Abstract
Purpose of review
Though fetal arrhythmias account for a small proportion of referrals to a fetal cardiologist, they may be associated with significant morbidity and mortality. The present review outlines the current literature with regard to the diagnosis and, in brief, some management strategies in fetal arrhythmias.
Recent findings
Advances in echocardiography have resulted in significant improvements in our ability to elucidate the mechanism of arrhythmia at the bedside. At the same time, fetal magnetocardiography is broadening our understanding of mechanisms of arrhythmia especially as it pertains to ventricular arrhythmias and congenital heart block. It provides a unique window to study electrical properties of the fetal heart, unlike what has been available to date. Recent reports of bedside use of fetal ECG make it a promising new technology. The underlying mechanisms resulting in immune-mediated complete heart block in a small subset of ‘at-risk’ fetuses is under investigation.
Summary
There have been great strides in noninvasive diagnosis of fetal arrhythmias. However, we still need to improve our knowledge of the electromechanical properties of the fetal heart as well as the mechanisms of arrhythmia to further improve outcomes. Multiinstitutional collaborative studies are needed to help answer some of the questions regarding patient, drug selection and management algorithms.
Keywords: fetal arrhythmia, fetal bradycardia, fetal tachycardia
Introduction
Abnormalities of fetal rhythm account for about 10–20% of referrals to fetal cardiologists. Though the mechanisms of arrhythmia in the fetus have many similarities to postnatal arrhythmia mechanisms, there are several developmental and functional aspects that are unique to the fetus. Our understanding of arrhythmia mechanisms in the fetus is gradually expanding in the current era, with the application of newer technologies such as magnetocardiography (MCG) and more evolved echocardiographic techniques to the study of fetal rhythm disturbances. There are several issues yet to be resolved. It is hoped that in the future there will be a more consistent and scientific approach to the management of fetal arrhythmias. The present review outlines the current approach to the diagnosis and management of fetal arrhythmias and recent research elucidating the mechanism of tachycardia and outlines some areas of future research.
Evaluation of fetal arrhythmias
Currently, echocardiography is the most widely used tool for diagnosis and follow-up of fetal arrhythmias in clinical practice. This involves inferring the electrical events by temporal mapping of mechanical events, such as atrial or ventricular contraction or both (by M-mode, Doppler tissue imaging), or their functional consequence, such as flow (by Doppler) or a combination of the two (by color M-mode). M-mode tracings obtained by incorporating atrial and ventricular contractions are widely used to determine the sequence of electrical events but are often limited by poor signal quality. Incorporating color flow information (color M-mode tracings) often allows for optimization of low-intensity atrial signals. Recent reviews outline the methodology in greater detail [1•]. Dancea et al. [2] and Fouron et al. [3] described the use of simultaneous Doppler signals from the superior vena cava (SVC) and aorta in arrhythmia analysis. Apart from the identification of temporal events, Doppler techniques lend themselves to the measurement of various time intervals and classification of supraventricular tachycardia (SVT) into ‘long’ or ‘short’ ventriculoatrial tachycardia, similar to the usage of ‘PR’ intervals on postnatal ECG (Fig. 1). It is sometimes difficult to obtain optimal ‘atrial’ tracings because of angle dependence and fetal position. Similar information can be obtained from a pulmonary vein and pulmonary artery [4]. Doppler evaluation is also used for the evaluation of ‘mechanical PR intervals’ as a surrogate for ‘PR’ interval measured on ECG or MCG [5,6,7•• ] (Fig. 2).
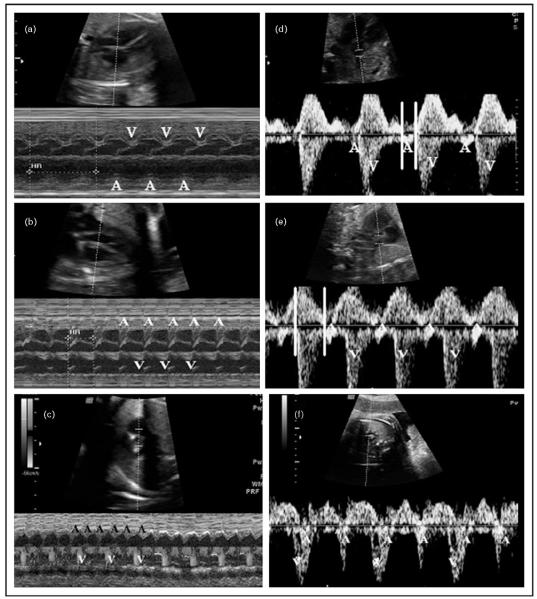
Figure 1. M-Mode and pulsed Doppler evaluation of fetal arrhythmias.
(a–c) M-mode recordings with representative SVC–Ao tracings from respective patients shown in (d–f). ‘A’ indicates atrial events and ‘V’ ventricular events. (a) M-mode recording in sinus rhythm. 1: 1 AV relationship is noted at a heart rate (HR) of 136 bpm (HR not shown). (b) M-mode recording in SVT with 1: 1 AV relation, fetal HR of 200 bpm is seen. (c) Color M-mode recording in atrial flutter with an atrial rate of 420 bpm and ventricular rate of 210 bpm indicating 2: 1 block. Here, flow in the aorta seen on color flow evaluation marked ‘V’ represents ventricular ejection. (d) SVC–Ao tracing in sinus rhythm. Parallel lines denote the mechanical PR interval, measured from beginning of atrial flow to the beginning of ventricular ejection. (e) SVC–Ao tracing in SVT. Parallel lines show the long ventriculoatrial interval of 170 ms (AV interval 130 ms). Gradual increase in HRs in tachycardia (not shown) indicated likely atrial ectopic tachycardia. (f) SVC–Ao tracing in atrial flutter showing 2: 1 block. Note: prominent ‘A’ waves. bpm, beats per minute; HR, heart rate; SVC–Ao, superior vena cava–aorta; SVT, supraventricular tachycardia.
Figure 2. Measurement of mechanical PR interval from simultaneous inflow-outflow Doppler obtained from the left ventricular outflow tract.
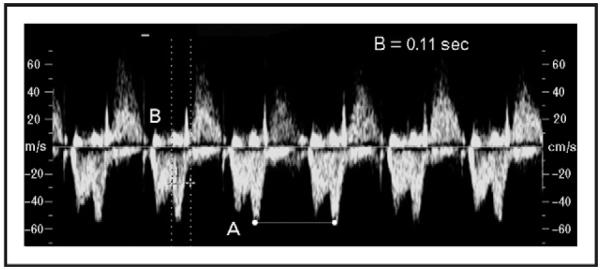
The mechanical PR interval, indicated by parallel lines (B), is calculated from the beginning of the mitral valve ‘A’ signal to the beginning of the aortic flow signal. In this example, it measures 0.11 s with a fetal heart rate of 146 beats per minute (A).
