Abstract
Once the topic of folklore and science fiction, the notion of restoring vision to the blind is now approaching a tractable reality. Technological advances have inspired numerous multidisciplinary groups worldwide to develop visual neuroprosthetic devices that could potentially provide useful vision and improve the quality of life of profoundly blind individuals. While a variety of approaches and designs are being pursued, they all share a common principle of creating visual percepts through the stimulation of visual neural elements using appropriate patterns of electrical stimulation. Human clinical trials are now well underway and initial results have been met with a balance of excitement and cautious optimism. As remaining technical and surgical challenges continue to be solved and clinical trials move forward, we now enter a phase of development that requires careful consideration of a new set of issues. Establishing appropriate patient selection criteria, methods of evaluating long-term performance and effectiveness, and strategies to rehabilitate implanted patients will all need to be considered in order to achieve optimal outcomes and establish these devices as viable therapeutic options.
“We can rebuild him…we have the technology.”
- from the television series “The Six Million Dollar Man”
Introduction: History, an Unmet Demand, and State of the Art
Our fascination with building a bionic human mirrors the technological advances that ubiquitously characterize the modern era. Today, this idea has become less the subject of science fiction and more the pursuit of intense scientific research. Advances within the realms of microfabrication, microelectronics, material science, wireless technology and high-speed computer processing power have allowed for the development of neuroprosthetic devices designed to assist individuals living with sensory loss and/or motor impairment. The basic premise underlying all neuroprosthetic approaches is that targeted and controlled delivery of electrical stimulation to nerves or muscles can potentially restore (to a certain degree) the physiological function of a damaged organ or limb (Marbach et al., 1982). The success of cochlear implants, developed over 30 years ago, serves as a well known example. This neuroprosthetic device has helped thousands of profoundly deaf individuals regain hearing and develop speech communication (Jones et al., 2008; Loeb, 1990). Similarly, sophisticated artificial limbs have led to improved walking mobility and even grasping skills for amputees (Allin et al., 2010; Craelius, 2002; Laferrier and Gailey, 2010). The continued development of brain-machine interfaces (BMIs) is also providing exciting hope for paralyzed patients. By recording neuronal signals from the brain that code for movement, these signals can be converted and used to control external devices such as a robotic limb prosthesis (Donoghue, 2002; Hochberg et al., 2006; Nicolelis, 2003). Rapid progress in all of these arenas continues and in many ways serves as inspiration for the development of a visual neuroprosthesis for the blind. Today, several device designs and approaches are being developed and human clinical trials are well underway (for extensive reviews see: (Chader et al., 2009; Dagnelie, 2006; Dowling, 2005; Humayun, 2007; Javaheri et al., 2006; Merabet et al., 2005)).
According to the World Health Organization, there are 314 million visually impaired individuals worldwide (2009 WHO fact sheet; http://www.who.int/mediacentre/factsheets/fs282/en/). While an astonishingly large number, it is worthy to note that that only a minority of individuals (approximately 45 million) are actually considered profoundly blind (defined as best-corrected visual acuity worse than 20/400 Snellen acuity) and have some degree of residual visual function. Furthermore, a tragic reality exists. The greatest portion of these individuals live in developing countries and the majority of the leading causes of blindness are actually avoidable and/or treatable (e.g. surgery for cataracts or antibiotic treatment for trachoma). Thus, the restoration of functional vision through a visual prosthesis will likely target only a restricted segment of the blind population. Moreover, it is important to realize that not all individuals and all forms of visual impairment could potentially benefit from a visual neuroprosthesis. As presently conceived, visual prosthetic devices have been designed for individuals with profound vision loss and who have had normal visual development (as opposed to congenital causes of blindness). Furthermore, as these devices are designed to interface with viable neuronal tissue, the site of damage and nature of pathology will largely dictate whether a prosthetic device can be feasibly implemented. For example, in conditions where the overall functional and structural integrity of the retina is compromised (e.g. trauma, glaucoma, or retinal complications related to diabetes), a retinal-based visual prosthesis is unlikely to be effective in restoring visual function (see discussion on various visual prosthesis approaches). These limitations notwithstanding, it is also important to highlight advances being made in other areas of biomedical research such as gene therapy and cell transplantation. These molecular based approaches may in time provide new treatments and help halt the progression of vision loss particularly with respect to hereditary causes of blindness (Acland et al., 2001; MacLaren et al., 2006). At the same time, blind individuals will continue to benefit from the use of sensory substitution devices (also discussed in this edition). These devices are specially designed to leverage sensory information obtained from the intact senses (e.g. hearing and touch) to substitute for the vision. This allows a blind user to interact with their surrounding environment (Bach-y-Rita, 2004; Bach-y-Rita and Kercel, 2003). Thus, the future rehabilitation of individuals with visual impairment will likely continue to encompass multi-disciplinary approaches and include molecular-based therapies designed to halt the progression of vision loss, the use of sensory substitution devices, and potentially restore a certain level of functional vision through the use of visual neuroprosthetic devices. Here, we will highlight advances and discuss future perspectives relating to visual neuroprosthesis development.
Summary of Visual Neuroprosthetic Approaches: From Retina to Cortex
Generally speaking, the operating premise underlying a visual neuroprosthesis is to artificially replace the function of damaged neuronal elements that make up the visual pathway (figure 1). Typically, patterned micro-electrical stimulation is delivered through an array of tiny microelectrodes to elicit the perception of organized patterns of light (however, see also the development of sub-millimeter, geometrically constrained microfluidic channels to deliver targeted and controlled release of neurotransmitters, (Peterman et al., 2004)). The electrical stimulation of these surviving visual neuronal elements evokes the subjective sensation of discrete points of light (referred to as “phosphenes”; (Gothe et al., 2002; Marg and Rudiak, 1994)). In principle, by delivering appropriate multi-site patterns of electrical stimulation (i.e. characterizing the shape of the intended visual target and reflecting the neural structure’s retinotopic organization), geometrical visual percepts can be generated. This allows for the perception of visual images (much akin to viewing a stadium electronic scoreboard or the images generated by an ink jet printer). The pattern of electrical stimulation delivered is determined by analyzing an image captured by a digital camera or in response to the images captured by the optics of the eye itself. With regards to visual perception, this “scoreboard” approach certainly represents a great oversimplification. It is clear that many attributes characterize a visual scene such as color, motion, and form. However, as currently conceived, visual prostheses are designed to address only one of the most basic components of vision, that is, spatial detail.
