Abstract
Aim
Studies characterizing treatment interventions in a naturalistic setting suggest antidepressant and antipsychotic medications may be equally effective in improving clinical outcome in individuals at high risk for first-episode psychosis. Of interest, both beneficial as well as potentially adverse effects have been observed following fluoxetine treatment in a mouse prenatal immune activation model of relevance to psychosis prevention. We sought to extend those findings by examining the effects of fluoxetine, as well as the antipsychotic medication aripiprazole, in a rat prenatal immune activation model.
Methods
Pregnant Sprague-Dawley rats were injected with poly I:C or saline on gestational day 14. Offspring of poly I:C and saline-treated dams received fluoxetine (10.0 mg/kg/d), aripiprazole (0.66 mg/kg/d), or vehicle from postnatal days 35 to 70. Locomotor responses to novelty, saline injection, and amphetamine (1 and 5 mg/kg) were determined at three months, i.e., 21 days following drug discontinuation.
Results
Both fluoxetine and aripiprazole had beneficial effects on behavioral response to amphetamine (1 mg/kg) at 3 months, ameliorating the impact of prenatal immune activation on offspring of poly I:C-treated dams. Significantly, both drugs also exerted effects in offspring of control (saline-treated) dams on locomotor response to injection.
Conclusions
Fluoxetine and aripiprazole pretreatment of poly I:C offspring from postnatal days 35 to 70 stabilized response to amphetamine exposure persisting through 3 months of age, similar to earlier findings in mice that fluoxetine treatment following prenatal immune activation prevented altered locomotor response to amphetamine. The current data also confirm earlier findings of potential adverse behavioral effects in offspring of control dams following treatment with fluoxetine and antipsychotic medications, highlighting the potential for both therapeutic as well as safety concerns with exposure to preventive pharmacological treatments over the course of adolescent development. Further study is needed to determine clinical and epidemiological consequences of these pre-clinical findings.
Keywords: maternal, schizophrenia, animal model, psychosis, dopamine, serotonin
1. Introduction
While the majority of pharmacological treatments for patients with established psychotic illness focus on the dopaminergic system, an evolving literature suggests a potential role for other neurotransmitter systems, including the serotonergic system. Cornblatt et al. developed a comprehensive assessment program for adolescents and young adults at risk for schizophrenia, including a naturalistic treatment model which showed success with antidepressant medications, predominantly selective serotonin reuptake inhibitors, in delaying psychosis onset [1, 2]. While limited sample size and high non-compliance rates suggest caution in generalizing those promising results, a recent study also observed decreased cortical 5-HT2A receptor density associated with first-episode psychosis risk in prodromal schizophrenia patients [3], further implicating opportunities for serotonergic targets for early intervention. Additionally, evidence suggests a protective effect mediated through actions at serotonin 5-HT2A receptors against abnormal locomotor response to amphetamine following neonatal hippocampal lesion, an animal model of relevance to schizophrenia [4]. In combination, these observations suggest the potential for serotonergic agents as a target for primary prevention, as they may also offer superior side effect, drug safety, and patient compliance profiles compared to antipsychotic medications.
Because of the tremendous human and economic burden of schizophrenia, primary prevention modalities of even modest impact would likely have significant public health consequence, and a growing number of studies have examined preventive treatment for individuals at high risk of developing first-episode psychosis [1, 2, 5-14]. In combination, these studies suggest that for many prodromal patients clinical need provides a rationale for early intervention, and that effective preventive intervention appears to be a feasible goal. Because side effects including weight gain/metabolic issues associated with atypical antipsychotic medications negatively impact medication compliance, the selective serotonin reuptake inhibitor fluoxetine and partial agonist atypical antipsychotic medication aripiprazole are frequently prescribed to adolescent patients. Both medications exert effects on serotonergic systems (fluoxetine through serotonin transporter inhibition, and aripiprazole through serotonin 5-HT1A, 5-HT2A, and 5-HT2C receptor interactions). Here we tested the long-term effects of fluoxetine and aripiprazole on behavioral outcomes following prenatal immune activation.
Prenatal immune activation provides a well-studied developmental model of relevance to schizophrenia, based upon elevated schizophrenia risk following prenatal infection [15]. Prenatal infection stimulates maternal cytokines, soluble polypeptides mediating the innate inflammatory response. Consequences to the offspring of maternal cytokine elevation have been studied in prenatal immune activation animal models using immunogens including the synthetic nucleic acid poly I:C, which stimulates cytokine expression through Toll-like receptor TLR3 activation [16]. When combined with the appropriate host genetic background [17], prenatal immune activation is a suspected environmental risk factor for schizophrenia [18]. Studies in multiple laboratories have identified cellular, neurochemical, structural, behavioral, and cognitive alterations of relevance to schizophrenia following prenatal immune activation with poly I:C, the bacterial endotoxin lipopolysaccharide (LPS), and with direct injection of pro-inflammatory cytokines [recently reviewed in [18-22]]. We and others [23-26] have previously observed protective effects of clozapine, haloperidol, risperidone, paliperidone and fluoxetine against the emergence of behavioral and structural abnormalities following prenatal immune activation. Persistent, potentially adverse effects of pharmacological treatment have also been observed in adult offspring of control animals. Here we sought to extend those findings by 1.) determining if treatment with fluoxetine in a rat prenatal immune activation model resulted in similar outcomes to those identified in a mouse prenatal immune activation model [24], and 2.) determining the long-term effects of treatment with the high affinity dopamine D2/D3 receptor partial antagonist aripiprazole following prenatal immune activation. We hypothesized treatment with fluoxetine or aripiprazole across adolescence and young adulthood would provide longstanding protection following prenatal immune activation against abnormal behavioral response to the indirect dopamine agonist amphetamine. We also sought to determine whether these treatments would result in long-standing behavioral effects in offspring of control dams.