Tissue Doppler analysis using myocardial depolarization signals with selected simultaneous sampling sites in the fetal atrium and ventricular myocardium has shown better correlation with intervals obtained by ECG [8-10].
Echocardiography also has an important role in sequential follow-up of fetuses with arrhythmias and in the assessment of functional consequences of arrhythmia such as myocardial function, valvular regurgitation and evolution of hydrops [11,12]. It is important to recognize that changes in venous Doppler may occur as a consequence of the arrhythmia itself [13,14].
Fetal electrocardiograms (fECGs) are hampered by the low intensity of fetal signal and the need for signal averaging to assess ‘P’ wave morphology [9]. This precludes their use in real-time arrhythmia analysis at this time, though commercial units with improved algorithms are now available in some countries and are being evaluated clinically [15•]. The STAN ECG system provides ST segment analysis but is only applicable using a direct scalp electrode during labor [16]. Arrhythmia mechanisms have also been studied using magnetocardiograms [7••,8-14,15•,16-21,22••,23•• ]. This technique detects small magnetic fields that are associated with cardiac electrical signals. Maternal signals are identified and subtracted, allowing for the identification of fetal magnetocardiograms (fMCGs) that are similar to fECG signals. Temporal data as well as signal-averaged and real-time fMCG tracings can then be analyzed (Fig. 3). The main limitation of the study remains the limited availability of the technique and the need for a magnetically shielded room for the study. Recent reports of success in an unshielded environment allow one to be hopeful of increasing mainstream application of this exciting technology [24,25].
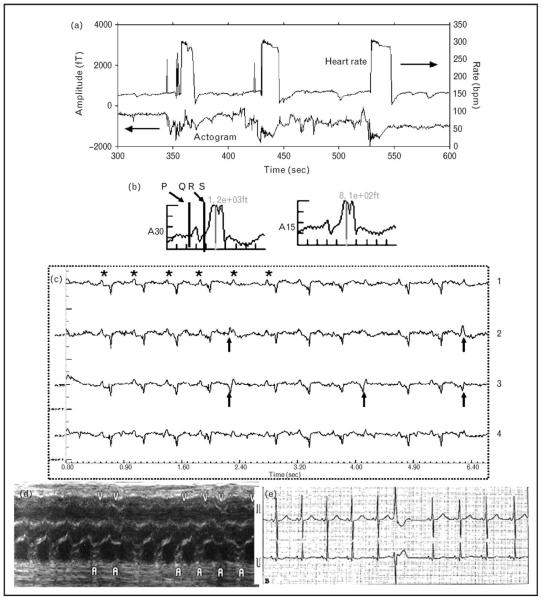
Figure 3. Fetus with intermittent supraventricular tachycardia and preexcitation noted on magnetocardiogram.
(a) Heart rate trend (top) and actogram (bottom). Intermittent supraventricular tachycardia at rates of approximately 300 beats per minute is seen. (b) Averaged ECG showing short PR and delta wave. (c) Real-time tracing obtained from magnetocardiogram. Line 4 represents a composite of maternal and fetal signals. Maternal signals have been averaged out in 1, 2 and 3. Arrowheads point to ectopic beats with a different morphology from QRS in sinus rhythm. In line 1, the ectopic QRS is isoelectric, revealing the hidden ‘P’ (*) wave buried in the QRS, indicating ventricular or aberrantly conducted junctional ectopic beats. (d) Prenatal M-mode with premature ventricular ‘V’ beats and a regular atrial rate (A). In this scenario, the possibility of ventricular tachycardia with 1: 1 conduction becomes difficult to rule out by M-mode analysis. (e) Postnatal rhythm strip with preexcitation and ventricular ectopy.
Irregular rhythms
An irregular rhythm is the most common cause of fetal arrhythmia seen in clinical practice. Most are caused by frequent ectopic beats. Ectopic beats are usually atrial in origin, though occasionally they are ventricular in origin. Atrial ectopic beats are most common in the late second trimester through term and are associated with a 2–3% risk of tachyarrhythmias or other comorbidity [26•,27•]. Some are conducted and some blocked, resulting in an irregular rhythm or a short pause, respectively.
Ventricular ectopic beats are much less common. In the absence of underlying heart defects, complete atrioventricular block (CAVB), long QT syndrome (LQTS) or myocarditis, they are thought to be benign [26•]. They are identified by the earliest activation being in the ventricle with either a regular atrial rate or a longer compensatory pause. Further studies of isolated premature ventricular complexes are needed.
Most ectopic beats are benign and do not need any specific intervention. An evaluation by a fetal cardiologist or perinatologist to rule out associated lesions is recommended. Cuneo et al. [28] noted a 2.6% incidence of conduction abnormalities as assessed by Doppler and fMCG in the setting of an irregular rhythm. Because of the small risk of sustained tachycardia, weekly Doppler assessment of heart rate (HR) in the obstetric office is recommended. Frequent atrial bigeminy or atrial couplets may suggest a higher risk for sustained tachyarrhythmia and merit reevaluation [29]. Kick countshelp assessfetal well-being.Anyrapidincrease in abdominal girth merits evaluation to rule out hydrops. If ectopypersiststhroughtheterm,anECGonthe neonate to assess for conduction abnormalities may be beneficial.
Tachyarrhythmia
Tachyarrhythmias are defined as fetal HRs of more than 180 beats per minute (bpm) [14,19,23••,26•,30]. These are broadly classified as sinus tachycardia, SVT, atrial flutter and ventricular tachycardia. Advances in noninvasive evaluation outlined above have helped distinguish likely mechanisms in most cases of fetal tachycardia, though there are several limitations. The algorithm for the evaluation of tachycardia mechanisms based on echocardiographic findings is outlined in Fig. 4.
Figure 4. Algorithm for evaluation of mechanism of tachyarrhythmia based on Doppler and relationship of atrial and ventricular events.