Figure 1.

Summary diagram illustrating various neuroprosthesis approaches to restore vision. Theoretically, any point along the visual pathway can be electrically stimulated to generate the perception of phosphenes and thus represents a potential site to implant a visual prosthesis. At the level of the retina, an implanted device generates electrical current to stimulate cells of the inner retina (i.e. ganglion and bipolar cells). Two approaches are possible: i) epi-retinal; in which the device is attached to the inner surface of the retina, and ii) sub-retinal; in which the device is placed within the underside of the retina. The optic nerve can be stimulated by implanting a cuff electrode around the nerve. In the cortical approach, electrodes are placed either intra-cortically or on the cortical surface in order to stimulate the visual cortex directly and thus bypassing afferent visual structures.
Amongst the biggest challenges of prosthetic vision is the puzzle of the neural code for perception. The complexity of the neural code suggests that prosthetic devices should rely on intact neural circuitry whenever possible in order to take advantage of any intact sensory processing available (Dagnelie and Schuchard, 2007). Thus, reducing the complexity of neural coding necessary could potentially be achieved by implanting the prosthetic device at the earliest point along the visual pathway that retains functional integrity. Following to this premise, the retina would represent the earliest site of potential neuronal interface.
Retinitis pigmentosa (RP) and age related macular degeneration (AMD) are two retinal disorders that contribute greatly to the incidence of inherited blindness and blindness in the elderly respectively (Bunker et al., 1984; Klein et al., 1997). Profound vision loss results largely due to the progressive degeneration of the light-capturing component of the outer-segment of the retina, that is, the photoreceptor cells. However, the remaining retinal elements within the inner retinal layers (e.g. the bipolar and ganglion cells that converge to form the optic nerve) appear to survive in large numbers. Furthermore, these elements remain responsive to electrical simulation even in highly advanced stages of the disease (Humayun et al., 1996). In essence, a retinal based visual prosthesis would replace the function of the degenerated photoreceptor cells by stimulating the surviving retinal neuronal machinery. A set of pivotal human experiments demonstrated that electrical stimulation of the retina of RP patients (Humayun et al., 1996; Rizzo et al., 2003b) as well as one patient with AMD (Humayun et al., 1999) led to the generation of phosphenes despite the fact that patients were profoundly blind for many years. Experiments lasted minutes to hours while patients remained awake in order to describe their visual experiences. Following electrical stimulation, patients reported visual patterned perceptions that were initially relatively crude. However, the gross geometric structure of the phosphene patterns could be altered in a controlled fashion by varying the position and number of the stimulating electrodes and the strength or duration of the delivered current (Humayun et al., 1996; Rizzo et al., 2003a, b). This demonstration of proof-of-principle has led many groups worldwide to pursue development of a variety of retinal-based designs and approaches. Currently, the retinal-based approach is arguably receiving the most attention as evidenced by size and number of on-going human clinical trials.
Two retinal-based approaches are being pursued that are largely differentiated by their location of implantation with respect to the retina. In the sub-retinal approach, the implant is placed in the region of degenerated photoreceptors by creating a pocket between the sensory retina and retinal pigment epithelium (RPE) layer. In the epi-retinal approach, the implant device is attached to the inner surface of the retina, close to the ganglion cell side (figure 1A).
The sub-retinal visual neuroprosthesis design is currently being pursued by the Boston Retinal Implant Project (a large joint collaborative effort that includes the Massachusetts Eye and Ear Infirmary and Harvard Medical School, the Massachusetts Institute of Technology, the Boston Veterans Affairs Healthcare System, and other partnering institutions) (see figure 2) (Shire et al., 2009). By virtue of being placed in juxtaposition to the nearest layer of surviving neurons (i.e. bipolar cells) the sub-retinal approach affords greater inherent mechanical stability. This is due to the fact that the ultra-thin electrode array is effectively “sandwiched” between the inner-segment of retina and the RPE layer. Furthermore, this approach has the theoretical advantage of not only being closer to surviving neuronal elements (thus potentially requiring lower amounts of electrical stimulating current) but also exploiting retinal signal pre-processing inherent to the bipolar cell layer. The placement of a sub-retinal device does require elaborate and complex surgical methods. For the Boston Retinal Implant device, this includes inserting an ultra-thin flexible microelectrode array through an incision made on the outside scleral wall of the ocular globe. This surgical approach is used so that the device resides within the sub-retinal space created (referred to as the “ab externo approach” as opposed to “ab interno”; where ones passes through the vitreous humor of the eye and inserts the device through an incision made directly in the retina, see (Javaheri et al., 2006)). Another feature of this configuration is that it leaves the bulk of the electronic hardware outside of the eye thus avoiding complications related to heat generation and corrosion and facilitates the exchange of electronic components as needed. For its operation, a miniature camera mounted on a pair of eyeglasses is used for image capture. These images are then analyzed by an externally worn portable microprocessor used to convert the image data into an electronic signal. The appropriate signal pulses (delivering data and power) are transferred to the implant wirelessly via radio frequency (RF) coils. The resulting signal is transmitted to the subretinal microelectrode array driving the surviving retinal neural elements (i.e. bipolar and ganglion cells) with appropriate patterned electrical stimulation. It is here that the signal processing begins and is further integrated as it passes down the optic nerve on to the visual cortex for final perception of the visual image. All electronic parts are hermetically sealed in a titanium case connected to an external flex circuit and the microelectronic array (Kelly et al., 2009). To date, the group has succeeded in developing a wireless retinal prosthesis prototype as the first step towards a human subretinal prosthesis implant. Initial studies in animal models have been successful in implanting active versions of the device and refining surgical techniques and mechanical design (Kelly et al., 2009). Human clinical trials are now being planned.
Figure 2.
