Abstract
Background:
Polycystic ovarian syndrome (PCOS), characterized by ovulatory infertility and hyperandrogenism, is associated with metabolic complications such as dyslipidemia, insulin resistance and endothelial dysfunction. Almost 70% PCOS women have abnormal serum lipid levels (dyslipidemia) and 50% of these women are obese. Several classes of pharmacological agents have been used to manage dyslipidemia. However, studies have shown adverse effects associated with these drugs. In the light of alternate therapy, many medicinal herbs have been reported to show hypoglycemic, anti-hyperlipidemic potential. Aloe barbadensis Mill. or Aloe vera is reported as one such herb. This study was to evaluate the lipid correcting effect of Aloe vera gel (AVG) in a PCOS rat model.
Materials and Methods:
PCOS was induced in Charles Foster female rats by oral administration of non-steroidal aromatase inhibitor letrozole (0.5 mg/kg body weight, 21 days). All rats were hyperglycemic and 90% rats also showed elevated plasma triglycerides, elevated LDL cholesterol levels, and lowered plasma HDL cholesterol levels indicative of a dyslipidemic profile. PCOS positive rats with an aberrant lipid profile were selected for treatment. An AVG formulation (1 ml (10 mg)/day, 30 days) was administered orally.
Results and Conclusion:
AVG treated PCOS rats exhibited significant reduction in plasma triglyceride and LDL cholesterol levels, with an increase in HDL cholesterol. The gel treatment also caused reversion of abnormal estrous cyclicity, glucose intolerance, and lipid metabolizing enzyme activities, bringing them to normal. In conclusion, AVG has phyto components with anti-hyperlipidemic effects and it has shown efficacy in management of not only PCOS but also the associated metabolic complication : dyslipidemia.
Keywords: Aloe vera gel, anovulation, anti-hyperlipidemic effect, dyslipidemia, hyperandrogenism, infertility, insulin resistance, letrozole, polycystic ovarian syndrome
INTRODUCTION
Polycystic ovarian syndrome (PCOS), one of the most common causes of infertility due to anovulation, affects up to one in fifteen women of reproductive age. There is a consistent 4–9% prevalence of PCOS seen in studies carried out across diverse populations in the world.[1–5] According to the National Institutes of Health (NIH), the diagnostic criteria for PCOS are (1) hyperandrogenism, (2) chronic oligo-anovulation along with (3) the exclusion of other causes of hyperandrogenism like Congenital Adrenal Hyperplasia (CAH).[6]
PCOS subjects demonstrate many features of the metabolic syndrome such as insulin resistance, hypertension and dyslipidemia.[7] Around 70% PCOS women have abnormal serum lipid levels or dyslipidemia, as confirmed by the National Cholesterol Education Program (NCEP), making it the most common metabolic abnormality associated with PCOS.[8] Furthermore, dyslipidemia-induced atherosclerosis is a primary determinant in the development of cardiovascular diseases (CVD), thus acting as a major risk factor in PCOS women for the development of CVD.[9]
Dyslipidemia in women with PCOS is characterized by decreased HDL-C (high-density lipoprotein cholesterol) levels and increased plasma triglycerides, increased LDL-C (Low-density lipoprotein cholesterol), and increased non-HDL cholesterol levels.[10] PCOS women also tend to have an increased waist-to-hip ratio, manifested as a result of visceral fat accumulation. Visceral fat tissue delivers high concentrations of free fatty acids and increases the production of very low density lipoprotein (VLDL) in the liver, thus contributing to the dyslipidemic profile in these women.[11]
Insulin resistance and dyslipidemia are almost concomitantly observed in most PCOS women. While the effects of hormonal changes on insulin secretion and function in PCOS women have been studied extensively, the interactions of metabolic and hormonal abnormalities on lipid metabolism in PCOS are not very well explored. Combination therapies of insulin sensitizing drugs such as metformin and thiazolidinediones along with lipid lowering drugs such as statins (HMG-CoA reductase inhibitors), nicotinic acid and fibric acid derivatives are used currently as a therapies for PCOS women.[12–15]
Several studies have shown that a prolonged use of some of these pharmacological agents have a variety of moderate to severe side effects.[16–18] Thus, current research is shifting its focus to alternative therapies in order to prevent these deleterious effects. In India and China, many medicinal plants are used for the treatment of various diseases. The goal of herbal remedy is to enable the body to readjust excess levels of hormones, bring them to ‘normal’ and establish normal physiological function. Medicinal plants such as Terminalia arjuna and Enicostemma littorale have been used for their anti-hyperlipidemic effects,[19,20] whereas Tecoma stans and Poliomintha longiflora have been used as therapy for their hypoglycemic properties.[21]
Aloe barbadensis Mill. (Aloe vera) has been popularly used as a medicinal plant and is known for its hypoglycemic, lipid lowering, anti-inflammatory, and anti-oxidant properties.[22–25] Our previous work has already shown that an Aloe vera gel (AVG) formulation causes partial reversion of estrous cyclicity and improves steroidogenic activity in the PCOS rat model.[26] Hence, this study was aimed to test the efficacy of the AVG formulation as a antihyperlipidemic and hypoglycemic agent in the PCOS condition, in order to establish its therapeutic use for PCOS–metabolic syndrome subjects.