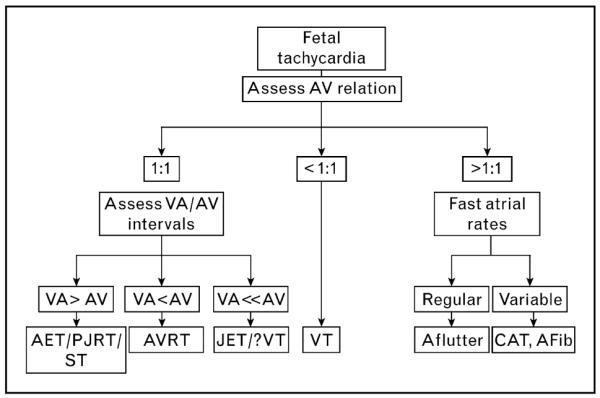
Cases of junctional tachycardia and ventricular tachycardia with retrograde conduction may present with 1: 1 AV relationship, but there is near-simultaneous depolarization of the ventricles and atria resulting in a very short VA interval and VA<<AV. AET, atrial ectopic tachycardia; Afib, atrial fibrillation; Aflutter, atrial flutter; AV, atrioventricular interval as measured from the beginning of atrial signal to beginning of arterial flow signal; AVRT, atrioventricular reentry tachycardia; CAT, chaotic atrial tachycardia; JET: junctional ectopic tachycardia; PJRT, paroxysmal junctional reciprocating tachycardia; ST, sinus tachycardia; VA interval, ventriculoatrial time duration as measured from the beginning of arterial flow to the beginning of atrial contraction.
Sinus tachycardia may rarely present with fetal HRs of 180–200 bpm. This may be seen in the setting of maternal pyrexia, use of stimulants, maternal thyrotoxicosis or fetal systemic disease such as anemia, fetal distress and rare infections [14,26•,31]. A gradual increase or decrease in fetal HR, lack of abrupt initiation or breaks and preserved HR variability in the setting of 1: 1 atrioventricular conduction and normal atrioventricular conduction times would suggest sinus tachycardia rather than SVT.
SVT as defined by nonsinus mechanism, 1: 1 atrioventricular conduction and HRs above 180 bpm account for the majority (70%) of fetal tachyarrhythmia. They encompass tachycardias because of different mechanisms.
Short ‘ventriculoatrial’ tachycardias, in which the ‘ventriculoatrial’ interval is less than half of RR interval, usually involve reentry mechanisms including atrioventricular reentry (AVRT) using a bypass tract or atrioventricular nodal reentry (AVNRT). As retrograde activation of the atria occurs shortly after ventricular activation, a short ‘ventriculoatrial’ interval is noted on Doppler evaluation. Using MCG, Strasburger et al. were able to show that complex mechanisms were involved in the initiation and maintenance of tachycardia in fetuses. Premature atrial contractions are also common [19]. Preexcitation is noted in about 30% of cases [32,33]. Tachycardia typically shows a sudden onset and cessation and may be intermittent or sustained (present >50% of time in a 24-h period).
Long ‘ventriculoatrial’ tachycardias include atrial ectopic tachycardia or paroxysmal junctional reciprocating tachycardia (PJRT). These can often be difficult to distinguish from each other with echocardiography, though use of tissue Doppler imaging (TDI) has shown promise. Atrial ectopic tachycardia is secondary to an automatic ectopic focus [31]. Gradual warm up and cool down and variable atrioventricular conduction times may be noted. PJRT, on the contrary, is due to reentry using a slow conducting parahisian pathway that conducts retrograde with resultant delayed atrial activation and reentrant tachycardia. PJRT is usually incessant and difficult to treat and may be associated with tachycardia-mediated cardiomyopathy and cardiac failure due to the incessant nature of the tachycardia.
Atrial flutter accounts for about 30% of cases of fetal tachycardia [31,34,35]. These are typically associated with high atrial rates of around 300–500 bpm and slower ventricular response in the setting of variable atrioventricular conduction. There is a high incidence of associated reentry in fetuses with atrial flutter, with reentry being noted in about 70% of cases [32,36,37]. Atrial flutter is usually noted later in gestation.
There are very few multicenter organized studies with regard to the management and drug therapy for SVT; however, several excellent institutional reviews and general guidelines are available, with more recent publications outlining a more systematic approach based on arrhythmia mechanism [23•• ,32,38–49]. The decision to treat or not to treat depends upon several factors including mechanism of tachycardia, persistence, fetal gestational age and well-being and the presence or absence of associated congenital heart disease (CHD). Sustained tachyarrhythmia may affect fetal well-being and result in hydrops due to impaired ventricular filling, altered flow patterns across the foramen ovale or cardiac dysfunction in the setting of incessant tachycardia. Left-sided path-ways and atrial flutter were noted to be associated with hydrops earlier, presumably secondary to altered flow across the foramen ovale [50].
In general three options are available: no treatment with close monitoring, transplacental drug therapy and, finally, delivery of fetus [51]. It is important to recognize that intermittent tachycardia in the fetus, especially later in gestation, is well tolerated and may not necessarily require transplacental therapy or emergent delivery in the absence of hydrops. In such cases, in-hospital monitoring of the fetus for 12–24 h to assess arrhythmia frequency and fetal well-being is recommended. Intermittent arrhythmias (<50% of time) in a healthy fetus can then be monitored on an outpatient basis. Close follow-up with repeat ultrasounds to assess rhythm and fetal well-being once or twice a week is recommended. On occasions, arrhythmia frequency may increase or concerns of fetal well-being may prompt hospital admission and institution of drug therapy or delivery if the fetus is near term. Rare cases of hydrops and neurologic sequelae have been reported in the setting of intermittent tachycardia [40,52].
The comparison of some of the studies on the management of fetal tachyarrhythmia is shown in Table 1 [38,40,41,43,45-48]. Over the recent years, there has been an increasing recognition of the potential risks of proarrhythmia to both the mother and the fetus. The efficacy of transplacental therapy is highly dependent on the pharmacokinetics of a given drug, its ability to cross the placenta and fetal bioavailability [23••,26•]. For example, transplacental transfer of digoxin is significantly impaired in the presence of hydrops fetalis, whereas sotalol has been shown to have good transfer. On the contrary, higher levels of flecainide have been noted in the fetal amniotic fluid and conduction delay noted on ECG in infancy [53-55]. In general, digoxin continues to be the first line of therapy in the absence of fetal hydrops. Second-line agents for refractory SVT or the presence of hydrops or both include sotalol, flecainide or amiodarone. Agents used include propranolol, propafenone and procainamide amongst others. Direct fetal therapy with intramuscular digoxin in the setting of fetal hydrops has been reported [41,56]. Intravenous adenosine by cordocentesis has been used to interrupt incessant reentrant tachycardia or to exclude atrial flutter. Adenosine has a transient effect and is typically used in association with other antiarrhythmic agents to control the tachycardia. Finally, delivery and postnatal management of the arrhythmia, if persistent, is an option in the mature near-term fetus. The underlying arrhythmia makes fetal monitoring during pregnancy and labor challenging at times. However, adding the risks of prematurity is not doing the fetus a favor, and hence this should be reserved for patients with persistent, difficult-to-treat arrhythmias and associated fetal hydrops, in which transplacental or direct therapy or both have failed and the fetus is of a reasonable gestational age. Because of the real potential for proarrhythmia and adverse outcomes, a coordinated multidisciplinary approach including perinatologists, fetal cardiologist, adult cardiologist and neonatologist is beneficial.