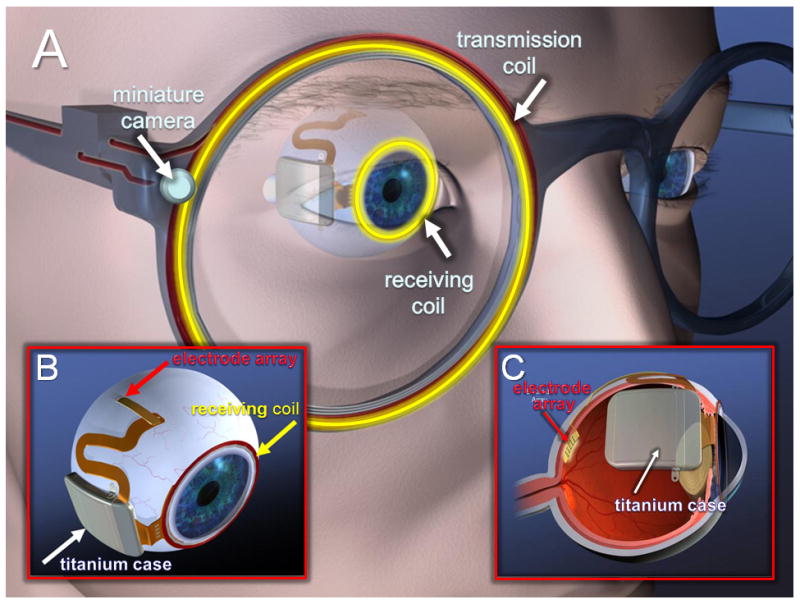
Artist conception of the Boston Retinal Implant device. A) Specially designed glasses house a miniature camera used for the capture of images. The image is analyzed by an image processing unit and appropriate stimulus pulse information and power are sent via a transmission coil. A secondary receiving coil (sutured around the iris of the eye) captures the wireless information transmitted. B) The transmitted information is relayed through a series of electronic components (hermetically sealed in a titanium case) and then ultimately to the stimulating electrode array that is inserted into subretinal space through a scleral flap created behind the eye. C) Cross-sectional view of the eye and implant device. Note that only the electrode array penetrates the sclera and that the bulk of the implant components lie outside the eye.
Variations of the sub-retinal implant design have also been pursued by several large consortia efforts. The Artificial Silicone Retina (ASR) developed by Optobionics Corporation contains approximately 5,000 micro-photodiodes, each containing its own stimulating electrode (Chow et al., 2004). When implanted under the retina, photocurrents generated by absorbed light stimulate adjacent retinal neurons in a multi-site fashion. In a phase 1 trial of safety and efficacy carried out in six patients with profound vision loss from RP (followed from 6 to 18 months after implantation), patients reported an improvement in visual function after implantation. These reports were evidenced by an increase in visual field size and the ability to name more letters using a standardized visual acuity chart (Chow et al., 2004). While the relatively simple design of this device was intuitively appealing (note that no camera and subsequent image processing is required with this device), the apparent improvement in vision was not attributed to true prosthetic vision per se, but rather to a potential neurotrophic (or “cell rescue”) effect related to micro-electric currents generated by the device (Pardue et al., 2005a; Pardue et al., 2005b). With this limitation in mind, a multilayered subretinal chip device incorporating signal amplification is now being pursued by a German consortium (Retina Implant AG). This device has recently been implanted in profoundly blind RP patients and recent results have been encouraging. Early human clinical trial data suggests that stable visual percepts can be obtained and implanted patients profoundly blind with RP have been able to identify objects and letters (Besch et al., 2008; Zrenner, 2002).
As a contrasting design approach, the epi-retinal strategy entails placing an electrode array along the inner surface of the retina to stimulate the underlying ganglion cells. This procedure employs more typical vitreo-retinal surgery techniques so as to affix the microelectrode array on to the retinal surface (e.g. using a retinal tacks). The Artificial Retina Project has been actively pursued by a collaborative effort between the Doheny Eye Institute (University of Southern California) and Second Sight Medical Products. Like the Boston Retinal Implant design, this device incorporates a digital camera mounted on a pair of eyeglass capturing an image that in turn is converted into an electrical signal that is delivered to the retina (Humayun et al., 2003). Initial testing with a 16 electrode device (Argus I) in human volunteers with advanced RP has been successful. A large-scale multi-centered phase II FDA-sponsored clinical trial is currently underway to evaluate a second generation implant (Argus II; 60 electrodes) in the largest cohort of visual prosthesis recipients to date. Results suggest that patients chronically implanted with this device can detect phosphenes at individual electrodes, discriminate crude shapes upon multiple electrode stimulation, and recognize simple stimuli presented via a head-mounted camera (Humayun et al., 2009; Weiland et al., 2004). Very recently, the group reported that implanted subjects showed a significant improvement in accuracy in a spatial visual-motor target localization task comparing performance in patients implanted with their second generation device. Subjects were instructed to locate and touch a high contrast square target presented on a monitor. Nearly all subjects (26/27) showed a significant improvement in accuracy (Ahuja et al., 2010). This is consistent with the observation that implanted subjects were able to develop appropriate head-scanning techniques and good “camera-hand” coordination in using their visual prosthetic device (Ahuja et al., 2010).
Other groups are also pursuing the epi-retinal approach including a variety of German based consortiums. While still in earlier stages of development, early results have also been encouraging (Eckmiller et al., 2005; Gerding, 2007; Gerding et al., 2007)
Other notable downstream approaches have been developed. A Belgian consortium has developed a prosthesis designed to stimulate the optic nerve using a four-electrode cuff electrode design and driven by stimuli captured by an external camera (figure 1B). Two patients have been chronically implanted to date. Reports from one blind volunteer demonstrated that electrical stimulation evoked the perception of localized, and often colored, phosphenes throughout the visual field (Veraart et al., 2003). After four months of psychophysical testing, the patient could recognize and distinguish orientations of lines, some shapes and even certain letters (Brelen et al., 2005; Veraart et al., 2003).
