Abstract
It is well established that iNKT cells can be activated by both exogenous and a limited number of endogenous glycolipids. However, although iNKT cells have been implicated in the immune response to transplanted organs, the mechanisms by which iNKT cells are activated in this context remains unknown. Here we demonstrate that iNKT cells are not activated by allogeneic cells per se, but expand, both in vitro and in vivo, in the presence of a concomitant conventional T cell response to alloantigen. This form of iNKT activation was found to occur independently of TCR-glycolipid/CD1d interactions but rather was a result of sequestration of IL-2 produced by conventional alloreactive T cells.
These results show for the first time that IL-2, produced by activated conventional T cells, can activate iNKT cells independently of glycolipid/CD1d recognition. Therefore, we propose that the well-documented involvement of iNKT cells in autoimmunity, the control of cancer as well as following transplantation need not involve recognition of endogenous or exogenous glycolipids but alternatively may be a consequence of specific adaptive immune responses.
Keywords: iNKT cells, Allorecognition, Bystander Activation, Transplantation
Introduction
iNKT cells are a small but highly conserved population of T cells that are able to respond at high frequency, due to limited TCR diversity, to certain glycolipids presented by the non-polymorphic MHC-class-I-like-molecule CD1d (1), as opposed to peptide-MHC complexes. Whilst conventional T cells respond to foreign transplants through the recognition of alloantigen in the form of intact or processed MHC molecules (2), it remains unknown how iNKT cells may be activated in this setting.
iNKT cells can be activated by a number of glycolipid ligands presented in the context of CD1d, such as that derived from Borrelia burgdorferi or the marine sponge-derived, α-galactosylceramide (α-GalCer) (3-8). In addition to TCR-mediated recognition of glycolipids, iNKT cells can be activated indirectly by stimulatory cytokines that are released by dendritic cells (DC) following toll-like receptor (TLR) signalling (i.e. interleukin (IL)-12 and type-1 interferons) (7, 9-11). The activation of iNKT cells by α-GalCer (3) has been shown to result in the release of a plethora of cytokines (such as IL-2,-3,-4,-6,-9,-10,-17,-21,GM-CSF,IFNγ and TNFα) within hours of TCR ligation (12) supporting a potential role for these cells in contributing to the local cytokine milieu thereby impacting adaptive immune responses. iNKT cells provide an important bridge between innate and adaptive responses. For example, iNKT cells can modulate the function of DC and B cells through the provision of CD40L signals, activate natural killer (NK) cells following IFNγ release and inhibit the activity of myeloid-derived-suppressor-cells (MDSC) and suppressive-IL-10-producing-neutrophils (13-21).
The importance of iNKT cell activation and cross-talk between innate and adaptive immunity was demonstrated in early studies using α-GalCer which identified a role for iNKT cells in tumour surveillance (3). In addition to mediating tumour immunosurveillance, iNKT cells have been implicated in preventing autoimmune diseases (22) and aiding the clearance of pathogens (23). In the context of transplantation there are robust data to support a role for iNKT cells in the immune response to transplanted tissue. For example, Seino et al, showed that monoclonal antibodies (mAbs) that interrupt either LFA-1/ICAM-1 or CD28/B7 interactions resulted in the induction of tolerance to cardiac allografts in wild-type (WT) but not in iNKT−/− recipient mice (24). Importantly, long-term graft survival could be restored upon the adoptive transfer of WT NKT cells to iNKT−/− recipients (24). Similar results have been found in other models where anti-CD154 mAb was used to induce tolerance to cardiac and islet allografts (25, 26). It is also worthwhile noting that iNKT cells have also been shown to be involved in the rejection of allogeneic (27) and destruction of syngeneic islets (28).
We and others have proposed that the microenvironment created by transplantation, incorporating inflammation generated by surgical trauma and ischemia as well as the induction of conventional alloresponses at sites of T cell priming and within the allograft itself, may promote iNKT cell activation (26, 29, 30). In support of this, Li and colleagues demonstrated that following ischemia-reperfusion-injury to the kidney, iNKT cells rapidly infiltrated the kidney, increasing the production of IFNγ and promoting the recruitment of neutrophils; a process that was significantly reduced in iNKT−/− mice (31). Likewise, iNKT cells have been shown to expand in draining lymph nodes following allogeneic but not syngeneic skin transplantation (29).
However, despite numerous reports implicating iNKT cells in transplant rejection and tolerance, little is known regarding how such cells are activated in this setting. Here we show that murine iNKT cells bear no intrinsic reactivity to allogeneic cells. Rather, iNKT cells undergo proliferation and secrete cytokine following bystander activation as a result of IL-2 produced by alloreactive T cells following activation.
Material and Methods
Mice
C57BL/6 (B6; H2b), C57BL/6 MHCII−/− (MHCII−/−; H2b), C57BL/6 TEa (TEa; H2b), BALB/cxB6 F1 (F1; H2dxb), BALB/c CD1d−/−xB6 F1 CD1d−/− (F1 CD1d−/−; H2dxb) mice were bred and maintained at the BMSU, John Radcliffe Hospital. NKT cell deficient mice colonies were obtained from Prof. V. Cerundolo (Oxford, UK) with permission from Prof. L Van Kaer (Nashville, USA) and TEa mice (CD4+ T cell TCR transgenic mice (Vα2 and Vβ6 chain); respond to IEαd peptide in the context of IAb) from Dr. W Gao and Prof. T Strom (Boston, USA). All mice were sex-matched and aged between 6-10 weeks at the time of first experimental procedure. All studies were carried out in accordance with the Animals (Scientific Procedures) Act 1986.
Skin Transplantation
Individual full-thickness tail skin grafts were prepared to fit the graft bed on the left lateral thorax of anesthetized recipients. The grafts were inspected regularly and harvested at day 10 post transplantation for analysis.
Flow Cytometry, Antibodies and Tetramer staining
Cell sorting and analysis was performed on a FACSAria, and flow-cytometric analyses were performed using FACS DIVA software (BD Biosciences, UK). All antibodies used in flow cytometry were purchased from BD PharMingen or eBioscience. Anti-CD1d mAb (3CII) was a kind gift from Dr J. Yewdell (NIH, Bethesda, Maryland, USA). CD1d tetramer conjugated with APC was provided pre-loaded with PBS-57 (National Institutes of Health (NIH) Tetramer Facility, Atlanta, USA). PBS-57 is an analogue of α-GalCer and CD1d-PBS57 tetramers stain iNKT cells comparably to CD1d-αGalCer tetramers (ProImmune, UK). To determine cell numbers by flow cytometry, a fixed number of 6μM synthetic fluorescent beads (BD Biosciences, UK) were added, as detailed previously (32).
