi: 10.1002/1097-0142(20000615)88:12+<2961::aid-cncr12>3.3.co;2-c. [DOI] [PubMed] [Google Scholar]
- 16.Hosking D. Pharmacological therapy of Paget’s and other metabolic bone diseases. Bone. 2006;38(Suppl. 2):S3–7. doi: 10.1016/j.bone.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Berenson JR, Vescio RA, Rosen LS, VonTeichert JM, Woo M, Swift R, Savage A, Givant E, Hupkes M, Harvey H, Lipton A. A phase I dose-ranging trial of monthly infusions of zoledronic acid for the treatment of osteolytic bone metastases. Clin Cancer Res. 2001;7:478–485. [PubMed] [Google Scholar]
- 18.Costa L, Lipton A, Coleman RE. Role of bisphosphonates for the management of skeletal complications and bone pain from skeletal metastases. Support Cancer Ther. 2006;3:143–153. doi: 10.3816/SCT.2006.n.012. [DOI] [PubMed] [Google Scholar]
- 19.Das H, Wang L, Kamath A, Bukowski JF. Vγ2Vδ2 T-cell receptor-mediated recognition of aminobisphosphonates. Blood. 2001;98:1616–1618. doi: 10.1182/blood.v98.5.1616. [DOI] [PubMed] [Google Scholar]
- 20.Kato Y, Tanaka Y, Tanaka H, Yamashita S, Minato N. Requirement of species-specific interactions for the activation of human γδ T cells by pamidronate. J Immunol. 2003;170:3608–3613. doi: 10.4049/jimmunol.170.7.3608. [DOI] [PubMed] [Google Scholar]
- 21.Miyagawa F, Tanaka Y, Yamashita S, Minato N. Essential requirement of antigen presentation by monocyte lineage cells for the activation of primary human γδ T cells by aminobisphosphonate antigen. J Immunol. 2001;166:5508–5514. doi: 10.4049/jimmunol.166.9.5508. [DOI] [PubMed] [Google Scholar]
- 22.Dieli F, Gebbia N, Poccia F, Caccamo N, Montesano C, Fulfaro F, Arcara C, Valerio MR, Meraviglia S, Di Sano C, Sireci G, Salerno A. Induction of γδ T-lymphocyte effector functions by bisphosphonate zoledronic acid in cancer patients in vivo. Blood. 2003;102:2310–2311. doi: 10.1182/blood-2003-05-1655. [DOI] [PubMed] [Google Scholar]
- 23.Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Stimulation of γδ T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood. 2000;96:384–392. [PubMed] [Google Scholar]
- 24.Wang H, Fang Z, Morita CT. Vγ2Vδ2 T cell receptor recognition of prenyl pyrophosphates is dependent on all CDRs. J Immunol. 2010;184:6209–6222. doi: 10.4049/jimmunol.1000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morita CT, Beckman EM, Bukowski JF, Tanaka Y, Band H, Bloom BR, Golan DE, Brenner MB. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human γδ T cells. Immunity. 1995;3:495–507. doi: 10.1016/1074-7613(95)90178-7. [DOI] [PubMed] [Google Scholar]
- 26.Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De Libero G. Human T cell receptor γδ cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197:163–168. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson K, Rogers MJ. Statins prevent bisphosphonate-induced γδ-T-cell proliferation and activation in vitro. J Bone Miner Res. 2004;19:278–288. doi: 10.1359/JBMR.0301230. [DOI] [PubMed] [Google Scholar]
- 28.Thompson K, Rojas-Navea J, Rogers MJ. Alkylamines cause Vγ9Vδ2 T-cell activation and proliferation by inhibiting the mevalonate pathway. Blood. 2006;107:651–654. doi: 10.1182/blood-2005-03-1025. [DOI] [PubMed] [Google Scholar]
- 29.Youssef S, Stüve O, Patarroyo JC, Ruiz PJ, Radosevich JL, Hur EM, Bravo M, Mitchell DJ, Sobel RA, Steinman L, Zamvil SS. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420:78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- 30.Ulivieri C, Fanigliulo D, Benati D, Pasini FL, Baldari CT. Simvastatin impairs humoral and cell-mediated immunity in mice by inhibiting lymphocyte homing, T-cell activation and antigen cross-presentation. Eur J Immunol. 2008;38:2832–2844. doi: 10.1002/eji.200838278. [DOI] [PubMed] [Google Scholar]
