Abstract
Purpose of review
In contrast to previous understanding, adipocytes are now known to produce an array of factors collectively termed “adipokines”, several of which have effects on the cardiovascular system. The marked rise in prevalence of obesity warrants investigation into the role of adipocyte-derived factors in the regulation of blood pressure. For example, dysregulated production of specific adipokines in the setting of obesity may contribute to hypertension commonly experienced in obese subjects. This editorial highlights current concepts for regulation of adipokine production by adipocytes and their potential role in blood pressure regulation.
Recent findings
Adipocytes synthesize and release several factors that have been linked to blood pressure control, including adiponectin, leptin, angiotensin, perivascular relaxation factors and resistin. Increasing evidence suggests that aberrant production and release of these factors from adipocytes may contribute to the high prevalence of hypertension in the obese population. However, additional studies are warranted to define precise mechanisms for blood pressure regulation by these factors, and to delineate their role in obesity-related hypertension.
Summary
Studies aimed at determining the role of adipocyte-derived factors in blood pressure regulation during normal physiology and in the setting of obesity are needed.
Keywords: adipokine, blood pressure, obesity, hypertension
Introduction
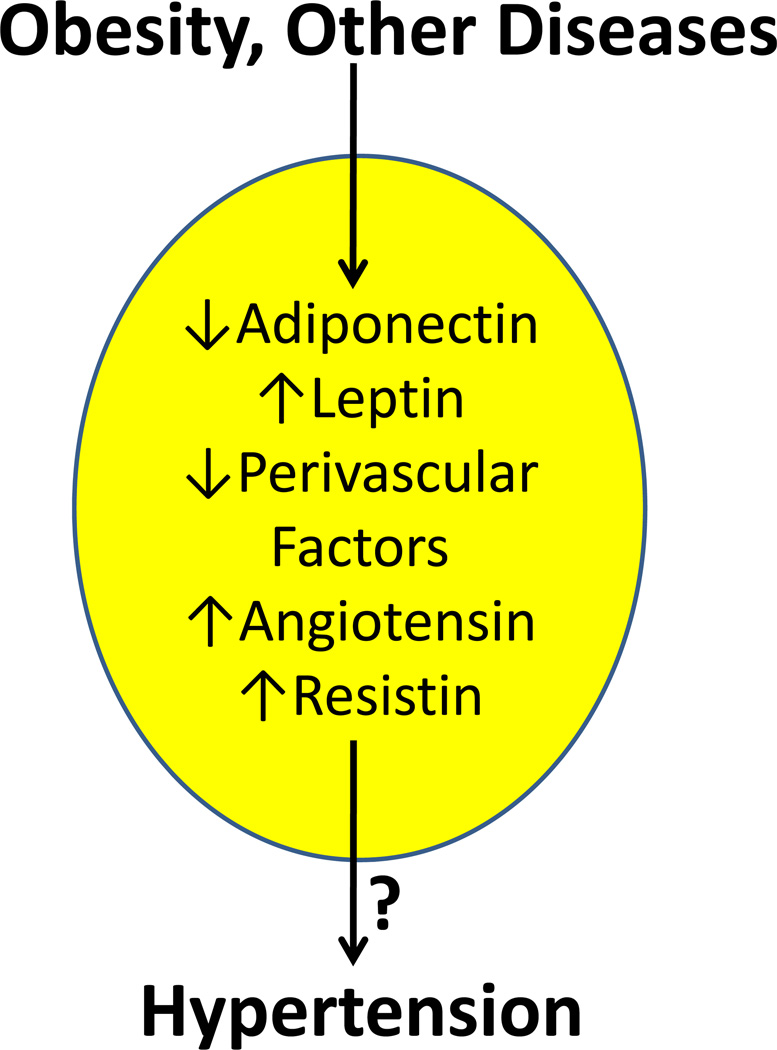
The long-held view that adipocytes function primarily to store and mobilize lipid for metabolic needs of the body has been revised by identification of a plethora of factors now known to be synthesized and secreted by adipocytes. Currently, over 50 different substances, which we will refer to in this review as adipocyte-derived factors, have been demonstrated to be synthesized by adipocytes. Many adipocyte-derived factors play a role in homeostasis of body weight, acting as a thermostat or sensor of lipid stores through receptor-mediated effects at specific neurons in the brain important in the control of food intake. Several different cytokines are produced by adipocytes (termed adipokines), many of which contribute to local or systemic inflammation. Importantly, several adipocyte-derived factors are beginning to be explored as regulators of blood pressure. This editorial provides a brief review of adipocyte-derived factors that have been implicated in blood pressure control, with a focus on potential contributions of these substances to the current epidemic of obesity-related hypertension (Figure 1).