2. Materials and Methods
2.1. Animals
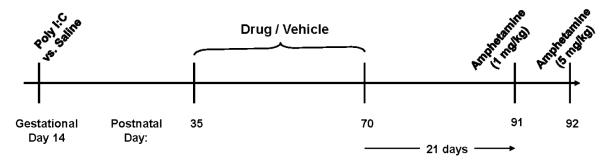
A summary of the experimental design is shown in Figure 1. Nulliparous female Sprague-Dawley rats for use as breeders were obtained from Harlan Laboratories (Indianapolis, IN) and male breeders were generated within our facility. Following a minimum two week acclimatization, males and females were co-housed overnight, with the following morning defined as gestational day 0 [27]. Pregnant rats, identified by weight gain of ≥ 40 g, were injected with the synthetic nucleic acid analogue poly I:C (Sigma P1530; 8 mg/kg, i.p.) or vehicle (saline, 1 ml/kg) on gestational day 14 to stimulate a maternal inflammatory response. The timing of poly I:C injection was based upon the work of Zuckerman and colleagues describing outcomes following poly I:C injection on varying gestational dates in rats [28]. (Note gestational day 15 in Zuckerman’s study [28] is defined as gestational day 14 in some widely-referenced text books [27]; in our study we use the [27] definitions). The poly I:C dosage was based upon dosage ranges used by other investigators for rat intraperitoneal injection [reported dose range 0.75 to 20 mg/kg; mean dose 10 mg/kg [[29, 30]]. Based upon a study describing anorexia and weight loss associated with maternal immune activation [29], weight change was determined in the pregnant dams over the 24 hour period following poly I:C injection. Offspring of poly I:C-injected dams without weight loss were excluded from study. Poly I:C-treated dams included in the study experienced weight loss of 3 or more grams. We have previously determined that offspring from poly I:C-injected dams without weight loss exhibit similar amphetamine-stimulated locomotion to offspring of saline-injected dams using this injection regimen [31]. Offspring from two poly I:C-treated dams and one saline-treated dam were excluded based on these weight change criteria. All experimental animals were born within a six-week period.
Figure 1. Experimental design summary.
Pregnant dams received poly I:C or saline injection on gestational day 14. Male and female offspring received drug or vehicle treatment in drinking water on postnatal days 35-70. Behavioral testing was performed at 3 months of age.
Offspring were weaned on postnatal day 21 and housed 2-3 /cage with same sex siblings. Animals were maintained at all times on the same 12-hour light:dark cycle (0600 on; 1800 off). All animal procedures were conducted in agreement with the Guide for the Care and Use of Laboratory Animals in accordance with NIH guidelines, and were approved by the Cincinnati Department of Veterans Affairs Medical Center Institutional Animal Care and Use Committee.
2.2. Drug treatment
Male and female rat pups were randomly assigned at birth to treatment with fluoxetine (10.0 mg/kg/day), aripiprazole (0.66 mg/kg/day) or vehicle via drinking water from postnatal days (PD) 35 to 70. Groups were balanced by birth cohort, and group sizes were balanced for sex. Group sample sizes were Poly I:C/Veh: n=13, Poly I:C/Fluox: n=13, Poly I:C/Arip: n=15; Sal/Veh: n=31, Sal/Fluox: n=10, Sal/Arip: n=10. The fluoxetine dose was chosen to be roughly comparable to a dosage range of 40 mg in humans, based on published values for oral dosages achieving comparable plasma fluoxetine levels in humans and rats [32]. The aripiprazole dose chosen for study has been previously identified as the oral ED50 dose in rat for effects upon prolactin release [33], and is also within a dose range exhibiting effects on neurotransmitter release in hippocampus, cortex, and nucleus accumbens when administered via a single i.p. injection [34]. The treatment period was selected in order to mirror the developmental exposure experienced by many patients who begin these medications in adolescence and continue into adulthood. In the rat, postnatal days 23 through approximately 42 are the period of pre-puberty, and postnatal days ~ 45 through postnatal day 60 represent the pubertal period of sexual maturation [35-38]. By convention, rats are considered adults at postnatal day 60, as reproductive behavior has matured by that time point [39, 40], while brain development continues beyond postnatal day 60. Animals received drug treatment administered in drinking water as previously described [41-43] in order to avoid the stress of repeated subcutaneous injections, or stress associated with the surgical implantation of minipumps. Drug administration in drinking water mimics oral administration in human patients. In more detail, following the general method of Parikh and colleagues [41], fluoxetine oral solution (20 mg/ 5 ml) and aripiprazole stock solution (7.5 mg/ml) were diluted to working solutions with deionized water. Water consumption and weight were measured daily for each animal, and the necessary volume of drug or vehicle stock solution was provided in the animal’s water bottle to supply the appropriate drug dosage.
Fluoxetine was supplied by Mallinckrodt, Inc. and aripiprazole supplied by Bristol-Myers Squibb. Both were supplied as liquid formulations, and were diluted with deionized water. D-amphetamine sulfate (Sigma, St. Louis, MO) was dissolved in 0.9% saline. Amphetamine concentration is described as free base. All injections were in a final volume of 1 ml/kg.
2.3. Behavioral Testing
Behavioral testing was performed in Residential Activity Chambers as we have previously described [44]. Locomotion was monitored with a 16 x16 photo beam array (San Diego Instruments, San Diego, CA) and is expressed as “crossovers”, defined as entry into any of the active zones of the chamber, as we and others have previously described [44-46]. Data were collected in ten-minute bins for behavioral analyses. Animals were maintained throughout behavioral testing on the same 12-hour light/dark cycle (0600 on; 1800 off) with ad lib access to food and water. Testing began at 3 months of age, i.e., 21 days following antipsychotic discontinuation. On Day 1 of testing between 0900 and 1000, animals were placed into the Residential Activity Chambers and locomotor response during the first hour in the novel environment was determined. Animals were then injected with saline (1.0 ml/kg subcutaneous), and locomotion response to injection was monitored for one hour. Animals were then injected with amphetamine (1.0 mg/kg subcutaneous), and locomotion recorded for one hour. They remained in the testing chambers overnight. The following day (Day 2) between 0900 and 1000, animals were injected with a higher amphetamine dose (5 mg/kg subcutaneous), and locomotion recorded for 7 hours. Two different amphetamine dosages were selected for study in order to observe both direct locomotion (1 mg/kg amphetamine), as well as stereotypy and post–stereotypy locomotion (5 mg/kg) effects [47, 48]. The rodent locomotor response to high dose amphetamine involves simultaneous expression of two competing behaviors, locomotion and stereotypy. Monitoring the period of elevated stereotypy, evidenced by decreased locomotion, as well as the post-stereotypy period of elevated locomotion therefore provides an additional opportunity to characterize changes in systems regulating behavioral response to dopamine transporter blockade by amphetamine [47, 48].