Finally, there have also been attempts to deliver electrical stimulation to the visual cortex itself (figure 1C). Historically, this represents the oldest approach in developing a visual neuroprosthesis. By stimulating the visual cortex directly (thus bypassing earlier visual structures), this strategy has the appealing feature of potentially helping all forms of blindness regardless of ocular pathology. Early seminal work in a profoundly blind volunteer demonstrated that electrical stimulation delivered to the cortex (using surface electrodes) evoked the perception of discrete phosphenes (Brindley and Lewin, 1968). While the phosphene perceptions were rather crude, their spatial location approximately corresponded to the known cortical retinotopic representation of visual space. Later efforts incorporated a digital video camera mounted onto a pair of glasses interfaced with a cortical stimulating array via a cable attached in the patient’s skull (Dobelle and Mladejovsky, 1974). Several blind volunteers have been implanted and reportedly, one patient could distinguish the outline of a person and identify the orientation of certain letters using this device (Dobelle et al., 1974). While certainly a pioneering effort, the cortical approach still faces several technical challenges. These include determining the appropriate encoding strategies that are necessary to generate patterns of stimulation, safety concerns due the inherent invasiveness of surgical implantation and the risk of focal seizures induced by direct cortical stimulation. However, new electrode designs (such as the 100-electrode array developed at the University of Utah; (Normann et al., 1999)) and advances in wireless technology have stimulated renewed interest and several groups are further pursuing this approach (Fernandez et al., 2005; Normann et al., 2009; Tehovnik et al., 2009; Troyk et al., 2003; Troyk et al., 2005).
Current Technical Challenges
As with all neuroprosthesis efforts, the development and realization of a visual neuroprosthetic device will require continued and extensive collaborative effort among basic scientists, engineers and clinicians. Despite great technical progress, certain technical challenges are immediately apparent and must be solved before a visual neuroprosthesis can be considered a viable clinical therapy (for further discussion see (Chader et al., 2009; Cohen, 2007a, b; Dagnelie and Schuchard, 2007; Winter et al., 2007)).
For example, electrode geometry poses inherent limitations that must be carefully considered. This is particularly true with regards to how closely electrodes can be placed next to each other thus impacting the theoretical resolution the visual prosthesis can provide. Furthermore, electrode geometry is intimately related to the amount of current that can be delivered safely to the target neuronal tissue (i.e. charge/density limits). As these neuroprosthetic devices are meant to be implanted and used for very long-periods of time, the effect of prolonged and focal electrical stimulation delivered to delicate (and even potentially further degenerating) neuronal tissue remains unknown. In this direction, new electrode designs, materials and coatings are being actively pursued in order to improve and expand safety profiles. One intriguing possibility is the development of pillar electrode arrays. Implanting this electrode array design has shown that penetrating pillars are able to attract neuronal elements (e.g. ganglion cells of the retina). The closer electrode-neuron interface may allow for lower currents to be used and thus safer injection of current for prolonged electrical stimulation (Butterwick et al., 2009).
There also exists the issue of how a captured image is co-registered with the natural movement of the eye. Inappropriate compensatory eye movements may lead to perceptual mismatch, causing the patient wearing the implant to mis-localize objects in the external world. This potential confound is particularly true of implant designs that incorporate the use of an external mounted camera. To solve this issue, sophisticated eye-tracking mechanisms have been proposed and designed to generate appropriate shifts in the image (e.g. (Palanker et al., 2005)). However, these solutions await further development. Interestingly, recent work with visual simulations suggests that following training, implanted patients may learn to carry out appropriate compensatory head and camera movements to generate more stable percepts (Chen et al., 2009; Srivastava et al., 2009).
Identifying appropriate candidates for implantation and determining the optimal placement of the implant are also of crucial importance (Merabet et al., 2007). Diagnostic techniques typically found in the clinical setting (such as the electroretinogram, visual evoked potentials and visual field perimetry) are certainly intuitive choices to help characterize the profoundness of an individual’s visual impairment. Establishing predictive testing methods that allow for correlations between objective measures of visual function and eventual implant success would be highly desirable (Bach et al., 2010; Dagnelie, 2008). Along these lines, work has been done to develop extensive methodologies aimed at determining “best candidates” for long-term implantation of a microelectronic retinal implant (specifically defined as those requiring lowest current levels delivered to the retina to elicit visual perceptions) (Yanai et al., 2003). This includes a series of preoperative visual, psychophysical and electrophysiological tests (including dark adapted bright flash and flicker electroretinograms and electrical evoked responses) (Yanai et al., 2003). Novel applications of other imaging techniques such as functional magnetic resonance imaging (fMRI), diffusion tensor imaging (DTI) and transcranial magnetic stimulation (TMS) may also prove helpful for the direct evaluation of overall visual cortical function and excitability (Fernandez et al., 2002; Merabet et al., 2007). With regard to retinal implants, the use of high resolution optical coherence tomography (OCT) provides detailed analysis and characterization of retinal laminar anatomy (Matsuo and Morimoto, 2007) (Figure 3A). This is particularly relevant in considering more recent detailed anatomical findings indicating that there is extensive retinal reorganization of cellular components and interconnections in patients with longstanding retinal pathologies such as RP (Marc et al., 2003). It would follow that a degenerating retina may respond very differently to electrical stimulation over time (Dagnelie, 2006). Implantation of a retinal prosthesis during stages of complete photoreceptor loss, but with minimally altered inner retinal structure, may prove beneficial in increasing the likelihood that a visual prosthesis will function. Thus, continued assessment of retinal structural and functional viability could assist in not only selecting appropriate candidates, but also identifying the optimal location and timing of implantation as a function of disease progression (figure 3B).
Figure 3.
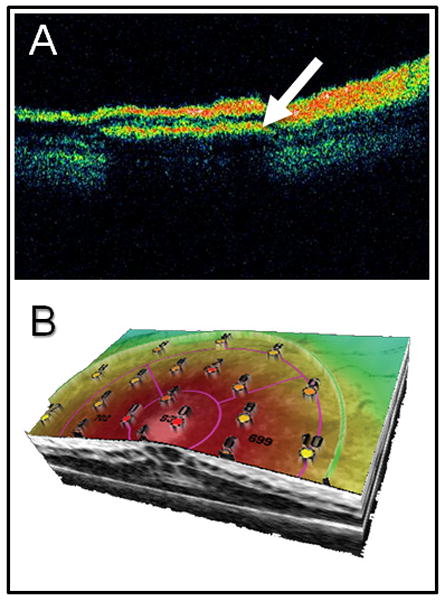
Use of advanced imaging methodology to ascertain implant positioning and retinal integrity. A) using optical coherence tomography (OCT), the cross-sectional position of the implanted microelectrode array (arrow) can be viewed in subretinal space. B) Three dimensional combined OCT with retinal microperimtery allows for simultaneous assessment of structure and function at specific points of retina. The resulting topographic map (values indicate luminance levels detected in decibels; dB) could potentially be used for post-operative evaluation as well as pre-operative assessment of candidate implant locations. Image generated using an OPKO/OTI combined optical coherence tomography and scanning laser ophthalmoscope with microperimetry feature (Opko Health Inc. Miami, FLA).