Figure 1.
Obesity, as well as other factors and/or diseases, results in regulation of a variety of adipocyte-derived factors, typically called adipokines. Several of these factors have been demonstrated to regulate blood pressure. Dysregulated production of adipokines may contribute to the link between obesity and hypertension. Arrows indicate regulation that is consistent with an increase in blood pressure.
Adiponectin
Adiponectin is an anti-inflammatory, insulin-sensitizing, and anti-atherogenic protein exclusively secreted by adipocytes. Previous studies have established adiponectin to be protective against hypertension through an endothelium-dependent mechanism as adiponectin-deficient mice show an impaired response to acetylcholine-induced vascular relaxation [1]. Also, adeno-viral over-expression of adiponectin in obese KKAy mice resulted in reduced blood pressure and reversed salt-induced hypertension [2]. In contrast, a recent study showed that Sprague-Dawley rats fed a high salt diet exhibited hypertension associated with elevated levels of adiponectin, suggesting that adiponectin does not play a protective role against salt-induced hypertension [3].
Two recent studies highlight a potentially important role for adiponectin in regulation of vascular tone. In gluteal fat biopsies from obese patients, the anti-contractile capacity of perivascular fat was abolished compared to lean controls [4]. Application of a fragment of the human type 1 adiponectin receptor to arteries from healthy patients abolished the anti-contractile effects of perivascular fat. Moreover, application of exogenous adiponectin extralumenally to mesenteric arteries of rats resulted in vasodilation [4]. In mice with adiponectin deficiency, endothelium-dependent relaxation of aortic rings was impaired, and was associated with increased dihydroethidium staining and superoxide production [5]. Treatment of adiponectin deficient mice with recombinant human adiponectin for 3 days reduced superoxide production and restored endothelium dependent relaxation. Collectively, these results suggest that adiponectin, either systemically-derived or from perivascular fat, promotes endothelial-dependent vasodilation and that these effects are diminished with obesity. Future studies in adiponectin deficient, as well as adiponectin receptor deficient mice should further our understanding of the impact of these findings on hypertension.
Recent population-based studies over the last year have further explored the relationship between adiponectin and blood pressure with conflicting results. A small study of 41 obese adolescents with or without type 2 diabetes mellitus found lower serum total adiponectin levels were indeed associated with higher 24-hour systolic and diastolic blood pressures [6]. In contrast, the Quebec Child and Adolescent Health and Social Survey conducted in 1999 showed no independent association between adiponectin Z-scores and systolic or diastolic blood pressures [7]. Patients with essential hypertension treated with telmisartan or perindopril exhibited similar reductions in blood pressure, but only treatment with telmisartan resulted in a temporary increase in plasma adiponectin concentrations [8]. Thus, while some studies show correlations between systemic adiponectin concentrations and blood pressure, there are conflicting data, making it difficult to determine if circulating adiponectin concentrations predict hypertension. While evidence supports a potential role for adiponectin in the regulation of blood pressure, more studies are needed at both the basic science and clinical level to establish adiponectin as a primary regulator of blood pressure.