- 31.Zeiser R, Maas K, Youssef S, Dürr C, Steinman L, Negrin RS. Regulation of different inflammatory diseases by impacting the mevalonate pathway. Immunology. 2009;127:18–25. doi: 10.1111/j.1365-2567.2008.03011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morita CT, Lee HK, Wang H, Li H, Mariuzza RA, Tanaka Y. Structural features of nonpeptide prenyl pyrophosphates that determine their antigenicity for human γδ T cells. J Immunol. 2001;167:36–41. doi: 10.4049/jimmunol.167.1.36. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka Y, Kobayashi H, Terasaki T, Toma H, Aruga A, Uchiyama T, Mizutani K, Mikami B, Morita CT, Minato N. Synthesis of pyrophosphate-containing compounds that stimulate Vγ2Vδ2 T cells: application to cancer immunotherapy. Med Chem. 2007;3:85–99. doi: 10.2174/157340607779317544. [DOI] [PubMed] [Google Scholar]
- 34.Amslinger S, Hecht S, Rohdich F, Eisenreich W, Adam P, Bacher A, Bauer S. Stimulation of Vγ9/Vδ2 T-lymphocyte proliferation by the isoprenoid precursor, (E)-1-hydroxy-2-methyl-but-2-enyl 4-diphosphate. Immunobiology. 2007;212:47–55. doi: 10.1016/j.imbio.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Sanders JM, Song Y, Chan JM, Zhang Y, Jennings S, Kosztowski T, Odeh S, Flessner R, Schwerdtfeger C, Kotsikorou E, Meints GA, Gomez AO, Gonzalez-Pacanowska D, Raker AM, Wang H, van Beek ER, Papapoulos SE, Morita CT, Oldfield E. Pyridinium-1-yl bisphosphonates are potent inhibitors of farnesyl diphosphate synthase and bone resorption. J Med Chem. 2005;48:2957–2963. doi: 10.1021/jm040209d. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Cao R, Yin F, Lin FY, Wang H, Krysiak K, No JH, Mukkamala D, Houlihan K, Li J, Morita CT, Oldfield E. Lipophilic pyridinium bisphosphonates: potent γδ T cell stimulators. Angew Chem Int Ed Engl. 2010;49:1136–1138. doi: 10.1002/anie.200905933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giner J-L. A convenient synthesis of (E)-4-hydroxy-3-methyl-2-butenyl pyrophosphate and its [4-13C]-labeled form. Tetrahedron Lett. 2002;43:5457–5459. [Google Scholar]
- 38.Song Y, Zhang Y, Wang H, Raker AM, Sanders JM, Broderick E, Clark A, Morita CT, Oldfield E. Synthesis of chiral phosphoantigens and their activity in γδ T cell stimulation. Bioorg Med Chem Lett. 2004;14:4471–4477. doi: 10.1016/j.bmcl.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 39.Morita CT, Parker CM, Brenner MB, Band H. T cell receptor usage and functional capabilities of human γδ T cells at birth. J Immunol. 1994;153:3979–3988. [PubMed] [Google Scholar]
- 40.Morita CT, Verma S, Aparicio P, Martinez-A C, Spits H, Brenner MB. Functionally distinct subsets of human γ/δ T cells. Eur J Immunol. 1991;21:2999–3007. doi: 10.1002/eji.1830211215. [DOI] [PubMed] [Google Scholar]
- 41.Spits H, Paliard X, Vandekerckhove Y, van Vlasselaer P, de Vries JE. Functional and phenotypic differences between CD4+ and CD4- T cell receptor-γδ clones from peripheral blood. J Immunol. 1991;147:1180–1188. [PubMed] [Google Scholar]
- 42.Leslie DS, Vincent MS, Spada FM, Das H, Sugita M, Morita CT, Brenner MB. CD1-mediated γ/δ T cell maturation of dendritic cells. J Exp Med. 2002;196:1575–1584. doi: 10.1084/jem.20021515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roncarolo MG, Yssel H, Touraine J-L, Bacchetta R, Gebuhrer L, de Vries JE, Spits H. Antigen recognition by MHC-incompatible cells of a human mismatched chimera. J Exp Med. 1988;168:2139–2152. doi: 10.1084/jem.168.6.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarikonda G, Wang H, Puan K-J, Liu X-H, Lee HK, Song Y, Distefano MD, Oldfield E, Prestwich GD, Morita CT. Photoaffinity antigens for human γδ T cells. J Immunol. 2008;181:7738–7750. doi: 10.4049/jimmunol.181.11.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morita CT, Li H, Lamphear JG, Rich RR, Fraser JD, Mariuzza RA, Lee HK. Superantigen recognition by γδ T cells: SEA recognition site for human Vγ2 T cell receptors. Immunity. 2001;14:331–344. doi: 10.1016/s1074-7613(01)00113-3. [DOI] [PubMed] [Google Scholar]