Moving Forward: New Challenges and Remaining Questions
Experimental evidence from numerous groups has demonstrated, at least in principle, that patterned electrical stimulation can evoke patterned light perceptions. However, as human clinical trials expand and patients continue to interact with these visual neuroprosthetic devices on a more long-term basis, we now move away from the goal of simply demonstrating proof-of-principle towards establishing the fact that a visual neuroprosthesis can indeed improve the quality of life of an implanted patient. In that direction, we also need to define what are tractable milestones of success. Overall success can certainly be interpreted differently particularly when taken from the perspective of the device user. Moving forward, the implementation and potential benefit of a visual prosthesis needs to consider outcome measures and performance assessments that translate directly into improvements in the quality of life of blind individuals (e.g. accurate recognition and grasping of objects or skillful navigation in an unfamiliar environment and carrying out activities of daily living) (Dagnelie, 2008). There is a clear need for new standardized testing and assessment of device efficacy that can be quantified in a manner that is scientifically testable and verifiable. In addition, the selection criteria for potential candidates must be clear. Not only would this would allow for easier comparison of results across design efforts, but also establish and evaluate patients’ visual demands and needs within the context of what a visual prosthesis can ultimately deliver.
A review of human testing reveals that implanted recipients experience difficulties in fully understanding the visual information provided by these visual prosthetic devices. In the initial studies, the reported patterns of visual percepts often did not correspond to what was predicted based on the patterns of electrical stimulation delivered (e.g. (Rizzo et al., 2003a, b)). This key observation suggests that our intuitive sense as to how to generate patterned visual percepts (i.e. the “scoreboard approach”) may not prove to be an adequate strategy (Fernandez et al., 2005). This might be related to the fact that stimulation is carried out on neuronal tissue that is severely degenerated and, therefore, physiologically compromised. Certainly, the quality of visual percepts is likely to improve as remaining technical challenges continue to be solved. However, there may arrive a point when engineering and surgical issues will no longer represent the greatest impediment to future progress. Rather, the greatest barrier will likely lie in our ignorance of how to introduce visual information that is meaningful to the visually deprived brain. It is a misconception that simple perceptions generated from patterned light are sufficient to generate meaningful vision. Furthermore, increasing the resolution of images (for example, by increasing the number of stimulating electrodes) with the goal of generating more complex perceptions would initially be perceptually meaningless rather than helpful.
Several studies have highlighted that following the loss of vision, the brain undergoes profound neuroplastic transformation and that the occipital visual cortex is a major site of these changes (Bavelier and Neville, 2002; Fine et al., 2003; Merabet et al., 2005; Pascual-Leone et al., 1999). The extent and magnitude of these neuroplastic changes is likely to be influenced by such factors as the cause, onset and duration of blindness. The plasticity of the visual system may allow for a considerable degree of adaptation. However, understanding the precise constraints of these neuroplastic processes will be crucial and have clear implications for rehabilitative training and progress in device development (Fernandez et al., 2005).
A better understanding of how the brain adapts to the loss of sight and how the remaining senses process information in the visually deprived brain are necessary to appropriately modulate restored visual input and, ultimately, to allow meaningful vision with a neuroprosthetic device. One possibility might be to envision a patient controlled system that coordinates and registers the visual perceptions generated by a visual prosthesis with the identification of objects perceived through other senses (such as touch and audition). Patients could learn to integrate these concordant sources of sensory stimuli into meaningful percepts and ultimately, gain the ability to identify objects in the visual world (Merabet et al., 2005). Related studies in the development of sensory-substitution devices will likely contribute greatly to our knowledge in this arena. Furthermore, these issues of training an implanted patient to “see again” are directly related to the realm of visual rehabilitation. The adaptive strategies and structured training necessary to interpret newly acquired visual percepts that ultimately translate to useful functional vision should not be left to chance (Dagnelie and Schuchard, 2007). This should be carried out within the context of a patient’s current rehabilitation program (such as sensory substitution devices as well as mobility aids such as a guide-dog) to ensure an appropriate functional overlap. Clearly, any functional advantage gained through the use of a visual prosthetic device should meet, if not exceed, current rehabilitative options. Ultimately, the implementation of a visual prosthesis should not interfere with an individual’s on-going rehabilitative program.
Conclusion
The loss of sight can have a devastatingly negative impact on the quality of life of an individual. The goal of restoring functional vision to blind, while certainly valiant, still faces formidable challenges before it will ever become a tractable reality. However, there are grounds to be cautiously optimistic and there is every reason to believe we are on the path to achieve this goal. It is also important to realize that the rehabilitation of the blind is a very complex problem, requiring extraordinarily diverse, lengthy and intimate collaborations among basic scientists, engineers, clinicians, educators and rehabilitative experts.
As technical challenges continue to be solved, there also remains the issue of understanding how the brain adapts to the loss of vision itself. Success in restoring functional vision depends on our understanding of how blindness affects the brain and what it means to “see” again. The neural changes that result from loss of vision need to be addressed if the restoration of visual input is to lead to functional vision. These issues of neuroplasticity also lead to questions regarding the feasibility of the visual prosthesis approach and its potential to benefit blind individuals. Therefore, it is essential that future research explores the mechanisms that underlie brain plasticity following the loss of vision. Such insight could help to develop and refine strategies for merging visual sensations that are generated by the prosthesis. Uncovering these adaptive strategies may ultimately assist in the rehabilitation process itself.