Leptin
Leptin is a hormone secreted almost exclusively by adipocytes in blood in proportion to adipose tissue mass. Leptin receptors are located mainly in the mediobasal hypothalamus, particularly the arcuate, ventromedial and dorsomedial nuclei. By acting at receptors in these hypothalamic nuclei, leptin decreases food intake and increases energy expenditure. Leptin-mediated elevations in energy expenditure result from increased sympathetic nerve system efferent outflow. Predictably, genetic mutation in either leptin or its receptor leads to the development of obesity in humans or rodents.
Leptin has been shown to increase blood pressure when infused chronically due to heightened sympathetic outflow [9–11]. However, the majority of studies examining leptin effects on blood pressure have been performed in rodents fed standard diets. Since obesity persists in patients with high circulating leptin concentrations, resistance to the metabolic effects of leptin have been proposed. However, it is unclear whether this resistance extends to the cardiovascular actions of leptin. Recent studies demonstrated elevated blood pressures in db/db mice with marked obesity [12,13], suggesting obesity-induced hypertension can occur in the absence of leptin receptor signaling. These findings raise the question whether elevated circulating leptin is associated with increased blood pressure due to leptin resistance or from leptin excess? To address this question, ob/ob mice were either treated with leptin or calorie-restricted to lower body weight [14]. Caloric restriction has been previously demonstrated to decrease plasma leptin concentrations [15]. Systolic blood pressure was significantly reduced in ob/ob mice treated with either leptin or calorie-restricted to decrease body weight compared to controls [14]. These results demonstrate that blood pressure reductions were achieved in ob/ob mice losing weight through leptin-dependent or independent mechanisms. Further studies, perhaps in high fat-fed mice exhibiting elevated circulating leptin concentrations and obesity-induced hypertension, are needed to discern the precise role of leptin in obesity-related hypertension.
Recent clinical studies examined free leptin index (ratio between the concentration of leptin and the soluble leptin receptor in plasma) as an indicator of pre-hypertensive status (termed masked hypertension) in nonobese subjects [16]. Results demonstrated that the free leptin index may provide prognostic value towards hypertension and cardiovascular events. Collectively, results suggest that adipocyte-derived leptin is capable of regulating blood pressure. However, whether leptin is indeed a pathologic contributor to different forms of hypertension has yet to be defined.
Perivascular Relaxation Factor
In 1991 our laboratory demonstrated that perivascular adipose tissue influences the contractile tone of rat aortic rings [17]. This finding remained relatively dormant until 2002, when Lohn et al.[18] demonstrated a transferable factor released from perivascular adipose tissue that activates K+ channels and tyrosine kinase in vascular smooth muscle to inhibit contraction. Several factors have recently been identified that are made and released by perivascular adipose tissue and are capable of influencing vascular contraction.
The vasodilator peptide angiotensin-(1–7) was localized by immunohistochemistry to brown and white adipocytes in perivascular adipose tissue surrounding the rat aorta [19]. Transfer of a donor solution incubated with perivascular adipose tissue to recipient aortic rings devoid of perivascular adipose tissue caused a relaxation response that was abolished when donor vessels were incubated with a mas receptor antagonist. Relaxation from perivascular adipose-derived angiotensin-(1–7) required the presence of the endothelium. It will be interesting to determine if production of this perivascular factor is influenced by obesity, and relates to the development of obesity-associated hypertension. It is also important to define if these factors can diffuse the distance of the vascular wall to exhibit tonic influences on vascular tone.
Hydrogen sulfide, a vasodilator that opens ATP-sensitive potassium channels to enhance potassium efflux, was recently demonstrated to be produced in perivascular adipose tissue [20]. Enzymes important in the production of hydrogen sulfide were localized to aortic perivascular adipose tissue from rats. An inhibitor of these enzymes prevented the vasodilator effects of perivascular adipose tissue in aortic rings, and vasoconstrictors were found to increase production of hydrogen sulfide by perivascular adipose tissue. Interestingly, in rats made hypertensive by abdominal aortic constriction, production of hydrogen sulfide was increased in perivascular adipose tissue, and transfer of perivascular adipose from normal donor rats to the stenotic area of the aorta resulted in a reduction in systolic blood pressure. It would be of interest to determine if production of hydrogen sulfide is altered in perivascular adipose tissue with obesity, since an increased mass of perivascular adipose surrounding the aorta has been reported in obese rats [21].