Table 1. This table depicts a few large series of fetal tachycardia or atrial flutter treatment.
| Year of publication | 1995 | 1998 | 1998 | 2000 | 2003 | 2004 | 2004 | 2008 |
|---|---|---|---|---|---|---|---|---|
| Patients | 35 | 110 | 14 | 20 | 25 | 24 | 29 | 22 |
| HF (%) | 37 | 39 | 57 | 40 | 100 | 93 | 27 | 33 |
| SVT, patients (n) | 35 | 105 | 14 | 10 | 21 | 15 | 22 | 21 |
| SVT HF patients converted (%) | 59 | 66 | 60 | 60 | 93 | 93 | ? | 93 |
| AF patients (n) | 0 | 22 | 0 | 10 | 4 | 9 | 7 | 1 |
| Atrial Flutter, HF converted atrial flutter (%) | 0 | 0 | 71 | 80 | 33 | 33 | 100 | 0 |
| Direct therapy (%) | ? | 3 (i.v.) | 0 | 0? | 0? | 86 (i.m.) | ? | 5 |
| HF (%), mortalitya | 46 | T27% D50% | 14 | 30 | 12 | 0 | 25 | 43 |
| Digoxin, patients (n) | 13+/6− | 40+/28− | 14 | 0 | 9 | 23− | 22+ | 14 |
| Verapamil, patients (n) | 0 | 24+/4− | 0 | 0 | 0 | 0 | 0 | 0 |
| Flecainide, patients (n) | 7+/4− | 20+/5− | 0 | 0 | 7+/3− | 0 | ||
| Sotalol, patients (n) | 0 | 10+/4− | 16+4− | 2− | 0 | 15 | 3 | |
| Amiodarone, patients (n) | 0 | 0 | 0 | 7+/4− | 12+/SVT,3+/6-AF | 1 | 0 | |
| Multidrug, patients (n) | 5− | 23+/11− | 0 | 0 | 0 | 3+SVT | 1 | 1 |
| Total fetuses treated (n) | 35 | 110 | 14 | 20 | 25 | 24 | 27 | 15 |
| Reference number | [43] | [38] | [46] | [40] | [45] | [41] | [3,47] | 48 |
AF, atrial flutter; D, direct; HF, hydrops fetalis; i.m., intramuscular; i.v., intravenous; SVT, supraventricular tachycardia; T, transplacental.
The mortality for fetuses with hydrops has been specified, as proarrhythmia accompanying antiarrhythmic treatment can be judged most effectively in the very ill. Multidrug, more than two drugs used in management. ? is used where the data is not clear from the article.Adapted with permission from [14].
Ventricular tachycardia is rare and may present with ventricular rates of more than 180 bpm in the setting of atrioventricular dissociation. Atrial rates are normal, or rarely 1: 1 retrograde ventriculo-atrial rates secondary to retrograde atrial activation are noted. This makes diagnosis by echocardiography challenging, though TDI has been helpful in identifying the earliest activation in the ventricular myocardium. Ventricular tachycardia is usually seen in the setting of underlying conduction abnormalities such as CAVB, fetal myocarditis or LQTS [14,22••,26•,57,58]. MCG has been very useful in the prenatal detection of LQTS and the quantification of arrhythmias [59]. Regional referral should be considered in cases with a strong family history of LQTS or in the presence of atypical echocardiographic findings or both. Prenatal transplacental therapy with lidocaine and magnesium sulphate, mexiletine and beta-blockers has been reported [57,59].
Bradyarrhythmias
Brief episodes of transient bradycardia that resolve within a couple of minutes are often noted and are benign. Fetal bradycardia, defined as persistent fetal HR of less than 100 bpm, may be secondary to sinus bradycardia, blocked atrial bigeminy or high-grade atrioventricular block. Bradycardia from different causes may present with similar effective HRs and hence needs echocardiographic evaluation to distinguish between them.
Sinus bradycardia
Persistent sinus bradycardia below 100 bpm is rare and can be seen in the setting of sinus node dysfunction associated with lower atrial focus in left atrial isomerism, fetal distress, hypoxia and acidosis, congenital LQTS and sinus node dysfunction. Sinus bradycardia is identified by one-to-one atrioventricular relationship on echocardiogram with a slow atrial rate. Atrioventricular conduction times are usually within normal limits in the absence of significant CHD. It is advisable to correlate with Doppler methods, as blocked premature atrial beats may be missed on M-mode, leading to a wrong diagnosis of sinus bradycardia.
Occasionally, frequent blocked premature atrial beats in atrial bigeminy or trigeminy may result in slow effective HRs in the range of 70–90 bpm. Typically, these resolve with fetal activity with normal acceleration of the sinus node and suppression of atrial ectopy. These are usually benign, and it is important to recognize the cause so as to avoid emergent delivery of a presumed fetus in distress. This needs to be differentiated from 2: 1 block, in which every other atrial beat is conducted to the ventricles but the atrial-atrial (AA) interval is relatively constant, whereas the premature beats show a shorter AA interval.
Second and third-degree atrioventricular block
Altered conduction of atrial impulses to the ventricles resulting in variable conduction as in second-degree block or complete dissociation of the atria and ventricle as in CAVB is an important cause of fetal bradycardia.
In about 50% of cases, CAVB is associated with complex CHD, in particular heterotaxy and endocardial cushion defect or discordant atrioventricular connections as in congenital corrected transposition of the great vessels. This is generally associated with poor outcomes, with a less than 20% neonatal survival being reported [60,61]. Survival was unlikely in the presence of ventricular rates of less than 60 bpm and hydrops. Currently there are few options for prenatal management of these fetuses. Administration of beta-sympathomimetics, such as oral terbutaline, may result in improved ventricular rates, but whether it results in improved survival is debatable [23••,27•,62•]. Preterm delivery in the setting of hydrops is a consideration but the compounding risk of added prematurity and need for pacing must be considered.