References
- Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, Pearce-Kelling SE, Anand V, Zeng Y, Maguire AM, Jacobson SG, Hauswirth WW, Bennett J. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28:92–95. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- Ahuja AK, Dorn JD, Caspi A, McMahon MJ, Dagnelie G, Dacruz L, Stanga P, Humayun MS, Greenberg RJ. Blind subjects implanted with the Argus II retinal prosthesis are able to improve performance in a spatial-motor task. Br J Ophthalmol. 2010 doi: 10.1136/bjo.2010.179622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allin S, Eckel E, Markham H, Brewer BR. Recent trends in the development and evaluation of assistive robotic manipulation devices. Phys Med Rehabil Clin N Am. 2010;21:59–77. doi: 10.1016/j.pmr.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Bach-y-Rita P. Tactile sensory substitution studies. Ann N Y Acad Sci. 2004;1013:83–91. doi: 10.1196/annals.1305.006. [DOI] [PubMed] [Google Scholar]
- Bach-y-Rita P, Kercel WS. Sensory substitution and the human-machine interface. Trends Cogn Sci. 2003;7:541–546. doi: 10.1016/j.tics.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Bach M, Wilke M, Wilhelm B, Zrenner E, Wilke R. Basic quantitative assessment of visual performance in patients with very low vision. Invest Ophthalmol Vis Sci. 2010;51:1255–1260. doi: 10.1167/iovs.09-3512. [DOI] [PubMed] [Google Scholar]
- Bavelier D, Neville HJ. Cross-modal plasticity: where and how? Nat Rev Neurosci. 2002;3:443–452. doi: 10.1038/nrn848. [DOI] [PubMed] [Google Scholar]
- Besch D, Sachs H, Szurman P, Gulicher D, Wilke R, Reinert S, Zrenner E, Bartz-Schmidt KU, Gekeler F. Extraocular surgery for implantation of an active subretinal visual prosthesis with external connections: feasibility and outcome in seven patients. Br J Ophthalmol. 2008;92:1361–1368. doi: 10.1136/bjo.2007.131961. [DOI] [PubMed] [Google Scholar]
- Brelen ME, Duret F, Gerard B, Delbeke J, Veraart C. Creating a meaningful visual perception in blind volunteers by optic nerve stimulation. J Neural Eng. 2005;2:S22–28. doi: 10.1088/1741-2560/2/1/004. [DOI] [PubMed] [Google Scholar]
- Brindley GS, Lewin WS. The sensations produced by electrical stimulation of the visual cortex. J Physiol. 1968;196:479–493. doi: 10.1113/jphysiol.1968.sp008519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunker CH, Berson EL, Bromley WC, Hayes RP, Roderick TH. Prevalence of retinitis pigmentosa in Maine. Am J Ophthalmol. 1984;97:357–365. doi: 10.1016/0002-9394(84)90636-6. [DOI] [PubMed] [Google Scholar]
- Butterwick A, Huie P, Jones BW, Marc RE, Marmor M, Palanker D. Effect of shape and coating of a subretinal prosthesis on its integration with the retina. Exp Eye Res. 2009;88:22–29. doi: 10.1016/j.exer.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Chader GJ, Weiland J, Humayun MS. Artificial vision: needs, functioning, and testing of a retinal electronic prosthesis. Prog Brain Res. 2009;175:317–332. doi: 10.1016/S0079-6123(09)17522-2. [DOI] [PubMed] [Google Scholar]
- Chen SC, Suaning GJ, Morley JW, Lovell NH. Simulating prosthetic vision: II. Measuring functional capacity. Vision Res. 2009;49:2329–2343. doi: 10.1016/j.visres.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Chow AY, Chow VY, Packo KH, Pollack JS, Peyman GA, Schuchard R. The artificial silicon retina microchip for the treatment of vision loss from retinitis pigmentosa. Arch Ophthalmol. 2004;122:460–469. doi: 10.1001/archopht.122.4.460. [DOI] [PubMed] [Google Scholar]
- Cohen ED. Prosthetic interfaces with the visual system: biological issues. J Neural Eng. 2007a;4:R14–31. doi: 10.1088/1741-2560/4/2/R02. [DOI] [PubMed] [Google Scholar]
- Cohen ED. Safety and effectiveness considerations for clinical studies of visual prosthetic devices. J Neural Eng. 2007b;4:S124–129. doi: 10.1088/1741-2560/4/1/S14. [DOI] [PubMed] [Google Scholar]
- Craelius W. The bionic man: restoring mobility. Science. 2002;295:1018–1021. doi: 10.1126/science.295.5557.1018. [DOI] [PubMed] [Google Scholar]
- Dagnelie G. Visual prosthetics 2006: assessment and expectations. Expert Rev Med Devices. 2006;3:315–325. doi: 10.1586/17434440.3.3.315. [DOI] [PubMed] [Google Scholar]
- Dagnelie G. Psychophysical evaluation for visual prosthesis. Annu Rev Biomed Eng. 2008;10:339–368. doi: 10.1146/annurev.bioeng.10.061807.160529. [DOI] [PubMed] [Google Scholar]
- Dagnelie G, Schuchard RA. The state of visual prosthetics--hype or promise? J Rehabil Res Dev. 2007;44:xi–xiv. doi: 10.1682/jrrd.2006.08.0098. [DOI] [PubMed] [Google Scholar]
- Dobelle WH, Mladejovsky MG. Phosphenes produced by electrical stimulation of human occipital cortex, and their application to the development of a prosthesis for the blind. J Physiol. 1974;243:553–576. doi: 10.1113/jphysiol.1974.sp010766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobelle WH, Mladejovsky MG, Girvin JP. Artifical vision for the blind: electrical stimulation of visual cortex offers hope for a functional prosthesis. Science. 1974;183:440–444. doi: 10.1126/science.183.4123.440. [DOI] [PubMed] [Google Scholar]
- Donoghue JP. Connecting cortex to machines: recent advances in brain interfaces. Nat Neurosci. 2002;5(Suppl):1085–1088. doi: 10.1038/nn947. [DOI] [PubMed] [Google Scholar]
- Dowling J. Artificial human vision. Expert Rev Med Devices. 2005;2:73–85. doi: 10.1586/17434440.2.1.73. [DOI] [PubMed] [Google Scholar]