Finally, visfatin, expressed and secreted by visceral fat (hence the name), was found to be released from perivascular adipose tissue of rats and monkeys [22]. Perivascular-derived visfatin did not influence contractile tone of the aorta, however, visfatin was found to promote smooth muscle cell proliferation. This effect of perivascular-derived visfatin was suggested to contribute to the development of atherosclerosis.
A surging interest in perivascular adipose tissue as a source of factors influencing vascular function is noteworthy, since almost all blood vessels and organs are surrounded by fat. However, it will be a challenge to define the role of perivascular-derived factors in blood pressure control, since there are currently no methods available that enable specific targeting of genes of interest within this adipose depot.
Renin-angiotensin system
Studies from several different laboratories have demonstrated the presence of a local renin angiotensin system (RAS) in adipose tissue, including production of angiotensin II (AngII), the most potent vasoactive peptide of the RAS [23]. Elevated expression of RAS components in adipose tissue, including AngII, have been demonstrated in experimental models of obesity and in adipose tissue from obese hypertensives [23]. However, mechanisms for an activated adipocyte RAS from obesity and the contributions of adipose-derived angiotensinogen/AngII to obesity-related hypertension are unknown.
Several studies over the last few years have focused on manipulation of the RAS in reference to regulation of body weight and fat mass. Interesting recent studies examined effects of administration of an ACE inhibitor (perindopril) administered from birth on body weight, adiposity and blood pressure in Sprague Dawley rats [24]. Rats administered perindopril had decreased food intake, body weight and adipose mass compared to vehicle, and these changes were associated with reductions in systolic blood pressure. This same group demonstrated similar reductions in body weight and fat mass in ACE deficient mice known to exhibit reduced blood pressure [24–26]. Inhibition of the RAS at another site, namely renin, by administration of Aliskiren to mice fed a low or high fat diet reduced body weight and fat mass [27]. Unfortunately, blood pressure was not measured in this study. It is unclear in the above described studies if reductions in adipose AngII production contributed to the observed effects of RAS inhibition on the regulation of body weight or fat mass. Moreover, blood pressure was not measured in studies aimed at defining effects of RAS manipulation on the regulation of body weight. Finally, current approaches cannot distinguish between the relative contribution of adipocyte-derived versus systemic angiotensin in the regulation of adipose mass and blood pressure with obesity.
AngII exerts its physiological responses by activating two subtypes of angiotensin receptors, termed AT1 and AT2 receptors. It is well established that the AT1 receptor mediates most of the known physiological functions of AngII, while the AT2 receptor typically opposes AngII/AT1 receptor responses. Interestingly, previous investigators have demonstrated that whole body deficiency of either AT1a receptors [28] or AT2 receptors [29] reduced the development of diet-induced obesity. In mice with AT1a receptor deficiency, blood pressure was decreased in mice fed low fat or high fat diets compared to control. Surprisingly, in AT2 receptor deficient mice, obesity-induced elevations in blood pressure were also abolished. It was unclear from these findings why deficiency of these angiotensin receptors typically mediating opposing effects would result in a similar resistance to the development of obesity and hypertension. Recent studies by the same group [30] examined effects of AT2 receptor deficiency on transgenic mice over-expressing angiotensinogen (AGT) as a model of increased fat mass and body weight. Increased body weight and fat mass in mice over-expressing AGT in adipose tissue were abolished by AT2 receptor deficiency. However, even though AT2 receptor deficiency prevented increases in body weight, elevations in blood pressure in mice with transgenic AGT expression in adipose tissue were augmented in AT2 receptor deficient mice. Elevated blood pressures in AT2 receptor deficient mice were attributed to stimulatory effects on kidney renin. These results suggest that adipocyte-derived AGT influences blood pressure through an interplay with AT2 receptors and through endocrine feedback regulation of the systemic RAS.