Isolated CAVB in the fetus in the absence of CHD is usually immune-mediated in association with transplacental transfer of circulating antibodies to Ro (SSA) and La (SSB) antigens from the mother [7••,22••,23••,29,62•,63]. Often, mothers are asymptomatic, and antibodies are detected upon evaluation for fetal bradycardia. The risk for acquired heart block in the fetus in the setting of maternal anti-SSA and anti-SSB antibodies is around 2–3%, with a recurrence risk of 14–17%. Varying degrees of conduction abnormalities, ranging from transient first-degree heart block to CAVB and hydrops, may be seen. Features of myocardial dysfunction, cardiomyopathy and atrial as well as ventricular endocardiofibroelastosis have been reported in utero, as well as in about 10–15% of offspring [7•• ,64,65].
The pathogenesis is thought to be secondary to immune-mediated inflammatory response and injury to the developing fetal myocardium and conduction tissue during a unique window in fetal cardiac development. The risk to the fetus appears to be maximal between 16 and 26 weeks of gestation. The precise mechanism by which an extracellular antibody meets with a normally intracellular antigen is currently the subject of intense research. The translocation of SSA/Ro and SSB/La to the surface in apoptotic cells has been shown. Clancy et al. [66,67] have proposed that inhibition of the physiologic clearance of apoptotic cells by resident cardiomyocytes in the fetus by antigen binding of the apoptotic cells results in the accumulation of apoptotic cells and resultant inflammation and cell injury. However, the exact cascade of events in vivo is still under investigation [68,69]. It is now well recognized that the mere presence of anti-SSA and anti-SSB antibodies is not enough, as evidenced by the low rates of involvement and discordant manifestations in twin pregnancies and the fetal-specific nature of the disease, that is, the lack of maternal involvement.
CAVB carries a significant mortality of about 18–40%, mainly fetal and neonatal, and morbidity (pacemaker and risk for congestive cardiomyopathy). Atrioventricular conduction times (mechanical PR interval) by Doppler techniques are commonly used in fetal surveillance in an attempt to diagnose the ‘at-risk’ fetus prior to the onset of complete heart block (Fig. 2). By definition, this would be longer than the electrical PR interval measured by ECG or MCG due to the inclusion of the isovolumic contraction phase of the ventricle [9]. The ‘normal’ values for mechanical PR interval in the fetus are 0.12±0.02, as obtained by the mitral A-wave/aortic outflow method [31]. There is controversy with regard to the ‘upper limit of normal’ for fetal mechanical PR intervals depending on a cutoff of +2 versus +3 SD. Using a more liberal cutoff of 0.14 s, Sonesson et al. [70] were able to show ‘PR’ prolongation, some transient, in as many as 30% of fetuses in pregnancies complicated by anti-SSA antibodies. Using MCG, Zhao et al. [22•• ] observed the presence of junctional ectopic tachycardia or ventricular tachycardia in about 30% of fetuses with recent-onset third-degree heart block likely representing a unique window in the pathogenesis. Cuneo et al. [71] were able to show differences in HR reactivity and response to terbutaline in those with immune-mediated CAVB compared with those associated with CHD.
Unfortunately, there are no reliable markers to predict which fetus will go on to develop immune-mediated CAVB in the setting of maternal anti-SSA and anti-SSB antibodies. In some, CAVB may set in rapidly within the course of a week, and the gradual progression through first to CAVB either is rapid or does not happen. Moderate tricuspid regurgitation, decreased cardiac function or atrial endocardiofibroelastosis or all three could be potential markers of an ‘at-risk’ fetus [7•• ]. There is some evidence that early use of anti-inflammatory agents such as dexamethasone or betamethasone, both fluorinated steroids that cross the placenta effectively, may modify the course of the disease by limiting the inflammation and resulting in improvement in rhythm, cardiac function and resolution of hydrops in CAVB. By using a systematic protocol, Jaeggi et al. [62•,72] were able to show an increase in 1-year survival from 47 to 95%. Transient or stable first-degree heart block or both in utero have been reported both with and without steroid use. Chronic steroid therapy does have significant associated risks [57]. Maternal and neonatal plasmapheresis has been used with variable success [23•• ,49]. Fetal ventricular pacing has been attempted without success because of the lack of atrioventricular synchrony and, presumably, myocardial dysfunction. No significant long-term sequelae were identified in fetuses surviving CAVB and hydrops in a small series, though others have shown persistent fetal and neonatal growth restriction [73,74].
Finally, in a small subset of fetuses, transient second-degree heart block may be seen in the absence of CHD or immune-mediated injury. In this subset, congenital LQTS should be evaluated, especially if associated with sinus bradycardia or intermittent ventricular arrhythmias. Most of these isolated cases of fetal 2: 1 block have had a good prognosis [75]. It is unknown if this represents a normal developmental maturational process, now noted because of enhanced surveillance.
Conclusion
In summary, the last decade has seen a significant advancement in methods of assessment of rhythm abnormalities available to the fetal cardiologists. Application of newer techniques such as MCG is advancing our knowledge of fetal electrophysiological properties. Recent reports of possible application of a portable MCG system, along with advances in fetal ECG recording, hold promise of extending these newer methods to routine clinical practice. Multicenter studies are required to evaluate different therapies for arrhythmia management, patient selection, and acute and long-term effects of antiarrhythmic use. Significant gaps still exist in our understanding of the unique electrophysiological and maturational properties of the fetal conduction systems. The role of repolarization abnormalities and their potential implications in sudden fetal death remain to be explored further.
Acknowledgement
The authors wish to thank Ivie Okundaye and Joban Vaishnav, research assistants, for their technical support.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
•of special interest
••of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 611-612).
- 1•.Hornberger LK. Echocardiographic assessment of fetal arrhythmias. Heart. 2007;93:1331–1333. doi: 10.1136/hrt.2006.108605. This article is a nice review of current echocardiographic techniques for the evaluation of fetal arrhythmias.
- 2.Dancea A, Fouron JC, Miro J, et al. Correlation between electrocardiographic and ultrasonographic time-interval measurements in fetal lamb heart. Pediatr Res. 2000;47:324–328. doi: 10.1203/00006450-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Fouron JC, Fournier A, Proulx F, et al. Management of fetal tachyarrhythmia based on superior vena cava/aorta Doppler flow recordings. Heart. 2003;89:1211–1216. doi: 10.1136/heart.89.10.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carvalho JS, Prefumo F, Ciardelli V, et al. Evaluation of fetal arrhythmias from simultaneous pulsed wave Doppler in pulmonary artery and vein. Heart. 2007;93:1448–1453. doi: 10.1136/hrt.2006.101659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenthal D, Friedman DM, Buyon J, et al. Validation of the Doppler PR interval in the fetus. J Am Soc Echocardiogr. 2002;15:1029–1030. doi: 10.1067/mje.2002.121438. [DOI] [PubMed] [Google Scholar]
- 6.Bergman G, Jacobsson LA, Wahren-Herlenius M, et al. Doppler echocardiographic and electrocardiographic atrioventricular time intervals in newborn infants: evaluation of techniques for surveillance of fetuses at risk for congenital heart block. Ultrasound Obstet Gynecol. 2006;28:57–62. doi: 10.1002/uog.2712. [DOI] [PubMed] [Google Scholar]
- 7•.Friedman DM, Kim MY, Copel JA, et al. Utility of cardiac monitoring in fetuses at risk for congenital heart block: the PR Interval and Dexamethasone Evaluation (PRIDE) prospective study. Circulation. 2008;117:485–493. doi: 10.1161/CIRCULATIONAHA.107.707661. This study reports the latest results of the PR Interval and Dexamethasone Evaluation study and issues with prenatal detection of the fetus at risk of congenital heart block (CHB) in the setting of anti-SSA/Ro antibodies.