- Eckmiller R, Neumann D, Baruth O. Tunable retina encoders for retina implants: why and how. J Neural Eng. 2005;2:S91–S104. doi: 10.1088/1741-2560/2/1/011. [DOI] [PubMed] [Google Scholar]
- Fernandez E, Alfaro A, Tormos JM, Climent R, Martinez M, Vilanova H, Walsh V, Pascual-Leone A. Mapping of the human visual cortex using image-guided transcranial magnetic stimulation. Brain Res Brain Res Protoc. 2002;10:115–124. doi: 10.1016/s1385-299x(02)00189-7. [DOI] [PubMed] [Google Scholar]
- Fernandez E, Pelayo F, Romero S, Bongard M, Marin C, Alfaro A, Merabet L. Development of a cortical visual neuroprosthesis for the blind: the relevance of neuroplasticity. J Neural Eng. 2005;2:R1–R12. doi: 10.1088/1741-2560/2/4/R01. [DOI] [PubMed] [Google Scholar]
- Fine I, Wade AR, Brewer AA, May MG, Goodman DF, Boynton GM, Wandell BA, MacLeod DI. Long-term deprivation affects visual perception and cortex. Nat Neurosci. 2003;6:915–916. doi: 10.1038/nn1102. [DOI] [PubMed] [Google Scholar]
- Gerding H. A new approach towards a minimal invasive retina implant. J Neural Eng. 2007;4:S30–37. doi: 10.1088/1741-2560/4/1/S05. [DOI] [PubMed] [Google Scholar]
- Gerding H, Benner FP, Taneri S. Experimental implantation of epiretinal retina implants (EPI-RET) with an IOL-type receiver unit. J Neural Eng. 2007;4:S38–49. doi: 10.1088/1741-2560/4/1/S06. [DOI] [PubMed] [Google Scholar]
- Gothe J, Brandt SA, Irlbacher K, Roricht S, Sabel BA, Meyer BU. Changes in visual cortex excitability in blind subjects as demonstrated by transcranial magnetic stimulation. Brain. 2002;125:479–490. doi: 10.1093/brain/awf045. [DOI] [PubMed] [Google Scholar]
- Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, Branner A, Chen D, Penn RD, Donoghue JP. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442:164–171. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- Humayun MS. Artificial sight : basic research, biomedical engineering, and clinical advances. Springer; New York: 2007. [Google Scholar]
- Humayun MS, de Juan E, Jr, Dagnelie G, Greenberg RJ, Propst RH, Phillips DH. Visual perception elicited by electrical stimulation of retina in blind humans. Arch Ophthalmol. 1996;114:40–46. doi: 10.1001/archopht.1996.01100130038006. [DOI] [PubMed] [Google Scholar]
- Humayun MS, de Juan E, Jr, Weiland JD, Dagnelie G, Katona S, Greenberg R, Suzuki S. Pattern electrical stimulation of the human retina. Vision Res. 1999;39:2569–2576. doi: 10.1016/s0042-6989(99)00052-8. [DOI] [PubMed] [Google Scholar]
- Humayun MS, Dorn JD, Ahuja AK, Caspi A, Filley E, Dagnelie G, Salzmann J, Santos A, Duncan J, daCruz L, Mohand-Said S, Eliott D, McMahon MJ, Greenberg RJ. Preliminary 6 month results from the Argus II epiretinal prosthesis feasibility study. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:4566–4568. doi: 10.1109/IEMBS.2009.5332695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humayun MS, Weiland JD, Fujii GY, Greenberg R, Williamson R, Little J, Mech B, Cimmarusti V, Van Boemel G, Dagnelie G, de Juan E. Visual perception in a blind subject with a chronic microelectronic retinal prosthesis. Vision Res. 2003;43:2573–2581. doi: 10.1016/s0042-6989(03)00457-7. [DOI] [PubMed] [Google Scholar]
- Javaheri M, Hahn DS, Lakhanpal RR, Weiland JD, Humayun MS. Retinal prostheses for the blind. Ann Acad Med Singapore. 2006;35:137–144. [PubMed] [Google Scholar]
- Jones S, Harris D, Estill A, Mikulec AA. Implantable hearing devices. Mo Med. 2008;105:235–239. [PubMed] [Google Scholar]
- Kelly SK, Shire DB, Chen J, Doyle P, Gingerich MD, Drohan WA, Theogarajan LS, Cogan SF, Wyatt JL, Rizzo JF., 3rd Realization of a 15-channel, hermetically-encased wireless subretinal prosthesis for the blind. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:200–203. doi: 10.1109/IEMBS.2009.5333619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1997;104:7–21. doi: 10.1016/s0161-6420(97)30368-6. [DOI] [PubMed] [Google Scholar]
- Laferrier JZ, Gailey R. Advances in lower-limb prosthetic technology. Phys Med Rehabil Clin N Am. 2010;21:87–110. doi: 10.1016/j.pmr.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Loeb GE. Cochlear prosthetics. Annu Rev Neurosci. 1990;13:357–371. doi: 10.1146/annurev.ne.13.030190.002041. [DOI] [PubMed] [Google Scholar]
- MacLaren RE, Pearson RA, MacNeil A, Douglas RH, Salt TE, Akimoto M, Swaroop A, Sowden JC, Ali RR. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444:203–207. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- Marbach WD, Zabarsky M, Hoban P, Nelson C. Building the bionic man. Newsweek. 1982;100:78–79. [PubMed] [Google Scholar]
- Marc RE, Jones BW, Watt CB, Strettoi E. Neural remodeling in retinal degeneration. Prog Retin Eye Res. 2003;22:607–655. doi: 10.1016/s1350-9462(03)00039-9. [DOI] [PubMed] [Google Scholar]
- Marg E, Rudiak D. Phosphenes induced by magnetic stimulation over the occipital brain: description and probable site of stimulation. Optom Vis Sci. 1994;71:301–311. doi: 10.1097/00006324-199405000-00001. [DOI] [PubMed] [Google Scholar]
- Matsuo T, Morimoto N. Visual acuity and perimacular retinal layers detected by optical coherence tomography in patients with retinitis pigmentosa. Br J Ophthalmol. 2007;91:888–890. doi: 10.1136/bjo.2007.114538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merabet LB, Rizzo JF, 3rd, Pascual-Leone A, Fernandez E. ‘Who is the ideal candidate?’: decisions and issues relating to visual neuroprosthesis development, patient testing and neuroplasticity. J Neural Eng. 2007;4:S130–135. doi: 10.1088/1741-2560/4/1/S15. [DOI] [PubMed] [Google Scholar]