Recent studies by our laboratory examined effects of diet-induced obesity on regulation of ACE2 expression in adipose tissue [31]. Initial elevations in ACE2 expression in adipose tissue from mice fed a high fat diet were hypothesized to protect against activation of the systemic RAS and the development of hypertension. However, with chronic high fat feeding, adipose ACE2 activity was no longer increased, systemic angiotensin peptide concentrations rose, and mice exhibited hypertension. Further studies are warranted to determine the role of ACE2 in obesity-related hypertension, and whether adipocyte-derived ACE2 is important in blood pressure control.
Resistin
Resistin was identified by screening for genes that are regulated during the process of adipocyte differentiation. Human resistin is a cysteine-rich 108 precursor that contains a 16 residue-N-terminal signal peptide. Circulating resistin exists most frequently as a hexamer, using disulphide bonds that inter-digitate N-terminal coiled coils. Resistin belongs to the FIZZ (found in inflammatory zones) family, and has been demonstrated to be expressed in human macrophages [32]. Sources of resistin differ across species, with resistin mRNA abundance greater in mouse compared to human adipocytes, while the majority of resistin expression in human adipose tissue arises from infiltrating macrophages [33]. These differences have led to debate over the precise role of resistin in insulin resistance. In rodents, over-expression of resistin results in hyperglycemia from increased hepatic glucose production, while reductions in resistin protect against obesity-induced hyperglycemia by improving hepatic insulin responsiveness [34–36]. In humans, data is conflicting showing either increased serum resistin levels associated with obesity [37,38], or no association between resistin and obesity [39,40].
Several recent studies have suggested a relationship between hyper-resistinemia and hypertension. Serum resistin concentrations correlated positively to mean blood pressure in type 2 diabetic patients, but not in non-diabetic hypertensive patients [41]. As a potential mechanism for resistin effects on blood pressure, resistin resulted in an increase in mRNA abundance of fatty acid binding protein in human coronary artery endothelial cells. Other potential mechanisms linking resistin to hypertension include an ability to promote smooth muscle cell proliferation [42] and vasoconstrictor properties of resistin [43]. Additional studies are needed to define whether resistin influences blood pressure. For example, even though resistin deficient mice have been developed and examined in the context of insulin resistance, no studies have defined blood pressure regulation in mice lacking resistin. Moreover, very few studies have directly examined effects of resistin on vascular tone.
Conclusions
Adipocytes produce a variety of factors that could contribute to the long-term regulation of blood pressure (Table 1). Moreover, production of adipocyte-derived factors is regulated in pathophysiologic states associated with hypertension, such as obesity. Some adipocyte-derived factors, such as leptin or angiotensin, have been well studied in the context of blood pressure control. However, in the case of leptin, additional studies using diet-induced obesity models with elevated circulating leptin concentrations and targeted deletion of leptin responsiveness would further define leptin’s role in blood pressure control. In the case of the RAS, targeted deletion in adipocytes will be required to discern the relative contribution of adipocyte-derived RAS components in blood pressure control. Moreover, more rigorous measurement of blood pressure in available mouse models with deficiency of individual adipocyte-derived factors is required to define their role in blood pressure regulation.
Table 1.
Adipocyte-derived factors and blood pressure control.
Acknowledgments
Disclosure of funding: This review was supported by NIH HL73085 to LAC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ouchi N, Ohishi M, Kihara S, Funahashi T, et al. Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension. 2003;42:231–234. doi: 10.1161/01.HYP.0000083488.67550.B8. [DOI] [PubMed] [Google Scholar]
- 2.Ohashi K, Kihara S, Ouchi N, et al. Adiponectin replenishment ameliorates obesity-related hypertension. Hypertension. 2006;47:1108–1116. doi: 10.1161/01.HYP.0000222368.43759.a1. [DOI] [PubMed] [Google Scholar]
- 3.Kamari Y, Shimoni N, Koren F, et al. High-salt diet increases plasma adiponectin levels independent of blood pressure in hypertensive rats: the role of the renin-angiotensin-aldosterone system. J Hypertens. 2009 doi: 10.1097/HJH.0b013e3283325eee. in press. [DOI] [PubMed] [Google Scholar]
- 4. Greenstein AS, Khavandi K, Withers SB, et al. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation. 2009;119:1661–1670. doi: 10.1161/CIRCULATIONAHA.108.821181. This article demonstrated that adiponectin, derived from perivascular adipose tissue in humans, regulates contractile tone and these effects are abolished in samples from obese patients.