Purification of NKT cells
Splenocytes were incubated with in house produced rat anti-mouse mAb specific for MHC class II (TIB120), CD8 (YTS169.4.2; gift from Prof. H. Waldmann, Oxford, UK), B cells (RA36B2), monocytes (M1/70) and CD62L (MEL14; BD Biosciences). Anti-rat immunoglobulin (Ig) DYNAL beads (Invitrogen, UK) were used to remove positive cells. The remaining cells were stained with anti-CD5 and NK1.1 (BD Biosciences, UK) or anti-CD5 and CD1d-PBS57 loaded tetramer (NIH Tetramer Facility, Atlanta, USA). CD5+NK1.1+ or CD5+CD1d-PBS57+ cells were gated and sorted twice using a FACS Aria (BD Biosciences, UK).
Proliferation Assay in vitro and in vivo
Responders: iNKT and TEa T cells were prepared and sorted. BALB/cxB6 F1 (H2dxb) or BALB/cxB6 F1 CD1d−/− (H2dxb) splenocytes were prepared and irradiated (3000 rad). MLRs were performed in 96 well round bottom plates with responder cells cultured (5×104) with irradiated stimulators (2.5×105). Proliferative responses were measure with 0.5μCi of 3H-thymidine (Amersham International, UK) after 3 days of culture. For blocking experiments of IL-2 signalling, anti-CD25 (clone PC61) or isotype were from ATCC.
For the in vivo proliferation assay iNKT-RAG mice were generated (S1). Briefly, iNKT cells were cell sorted by FACS twice using splenocytes derived from MHCII−/− using CD1d-PBS57 loaded tetramer and CD5 mAb to a purify exceeding 99.7%. iNKT cells (1×105) were adoptive transfer i.v. to B6 RAG−/− mice and received 2μg α-GalCer i.p. on day+1 and day+14, then rested for 14 days (herein referred to as iNKT-RAG mice). For in vivo MLR, purified TEa T cells (>99%; 1×105) were delivered i.v. on day −1 to iNKT-RAG mice and RAG−/− mice, followed by F1 splenocyes (1×107) on day 0. Splenocytes were enumerated for TEa T cell (CD3+Vα2+CD4+) and iNKT cell (CD3+CD1d-PBS57 Tetramer+) number on day 7 using a FACS Aria (BD Biosciences, UK).
Real-time PCR
Skin grafts were harvested at day 10 post transplant and snap frozen. DNase I-treated total RNA was later isolated by using the Absolutely RNA Miniprep kit (Stratagene, UK) and reverse-transcribed by the M-MLV reverse transcriptase (Invitrogen, UK) as previously described (33). Real-time quantification was performed by using the PRISM 7700 sequence detection system (AppliedBiosystem, UK) using a fluorogenic probe as described previously (33). Specific primers and probes for HPRT and Vα14Jα218 were used as previously described (26, 33). Samples were standardised for HPRT and quantification of the gene of interest calculated as previously described (34).
ELISA
ELISA were conducted in 96 well Maxisorp plates (Nunc, Denmark) and analysed using an Emax precision microplate reader (Molecular Devices) and Softmax software (Molecular Devices). Samples and doubling dilutions of IFN-γ standards (BD Pharmingen) were analyzed in triplicate. IFN-γ capture mAb (R46A2) was grown and purified in the laboratory. IFN-γ was detected using a biotin-labelled anti-IFN-γ mAb (XMG1.2; BD Pharmingen) and developed using avidin HRP (Vector Laboratories) followed by ABTS substrate and hydrogen peroxidise (Sigma, UK).
Cytokine Bead Array
Cytokine bead array kits were used to detect Th1 and Th2 associated cytokines (IL-2, IL-4, IL-5, IFN-γ, TNF) following the manufacturer’s protocol (BD Biosciences, UK). Samples were acquired on a FACS Aria (BD Biosciences, UK).
Statistical analysis
All statistical analyses were performed using Graphpad Prism software version 4.0. The Student’s t test with two-tailed analysis was used to compare the level of significance between data sets. All p values <0.05 were considered significant.
Results
iNKT cells are activated by conventional alloreactive T cells
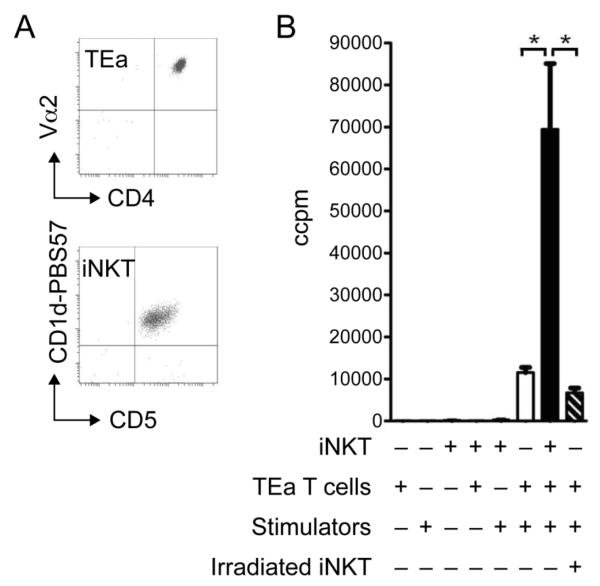
The ability of iNKT cells to recognise and respond to allogeneic cells was first investigated in vitro. iNKT cells were sorted by flow cytometry (Figure 1A;CD5+ CD1d-PBS57+) from C57BL/6 mice before being cultured with semi-allogeneic (B6xBALB/c F1;H2bxd) splenocytes for 3 days. Upon culture, iNKT cells did not proliferate (Figure 1B) or show signs of activation (CD25/CD69 upregulation; data not shown) and no IFNγ was found in culture supernatants (data not shown). Identical results were observed using a range of allogeneic splenocytes (BALB/c;H2d and CBA;H2k: data not shown). Despite this, we examined whether iNKT cells had any impact on a conventional alloreactive T cell response to alloantigen. To this end, a mixed lymphocyte reaction (MLR) was developed using naïve, alloreactive CD4+ TCR-transgenic T cells (TEa;H2b; reactive to BALB/cxB6 F1 stimulator cells; Figure 1A (35)). TEa T cells and irradiated F1 spleen cells were cultured with and without the addition of sorted iNKT cells. The co-culture of TEa T cells and iNKT cells with allogeneic stimulators resulted in a marked increase in proliferation; 5.7 fold higher than TEa T cells and stimulators alone (12037±786 TEa/stim vs 69451±15461 iNKT/TEa/stim, p=0.003; Figure 1B). The irradiation of iNKT cells prior to culture was found to abolish the increase in proliferation seen with non-irradiated iNKT cells (69451±15461, iNKT/TEa/stim vs 6715±1974 CCPM, irradiated iNKT/TEa/stim, p=0.002; Figure 1B).