- Merabet LB, Rizzo JF, Amedi A, Somers DC, Pascual-Leone A. What blindness can tell us about seeing again: merging neuroplasticity and neuroprostheses. Nat Rev Neurosci. 2005;6:71–77. doi: 10.1038/nrn1586. [DOI] [PubMed] [Google Scholar]
- Nicolelis MA. Brain-machine interfaces to restore motor function and probe neural circuits. Nat Rev Neurosci. 2003;4:417–422. doi: 10.1038/nrn1105. [DOI] [PubMed] [Google Scholar]
- Normann RA, Greger B, House P, Romero SF, Pelayo F, Fernandez E. Toward the development of a cortically based visual neuroprosthesis. J Neural Eng. 2009;6:035001. doi: 10.1088/1741-2560/6/3/035001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normann RA, Maynard EM, Rousche PJ, Warren DJ. A neural interface for a cortical vision prosthesis. Vision Res. 1999;39:2577–2587. doi: 10.1016/s0042-6989(99)00040-1. [DOI] [PubMed] [Google Scholar]
- Palanker D, Vankov A, Huie P, Baccus S. Design of a high-resolution optoelectronic retinal prosthesis. J Neural Eng. 2005;2:S105–120. doi: 10.1088/1741-2560/2/1/012. [DOI] [PubMed] [Google Scholar]
- Pardue MT, Phillips MJ, Yin H, Fernandes A, Cheng Y, Chow AY, Ball SL. Possible sources of neuroprotection following subretinal silicon chip implantation in RCS rats. J Neural Eng. 2005a;2:S39–47. doi: 10.1088/1741-2560/2/1/006. [DOI] [PubMed] [Google Scholar]
- Pardue MT, Phillips MJ, Yin H, Sippy BD, Webb-Wood S, Chow AY, Ball SL. Neuroprotective effect of subretinal implants in the RCS rat. Invest Ophthalmol Vis Sci. 2005b;46:674–682. doi: 10.1167/iovs.04-0515. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Hamilton R, Tormos JM, Keenan JP, Catala MD. Neuroplasticity in the adjustment to blindness. In: Grafman J, Christen Y, editors. Neuronal Plasticity: Building a Bridge from the laboratory to the Clinic. Springer-Verlag; Berlin Heidelber New York: 1999. [Google Scholar]
- Peterman MC, Noolandi J, Blumenkranz MS, Fishman HA. Localized chemical release from an artificial synapse chip. Proc Natl Acad Sci U S A. 2004;101:9951–9954. doi: 10.1073/pnas.0402089101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo JF, 3rd, Wyatt J, Loewenstein J, Kelly S, Shire D. Methods and perceptual thresholds for short-term electrical stimulation of human retina with microelectrode arrays. Invest Ophthalmol Vis Sci. 2003a;44:5355–5361. doi: 10.1167/iovs.02-0819. [DOI] [PubMed] [Google Scholar]
- Rizzo JF, 3rd, Wyatt J, Loewenstein J, Kelly S, Shire D. Perceptual efficacy of electrical stimulation of human retina with a microelectrode array during short-term surgical trials. Invest Ophthalmol Vis Sci. 2003b;44:5362–5369. doi: 10.1167/iovs.02-0817. [DOI] [PubMed] [Google Scholar]
- Shire DB, Kelly SK, Chen J, Doyle P, Gingerich MD, Cogan SF, Drohan WA, Mendoza O, Theogarajan L, Wyatt JL, Rizzo JF. Development and implantation of a minimally invasive wireless subretinal neurostimulator. IEEE Trans Biomed Eng. 2009;56:2502–2511. doi: 10.1109/TBME.2009.2021401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava NR, Troyk PR, Dagnelie G. Detection, eye-hand coordination and virtual mobility performance in simulated vision for a cortical visual prosthesis device. J Neural Eng. 2009;6:035008. doi: 10.1088/1741-2560/6/3/035008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehovnik EJ, Slocum WM, Smirnakis SM, Tolias AS. Microstimulation of visual cortex to restore vision. Prog Brain Res. 2009;175:347–375. doi: 10.1016/S0079-6123(09)17524-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyk P, Bak M, Berg J, Bradley D, Cogan S, Erickson R, Kufta C, McCreery D, Schmidt E, Towle V. A model for intracortical visual prosthesis research. Artif Organs. 2003;27:1005–1015. doi: 10.1046/j.1525-1594.2003.07308.x. [DOI] [PubMed] [Google Scholar]
- Troyk PR, Bradley D, Bak M, Cogan S, Erickson R, Hu Z, Kufta C, McCreery D, Schmidt E, Sung S, Towle V. Intracortical visual prosthesis research - approach and progress. Conf Proc IEEE Eng Med Biol Soc. 2005;7:7376–7379. doi: 10.1109/IEMBS.2005.1616216. [DOI] [PubMed] [Google Scholar]
- Veraart C, Wanet-Defalque MC, Gerard B, Vanlierde A, Delbeke J. Pattern recognition with the optic nerve visual prosthesis. Artif Organs. 2003;27:996–1004. doi: 10.1046/j.1525-1594.2003.07305.x. [DOI] [PubMed] [Google Scholar]
- Weiland JD, Yanai D, Mahadevappa M, Williamson R, Mech BV, Fujii GY, Little J, Greenberg RJ, de Juan E, Jr, Humayun MS. Visual task performance in blind humans with retinal prosthetic implants. Conf Proc IEEE Eng Med Biol Soc. 2004;6:4172–4173. doi: 10.1109/IEMBS.2004.1404164. [DOI] [PubMed] [Google Scholar]
- Winter JO, Cogan SF, Rizzo JF., 3rd Retinal prostheses: current challenges and future outlook. J Biomater Sci Polym Ed. 2007;18:1031–1055. doi: 10.1163/156856207781494403. [DOI] [PubMed] [Google Scholar]
- Yanai D, Lakhanpal RR, Weiland JD, Mahadevappa M, Van Boemel G, Fujii GY, Greenberg R, Caffey S, de Juan E, Jr, Humayun MS. The value of preoperative tests in the selection of blind patients for a permanent microelectronic implant. Trans Am Ophthalmol Soc. 2003;101:223–228. discussion 228–230. [PMC free article] [PubMed] [Google Scholar]
- Zrenner E. The subretinal implant: can microphotodiode arrays replace degenerated retinal photoreceptors to restore vision? Ophthalmologica. 2002;216(Suppl 1):8–20. doi: 10.1159/000064650. discussion 52–23. [DOI] [PubMed] [Google Scholar]