- 5.Cao Y, Tao L, Yuan Y, et al. Endothelial dysfunction in adiponectin deficiency and its mechanisms involved. J Mol Cell Cardiol. 2009;46:413–419. doi: 10.1016/j.yjmcc.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shatat IF, Freeman KD, Vuguin PM, et al. Relationship between adiponectin and ambulatory blood pressure in obese adolescents. Pediatr Res. 2009;65:691–695. doi: 10.1203/PDR.0b013e31819ea776. [DOI] [PubMed] [Google Scholar]
- 7.Lambert M, O'Loughlin J, Delvin EE, et al. Association between insulin, leptin, adiponectin and blood pressure in youth. J Hypertens. 2009;27:1025–1032. doi: 10.1097/HJH.0b013e32832935b6. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura T, Kawachi K, Saito Y, et al. Effects of ARB or ACE-inhibitor administration on plasma levels of aldosterone and adiponectin in hypertension. Int Heart J. 2009;50:501–512. doi: 10.1536/ihj.50.501. [DOI] [PubMed] [Google Scholar]
- 9.Haynes WG, Sivitz WI, Morgan DA, et al. Sympathetic and cardiorenal actions of leptin. Hypertension. 1997;30:619–623. doi: 10.1161/01.hyp.30.3.619. [DOI] [PubMed] [Google Scholar]
- 10.Dunbar JC, Hu Y, Lu H. Intracerebroventricular leptin increases lumbar and renal sympathetic nerve activity and blood pressure in normal rats. Diabetes. 1997;46:2040–2043. doi: 10.2337/diab.46.12.2040. [DOI] [PubMed] [Google Scholar]
- 11.Rahmouni K, Morgan DA. Hypothalamic arcuate nucleus mediates the sympathetic and arterial pressure responses to leptin. Hypertension. 2007;49:647–652. doi: 10.1161/01.HYP.0000254827.59792.b2. [DOI] [PubMed] [Google Scholar]
- 12.Senador D, Kanakamedala K, Irigoyen MC, et al. Cardiovascular and autonomic phenotype of db/db diabetic mice. Exp Physiol. 2009;94:648–658. doi: 10.1113/expphysiol.2008.046474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su W, Guo Z, Randall DC, et al. Hypertension and disrupted blood pressure circadian rhythm in type 2 diabetic db/db mice. Am J Physiol Heart Circ Physiol. 2008;295:H1634–H1641. doi: 10.1152/ajpheart.00257.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sikka G, Yang R, Reid S, et al. Leptin is essential in maintaining normal vascular compliance independent of body weight. Int J Obes (Lond) 2009 doi: 10.1038/ijo.2009.208. in press. This article demonstrated a dissociation between leptin and blood pressure in ob/ob mice.