Figure 1. The presence of iNKT cells promotes proliferation following in vitro co-culture with conventional T cells and stimulators.
TEa T cells (CD4+Vα2+) and iNKT cells (CD5+CD1d-PBS57+) were purified by cell sorting (A). MLR were carried out using TEa T cells and iNKT cells cultured alone, or with irradiated stimulators (BALB/cxB6F1) or together, and proliferation determined on day 3 (B). In addition iNKT cells were sorted and then irradiated and co-cultured with TEa T cells and stimulators (B). The data is presented as the mean of triplicate cultures ± s.d. of one experiment. *p=<0.05. Data is representative of >3 independent experiments.
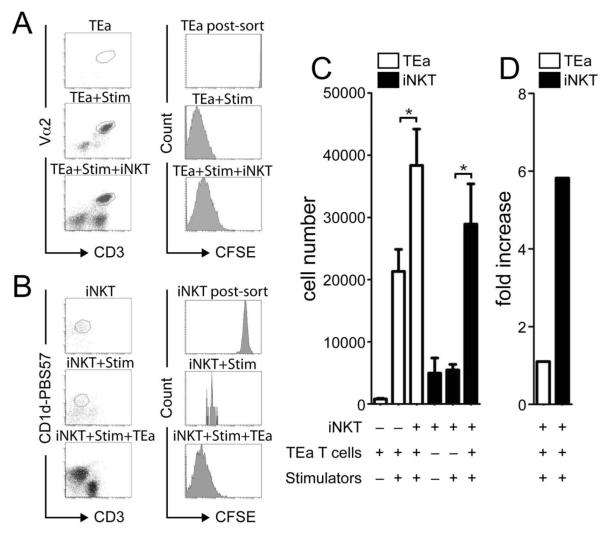
In order to determine which population of cells was responsible for the increased proliferation seen in cultures of TEa T cells and iNKT cells with irradiated stimulators, responder cells were CFSE labelled. iNKT cells and TEa T cells were cultured for four days, independently or together, with irradiated F1 spleen cells (Figure 2A-B). TEa T cells when cultured alone died, however when cultured with stimulators, with or without iNKT cells, TEa T cells survived and had diluted out detectable CFSE indicating extensive proliferation by day 4 of culture (Figure 2A). Importantly, the vast majority of iNKT cells died when cultured either alone or with allogeneic stimulators but were found to have survived, proliferated (iNKT cells were found to be CFSE− by day 4 of culture) and markedly expanded when cultured with TEa T cells and stimulators (Figure 2B). Analysis of cultures 4 days after initiation revealed that although the number of TEa T cells had increased 1.8 fold with the addition of iNKT cells (p=0.01; Figure 2C), remarkably the number of iNKT cells had increased 5.8 fold compared to cultures containing iNKT and stimulators only (28920±6476, iNKT/TEa/stim vs 5407±892, iNKT/stim p=0.003; Figure 2D). Therefore, these data show that despite iNKT cells demonstrating little intrinsic alloreactivity, in the presence of a conventional CD4+ T cell to alloantigen, iNKT cells became activated and proliferated.
Figure 2. iNKT cells are expanded in the presence of conventional T cells alloresponses.
The proportion of TEa T cells and iNKT cells proliferating during co-culture with stimulators was determined by CFSE labelling. On day 4 post culture, TEa T cells and iNKT cells CFSE profile was determined (A-B) and both cell number (C) and fold expansion (D) calculated following culturing alone, with stimulators or together with stimulators. Data is presented as the mean of triplicate cultures ± s.d. of one experiment. *p=<0.05. Data is representative of 3 independent experiments.
iNKT cells are activated independently of CD1d interactions
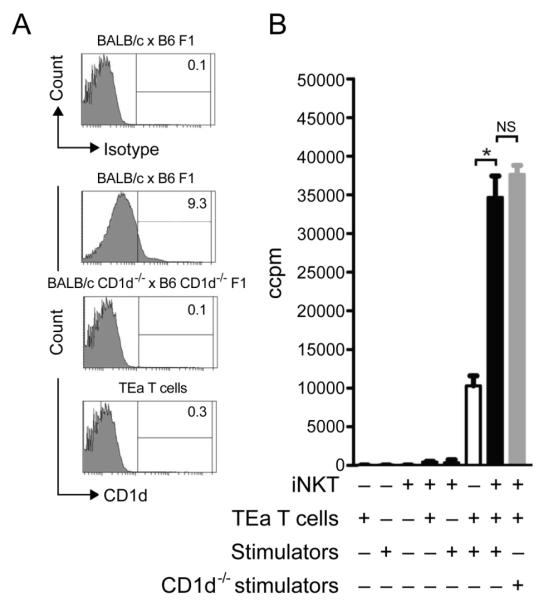
The activation of iNKT cells by CD1d-glycolipid is one possible mechanism which may facilitate iNKT cell proliferation during co-culture with TEa T cells and alloantigen. Therefore, the iNKT cell response was assessed in cultures containing TEa T cells and either WT or CD1d−/− BALB/cxB6 F1 stimulator cells where the absence of CD1d on BALB/cxB6 F1 CD1d−/− splenocytes was confirmed by staining with an anti-CD1d mAb (Figure 3A). No difference in proliferation was observed between such cultures (34655±2782 iNKT/TEa/F1 CD1d+ vs 37658±1168 iNKT/TEa/F1 CD1d−; p=0.15; Figure 3B). It remained possible that TEa T cells expressed CD1d and therefore provided a source of TCR-mediated recognition for the iNKT cells. However, this was ruled out as CD1d was not detected on TEa T cells (Figure 3A).
Figure 3. iNKT cells do not require CD1d to expand in the presence of alloreactive T cells.