2.4. Statistical analysis
Locomotor data were log-transformed and analyzed by three-way ANOVA with repeated measures, with Pre-treatment (Poly I:C vs. Saline), Post-treatment (Fluoxetine vs. Aripiprazole vs. Vehicle) and Sex as main factors, Time (10-minute interval) as the repeated measure, and locomotion as dependent measure using the MIXED procedure of SAS System for Windows, (SAS Institute, Cary, NC) with statistical significance set at p < .05. Where significant interactions with Time were identified, further analyses of simple effects were conducted (slice ANOVA) using the MIXED procedure and slice option. Treatment effects within each 10-minute interval were subsequently adjusted together for multiple planned comparisons by False Discovery Rate (error rate = 0.05). Tukey-Kramer was used for post hoc analysis of significant ANOVA effects; the method takes into account the fact that several comparisons are performed simultaneously. For all analyses, treatment differences were considered statistically significant at p <0.05.
3. Results
Locomotor response to novelty
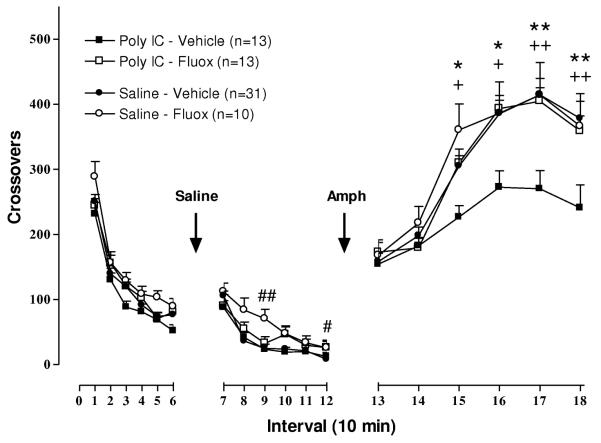
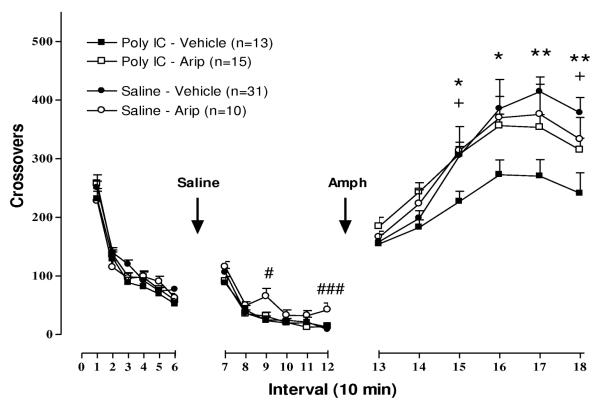
Similar to previous observations by other investigators [28, 49], ANOVA did not identify a significant Sex interaction with Pre-treatment or Post-treatment effects on novelty-stimulated locomotion, and therefore behavioral response of both sexes was combined in analysis of group effects. ANOVA identified significant effects of time [F(1,86) = 677.27, P < .0001], Pre-treatment × time interaction [F(1,86) = 5.37, P< .05] and a trend towards Post-treatment × time interaction [F (2,86) =2.84, P = .064]. Planned comparisons at each time interval did not identify any significant differences between groups, as seen in Figures 2 and 3, left panels.
Figure 2. Effect of fluoxetine on locomotor response to novelty, saline injection, and low dose amphetamine (1 mg/kg) following prenatal immune activation.
Statistically significant effects identified by slice ANOVA with False Discovery Rate adjustment are indicated. + P < .05, + +P < .01 between poly I:C/Veh and Poly I:C/Fluox; # P < .05, ## P < .01 between Sal/Veh and Sal/Fluox; * P < .05, ** P < .01 between poly I:C/Veh and Sal/Veh.
Figure 3. Effect of aripiprazole on locomotor response to novelty, saline injection, and low dose amphetamine (1 mg/kg) following prenatal immune activation.
Statistically significant effects identified by slice ANOVA with False Discovery Rate adjustment are indicated. + P < .05 between poly I:C/Veh and Poly I:C/Arip; # P < .05, ### P < .001 between Sal/Veh and Sal/Arip; * P < .05, ** P < .01 between poly I:C/Veh and Sal/Veh.
Locomotor response to saline injection
ANOVA did not identify a significant Sex interaction with Pre-treatment or Post-treatment effects on saline injection-stimulated locomotion, and therefore behavioral response of both sexes was combined in analysis of group effects. ANOVA identified a significant effect of time [F (1,86) = 318.55, P < .0001], Post-treatment × time interaction [F (2,86) = 10.19, P < .0001], and Pre-treatment × post-treatment × time interactions [F (2,86) = 4.62, P< .05].
Fluoxetine
Locomotor response to saline injection at 3 months of age is illustrated in Figure 2 (middle panel). Sal/Fluox rats exhibited elevated locomotion compared to the Sal/Veh group at the 30 and 60 minute time points following injection (intervals 9 and 12). In contrast, Poly I:C/Fluox rats did not differ from the Poly I:C/Veh or Sal/Veh treatment groups. Poly I:C/ Vehicle and Saline/ Vehicle rats did not differ in their response to a saline injection.
Aripiprazole
The effect of aripiprazole pretreatment on locomotor response to a saline injection is shown in Figure 3, middle panel. Similar to the effects of fluoxetine, Sal/Arip rats exhibited elevated locomotion compared to the Sal/Veh group at the 30 and 60 minute time points following injection (intervals 9 and 12). Poly I:C/Arip did not differ from Poly I:C/Veh or Sal/Veh groups.
Locomotor response to amphetamine (1 mg/kg)
ANOVA identified a significant Sex interaction with Pre-treatment effects on amphetamine-stimulated locomotion [F (1,80) = 6.47, P< .05], however Post-hoc Tukey-Kramer tests demonstrated no differences in comparisons between relevant groups and therefore behavioral response of both sexes was combined in analysis of group effects. ANOVA identified a significant effect of time [F (1,86) = 44.46, P <.0001], and Pre-treatment × post-treatment × time interaction [F (2,86) = 3.42, P < .05].