- 15.Chiba T, Yamaza H, Higami Y, et al. Anti-aging effects of caloric restriction: Involvement of neuroendocrine adaptation by peripheral signaling. Microsc Res Tech. 2002;59:317–324. doi: 10.1002/jemt.10211. [DOI] [PubMed] [Google Scholar]
- 16.Thomopoulos C, Papadopoulos DP, Papazachou O, et al. Free leptin is associated with masked hypertension in nonobese subjects: a cross-sectional study. Hypertension. 2009;53:965–972. doi: 10.1161/HYPERTENSIONAHA.108.128041. [DOI] [PubMed] [Google Scholar]
- 17.Soltis EE, Cassis LA. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin Exp Hypertens. 1991;A 13:277–296. doi: 10.3109/10641969109042063. [DOI] [PubMed] [Google Scholar]
- 18.Lohn M, Dubrovska G, Lauterbach B, et al. Periadventitial fat releases a vascular relaxing factor. FASEB J. 2002;16:1057–1063. doi: 10.1096/fj.02-0024com. [DOI] [PubMed] [Google Scholar]
- 19.Lee RM, Lu C, Su LY, Gao YJ. Endothelium-dependent relaxation factor released by perivascular adipose tissue. J Hypertens. 2009;27:782–790. doi: 10.1097/HJH.0b013e328324ed86. [DOI] [PubMed] [Google Scholar]
- 20. Fang L, Zhao J, Chen Y, et al. Hydrogen sulfide derived from periadventitial adipose tissue is a vasodilator. J Hypertens. 2009 doi: 10.1097/HJH.0b013e328330a900. in press. This article demonstrated that perivascular adipose tissue releases hydrogen sulfide to regulate vascular tone.
- 21.Henrichot E, Juge-Aubry CE, Pernin A, et al. Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? Arterioscler Thromb Vasc Biol. 2005;25:2594–2599. doi: 10.1161/01.ATV.0000188508.40052.35. [DOI] [PubMed] [Google Scholar]
- 22.Wang P, Xu TY, Guan YF, et al. Perivascular adipose tissue-derived visfatin is a vascular smooth muscle cell growth factor: role of nicotinamide mononucleotide. Cardiovasc Res. 2009;81:370–380. doi: 10.1093/cvr/cvn288. [DOI] [PubMed] [Google Scholar]
- 23.Thatcher S, Yiannikouris F, Gupte M, et al. The adipose renin-angiotensin system: role in cardiovascular disease. Mol Cell Endocrinol. 2009;302:111–117. doi: 10.1016/j.mce.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jayasooriya AP, Mathai ML, Walke LL, et al. Mice lacking angiotensin-converting enzyme have increased energy expenditure, with reduced fat mass and improved glucose clearance. Proc Natl Acad Sci U S A. 2008;105:6531–6536. doi: 10.1073/pnas.0802690105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krege JH, John SW, Langenbach LL, et al. Male-female differences in fertility and blood pressure in ACE-deficient mice. Nature. 1995;375:146–148. doi: 10.1038/375146a0. [DOI] [PubMed] [Google Scholar]
- 26.Esther CR, Jr, Howard TE, Marino EM, et al. Mice lacking angiotensin-converting enzyme have low blood pressure, renal pathology, and reduced male fertility. Lab Invest. 1996;74:953–965. [PubMed] [Google Scholar]
- 27.Stucchi P, Cano V, Ruiz-Gayo M, Fernandez-Alfonso MS. Aliskiren reduces body-weight gain, adiposity and plasma leptin during diet-induced obesity. Br J Pharmacol. 2009;158:771–778. doi: 10.1111/j.1476-5381.2009.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kouyama R, Suganami T, Nishida J, et al. Attenuation of diet-induced weight gain and adiposity through increased energy expenditure in mice lacking angiotensin II type 1a receptor. Endocrinology. 2005;146:3481–3489. doi: 10.1210/en.2005-0003. [DOI] [PubMed] [Google Scholar]
- 29.Yvan-Charvet L, Even P, Bloch-Faure M, et al. Deletion of the angiotensin type 2 receptor (AT2R) reduces adipose cell size and protects from diet-induced obesity and insulin resistance. Diabetes. 2005;54:991–999. doi: 10.2337/diabetes.54.4.991. [DOI] [PubMed] [Google Scholar]
- 30. Yvan-Charvet L, Massiera F, Lamande N, et al. Deficiency of angiotensin type 2 receptor rescues obesity but not hypertension induced by overexpression of angiotensinogen in adipose tissue. Endocrinology. 2009;150:1421–1428. doi: 10.1210/en.2008-1120. This article demonstrated an interplay between adipocyte-derived angiotensinogen and AT2 receptors in the regulation of body weight, but not blood pressure.