The requirements of iNKT cell-CD1d interactions were investigated by generating CD1d−/− stimulators and confirming that TEa T cells lacked CD1d molecules by flow cytometry (A). iNKT cells were co-cultured with TEa T cells and stimulators that either express CD1d or are deficient in CD1d and proliferation determined after 3 days of culture (B). Data is presented as the mean of triplicate cultures ± s.d. of one experiment. *p=<0.05. Data is representative of 2 independent experiments.
Activated alloreactive T cells facilitate iNKT cell activation in vivo
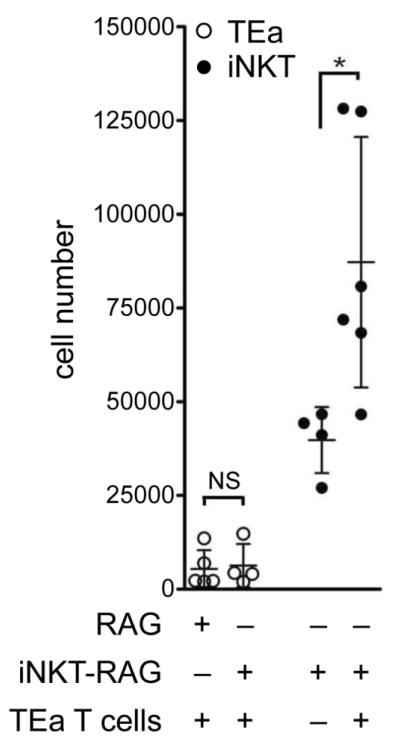
Thus far we have shown in vitro that upon co-culture with allogeneic stimulators iNKT cell proliferation was markedly increased when both conventional T cells and iNKT cells were responding in concert. Next, we examined whether these findings could be recapitulated in vivo by adoptively transferring TEa T cells to either C57BL/6 RAG−/− mice or C57BL/6 RAG−/− mice that had been seeded with iNKT cells (iNKT-RAG mice generated as described in material and methods/S1). One day after TEa T cell adoptive transfer such mice received an i.v. challenge with 1×107 BALB/cxB6 F1 splenocytes and 7 days later spleens were harvested and the number of iNKT cells and TEa T cells determined. In agreement with in vitro findings, activating TEa T cells with alloantigen was found to have a significant impact on iNKT cell expansion, increasing the number of iNKT cells in RAG-iNKT mice to a mean of 87214 ± 33393 iNKT cells as opposed to iNKT-RAG mice that received 1×107 BALB/cxB6 F1 splenocytes but no TEa T cells (39799±8814; p=0.02; Figure 4). Unmanipulated iNKT-RAG mice had an average of 33500±13800 splenic iNKT cells (n=27; S1) indicating that iNKT cells did not respond to alloantigen in the absence of TEa T cells. Unlike in the in vitro MLR, we found no evidence that TEa T cell expansion was altered by the presence of iNKT cells (Figure 4).
Figure 4. iNKT cells expand in vivo during conventional T cell alloresponses.
The expansion of iNKT cells following an in vivo MLR using TEa T cells was investigated using RAG−/− and iNKT-RAG mice. Briefly, iNKT-RAG mice were generated by sorting highly purified iNKT cells (99.7%) and expanded in vivo following adoptive transfer into RAG−/− mice (i.e lacking T/B cells) and given 2μg α-GalCer i.p. on day +1 and +14 (iNKT-RAG mice were used for experiments 30 days after iNKT cell adoptive transfer). TEa T cells were purified by cell sorting and adoptively transferred by i.v. into either RAG−/− or iNKT-RAG mice. The following day 107 stimulators (BALB/cxB6 F1 splenocytes) were delivered i.v. and the expansion of TEa T cells and iNKT cells determined by flow cytometry 7 days later. Dots represent individual animals (n= 4-6 per group). Bars indicate the mean cell number ± s.d. *p=0.02. Data pooled from 2 independent experiments.
Conventional T cell-derived IL-2 mediates bystander activation of iNKT cells
In order to examine the mechanism by which activated alloreactive T cells (TEa) facilitate iNKT cell expansion in vitro and in vivo we tested whether this form of “help” was dependent on a soluble molecule or required cell-cell contact. To this end, supernatant was collected from TEa T cells cultured with stimulators for 2 days and then added to cultures of iNKT cells alone or together with allogeneic stimulators. The previously observed expansion of iNKT cells when cultured with TEa T cells and stimulators, was found to be completely replicated when iNKT cells were cultured in the presence of supernatant with or without allogeneic spleen cells (Figure 5A). As with NKT cells cultured with TEa conventional T cells (Figure 3), NKT cell proliferation and expansion to TEa supernatant was found to occur independently of CD1d-TCR interactions (S2).
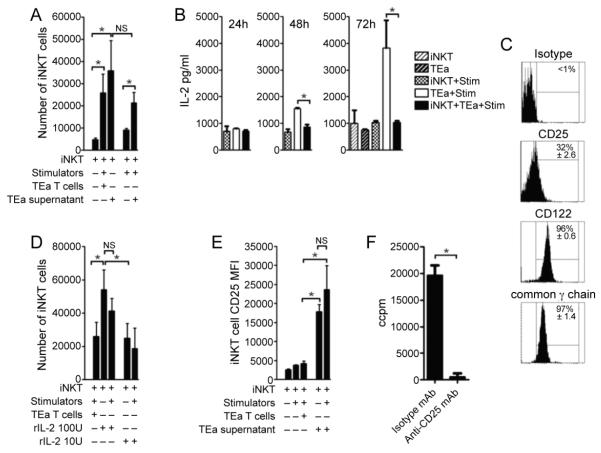
Figure 5. iNKT cells sequester IL-2 derived from conventional T cell alloresponses and proliferate.
TEa T cells and iNKT cells were purified by cell sorting. MLR were carried out with iNKT cells cultured in the presence of TEa T cell and stimulators or alone with TEa T cell derived supernatant (TEa+Stim taken at day 2) in the presence or absence of stimulators (A). Supernatants from TEa T cells and iNKT cells cultured alone, or with irradiated stimulators (BALB/cxB6F1) or together, were obtained at 24, 48 and 72 hours and the presence of IL-2 (B) determined by cytokine bead array (B). The basal expression of IL-2 receptor subunits (CD25, CD122 and common γ chain) was determined on freshly isolated iNKT cells by flow cytometry (C). The expansion of iNKT cells cultured in the presence of rIL-2 was determined following culture alone or with stimulators (D) and CD25 expression (E) determined by flow cytometry. iNKT cells were cultured with of TEa T cell derived supernatant (TEa+Stim taken at day 2) in the presence of anti-CD25 or isotype mAb and proliferation determined (F). Data is presented as the mean of triplicate cultures ± s.d. of one experiment. *p=<0.05. Data is representative of 2 independent experiments.