Fluoxetine
Post-hoc tests identified significant effects of 1.) prenatal immune activation on behavior, and 2.) fluoxetine ameliorating the impact of prenatal immune activation on behavioral outcome. As shown in Figure 2 (right panel), locomotor response of Poly I:C/Veh and Sal/Veh treatment groups differed significantly, with attenuated amphetamine-stimulated locomotion in Poly I:C/Veh rats over intervals 15-18 (30-60 minutes following amphetamine injection). The effect of poly I:C on locomotor response was ameliorated by fluoxetine post-treatment during postnatal days 35-70. Locomotor response of Poly I:C/Fluox and Poly I:C/Veh groups differed significantly from intervals 15-18 (30-60 mins following amphetamine injection). Locomotor response of Poly I:C/Fluox rats did not differ from Sal/Fluox or Sal/Veh groups. Sal/Fluox and Sal/Veh groups were also not significantly different.
Aripiprazole
As shown in Figure 3 (right panel), the reduction in amphetamine-stimulated locomotion resulting from prenatal immune activation was attenuated by aripiprazole post-treatment during postnatal days 35-70. Locomotor response of Poly I:C/Arip and Poly I:C/Veh groups differed significantly at intervals 15 and 18, 30 and 60 minutes following injection, and their was a trend towards statistical significant differences between the two groups over the intervening intervals 16 (P = .055) and 17 (P = .051). The locomotor response of the Poly I:C/Arip group did not differ from Sal/Arip or Sal/Veh groups, and there was no difference in amphetamine-stimulated locomotor response of Sal/Arip and Sal/Veh groups.
Locomotor response to amphetamine (5 mg/kg)
The rat behavioral response to high-dose amphetamine (5 mg/kg) is complicated by the simultaneous expression of two competing behaviors, locomotion and stereotypy, with an initial period of elevated locomotion followed by a period of elevated stereotypy, evidenced as decreased locomotion. The period of stereotyped behavior is then followed by a post-stereotypy period of elevated locomotion [47, 48]. In order to analyze the periods of predominantly stereotyped behavior and predominantly elevated locomotion separately, behavioral data were analyzed by ANOVA separately for intervals 0 through 20, and 21 through 40. ANOVA did not identify a significant interaction of Sex with the Pre-treatment effect on amphetamine-stimulated locomotion, and therefore behavioral response of both sexes was combined in analysis of group effects. For intervals 0 through 20 ANOVA identified significant effects of time [F (1,86) = 62.03, P < .0001] and Pre-treatment [F (1,80.2) = 16.45, P < .0001]. Tukey-Kramer post hoc comparisons identified significant differences between poly I:C and saline pre-treatment groups (P < .05). For intervals 21 through 40, ANOVA identified a significant effect of time [F (1,86) = 1349.51.44, P < .0001]. Neither fluoxetine nor aripiprazole had a significant effect on locomotor response to amphetamine (5 mg/kg) injection in poly I:C or saline pre-treatment groups (data not shown).
4. Discussion
Prenatal immune activation resulted in a blunted locomotor response to low dose (1 mg/kg) amphetamine, and also altered the locomotor response (crossovers) to a higher amphetamine dosage (5 mg/kg). While alterations in both locomotion and more complex stereotyped behavioral response to amphetamine following prenatal immune activation are suggested, effects upon behavioral sensitization, or an interaction with acclimation to multiple amphetamine exposures can’t be excluded since the higher amphetamine dose was the second stimulant drug exposure. The data above provide evidence for longstanding consequences of developmental exposure to the selective serotonin reuptake inhibitor fluoxetine, and the partial agonist atypical antipsychotic medication aripiprazole in offspring of both Poly I:C and saline-treated dams. The altered behavioral response following fluoxetine or aripiprazole exposure was observed in early adulthood, well beyond the cessation of the medication. Both fluoxetine and aripiprazole treatment also resulted in augmentation of the locomotor response to a saline injection in offspring of control (Saline – treated) dams. In contrast, in offspring of poly I:C– treated dams the blunted locomotor response to low dose amphetamine (1 mg/kg) following prenatal immune activation was normalized by prior fluoxetine and aripiprazole treatment (Figures 2-3), while the locomotor response to a saline injection was unaffected in offspring of poly I:C– treated dams.
An important contrast between our study and previously published findings is that prenatal immune activation blunted the locomotor response to amphetamine in our protocol, while adult rodents display enhanced locomotor response to amphetamine following prenatal immune activation in other published studies [reviewed in [18, 19, 21, 22]]. While the mechanisms underlying these different directions in response– elevated vs. depressed – are not known, the progression of dopamine system development following prenatal immune activation provides potential candidates. Similar to the development of mesolimbic abnormalities in schizophrenia, alterations in the mesolimbic dopamine system following prenatal immune activation exhibit a developmental progression. Indices of dopamine function are decreased early in development, with a transition to elevated dopamine function in adulthood in most but not all conditions [50]. For example, tyrosine hydroxylase and dopamine transporter immunoreactivity are decreased in caudate putamen, nucleus accumbens core, and nucleus accumbens shell in offspring of poly I:C injected dams relative to control mice at PD 35. In contrast, tyrosine hydroxylase immunoreactivity was increased at PD 70 in nucleus accumbens core and shell subregions [50]. In similar fashion, dopamine levels were decreased at postnatal day 40 following prenatal immune activation, while dopamine and DOPAC levels were similar in offspring of prenatal immune-activated and control rats in early adulthood (PD 70) [51]. Dopamine levels were elevated later in adulthood (beyond PD 170) following prenatal immune activation in one study [52], while nucleus accumbens dopamine levels remained reduced in adult offspring of rats following prenatal immune activation in a second study [53]. The locomotor response to stimulant drugs blocking dopamine transport follows a similar developmental progression. Locomotor response to amphetamine or methamphetamine was similar during the peripubertal period in offspring of prenatal immune-activated and control dams [28, 49], while in adult rodents elevated locomotor response to amphetamine and methamphetamine has been observed following prenatal immune activation [24, 28, 49, 54]. In combination, these observations suggest an adaptive interaction between a hypo-functional mesolimbic dopamine system following prenatal immune activation and developmental triggers leading to the capacity for elevated mesoaccumbens dopamine function in adulthood. The blunted response observed both in our data, and by Bakos et al. [53], may therefore result from a failure to trigger a developmental transition to elevated striatal dopamine function observed by others. Importantly, our data add to a growing literature demonstrating both hyper-responsive and hypo-responsive mesolimbic dopamine system function can be stabilized by developmental exposure to medication administration following prenatal immune activation [24-26, 55].