- 8.Rein AJ, O’Donnell C, Geva T, et al. Use of tissue velocity imaging in the diagnosis of fetal cardiac arrhythmias. Circulation. 2002;106:1827–1833. doi: 10.1161/01.cir.0000031571.92807.cc. [DOI] [PubMed] [Google Scholar]
- 9.Nii M, Hamilton RM, Fenwick L, et al. Assessment of fetal atrioventricular time intervals by tissue Doppler and pulse Doppler echocardiography: normal values and correlation with fetal electrocardiography. Heart. 2006;92:1831–1837. doi: 10.1136/hrt.2006.093070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nii M, Roman KS, Kingdom J, et al. Assessment of the evolution of normal fetal diastolic function during mid and late gestation by spectral Doppler tissue echocardiography. J Am Soc Echocardiogr. 2006;19:1431–1437. doi: 10.1016/j.echo.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 11.Hofstaetter C, Hansmann M, Eik-Nes SH, et al. A cardiovascular profile score in the surveillance of fetal hydrops. J Matern Fetal Neonatal Med. 2006;19:407–413. doi: 10.1080/14767050600682446. [DOI] [PubMed] [Google Scholar]
- 12.Huhta JC. Right ventricular function in the human fetus. J Perinat Med. 2001;29:381–389. doi: 10.1515/JPM.2001.054. [DOI] [PubMed] [Google Scholar]
- 13.Simpson JM. Fetal arrhythmias. Ultrasound Obstet Gynecol. 2006;27:599–606. doi: 10.1002/uog.2819. [DOI] [PubMed] [Google Scholar]
- 14.Strasburger JF. Prenatal diagnosis of fetal arrhythmias. Clin Perinatol. 2005;32:891–912. viii. doi: 10.1016/j.clp.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 15•.Gardiner HM, Belmar C, Pasquini L, et al. Fetal ECG: a novel predictor of atrioventricular block in anti-Ro positive pregnancies. Heart. 2007;93:1454–1460. doi: 10.1136/hrt.2006.099242. Application of fECG in the detection of fetuses at risk for CHB.
- 16.Doria V, Papageorghiou AT, Gustafsson A, et al. Review of the first 1502 cases of ECG-ST waveform analysis during labour in a teaching hospital. BJOG. 2007;114:1202–1207. doi: 10.1111/j.1471-0528.2007.01480.x. [DOI] [PubMed] [Google Scholar]
- 17.Menendez T, Achenbach S, Beinder E, et al. Magnetocardiography for the investigation of fetal arrhythmias. Am J Cardiol. 2001;88:334–336. doi: 10.1016/s0002-9149(01)01658-7. [DOI] [PubMed] [Google Scholar]
- 18.Van Leeuwen P, Hailer B, Bader W, et al. Magnetocardiography in the diagnosis of fetal arrhythmia. BJOG. 1999;11:1200–1208. doi: 10.1111/j.1471-0528.1999.tb08149.x. [DOI] [PubMed] [Google Scholar]
- 19.Wakai RT, Strasburger JF, Li Z, et al. Magnetocardiographic rhythm patterns at initiation and termination of fetal supraventricular tachycardia. Circulation. 2003;107:307–312. doi: 10.1161/01.cir.0000043801.92580.79. [DOI] [PubMed] [Google Scholar]
- 20.Comani S, Alleva G. Fetal cardiac time intervals estimated on fetal magnetocardiograms: single cycle analysis versus average beat inspection. Physiol Meas. 2007;28:49–60. doi: 10.1088/0967-3334/28/1/005. [DOI] [PubMed] [Google Scholar]
- 21.Horigome H, Ogata K, Kandori A, et al. Standardization of the PQRST waveform and analysis of arrhythmias in the fetus using vector magnetocardiography. Pediatr Res. 2006;59:121–125. doi: 10.1203/01.pdr.0000190578.81426.fc. [DOI] [PubMed] [Google Scholar]
- 22••.Zhao H, Cuneo BF, Strasburger JF, et al. Electrophysiological characteristics of fetal atrioventricular block. J Am Coll Cardiol. 2008;51:77–84. doi: 10.1016/j.jacc.2007.06.060. This study shows the association of complex ventricular arrhythmias with CHB using MCG and offers a unique perspective in the study of fetal CHB.
- 23••.Hornberger LK, Collins K. New insights into fetal atrioventricular block using fetal magnetocardiography. J Am Coll Cardiol. 2008;51:85–86. doi: 10.1016/j.jacc.2007.09.016. This is a review of current methods of assessment of the fetus with atrioventricular block.
- 24.Verklan MT, Padhye NS, Brazdeikis A. Analysis of fetal heart rate variability obtained by magnetocardiography. J Perinat Neonatal Nurs. 2006;20:343–348. doi: 10.1097/00005237-200610000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Seki Y, Kandori A, Kumagai Y, et al. Unshielded fetal magnetocardiography system using two-dimensional gradiometers. Rev Sci Instruments. 2008;79:036106. doi: 10.1063/1.2897588. [DOI] [PubMed] [Google Scholar]
- 26•.Strasburger JF, Cheulkar B, Wichman HJ. Perinatal arrhythmias: diagnosis and management. Clin Perinatol. 2007;34:627–652. vii–viii. doi: 10.1016/j.clp.2007.10.002. This is a nice review of fetal and neonatal arrhythmias with emphasis on MCGs.
- 27•.Cuneo BF, Zhao H, Strasburger JF, et al. Atrial and ventricular rate response and patterns of heart rate acceleration during maternal-fetal terbutaline treatment of fetal complete heart block. Am J Cardiol. 2007;100:661–665. doi: 10.1016/j.amjcard.2007.03.081. This study highlights the variability of fetal response to sympathomimetic agents on the basis of underlying causes.