Next, we investigated the possibility that this “help” was due to sequestration of IL-2 generated by conventional alloreactive T cells. To this end IL-2 levels in supernatants were determined in cultures that contained TEa T cells and/or iNKT cells and allogeneic stimulators at 24, 48 and 72 hours post initiation of cultures (Figure 5B). TEa T cells cultured with stimulators were found to produce IL-2 on day 2, which was further increased by day 3 (Figure 5B). In comparison, when TEa T cells were cultured with stimulators and iNKT cells, IL-2 levels were significantly reduced and no different from negative control cultures which is consistent with sequestration of IL-2 by iNKT cells. We tested whether adding exogenous IL-2 would also result in the proliferation and expansion of iNKT cells given that even in a resting state, iNKT cells express all constituent subunits of the IL-2 receptor-complex (Figure 5C). The addition of IL-2 was found to drive iNKT cell expansion in a dose-dependent manner (Figure 5D). Interestingly, the expression of the high affinity α-subunit of the IL-2 receptor, CD25, was greatly increased when iNKT cells were cultured with either exogenous IL-2 or TEa T cell supernatant, suggesting that iNKT cells operate the same positive feedback loop for IL-2-IL-2 receptor expression as has been reported for conventional T cells (36). Importantly, in the presence of anti-CD25 blocking mAb, iNKT cells fail to proliferate and expand when cultured in supernatant derived from TEa T cells cultures with stimulators (Figure 5F).
Bystander activation of iNKT cells results in cytokine secretion
The rapid production of cytokines is one of the hallmarks of iNKT cell activation. Having shown that iNKT cells proliferate and expand in vitro and in vivo when responding in concert with conventional alloreactive T cells through sequestration of IL-2, we next sought to examine whether bystander activation also resulted in cytokine secretion. To this end IL-4, IL-5, IFNγ and TNF levels in supernatants were determined in cultures that contained TEa T cells and/or iNKT cells and allogeneic stimulators at 24, 48 and 72 hours post initiation of cultures (Figure 6A). IFNγ was only detected following co-culture of iNKT cells and TEa T cells with stimulators (Figure 6A). IL-4 was produced at low levels when iNKT cells were cultured, irrespective of the presence of stimulator and no IL-5 or TNF was detected in any supernatants (data not shown).
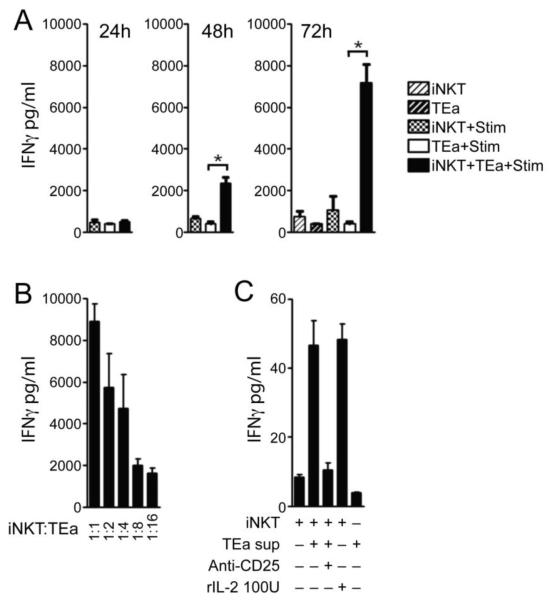
Figure 6. iNKT cells activation by conventional T cell alloresponses promotes effector cytokine release.
Supernatants from TEa T cells and iNKT cells cultured alone, or with irradiated stimulators (BALB/cxB6F1) or together, were obtained at 24, 48 and 72 hours and the presence of IFNγ determined by cytokine bead array (A). TEa T cells and iNKT cells were purified by cell sorting. MLR were carried out with iNKT cells cultured in a titration with TEa T cells (1:1-1:16) with stimulators and IFNγ measured in supernatants by ELISA on day 4 (B). iNKT cells were cell sorted and cultured in the presence TEa supernatant and anti-CD25 or isotype mAb and IL-2 and IFNγ measured by CBA (C). Data is presented as the mean of triplicate cultures ± s.d. of one experiment. *p=<0.05. Data is representative of 2-3 independent experiments.
Critically, the production of IFNγ was dependent upon the presence of iNKT cells, since a titration of the number of iNKT cells in cultures of TEa T cells and stimulators resulted in a reduction of IFNγ production (Figure 6B). In addition, culturing iNKT cells in the presence of TEa supernatant or rIL-2 induced IFNγ release (Figure 6C). Importantly, anti-CD25 blocking mAb completely abrogated IFNγ production when iNKT cells were cultured in the presence of TEa supernatant, demonstrating the requirement of IL-2 signalling for effector iNKT cell responses (Figure 6C).
Discussion
The activation of iNKT cells has largely focused on endogenous and exogenous ligands, presented in the context of CD1d. In this study we describe an alternative mechanism of iNKT cell activation which operates independently of CD1d, following the activation of conventional T cell by alloantigen.
We were unable to show that murine iNKT cells harbour alloreactivity (Figure 1B) and only one publication using human cloned iNKT cells has shown proliferation to allogeneic but not autologous monocytes, in a TCR dependent manner (37). It remains unclear whether this is a feature of cloned human iNKT cells and what glycolipid (if any) is being presented by allogeneic but not syngeneic CD1d molecules to drive the iNKT cell response. In contrast to these findings, in mouse models of skin transplantation, iNKT cells have been shown to proliferate in the draining lymph node following sex-mismatched but not syngeneic grafts and were also found within the allografts (29). Indeed, we have confirmed these finding using fully MHC mismatched skin allografts (S3). Importantly, such responses occurred independently of donor CD1d suggesting that murine iNKT cells have no direct TCR-mediated alloreactivity and that the microenvironment following transplantation provides the dominate signal for iNKT cell activation (29). We investigated this possibility in vitro and demonstrated that despite the lack of evidence supporting direct iNKT cell activation following recognition of allogeneic cells, co-culture of iNKT cells with alloreactive TEa T cells was found to drive iNKT cells to proliferate both in vitro (Figure 1-2) and in vivo (Figure 4) and was associated with secretion of effector cytokine (IFNγ; Figure 6). This is likely to have been produced by iNKT cells, since even after 3 days culture in the absence of iNKT cells, stimulated TEa T cells produced only low levels of IFNγ typical of the lag in effector cytokine secretion seen upon stimulation of naive T cells (Figure 6A). In support of this, the production of IFNγ was found to be directly correlated with iNKT cell number (Figure 6B) and replicated when iNKT cells were cultured alone in the presence of supernatant from TEa T cells and stimulator, removed after 2 days of culture (Figure 6C ).