- 31. Gupte MN, Boustany-Kari CM, Bharadwaj K, et al. ACE2 is Expressed in Mouse Adipocytes and Regulated by a High Fat Diet. Am J Physiol Regul Integr Comp Physiol. 2008;295:R781–R788. doi: 10.1152/ajpregu.00183.2008. This article demonstrated that ACE2 is expressed by adipocytes and regulated by high fat feeding in a manner that promotes obesity-related hypertension.
- 32.Yang RZ, Huang Q, Xu A, McLenithan JC, et al. Comparative studies of resistin expression and phylogenomics in human and mouse. Biochem Biophys Res Commun. 2003;310:927–935. doi: 10.1016/j.bbrc.2003.09.093. [DOI] [PubMed] [Google Scholar]
- 33.Curat CA, Wegner V, Sengenes C, et al. Macrophages in human visceral adipose tissue: increased accumulation in obesity and a source of resistin and visfatin. Diabetologia. 2006;49:744–747. doi: 10.1007/s00125-006-0173-z. [DOI] [PubMed] [Google Scholar]
- 34.Banerjee RR, Rangwala SM, Shapiro JS, et al. Regulation of fasted blood glucose by resistin. Science. 2004;303:1195–1198. doi: 10.1126/science.1092341. [DOI] [PubMed] [Google Scholar]
- 35.Qi Y, Nie Z, Lee YS, et al. Loss of resistin improves glucose homeostasis in leptin deficiency. Diabetes. 2006;55:3083–3090. doi: 10.2337/db05-0615. [DOI] [PubMed] [Google Scholar]
- 36.Muse ED, Obici S, Bhanot S, et al. Role of resistin in diet-induced hepatic insulin resistance. J Clin Invest. 2004;114:232–239. doi: 10.1172/JCI21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pagano C, Marin O, Calcagno A, et al. Increased serum resistin in adults with prader-willi syndrome is related to obesity and not to insulin resistance. J Clin Endocrinol Metab. 2005;90:4335–4340. doi: 10.1210/jc.2005-0293. [DOI] [PubMed] [Google Scholar]
- 38.Burnett MS, Devaney JM, Adenika RJ, et al. Cross-sectional associations of resistin, coronary heart disease, and insulin resistance. J Clin Endocrinol Metab. 2006;91:64–68. doi: 10.1210/jc.2005-1653. [DOI] [PubMed] [Google Scholar]
- 39.Savage DB, Sewter CP, Klenk ES, et al. Resistin / Fizz3 expression in relation to obesity and peroxisome proliferator-activated receptor-gamma action in humans. Diabetes. 2001;50:2199–2202. doi: 10.2337/diabetes.50.10.2199. [DOI] [PubMed] [Google Scholar]
- 40.Sentinelli F, Romeo S, Arca M, et al. Human resistin gene, obesity, and type 2 diabetes: mutation analysis and population study. Diabetes. 2002;51:860–862. doi: 10.2337/diabetes.51.3.860. [DOI] [PubMed] [Google Scholar]
- 41.Takata Y, Osawa H, Kurata M, et al. Hyperresistinemia is associated with coexistence of hypertension and type 2 diabetes. Hypertension. 2008;51:534–539. doi: 10.1161/HYPERTENSIONAHA.107.103077. [DOI] [PubMed] [Google Scholar]
- 42.Calabro P, Samudio I, Willerson JT, Yeh ET. Resistin promotes smooth muscle cell proliferation through activation of extracellular signal-regulated kinase 1/2 and phosphatidylinositol 3-kinase pathways. Circulation. 2004;110:3335–3340. doi: 10.1161/01.CIR.0000147825.97879.E7. [DOI] [PubMed] [Google Scholar]
- 43.Teng X, Li D, Champion HC, Johns RA. FIZZ1/RELMalpha, a novel hypoxia-induced mitogenic factor in lung with vasoconstrictive and angiogenic properties. Circ Res. 2003;92:1065–1067. doi: 10.1161/01.RES.0000073999.07698.33. [DOI] [PubMed] [Google Scholar]