Because mechanism(s) underlying transition from hypo-function to elevated mesolimbic dopamine function are directly relevant to the etiology of convergence to psychosis, it will be of interest to identify methodological differences between the present study and previous investigations contributing to these differences. Experimental variables which differ between prenatal immune activation studies include agent used for induction of prenatal immune activation; dose; route of administration; use of anesthesia; timing of prenatal immune activation during gestational phase; animal species and strain; and monitoring of maternal immune activation. While the dose of poly I:C used in the current study is higher than doses administered in some other studies, drug absorption differences between intravenous drug administration in other studies compared to i.p. administration in our animals makes it difficult to directly compare the placental drug exposure achieved in these different studies. Future studies dissecting the distinct components of dopamine synthesis, release, reuptake, and individual dopamine receptor subtype contributions to the behavioral response in this model may also be of interest.
The findings presented are relevant both to pharmacological strategies for prevention of prodromal psychosis, as well as safety issues in children at-risk but not yet ill. Similar to our findings, in a prior study treatment of mice with fluoxetine or the antipsychotic medication haloperidol during postnatal days 35 through 65 following prenatal immune activation prevented altered locomotor response to amphetamine (2.5 mg/kg) in adulthood. Treatment with fluoxetine and clozapine also prevented the emergence of abnormalities in prepulse inhibition of startle [24]. Of particular interest in the context of safety concerns for preventive treatments administered to individuals at-risk, but not yet diagnosed with a disease, behavioral abnormalities were observed in control mice treated with drugs compared to control placebo-treated mice. Offspring of control mice treated with fluoxetine did not exhibit the normally observed latent inhibition effect, and also exhibited elevated locomotor response to MK-801 (0.15 mg/kg) injection. Additionally, while offspring of control mice treated with haloperidol exhibited an elevated locomotor response following saline injection, offspring of control mice treated with fluoxetine did not exhibit a comparable elevation in locomotor response to saline injection [24].
Protective effects following prenatal immune activation have also been observed in rats using clozapine [25] and risperidone [26], targeting the emergence of ventricular enlargement and reduced hippocampal volume in adulthood. Pretreatment with clozapine, risperidone and paliperidone also prevented emergence of altered locomotor response to amphetamine in adult offspring of poly I:C treated dams [25, 26, 55]. In summary, the current data provide support for earlier findings by confirming a protective effect of fluoxetine against altered locomotor response to amphetamine in adulthood, and identifying a similar protective effect for aripiprazole. Additionally, these data extend earlier findings by identifying adverse effects for both fluoxetine and aripiprazole in offspring of control dams through elevation of the locomotor response to a saline injection. In combination, this literature highlights the potential for both therapeutic as well as safety concerns with exposure to preventive pharmacological treatments over the course of adolescent and early adult development. Further study is needed to determine clinical and epidemiological consequences of these pre-clinical findings.
Both aripiprazole and fluoxetine reversed the locomotor response abnormality elicited by low dose amphetamine, while neither corrected the behavioral abnormality observed at higher amphetamine dose. The mesolimbic dopamine system plays a key role in modulation of the locomotor response to low dose amphetamine [56, 57], while the nigrostriatal dopamine system is required for modulating stereotyped behavioral responses to high dose amphetamine [58-60]. Additional studies are needed to determine if differing effects on stabilization of mesolimbic vs. nigrostriatal circuits underlie the differences in correction of the two behaviors.
Both aripiprazole and fluoxetine exert effects on serotonergic systems. Fluoxetine is a serotonin transport inhibitor, and by increasing synaptic serotonin concentrations is an indirect serotonin receptor agonist. Aripiprazole is a partial agonist at serotonin 5-HT1A and 5-HT2C receptors, and is a functional serotonin 5-HT2A receptor antagonist [34, 61]. While the mechanism(s) underlying the protective effects of fluoxetine and aripiprazole are not known, shared effects on serotonin 5-HT1A receptor stimulation, which indirectly elevate medial prefrontal cortex dopamine release [34, 62], provides one potential mechanism. The developmental period of drug administration overlaps a time period of dramatic synaptic remodeling within prefrontal cortex. Excess synapse formation occurs during early puberty in cortical brain regions, followed later in adolescence by prominent pruning of synaptic connections [37, 63, 64]. Fluoxetine and aripiprazole might exert protective effects through a shared modulation of this remodeling. Such an influence on synaptic remodeling could therefore normalize aberrant connectivity following prenatal immune activation, but result in abnormal synaptic pruning when control rats are exposed to these drugs. NMDA glutamate receptor signaling is a central component of this synaptic remodeling [64], suggesting fluoxetine and aripiprazole might modulate synaptic remodeling via influences on glutamate system function. Glutamate system abnormalities have previously been identified following early developmental immune activation [31, 65-67]. As functional and physical connections between glutamate mGluR2 receptors and serotonin 5-HT2A receptors have previously been described [68], serotonergic effects of fluoxetine and aripiprazole may contribute to the observed outcome. Further study will be needed to determine the accuracy of this speculative model.
Highlights.