- 28.Cuneo BF, Strasburger JF, Wakai RT, et al. Conduction system disease in fetuses evaluated for irregular cardiac rhythm. Fetal Diagn Ther. 2006;21:307–313. doi: 10.1159/000091362. [DOI] [PubMed] [Google Scholar]
- 29.Li Z, Strasburger JF, Cuneo BF, et al. Giant fetal magnetocardiogram P waves in congenital atrioventricular block: a marker of cardiovascular compensation? Circulation. 2004;110:2097–2101. doi: 10.1161/01.CIR.0000144302.30928.AA. [DOI] [PubMed] [Google Scholar]
- 30.Allan L. The normal fetal heart. In: Allan L, Hornberger L, Sharland G, editors. Textbook of fetal cardiology. Greenwich Medical Media Limited; London, England: 2000. pp. 55–102. [Google Scholar]
- 31.Jaeggi ET, Nii M. Fetal brady- and tachyarrhythmias: new and accepted diagnostic and treatment methods. Semin Fetal Neonatal Med. 2005;10:504–514. doi: 10.1016/j.siny.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Naheed Z, Strasburger J, Deal B, et al. Fetal tachycardia: mechanisms and predictors of hydrops fetalis. J Am Coll Cardiol. 1996;27:1736–1740. doi: 10.1016/0735-1097(96)00054-x. [DOI] [PubMed] [Google Scholar]
- 33.Strasburger JF, Wakai RT, Zhimin L, et al. Prenatal preexcitation: magnetocardiographic assessment of Wolff-Parkinson-White syndrome in the fetus. PACE. 2002;24(4p2):602. [Google Scholar]
- 34.Krapp M, Kohl T, Simpson JM, et al. Review of diagnosis, treatment, and outcome of fetal atrial flutter compared with supraventricular tachycardia. Heart. 2003;89:913–917. doi: 10.1136/heart.89.8.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cuneo BF. Outcome of fetal cardiac defects. Curr Opin Pediatr. 2006;18:490–496. doi: 10.1097/01.mop.0000245348.52960.11. [DOI] [PubMed] [Google Scholar]
- 36.Johnson W, Dunnigan A, Fehr P, et al. Association of atrial flutter with orthodromic reciprocating fetal tachycardia. Am J Cardiol. 1987;59:374–375. doi: 10.1016/0002-9149(87)90823-x. [DOI] [PubMed] [Google Scholar]
- 37.Casey F, McCrindle B, Hamilton R, et al. Neonatal atrial flutter: significant early morbidity and excellent long-term prognosis. Am Heart J. 1997;133:302–306. doi: 10.1016/s0002-8703(97)70224-2. [DOI] [PubMed] [Google Scholar]
- 38.Simpson J. Fetal tachycardias: management and outcome of 127 consecutive cases. Heart. 1998;79:576–581. [PMC free article] [PubMed] [Google Scholar]
- 39.Van Engelen A, Weijtens O, Brenner J, et al. Management outcome and follow-up of fetal tachycardia. J Am Coll Cardiol. 1994;24:1371–1375. doi: 10.1016/0735-1097(94)90122-8. [DOI] [PubMed] [Google Scholar]
- 40.Oudijk MA, Michon MM, Kleinman CS, et al. Sotalol in the treatment of fetal dysrhythmias. Circulation. 2000;101:2721–2726. doi: 10.1161/01.cir.101.23.2721. [DOI] [PubMed] [Google Scholar]
- 41.Strasburger JF, Cuneo BF, Michon MM, et al. Amiodarone therapy for drug-refractory fetal tachycardia. Circulation. 2004;109:375–379. doi: 10.1161/01.CIR.0000109494.05317.58. [DOI] [PubMed] [Google Scholar]
- 42.Allan L, Chita S, Sharland G, et al. Flecainide in the treatment of fetal tachycardias. Heart. 1991;65:46–48. doi: 10.1136/hrt.65.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frohn-Mulder IM, Stewart PA, Witsenburg M, et al. The efficacy of flecainide versus digoxin in the management of fetal supraventricular tachycardia. Prenat Diagn. 1995;15:1297–1302. doi: 10.1002/pd.1970151309. [DOI] [PubMed] [Google Scholar]
- 44.Hansmann M, Gembruch U, Bald R, et al. Fetal tachyarrhythmias: transplacental and direct treatment of the fetus – a report of 60 cases. Ultrasound Obstet Gynecol. 1991;1:162–170. doi: 10.1046/j.1469-0705.1991.01030162.x. [DOI] [PubMed] [Google Scholar]
- 45.Jouannic JM, Delahaye S, Fermont L, et al. Fetal supraventricular tachycardia: a role for amiodarone as second-line therapy? Prenat Diagn. 2003;23:152–156. doi: 10.1002/pd.542. [DOI] [PubMed] [Google Scholar]
- 46.Sonesson SE, Fouron JC, Wesslen-Eriksson E, et al. Foetal supraventricular tachycardia treated with sotalol. Acta Paediatr. 1998;87:584–587. doi: 10.1080/08035259850158335. [DOI] [PubMed] [Google Scholar]
- 47.Furon JC. Fetal Arrhythmias: the Saint-Justine hospital experience. Prenat Diagn. 2004;24:1068–1080. doi: 10.1002/pd.1064. [DOI] [PubMed] [Google Scholar]
- 48.D’Alto M, Russo MG, Paladini D, et al. The challenge of fetal dysrhythmias: echocardiographic diagnosis and clinical management. J Cardiovasc Med. 2008;9:153–160. doi: 10.2459/JCM.0b013e3281053bf1. [DOI] [PubMed] [Google Scholar]
- 49.Hornberger LK, Sahn DJ. Rhythm abnormalities of the fetus. Heart. 2007;93:1294–1300. doi: 10.1136/hrt.2005.069369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kannankeril PJ, Gotteiner NL, Deal BJ, et al. Location of accessory connection in infants presenting with supraventricular tachycardia in utero: clinical correlations. Am J Perinatol. 2003;20:115–120. doi: 10.1055/s-2003-40014. [DOI] [PubMed] [Google Scholar]
- 51.Cuneo B, Strasburger JF. Management strategies for fetal tachycardia. Obstet Gynecol. 2000;96:575–581. doi: 10.1016/s0029-7844(00)00996-0. [DOI] [PubMed] [Google Scholar]
- 52.Schade R, Stoutenbeek P, de Vires L, et al. Neurological morbidity after fetal supraventricular tachyarrhythmia. Ultrasound Obstet Gynecol. 1999;13:43–47. doi: 10.1046/j.1469-0705.1999.13010043.x. [DOI] [PubMed] [Google Scholar]
- 53.Hall CM, Ward Platt MP. Neonatal flecainide toxicity following supraventricular tachycardia treatment. Ann Pharmacother. 2003;37:1343–1344. doi: 10.1345/aph.1C487. [DOI] [PubMed] [Google Scholar]