Collectively, to our knowledge, this is the first example, in the absence of exogenous synthetic ligands, to show that iNKT cells can become activated, expand and secrete cytokine as an indirect consequence of an adaptive immune response. Given the lack of requirement for CD1d-TCR mediated recognition for iNKT cell responses in this setting (Figure 3, S2), we propose that the production of IL-2 by activated alloreactive T cells drives iNKT cell activation and effector function following transplantation. Interestingly, this mechanism of iNKT cell expansion has been reported using experimental IL-2-mAb-immunocomplexes (38) and as a side effect of IL-2 therapy, for the treatment of HIV-1 patients (39).
In the context of transplantation, we and others have proposed that the cytokine milieu at the site of T cell priming is more important for the activation of iNKT cells than their activation through specific TCR-mediated interactions (26, 29, 30). These data provide one possible explanation of how iNKT cells are activated following transplantation, i.e. through bystander activation as a result of a conventional T cell response to alloantigen. Once activated, iNKT cells may in turn be able to contribute to and influence the local cytokine microenvironment which may be either detrimental or beneficial to transplant survival, since iNKT cells can produce a plethora of both pro- and anti-inflammatory cytokines upon activation (30).
Indeed, there remains debate whether iNKT cell responses aid the induction of tolerance or exacerbate rejection following transplantation of allogeneic organs. For example, iNKT cells have been shown to be essential for the tolerance of heart allografts using anti-CD154 mAb (24, 40, 41) and aid in the acceptance of allogeneic corneal and bone marrow transplants, in the absence of immunosuppression (42-44). However, iNKT cells have also been implicated in mediating the rejection of islet, when delivered via the portal vein, in a mechanism involving the recruitment of neutrophils to the allograft site (27, 28). Jiang et al have reported that splenic iNKT cells harvested from either rejection or tolerant cardiac allografts, and subsequently cultured with α-GalCer, produce signature cytokines, IFNγ or IL-10 respectively (41). However, in other instances the production of IFNγ by iNKT cells has been shown to be required for transplant tolerance of cardiac and islet allografts (24).
As we have shown that iNKT cells can be activated and produce IFNγ following conventional T cell alloresponses, it is tempting to speculate that this mechanism of activation may promote the induction of regulatory mechanisms. Although, IFNγ is classically thought of as a pro-inflammatory cytokine we and others have shown that the production of IFNγ is essential for the function of regulatory T cells (Treg) and that IFNγ can be immunosuppressive under certain circumstances (45, 46).
Despite the tight physiological control of CD25 expression (36), even at a resting state iNKT cells express all of the subunits that make up the IL-2 receptor complex (Figure 5C) and markedly increase expression of CD25 in the presence of rIL-2 (Figure 5E). Therefore, it is possible that iNKT cells and Treg unite to consume IL-2 from the microenvironment diminishing that which is available for responding conventional T cells. It will be intriguing to investigate these possibilities further to explain how the cytokine microenvironments can polarise iNKT cell responses during transplant rejection or tolerance.
Although our data shows that iNKT cells can be activated by IL-2 secreted by alloreactive T cells it is possible that iNKT cells are activated by IL-2 in other disease settings such as autoimmunity and cancer. Such bystander activation may occur either without the need for recognition of glycolipid-CD1d or where the basal self-glycolipid/CD1d interactions that maintain iNKT cells in a semi-activated state are able to fully activate iNKT cells, in concert with IL-2 receptor signalling, in response to IL-2 secreted by activated conventional cells. Indeed, this emphasis of TCR-independent activation of iNKT cells has been well described in the context of microbial infection (23, 47, 48). For example, Brigl et al have recently shown that iNKT cells exposed to microbes containing previously defined iNKT cell ligands are activated predominantly through IL-12/STAT-4 signalling, rather than directly through TCR signalling (48). This study compliments our findings, as we now provide evidence that iNKT cells can be activated by IL-2 produced by T cells during an adaptive immune response in addition to cytokines such as IL-12 secreted by innate immune cells following TLR stimulation.
In summary, these data offer another explanation of how iNKT cells can contribute to immunological processes by TCR-independent mechanisms, in the absence of potent agonists, where effector cell derived IL-2 may be the dominant signal that promotes iNKT cell activation in many immune-mediated diseases.
Supplementary Material
Description of Supporting Information: The supporting information is composed of three additional figures (S1, S2 and S3). In S1 we provide information for the development of the iNKT-RAG model. In this model we demonstrate the successfully generation of RAG deficient mice (i.e. lacking T and B cells) that can be seeded with a population of iNKT cells. In S2 we show that the proliferation and expansion of iNKT cells to IL-2 does not require CD1d-TCR interactions. In S3 we confirm the previously published finding of Oh et al that iNKT cells expand in lymph nodes that are draining alloantigen following skin transplantation and traffic to allogeneic but not syngeneic skin grafts (29). However, we have included this figure as we are using a different strain of donor. Oh et al performed skin transplantation between C57 BL/6 and sex-mistmatched recipients, whereas in S3 we are using major-MHC-mismatched donors (BALB/c).
Acknowledgements
We would like to thank Dr W. Gao and Prof. T. Strom (Boston, USA) for the kind gift of TEa mice and Dr L. van Kaer (Nashville, USA) for the gift of the CD1d−/− mice. This work was funded by the Medical Research Council Studentship and the Dr Clarke Memorial Fund (J-P.J.); a Wellcome Trust programme grant (K.J.W.); and a Kidney Research U.K. Senior Fellowship (N.D.J.). CD1d-PBS57 loaded tetramer was kindly provided by the National Institutes of Health Tetramer Facility (Atlanta, USA). We thank Dr Parveen Dhaliwal, Gillian Kinnear and Radhika Chadha for technical assistance.
Footnotes
The authors have no conflicting financial interests.