Fluoxetine and aripiprazole treatment normalized locomotor response to amphetamine (1 mg/kg) in poly I:C offspring
Fluoxetine and aripiprazole treatment cause abnormal locomotor response to saline injection in control offspring
These findings highlight potential for both therapeutic and safety concerns with exposure to preventive pharmacological treatments over the course of adolescent development
Acknowledgements
This work was supported by the Department of Veterans Affairs Medical Research Service and National Institute of Mental Health (R21MH083192-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Cornblatt BA. The New York high risk project to the Hillside recognition and prevention (RAP) program. Am. J. Med. Genet. 2002;114:956–966. doi: 10.1002/ajmg.b.10520. [DOI] [PubMed] [Google Scholar]
- 2.Cornblatt BA, Lencz T, Smith CW, Olsen R, Auther AM, Nakayama E, Lesser ML, Tai JY, Shah MR, Foley CA, Kane JM, Correll CU. Can antidepressants be used to treat the schizophrenia prodrome? Results of a prospective, naturalistic treatment study of adolescents. J Clin Psychiatry. 2007;68:546–557. doi: 10.4088/jcp.v68n0410. [DOI] [PubMed] [Google Scholar]
- 3.Hurlemann R, Matusch A, Kuhn KU, Berning J, Elmenhorst D, Winz O, Kolsch H, Zilles K, Wagner M, Maier W, Bauer A. 5-HT2A receptor density is decreased in the at-risk mental state. Psychopharmacology (Berl) 2008;195:579–590. doi: 10.1007/s00213-007-0921-x. [DOI] [PubMed] [Google Scholar]
- 4.Richtand NM, Taylor B, Welge JA, Ahlbrand R, Ostrander MM, Burr J, Hayes S, Coolen LM, Pritchard LM, Logue A, Herman JP, McNamara RK. Risperidone Pretreatment Prevents Elevated Locomotor Activity Following Neonatal Hippocampal Lesions. Neuropsychopharmacology. 2006;31:77–89. doi: 10.1038/sj.npp.1300791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGorry PD, Yung AR, Phillips LJ, Yuen HP, Francey S, Cosgrave EM, Germano D, Bravin J, McDonald T, Blair A, Adlard S, Jackson H. Randomized controlled trial of interventions designed to reduce the risk of progression to first-episode psychosis in a clinical sample with subthreshold symptoms. Arch. Gen. Psychiatry. 2002;59:921–928. doi: 10.1001/archpsyc.59.10.921. [DOI] [PubMed] [Google Scholar]
- 6.Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, Seidman LJ, Perkins D, Tsuang M, McGlashan T, Heinssen R. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch. Gen. Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Addington J, Cadenhead KS, Cannon TD, Cornblatt B, McGlashan TH, Perkins DO, Seidman LJ, Tsuang M, Walker EF, Woods SW, Heinssen R. North American Prodrome Longitudinal Study: a collaborative multisite approach to prodromal schizophrenia research. Schizophr. Bull. 2007;33:665–672. doi: 10.1093/schbul/sbl075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woods SW, Tully EM, Walsh BC, Hawkins KA, Callahan JL, Cohen SJ, Mathalon DH, Miller TJ, McGlashan TH. Aripiprazole in the treatment of the psychosis prodrome: an open-label pilot study. Br. J Psychiatry Suppl. 2007;51:s96–101. doi: 10.1192/bjp.191.51.s96. [DOI] [PubMed] [Google Scholar]
- 9.McGlashan TH, Zipursky RB, Perkins D, Addington J, Miller T, Woods SW, Hawkins KA, Hoffman RE, Preda A, Epstein I, Addington D, Lindborg S, Trzaskoma Q, Tohen M, Breier A. Randomized, double-blind trial of olanzapine versus placebo in patients prodromally symptomatic for psychosis. Am. J Psychiatry. 2006;163:790–799. doi: 10.1176/ajp.2006.163.5.790. [DOI] [PubMed] [Google Scholar]
- 10.McGlashan TH. Early detection and intervention in schizophrenia: research. Schizophr. Bull. 1996;22:327–345. doi: 10.1093/schbul/22.2.327. [DOI] [PubMed] [Google Scholar]
- 11.Tsuang MT, Stone WS, Tarbox SI, Faraone SV. Treatment of nonpsychotic relatives of patients with schizophrenia: six case studies. Am. J. Med. Genet. 2002;114:943–948. doi: 10.1002/ajmg.10363. [DOI] [PubMed] [Google Scholar]
- 12.Woods SW, Breier A, Zipursky RB, Perkins DO, Addington J, Miller TJ, Hawkins KA, Marquez E, Lindborg SR, Tohen M, McGlashan TH. Randomized trial of olanzapine versus placebo in the symptomatic acute treatment of the schizophrenic prodrome. Biol. Psychiatry. 2003;54:453–464. doi: 10.1016/s0006-3223(03)00321-4. [DOI] [PubMed] [Google Scholar]
- 13.Morrison AP, French P, Walford L, Lewis SW, Kilcommons A, Green J, Parker S, Bentall RP. Cognitive therapy for the prevention of psychosis in people at ultra-high risk: randomised controlled trial. Br. J Psychiatry. 2004;185:291–297. doi: 10.1192/bjp.185.4.291. [DOI] [PubMed] [Google Scholar]
- 14.Hafner H, Maurer K, Ruhrmann S, Bechdolf A, Klosterkotter J, Wagner M, Maier W, Bottlender R, Moller HJ, Gaebel W, Wolwer W. Early detection and secondary prevention of psychosis: facts and visions. Eur. Arch. Psychiatry Clin Neurosci. 2004;254:117–128. doi: 10.1007/s00406-004-0508-z. [DOI] [PubMed] [Google Scholar]
- 15.Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am. J Psychiatry. 2010;167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suh HS, Brosnan CF, Lee SC. Toll-like receptors in CNS viral infections. Curr. Top. Microbiol. Immunol. 2009;336:63–81. doi: 10.1007/978-3-642-00549-7_4. [DOI] [PubMed] [Google Scholar]
- 17.Clarke MC, Tanskanen A, Huttunen M, Whittaker JC, Cannon M. Evidence for an interaction between familial liability and prenatal exposure to infection in the causation of schizophrenia. Am. J Psychiatry. 2009;166:1025–1030. doi: 10.1176/appi.ajp.2009.08010031. [DOI] [PubMed] [Google Scholar]