- 46.Jauhiainen M, Mönkkönen H, Räikkönen J, Mönkkönen J, Auriola S. Analysis of endogenous ATP analogs and mevalonate pathway metabolites in cancer cell cultures using liquid chromatography-electrospray ionization mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:2967–2975. doi: 10.1016/j.jchromb.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 47.Bukowski JF, Morita CT, Tanaka Y, Bloom BR, Brenner MB, Band H. Vγ2Vδ2 TCR-dependent recognition of non-peptide antigens and Daudi cells analyzed by TCR gene transfer. J Immunol. 1995;154:998–1006. [PubMed] [Google Scholar]
- 48.Wang H, Lee HK, Bukowski JF, Li H, Mariuzza RA, Chen ZW, Nam K-H, Morita CT. Conservation of nonpeptide antigen recognition by rhesus monkey Vγ2Vδ2 T cells. J Immunol. 2003;170:3696–3706. doi: 10.4049/jimmunol.170.7.3696. [DOI] [PubMed] [Google Scholar]
- 49.Kamath AB, Wang L, Das H, Li L, Reinhold VN, Bukowski JF. Antigens in tea-beverage prime human Vγ2Vδ2 T cells in vitro and in vivo for memory and nonmemory antibacterial cytokine responses. Proc Natl Acad Sci USA. 2003;100:6009–6014. doi: 10.1073/pnas.1035603100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castella B, Riganti C, Fiore F, Pantaleoni F, Canepari ME, Peola S, Foglietta M, Palumbo A, Bosia A, Coscia M, Boccadoro M, Massaia M. Immune modulation by zoledronic acid in human myeloma: an advantageous cross-talk between Vγ9Vδ2 T cells, αβ CD8+ T cells, regulatory T cells, and dendritic cells. J Immunol. 2011;187:1578–1590. doi: 10.4049/jimmunol.1002514. [DOI] [PubMed] [Google Scholar]
- 51.Vantourout P, Mookerjee-Basu J, Rolland C, Pont F, Martin H, Davrinche C, Martinez LO, Perret B, Collet X, Périgaud C, Peyrottes S, Champagne E. Specific requirements for Vγ9Vδ2 T cell stimulation by a natural adenylated phosphoantigen. J Immunol. 2009;183:3848–3857. doi: 10.4049/jimmunol.0901085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zgani I, Menut C, Seman M, Gallois V, Laffont V, Liautard J, Liautard J-P, Criton M, Montero J-L. Synthesis of prenyl pyrophosphonates as new potent phosphoantigens inducing selective activation of human Vγ9Vδ2 T lymphocytes. J Med Chem. 2004;47:4600–4612. doi: 10.1021/jm049861z. [DOI] [PubMed] [Google Scholar]
- 53.Boedëc A, Sicard H, Dessolin J, Herbette G, Ingoure S, Raymond C, Belmant C, Kraus J-L. Synthesis and biological activity of phosphonate analogues and geometric isomers of the highly potent phosphoantigen (E)-1-hydroxy-2-methylbut-2-enyl 4-diphosphate. J Med Chem. 2008;51:1747–1754. doi: 10.1021/jm701101g. [DOI] [PubMed] [Google Scholar]
- 54.Reichenberg A, Hintz M, Kletschek Y, Kuhl T, Haug C, Engel R, Moll J, Ostrovsky DN, Jomaa H, Eberl M. Replacing the pyrophosphate group of HMB-PP by a diphosphonate function abrogates its potential to activate human γδ T cells but does not lead to competitive antagonism. Bioorg Med Chem Lett. 2003;13:1257–1260. doi: 10.1016/s0960-894x(03)00138-0. [DOI] [PubMed] [Google Scholar]
- 55.Belmant C, Espinosa E, Halary F, Tang Y, Peyrat M-A, Sicard H, Kozikowski A, Buelow R, Poupot R, Bonneville M, Fournié J-J. A chemical basis for selective recognition of nonpeptide antigens by human γδ T cells. FASEB J. 2000;14:1669–1670. doi: 10.1096/fj.99-0909fje. [DOI] [PubMed] [Google Scholar]
- 56.Li J, Herold MJ, Kimmel B, Muller I, Rincon-Orozco B, Kunzmann V, Herrmann T. Reduced expression of the mevalonate pathway enzyme farnesyl pyrophosphate synthase unveils recognition of tumor cells by Vγ9Vδ2 T cells. J Immunol. 2009;182:8118–8124. doi: 10.4049/jimmunol.0900101. [DOI] [PubMed] [Google Scholar]