References
- 1.Balk SP, Bleicher PA, Terhorst C. Isolation and characterization of a cDNA and gene coding for a fourth CD1 molecule. Proc Natl Acad Sci U S A. 1989;86(1):252–256. doi: 10.1073/pnas.86.1.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Game DS, Lechler RI. Pathways of allorecognition: implications for transplantation tolerance. Transpl Immunol. 2002;10(2-3):101–108. doi: 10.1016/s0966-3274(02)00055-2. [DOI] [PubMed] [Google Scholar]
- 3.Morita M, Motoki K, Akimoto K, Natori T, Sakai T, Sawa E, et al. Structure-activity relationship of alpha-galactosylceramides against B16-bearing mice. J Med Chem. 1995;38(12):2176–2187. doi: 10.1021/jm00012a018. [DOI] [PubMed] [Google Scholar]
- 4.Amprey JL, Im JS, Turco SJ, Murray HW, Illarionov PA, Besra GS, et al. A subset of liver NK T cells is activated during Leishmania donovani infection by CD1d-bound lipophosphoglycan. J Exp Med. 2004;200(7):895–904. doi: 10.1084/jem.20040704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer K, Scotet E, Niemeyer M, Koebernick H, Zerrahn J, Maillet S, et al. Mycobacterial phosphatidylinositol mannoside is a natural antigen for CD1d-restricted T cells. Proc Natl Acad Sci U S A. 2004;101(29):10685–10690. doi: 10.1073/pnas.0403787101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434(7032):520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 7.Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, 3rd, Zhou D, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434(7032):525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 8.Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, Benhnia MR, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7(9):978–986. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- 9.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4(12):1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 10.Paget C, Mallevaey T, Speak AO, Torres D, Fontaine J, Sheehan KC, et al. Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity. 2007;27(4):597–609. doi: 10.1016/j.immuni.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 11.Salio M, Speak AO, Shepherd D, Polzella P, Illarionov PA, Veerapen N, et al. Modulation of human natural killer T cell ligands on TLR-mediated antigen-presenting cell activation. Proc Natl Acad Sci U S A. 2007;104(51):20490–20495. doi: 10.1073/pnas.0710145104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuda JL, Mallevaey T, Scott-Browne J, Gapin L. CD1d-restricted iNKT cells, the ‘Swiss-Army knife’ of the immune system. Curr Opin Immunol. 2008;20(3):358–368. doi: 10.1016/j.coi.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carnaud C, Lee D, Donnars O, Park SH, Beavis A, Koezuka Y, et al. Cutting edge: Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999;163(9):4647–4650. [PubMed] [Google Scholar]
- 14.Eberl G, MacDonald HR. Selective induction of NK cell proliferation and cytotoxicity by activated NKT cells. Eur J Immunol. 2000;30(4):985–992. doi: 10.1002/(SICI)1521-4141(200004)30:4<985::AID-IMMU985>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 15.Vincent MS, Leslie DS, Gumperz JE, Xiong X, Grant EP, Brenner MB. CD1-dependent dendritic cell instruction. Nat Immunol. 2002;3(12):1163–1168. doi: 10.1038/ni851. [DOI] [PubMed] [Google Scholar]
- 16.Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198(2):267–279. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hermans IF, Silk JD, Gileadi U, Salio M, Mathew B, Ritter G, et al. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J Immunol. 2003;171(10):5140–5147. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]
- 18.Galli G, Nuti S, Tavarini S, Galli-Stampino L, De Lalla C, Casorati G, et al. Innate immune responses support adaptive immunity: NKT cells induce B cell activation. Vaccine. 2003;21(Suppl 2):S48–54. doi: 10.1016/s0264-410x(03)00200-7. [DOI] [PubMed] [Google Scholar]
- 19.Silk JD, Hermans IF, Gileadi U, Chong TW, Shepherd D, Salio M, et al. Utilizing the adjuvant properties of CD1d-dependent NK T cells in T cell-mediated immunotherapy. J Clin Invest. 2004;114(12):1800–1811. doi: 10.1172/JCI22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Santo C, Salio M, Masri SH, Lee LY, Dong T, Speak AO, et al. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. J Clin Invest. 2008;118(12):4036–4048. doi: 10.1172/JCI36264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Santo C, Arscott R, Booth S, Karydis I, Jones M, Asher R, et al. Invariant NKT cells modulate the suppressive activity of IL-10-secreting neutrophils differentiated with serum amyloid A. Nat Immunol. 2010;11(11):1039–1046. doi: 10.1038/ni.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Kaer L. Natural killer T cells as targets for immunotherapy of autoimmune diseases. Immunol Cell Biol. 2004;82(3):315–322. doi: 10.1111/j.0818-9641.2004.01252.x. [DOI] [PubMed] [Google Scholar]
- 23.Brigl M, Brenner MB. How invariant natural killer T cells respond to infection by recognizing microbial or endogenous lipid antigens. Semin Immunol. 2009 doi: 10.1016/j.smim.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Seino KI, Fukao K, Muramoto K, Yanagisawa K, Takada Y, Kakuta S, et al. Requirement for natural killer T (NKT) cells in the induction of allograft tolerance. Proc Natl Acad Sci U S A. 2001;98(5):2577–2581. doi: 10.1073/pnas.041608298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikehara Y, Yasunami Y, Kodama S, Maki T, Nakano M, Nakayama T, et al. CD4(+) Valpha14 natural killer T cells are essential for acceptance of rat islet xenografts in mice. J Clin Invest. 2000;105(12):1761–1767. doi: 10.1172/JCI8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang SH, Jin JZ, Lee SH, Park H, Kim CH, Lee DS, et al. Role of NKT cells in allogeneic islet graft survival. Clin Immunol. 2007;124(3):258–266. doi: 10.1016/j.clim.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Toyofuku A, Yasunami Y, Nabeyama K, Nakano M, Satoh M, Matsuoka N, et al. Natural killer T-cells participate in rejection of islet allografts in the liver of mice. Diabetes. 2006;55(1):34–39. [PubMed] [Google Scholar]