- 18.Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav. Brain Res. 2009;204:313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 19.Boksa P. Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain Behav. Immun. 2010;24:881–897. doi: 10.1016/j.bbi.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer U, Feldon J. Neural basis of psychosis-related behaviour in the infection model of schizophrenia. Behav. Brain Res. 2009;204:322–334. doi: 10.1016/j.bbr.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 22.Meyer U, Feldon J. Epidemiology-driven neurodevelopmental animal models of schizophrenia. Prog. Neurobiol. 2010;90:285–326. doi: 10.1016/j.pneurobio.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 23.Piontkewitz Y, Arad M, Weiner I. Tracing the development of psychosis and its prevention: What can be learned from animal models. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 24.Meyer U, Spoerri E, Yee BK, Schwarz MJ, Feldon J. Evaluating Early Preventive Antipsychotic and Antidepressant Drug Treatment in an Infection-Based Neurodevelopmental Mouse Model of Schizophrenia. Schizophr. Bull. 2010;36:607–623. doi: 10.1093/schbul/sbn131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piontkewitz Y, Assaf Y, Weiner I. Clozapine administration in adolescence prevents postpubertal emergence of brain structural pathology in an animal model of schizophrenia. Biol. Psychiatry. 2009;66:1038–1046. doi: 10.1016/j.biopsych.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Piontkewitz Y, Arad M, Weiner I. Risperidone Administered During Asymptomatic Period of Adolescence Prevents the Emergence of Brain Structural Pathology and Behavioral Abnormalities in an Animal Model of Schizophrenia. Schizophr. Bull. 2010 doi: 10.1093/schbul/sbq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor P. Practical Teratology. Academic Press; London: 1986. [Google Scholar]
- 28.Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology. 2003;28:1778–1789. doi: 10.1038/sj.npp.1300248. [DOI] [PubMed] [Google Scholar]
- 29.Fortier ME, Kent S, Ashdown H, Poole S, Boksa P, Luheshi GN. The viral mimic, polyinosinic:polycytidylic acid, induces fever in rats via an interleukin-1-dependent mechanism. Am. J Physiol Regul. Integr. Comp Physiol. 2004;287:R759–R766. doi: 10.1152/ajpregu.00293.2004. [DOI] [PubMed] [Google Scholar]
- 30.Gilmore JH, Jarskog LF, Vadlamudi S. Maternal poly I:C exposure during pregnancy regulates TNF alpha, BDNF, and NGF expression in neonatal brain and the maternal-fetal unit of the rat. J Neuroimmunol. 2005;159:106–112. doi: 10.1016/j.jneuroim.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 31.Bronson SL, Ahlbrand R, Horn PS, Kern JR, Richtand NM. Individual differences in maternal response to immune challenge predict offspring behavior: Contribution of environmental factors. Behav Brain Res. 2011;220:55–64. doi: 10.1016/j.bbr.2010.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hervas I, Vilaro MT, Romero L, Scorza MC, Mengod G, Artigas F. Desensitization of 5-HT(1A) autoreceptors by a low chronic fluoxetine dose effect of the concurrent administration of WAY-100635. Neuropsychopharmacology. 2001;24:11–20. doi: 10.1016/S0893-133X(00)00175-5. [DOI] [PubMed] [Google Scholar]
- 33.Cosi C, Carilla-Durand E, Assie MB, Ormiere AM, Maraval M, Leduc N, Newman-Tancredi A. Partial agonist properties of the antipsychotics SSR181507, aripiprazole and bifeprunox at dopamine D2 receptors: G protein activation and prolactin release. Eur. J Pharmacol. 2006;535:135–144. doi: 10.1016/j.ejphar.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 34.Li Z, Ichikawa J, Dai J, Meltzer HY. Aripiprazole, a novel antipsychotic drug, preferentially increases dopamine release in the prefrontal cortex and hippocampus in rat brain. Eur. J Pharmacol. 2004;493:75–83. doi: 10.1016/j.ejphar.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 35.Fernandez-Fernandez R, Navarro VM, Barreiro ML, Vigo EM, Tovar S, Sirotkin AV, Casanueva FF, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Effects of chronic hyperghrelinemia on puberty onset and pregnancy outcome in the rat. Endocrinology. 2005;146:3018–3025. doi: 10.1210/en.2004-1622. [DOI] [PubMed] [Google Scholar]
- 36.Koros C, Boukouvalas G, Gerozissis K, Kitraki E. Fat diet affects leptin receptor levels in the rat cerebellum. Nutrition. 2009;25:85–87. doi: 10.1016/j.nut.2008.06.033. [DOI] [PubMed] [Google Scholar]
- 37.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav. Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 38.Zicha J, Kunes J. Ontogenetic aspects of hypertension development: analysis in the rat. Physiol. Rev. 1999;79:1227–1282. doi: 10.1152/physrev.1999.79.4.1227. [DOI] [PubMed] [Google Scholar]
- 39.Sodersten P. Receptive behavior in developing female rats. Horm. Behav. 1975;6:307–317. doi: 10.1016/0018-506x(75)90001-x. [DOI] [PubMed] [Google Scholar]
- 40.Sodersten P, Damassa DA, Smith ER. Sexual behavior in developing male rats. Horm. Behav. 1977;8:320–341. doi: 10.1016/0018-506x(77)90006-x. [DOI] [PubMed] [Google Scholar]
- 41.Parikh V, Terry AV, Khan MM, Mahadik SP. Modulation of nerve growth factor and choline acetyltransferase expression in rat hippocampus after chronic exposure to haloperidol, risperidone, and olanzapine. Psychopharmacology (Berl) 2004;172:365–374. doi: 10.1007/s00213-003-1669-6. [DOI] [PubMed] [Google Scholar]
- 42.Rosengarten H, Quartermain D. Effect of prenatal administration of haloperidol, risperidone, quetiapine and olanzapine on spatial learning and retention in adult rats. Pharmacol. Biochem. Behav. 2002;72:575–579. doi: 10.1016/s0091-3057(02)00727-x. [DOI] [PubMed] [Google Scholar]
- 43.Terry AV, Jr., Gearhart DA, Mahadik SP, Warsi S, Davis LW, Waller JL. Chronic exposure to typical or atypical antipsychotics in rodents: temporal effects on central alpha7 nicotinic acetylcholine receptors. Neuroscience. 2005;136:519–529. doi: 10.1016/j.neuroscience.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 44.Pritchard LM, Logue AD, Hayes S, Welge JA, Xu M, Zhang J, Berger SP, Richtand NM. 7-OH-DPAT and PD 128907 Selectively Activate the D3 Dopamine Receptor in a Novel Environment. Neuropsychopharmacology. 2003;28:100–107. doi: 10.1038/sj.npp.1300018. [DOI] [PubMed] [Google Scholar]