- 57.Thompson K, Dunford JE, Ebetino FH, Rogers MJ. Identification of a bisphosphonate that inhibits isopentenyl diphosphate isomerase and farnesyl diphosphate synthase. Biochem Biophys Res Commun. 2002;290:869–873. doi: 10.1006/bbrc.2001.6289. [DOI] [PubMed] [Google Scholar]
- 58.DeBose-Boyd RA. Feedback regulation of cholesterol synthesis: sterol-accelerated ubiquitination and degradation of HMG CoA reductase. Cell Res. 2008;18:609–621. doi: 10.1038/cr.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Istvan ES, Palnitkar M, Buchanan SK, Deisenhofer J. Crystal structure of the catalytic portion of human HMG-CoA reductase: insights into regulation of activity and catalysis. EMBO J. 2000;19:819–830. doi: 10.1093/emboj/19.5.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jo Y, Debose-Boyd RA. Control of cholesterol synthesis through regulated ER-associated degradation of HMG CoA reductase. Crit Rev Biochem Mol Biol. 2010;45:185–198. doi: 10.3109/10409238.2010.485605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Omkumar RV, Rodwell VW. Phosphorylation of Ser871 impairs the function of His865 of Syrian hamster 3-hydroxy-3-methylglutaryl-CoA reductase. J Biol Chem. 1994;269:16862–16866. [PubMed] [Google Scholar]
- 62.Brinkkoetter P-T, Gottmann U, Schulte J, van der Woude FJ, Braun C, Yard BA. Atorvastatin interferes with activation of human CD4+ T cells via inhibition of small guanosine triphosphatase (GTPase) activity and caspase-independent apoptosis. Clin Exp Immunol. 2006;146:524–532. doi: 10.1111/j.1365-2249.2006.03217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blank N, Schiller M, Krienke S, Busse F, Schätz B, Ho AD, Kalden JR, Lorenz H-M. Atorvastatin inhibits T cell activation through 3-hydroxy-3-methylglutaryl coenzyme A reductase without decreasing cholesterol synthesis. J Immunol. 2007;179:3613–3621. doi: 10.4049/jimmunol.179.6.3613. [DOI] [PubMed] [Google Scholar]
- 64.Aktas O, Waiczies S, Smorodchenko A, Dörr J, Seeger B, Prozorovski T, Sallach S, Endres M, Brocke S, Nitsch R, Zipp F. Treatment of relapsing paralysis in experimental encephalomyelitis by targeting Th1 cells through atorvastatin. J Exp Med. 2003;197:725–733. doi: 10.1084/jem.20021425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simoni D, Gebbia N, Invidiata FP, Eleopra M, Marchetti P, Rondanin R, Baruchello R, Provera S, Marchioro C, Tolomeo M, Marinelli L, Limongelli V, Novellino E, Kwaasi A, Dunford J, Buccheri S, Caccamo N, Dieli F. Design, synthesis, and biological evaluation of novel aminobisphosphonates possessing an in vivo antitumor activity through a γδ-T lymphocytes-mediated activation mechanism. J Med Chem. 51:6800–6807. doi: 10.1021/jm801003y. [DOI] [PubMed] [Google Scholar]
- 66.Sato K, Kimura S, Segawa H, Yokota A, Matsumoto S, Kuroda J, Nogawa M, Yuasa T, Kiyono Y, Wada H, Maekawa T. Cytotoxic effects of γδ T cells expanded ex vivo by a third generation bisphosphonate for cancer immunotherapy. Int J Cancer. 116:94–99. doi: 10.1002/ijc.20987. [DOI] [PubMed] [Google Scholar]
- 67.Roelofs AJ, Jauhiainen M, Mönkkönen H, Rogers MJ, Mönkkönen J, Thompson K. Peripheral blood monocytes are responsible for γδ T cell activation induced by zoledronic acid through accumulation of IPP/DMAPP. Br J Haematol. 2009;144:245–250. doi: 10.1111/j.1365-2141.2008.07435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin JH. Bisphosphonates: a review of their pharmacokinetic properties. Bone. 1996;18:75–85. doi: 10.1016/8756-3282(95)00445-9. [DOI] [PubMed] [Google Scholar]
- 69.Chen T, Berenson J, Vescio R, Swift R, Gilchick A, Goodin S, LoRusso P, Ma P, Ravera C, Deckert F, Schran H, Seaman J, Skerjanec A. Pharmacokinetics and pharmacodynamics of zoledronic acid in cancer patients with bone metastases. J Clin Pharmacol. 2002;42:1228–1236. doi: 10.1177/009127002762491316. [DOI] [PubMed] [Google Scholar]