- 54.Rasheed A, Simpson J, Rosenthal E. Neonatal ECG changes caused by supratherapeutic flecainide following treatment for fetal supraventricular tachycardia. Heart. 2003;89:470. doi: 10.1136/heart.89.4.470-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trotter A, Kaestner M, Pohlandt F, et al. Unusual electrocardiogram findings in a preterm infant after fetal tachycardia with hydrops fetalis treated with flecainide. Pediatr Cardiol. 2000;21:259–262. doi: 10.1007/s002460010053. [DOI] [PubMed] [Google Scholar]
- 56.Parilla B, Strasburger J, Socol M. Fetal supraventricular tachycardia complicated by hydrops fetalis: a role for direct fetal intramuscular therapy. Am J Perinatol. 1996;13:483–486. doi: 10.1055/s-2007-994432. [DOI] [PubMed] [Google Scholar]
- 57.Cuneo BF, Ovadia M, Strasburger JF, et al. Prenatal diagnosis and in utero treatment of torsades de pointes associated with congenital long QT syndrome. Am J Cardiol. 2003;91:1395–1398. doi: 10.1016/s0002-9149(03)00343-6. [DOI] [PubMed] [Google Scholar]
- 58.Zhao H, Strasburger JF, Cuneo BF, et al. Fetal cardiac repolarization abnormalities. Am J Cardiol. 2006;98:491–496. doi: 10.1016/j.amjcard.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 59.Horigome H, Iwashita H, Yoshinaga M, et al. Magnetocardiographic demonstration of torsade de pointes in fetus with congenital long QT syndrome. J Cardiovasc Electrophysiol. 2008;19:334–335. doi: 10.1111/j.1540-8167.2007.01026.x. [DOI] [PubMed] [Google Scholar]
- 60.Eronen M, Siren MK, Ekblad H, et al. Short- and long-term outcome of children with congenital complete heart block diagnosed in utero or as a newborn. Pediatrics. 2000;106(1 Pt 1):86–91. doi: 10.1542/peds.106.1.86. [DOI] [PubMed] [Google Scholar]
- 61.Ferrer PL. Fetal arrhythmias. In: Deal B, Wolff GS, Gelband H, editors. Current concepts in diagnosis and treatment of arrhythmias in infants and children. Futura Publishing Company, Inc; Armonk, New York: 1998. pp. 17–63. [Google Scholar]
- 62•.Jaeggi ET, Friedberg MK. Diagnosis and management of fetal bradyarrhythmias. Pacing Clin Electrophysiol. 2008;31(Suppl 1):S50–S53. doi: 10.1111/j.1540-8159.2008.00957.x. A short review of the diagnosis and management algorithms for fetal bradycardia.
- 63.Buyon JP, Clancy RM. Neonatal lupus: basic research and clinical perspectives. Rheum Dis Clin North Am. 2005;31:299–313. vii. doi: 10.1016/j.rdc.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 64.Moak JP, Barron KS, Hougen TJ, et al. Congenital heart block: development of late-onset cardiomyopathy, a previously underappreciated sequela. J Am Coll Cardiol. 2001;37:238–242. doi: 10.1016/s0735-1097(00)01048-2. [DOI] [PubMed] [Google Scholar]
- 65.Villain E, Marijon E, Georgin S. Is isolated congenital heart block with maternal antibodies a distinct and more severe form of the disease in childhood? Heart Rhythm. 2005;2(1S):S45. [Google Scholar]
- 66.Clancy RM, Buyon JP. More to death than dying: apoptosis in the pathogenesis of SSA/Ro-SSB/La-associated congenital heart block. Rheum Dis Clin North Am. 2004;30:589–602. x. doi: 10.1016/j.rdc.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 67.Clancy RM, Neufing PJ, Zheng P, et al. Impaired clearance of apoptotic cardiocytes is linked to anti-SSA/Ro and -SSB/La antibodies in the pathogenesis of congenital heart block. J Clin Invest. 2006;116:2413–2422. doi: 10.1172/JCI27803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wahren-Herlenius M, Sonesson SE. Specificity and effector mechanisms of autoantibodies in congenital heart block. Curr Opin Immunol. 2006;18:690–696. doi: 10.1016/j.coi.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 69.Tzioufas AG, Moutsopoulos HM. Predicting autoimmune congenital heart block: is it feasible and how? Rheumatology (Oxford) 2007;46:1221–1222. doi: 10.1093/rheumatology/kem148. [DOI] [PubMed] [Google Scholar]
- 70.Sonesson SE, Salomonsson S, Jacobsson LA, et al. Signs of first degree heart block occur in one-third of fetuses of pregnant women with anti-SSA/Ro 52 kd antibodies. Arthritis Rheum. 2004;50:1253–1261. doi: 10.1002/art.20126. [DOI] [PubMed] [Google Scholar]
- 71.Cuneo BF, Strasburger JF, Zhao H, et al. Electrophysiologic patterns of fetal heart rate augmentation with terbutaline in complete AV block. Heart Rhythm. 2005;2(1S):S45. [Google Scholar]
- 72.Jaeggi ET, Fouron JC, Silverman ED, et al. Transplacental fetal treatment improves the outcome of prenatally diagnosed complete atrioventricular block without structural heart disease. Circulation. 2004;110:1542–1548. doi: 10.1161/01.CIR.0000142046.58632.3A. [DOI] [PubMed] [Google Scholar]
- 73.Breur JM, Gooskens RH, Kapusta L, et al. Neurological outcome in isolated congenital heart block and hydrops fetalis. Fetal Diagn Ther. 2007;22:457–461. doi: 10.1159/000106355. [DOI] [PubMed] [Google Scholar]
- 74.Skog A, Wahren-Herlenius M, Sundstrom B, et al. Outcome and growth of infants fetally exposed to heart block-associated maternal anti-Ro52/SSA autoantibodies. Pediatrics. 2008;121:e803–e809. doi: 10.1542/peds.2007-1659. [DOI] [PubMed] [Google Scholar]
- 75.Chang YL, Hsieh PC, Chang SD, et al. Perinatal outcome of fetus with isolated congenital second degree atrioventricular block without maternal anti-SSA/Ro-SSB/La antibodies. Eur J Obstet Gynecol Reprod Biol. 2005;122:167–171. doi: 10.1016/j.ejogrb.2005.01.013. [DOI] [PubMed] [Google Scholar]