- 45.Carrera MR, Ashley JA, Wirsching P, Koob GF, Janda KD. A second-generation vaccine protects against the psychoactive effects of cocaine. Proc. Natl. Acad. Sci. U. S. A. 2001;98:1988–1992. doi: 10.1073/pnas.041610998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richtand NM. Laboratory analysis of behavioral effects of drugs of abuse in rodents. Methods Mol. Med. 2003;79:475–480. doi: 10.1385/1-59259-358-5:475. [DOI] [PubMed] [Google Scholar]
- 47.Welge JA, Richtand NM. Regression modeling of rodent locomotion data. Behav. Brain Res. 2002;128:61–69. doi: 10.1016/s0166-4328(01)00311-4. [DOI] [PubMed] [Google Scholar]
- 48.Segal DS, Kuczenski R. Behavioral pharmacology of amphetamine. In: Cho AK, Segal DS, editors. Amphetamine and its analogues: pharmacology, toxicology, and abuse. Academic Press; Orlando, FL: 1994. pp. p115–150. [Google Scholar]
- 49.Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, Iyo M. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biol. Psychiatry. 2006;59:546–554. doi: 10.1016/j.biopsych.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 50.Vuillermot S, Weber L, Feldon J, Meyer U. A longitudinal examination of the neurodevelopmental impact of prenatal immune activation in mice reveals primary defects in dopaminergic development relevant to schizophrenia. J. Neurosci. 2010;30:1270–1287. doi: 10.1523/JNEUROSCI.5408-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Romero E, Guaza C, Castellano B, Borrell J. Ontogeny of sensorimotor gating and immune impairment induced by prenatal immune challenge in rats: implications for the etiopathology of schizophrenia. Mol. Psychiatry. 2008 doi: 10.1038/mp.2008.44. [DOI] [PubMed] [Google Scholar]
- 52.Romero E, Guaza C, Castellano B, Borrell J. Ontogeny of sensorimotor gating and immune impairment induced by prenatal immune challenge in rats: implications for the etiopathology of schizophrenia. Mol. Psychiatry. 2008 doi: 10.1038/mp.2008.44. [DOI] [PubMed] [Google Scholar]
- 53.Bakos J, Duncko R, Makatsori A, Pirnik Z, Kiss A, Jezova D. Prenatal immune challenge affects growth, behavior, and brain dopamine in offspring. Ann. N. Y. Acad. Sci. 2004;1018:281–287. doi: 10.1196/annals.1296.033. [DOI] [PubMed] [Google Scholar]
- 54.Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav. Immun. 2008;22:469–486. doi: 10.1016/j.bbi.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 55.Richtand NM, Ahlbrand R, Horn P, Stanford K, Bronson SL, McNamara RK. Effects of risperidone and paliperidone pre-treatment on locomotor response following prenatal immune activation. J. Psychiatr. Res. 2011;45:1194–1201. doi: 10.1016/j.jpsychires.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Costall B, Naylor RJ. Extrapyramidal and mesolimbic involvement with the stereotypic activity of D- and L-amphetamine. Eur. J. Pharmacol. 1974;25:121–129. doi: 10.1016/0014-2999(74)90039-9. [DOI] [PubMed] [Google Scholar]
- 57.Pijnenburg AJ, Honig WM, Van Rossum JM. Inhibition of d-amphetamine-induced locomotor activity by injection of haloperidol into the nucleus accumbens of the rat. Psychopharmacologia. 1975;41:87–95. doi: 10.1007/BF00421062. [DOI] [PubMed] [Google Scholar]
- 58.Creese I, Iversen SD. Amphetamine response in rat after dopamine neurone destruction. Nature New Biol. 1972;238:247–248. doi: 10.1038/newbio238247a0. [DOI] [PubMed] [Google Scholar]
- 59.Creese I, Iversen SD. The pharmacological and anatomical substrates of the amphetamine response in the rat. Brain Res. 1975;83:419–436. doi: 10.1016/0006-8993(75)90834-3. [DOI] [PubMed] [Google Scholar]
- 60.Fog R, Randrup A, Pakkenberg H. Lesions in corpus striatum and cortex of rat brains and the effect on pharmacologically induced stereotyped, aggressive and cataleptic behaviour. Psychopharmacologia. 1970;18:346–356. doi: 10.1007/BF00402761. [DOI] [PubMed] [Google Scholar]
- 61.Shapiro DA, Renock S, Arrington E, Chiodo LA, Liu LX, Sibley DR, Roth BL, Mailman R. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology. 2003;28:1400–1411. doi: 10.1038/sj.npp.1300203. [DOI] [PubMed] [Google Scholar]
- 62.Ichikawa J, Li Z, Dai J, Meltzer HY. Atypical antipsychotic drugs, quetiapine, iloperidone, and melperone, preferentially increase dopamine and acetylcholine release in rat medial prefrontal cortex: role of 5-HT1A receptor agonism. Brain Res. 2002;956:349–357. doi: 10.1016/s0006-8993(02)03570-9. [DOI] [PubMed] [Google Scholar]
- 63.Andersen SL, Teicher MH. Desperately driven and no brakes: developmental stress exposure and subsequent risk for substance abuse. Neurosci. Biobehav. Rev. 2009;33:516–524. doi: 10.1016/j.neubiorev.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol. Biochem. Behav. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lante F, Meunier J, Guiramand J, Maurice T, Cavalier M, Jesus Ferreira MC, Aimar R, Cohen-Solal C, Vignes M, Barbanel G. Neurodevelopmental damage after prenatal infection: role of oxidative stress in the fetal brain. Free Radic. Biol. Med. 2007;42:1231–1245. doi: 10.1016/j.freeradbiomed.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 66.Roenker N, Gudelsky GA, Ahlbrand RL, Bronson SL, Kern JR, Waterman H, Richtand NM. Effect of paliperidone and risperidone on extracellular glutamate in the prefrontal cortex of rats exposed to prenatal immune activation or MK-801. Neurosci Lett. 2011;500:167–171. doi: 10.1016/j.neulet.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ibi D, Nagai T, Kitahara Y, Mizoguchi H, Koike H, Shiraki A, Takuma K, Kamei H, Noda Y, Nitta A, Nabeshima T, Yoneda Y, Yamada K. Neonatal polyI:C treatment in mice results in schizophrenia-like behavioral and neurochemical abnormalities in adulthood. Neurosci Res. 2009;64:297–305. doi: 10.1016/j.neures.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 68.Gonzalez-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, Lopez-Gimenez JF, Zhou M, Okawa Y, Callado LF, Milligan G, Gingrich JA, Filizola M, Meana JJ, Sealfon SC. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]