- 70.Kondo M, Sakuta K, Noguchi A, Ariyoshi N, Sato K, Sato S, Sato K, Hosoi A, Nakajima J, Yoshida Y, Shiraishi K, Nakagawa K, Kakimi K. Zoledronate facilitates large-scale ex vivo expansion of functional γδ T cells from cancer patients for use in adoptive immunotherapy. Cytotherapy. 2008;10:842–856. doi: 10.1080/14653240802419328. [DOI] [PubMed] [Google Scholar]
- 71.Abe Y, Muto M, Nieda M, Nakagawa Y, Nicol A, Kaneko T, Goto S, Yokokawa K, Suzuki K. Clinical and immunological evaluation of zoledronate-activated Vγ9γδ T-cell-based immunotherapy for patients with multiple myeloma. Exp Hematol. 2009;37:956–968. doi: 10.1016/j.exphem.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 72.Mehrle S, Watzl C, von Lilienfeld-Toal M, Amoroso A, Schmidt J, Märten A. Comparison of phenotype of γδ T cells generated using various cultivation methods. Immunol Lett. 2009;125:53–58. doi: 10.1016/j.imlet.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 73.Nicol AJ, Tokuyama H, Mattarollo SR, Hagi T, Suzuki K, Yokokawa K, Nieda M. Clinical evaluation of autologous gamma delta T cell-based immunotherapy for metastatic solid tumours. Br J Cancer. 2011;105:778–786. doi: 10.1038/bjc.2011.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sakamoto M, Nakajima J, Murakawa T, Fukami T, Yoshida Y, Murayama T, Takamoto S, Matsushita H, Kakimi K. Adoptive immunotherapy for advanced non-small cell lung cancer using zoledronate-expanded γδ T cells: a phase I clinical study. J Immunother. 2011;34:202–211. doi: 10.1097/CJI.0b013e318207ecfb. [DOI] [PubMed] [Google Scholar]
- 75.Noguchi A, Kaneko T, Kamigaki T, Fujimoto K, Ozawa M, Saito M, Ariyoshi N, Goto S. Zoledronate-activated Vγ9 γδ T cell-based immunotherapy is feasible and restores the impairment of γδ T cells in patients with solid tumors. Cytotherapy. 2011;13:92–97. doi: 10.3109/14653249.2010.515581. [DOI] [PubMed] [Google Scholar]
- 76.Dunford JE, Kwaasi AA, Rogers MJ, Barnett BL, Ebetino FH, Russell RG, Oppermann U, Kavanagh KL. Structure-activity relationships among the nitrogen containing bisphosphonates in clinical use and other analogues: time-dependent inhibition of human farnesyl pyrophosphate synthase. J Med Chem. 2008;51:2187–2195. doi: 10.1021/jm7015733. [DOI] [PubMed] [Google Scholar]
- 77.Dunford JE, Thompson K, Coxon FP, Luckman SP, Hahn FM, Poulter CD, Ebetino FH, Rogers MJ. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J Pharmacol Exp Ther. 2001;296:235–242. [PubMed] [Google Scholar]
- 78.Bentinger M, Grünler J, Peterson E, Swiezewska E, Dallner G. Phosphorylation of farnesol in rat liver microsomes: properties of farnesol kinase and farnesyl phosphate kinase. Arch Biochem Biophys. 1998;353:191–198. doi: 10.1006/abbi.1998.0611. [DOI] [PubMed] [Google Scholar]
- 79.Crick DC, Andres DA, Waechter CJ. Novel salvage pathway utilizing farnesol and geranylgeraniol for protein isoprenylation. Biochem Biophys Res Commun. 1997;237:483–487. doi: 10.1006/bbrc.1997.7145. [DOI] [PubMed] [Google Scholar]
- 80.Thai L, Rush JS, Maul JE, Devarenne T, Rodgers DL, Chappell J, Waechter CJ. Farnesol is utilized for isoprenoid biosynthesis in plant cells via farnesyl pyrophosphate formed by successive monophosphorylation reactions. Proc Natl Acad Sci USA. 1999;96:13080–13085. doi: 10.1073/pnas.96.23.13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shridas P, Waechter CJ. Human dolichol kinase, a polytopic endoplasmic reticulum membrane protein with a cytoplasmically oriented CTP-binding site. J Biol Chem. 2006;281:31696–31704. doi: 10.1074/jbc.M604087200. [DOI] [PubMed] [Google Scholar]
- 82.Ischebeck T, Zbierzak AM, Kanwischer M, Dörmann P. A salvage pathway for phytol metabolism in Arabidopsis. J Biol Chem. 2006;281:2470–2477. doi: 10.1074/jbc.M509222200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