- 28.Yasunami Y, Kojo S, Kitamura H, Toyofuku A, Satoh M, Nakano M, et al. Valpha14 NK T cell-triggered IFN-gamma production by Gr-1+CD11b+ cells mediates early graft loss of syngeneic transplanted islets. J Exp Med. 2005;202(7):913–918. doi: 10.1084/jem.20050448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oh K, Kim S, Park SH, Gu H, Roopenian D, Chung DH, et al. Direct regulatory role of NKT cells in allogeneic graft survival is dependent on the quantitative strength of antigenicity. J Immunol. 2005;174(4):2030–2036. doi: 10.4049/jimmunol.174.4.2030. [DOI] [PubMed] [Google Scholar]
- 30.Jukes JP, Wood KJ, Jones ND. Natural killer T cells: a bridge to tolerance or a pathway to rejection? Transplantation. 2007;84(6):679–681. doi: 10.1097/01.tp.0000280551.78156.ac. [DOI] [PubMed] [Google Scholar]
- 31.Li L, Huang L, Sung SS, Lobo PI, Brown MG, Gregg RK, et al. NKT cell activation mediates neutrophil IFN-gamma production and renal ischemia-reperfusion injury. J Immunol. 2007;178(9):5899–5911. doi: 10.4049/jimmunol.178.9.5899. [DOI] [PubMed] [Google Scholar]
- 32.Jones ND, Carvalho-Gaspar M, Luo S, Brook MO, Martin L, Wood KJ. Effector and memory CD8+ T cells can be generated in response to alloantigen independently of CD4+ T cell help. J Immunol. 2006;176(4):2316–2323. doi: 10.4049/jimmunol.176.4.2316. [DOI] [PubMed] [Google Scholar]
- 33.Carvalho-Gaspar M, Billing JS, Spriewald BM, Wood KJ. Chemokine gene expression during allograft rejection: comparison of two quantitative PCR techniques. J Immunol Methods. 2005;301(1-2):41–52. doi: 10.1016/j.jim.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 35.Grubin CE, Kovats S, deRoos P, Rudensky AY. Deficient positive selection of CD4 T cells in mice displaying altered repertoires of MHC class II-bound self-peptides. Immunity. 1997;7(2):197–208. doi: 10.1016/s1074-7613(00)80523-3. [DOI] [PubMed] [Google Scholar]
- 36.Malek TR, Castro I. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity. 2010;33(2):153–165. doi: 10.1016/j.immuni.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patterson S, Chaidos A, Neville DC, Poggi A, Butters TD, Roberts IA, et al. Human invariant NKT cells display alloreactivity instructed by invariant TCR-CD1d interaction and killer Ig receptors. J Immunol. 2008;181(5):3268–3276. doi: 10.4049/jimmunol.181.5.3268. [DOI] [PubMed] [Google Scholar]
- 38.Tomala J, Chmelova H, Strohalm J, Ulbrich K, Sirova M, Rihova B, et al. Antitumor activity of IL-2/anti-IL-2 mAb immunocomplexes synergizes with that of HPMA copolymer-bound doxorubicin conjugate due to its low immunosuppressive activity. Int J Cancer. 2010 doi: 10.1002/ijc.25859. [DOI] [PubMed] [Google Scholar]
- 39.Moll M, Snyder-Cappione J, Spotts G, Hecht FM, Sandberg JK, Nixon DF. Expansion of CD1d-restricted NKT cells in patients with primary HIV-1 infection treated with interleukin-2. Blood. 2006;107(8):3081–3083. doi: 10.1182/blood-2005-09-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang X, Shimaoka T, Kojo S, Harada M, Watarai H, Wakao H, et al. Cutting edge: critical role of CXCL16/CXCR6 in NKT cell trafficking in allograft tolerance. J Immunol. 2005;175(4):2051–2055. doi: 10.4049/jimmunol.175.4.2051. [DOI] [PubMed] [Google Scholar]
- 41.Jiang X, Kojo S, Harada M, Ohkohchi N, Taniguchi M, Seino KI. Mechanism of NKT cell-mediated transplant tolerance. Am J Transplant. 2007;7(6):1482–1490. doi: 10.1111/j.1600-6143.2007.01827.x. [DOI] [PubMed] [Google Scholar]
- 42.Zeng D, Lewis D, Dejbakhsh-Jones S, Lan F, Garcia-Ojeda M, Sibley R, et al. Bone marrow NK1.1(−) and NK1.1(+) T cells reciprocally regulate acute graft versus host disease. J Exp Med. 1999;189(7):1073–1081. doi: 10.1084/jem.189.7.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sonoda KH, Taniguchi M, Stein-Streilein J. Long-term survival of corneal allografts is dependent on intact CD1d-reactive NKT cells. J Immunol. 2002;168(4):2028–2034. doi: 10.4049/jimmunol.168.4.2028. [DOI] [PubMed] [Google Scholar]
- 44.Leveson-Gower DB, Olson JA, Sega EI, Luong RH, Baker J, Zeiser R, et al. Low doses of natural killer T cells provide protection from acute graft-versus-host disease via an IL-4-dependent mechanism. Blood. 2011;117(11):3220–3229. doi: 10.1182/blood-2010-08-303008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Konieczny BT, Dai Z, Elwood ET, Saleem S, Linsley PS, Baddoura FK, et al. IFN-gamma is critical for long-term allograft survival induced by blocking the CD28 and CD40 ligand T cell costimulation pathways. J Immunol. 1998;160(5):2059–2064. [PubMed] [Google Scholar]
- 46.Wood KJ, Sawitzki B. Interferon gamma: a crucial role in the function of induced regulatory T cells in vivo. Trends Immunol. 2006;27(4):183–187. doi: 10.1016/j.it.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 47.Reilly EC, Wands JR, Brossay L. Cytokine dependent and independent iNKT cell activation. Cytokine. 2010;51(3):227–231. doi: 10.1016/j.cyto.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brigl M, Tatituri RV, Watts GF, Bhowruth V, Leadbetter EA, Barton N, et al. Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J Exp Med. 2011;208(6):1163–1177. doi: 10.1084/jem.20102555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Supporting Information: The supporting information is composed of three additional figures (S1, S2 and S3). In S1 we provide information for the development of the iNKT-RAG model. In this model we demonstrate the successfully generation of RAG deficient mice (i.e. lacking T and B cells) that can be seeded with a population of iNKT cells. In S2 we show that the proliferation and expansion of iNKT cells to IL-2 does not require CD1d-TCR interactions. In S3 we confirm the previously published finding of Oh et al that iNKT cells expand in lymph nodes that are draining alloantigen following skin transplantation and traffic to allogeneic but not syngeneic skin grafts (29). However, we have included this figure as we are using a different strain of donor. Oh et al performed skin transplantation between C57 BL/6 and sex-mistmatched recipients, whereas in S3 we are using major-MHC-mismatched donors (BALB/c).