MATERIALS AND METHODS
Preparation of Aloe vera gel (AVG) formulation
Aloe barbadensis Mill. (Voucher no. PSN 723) was compared with the specimen (Bhatt 2486, 653, 279, JVJ 448) located at the nationally recognized BARO Herbaria of the Department of Botany, The M.S. University of Baroda, Vadodara, Gujarat, India. The Aloe vera formulation was prepared under aseptic conditions. Fresh mature Aloe vera leaves (3.5 years old) were taken and washed with water. The epidermis (outer green portion) was removed with a sterilized knife in order to remove the aloin. The gel (colorless inner substance) was allowed to stand for 2 h and then later sonicated into a homogenous gel. Natural preservatives : turmeric (Curcuma longa L. [0.1 g/l]), Karaya gum (Sterculia urens Roxb. [10% g/l]), and lemon juice (Citrus limon L. [0.1 %]) were added. The concentration was decided after vigorous studies on the stability of Aloe vera juice with the stated concentration of preservative for its anti-microbial and anti-fungal properties. The concentration of aloin was checked by comparing the Aloe formulation prepared with the aloin A and B standards procured from Sigma (B6906) using HPTLC. The turmeric added to AVG had 4–5% curcumin. All precautions taken while preparing Aloe vera juice were as per the WHO guidelines (WHO monograph on selected medicinal plants volume I/ref. no. 43).
Induction of letrozole-induced PCOS
Adult virgin female Charles Foster rats (3–4 months) showing regular estrous cyclicity, weighing 200–225 g under controlled conditions of light and temperature, with free access to diet and water were chosen for the induction of the PCOS model. Animals were treated orally with letrozole (0.5 mg/kg body weight) every morning for 21 days,[27] while different control groups were administered their respective vehicle substances. All PCOS positive animals which exhibited glucose intolerance, irregular ovarian cyclicity with the presence of cysts, altered steroidogenic activity, increase in body weight and altered lipid profile were considered and chosen for the study. Proposal No : BC/03/2010 was administered by the M.S. University of Baroda Animal Ethics Committee (Registration no. 38/A06/CPCSEA) which approved all the protocols.
Experimental set-up
Induction of model: The rats were divided into three groups namely (a) CMC control group (treated with 1% carboxymethyl cellulose or CMC daily for 21 days), (b) Aloe control group (treated with AVG formulation—1 ml(10mg)/day daily for 21 days), and (c) letrozole-induced PCOS positive rats (treated with letrozole—0.5 mg/kg body weight daily for 21 days). The CMC and AVG control groups had six to eight animals each whereas the letrozole-induced PCOS group had 32 animals.
Treatment: On induction of PCOS model, the CMC and AVG control groups were continued with the same treatment for 30 days. The PCOS positive group was divided into four groups: (i) PCOS positive group or PCOS control group (no treatment given), (ii) AVG treated PCOS rats (treated AVG formulation—1 ml (10mg)//day daily for 30 days), (iii) PCOS rats treated with Metformin (500 mg/kg body weight daily for 30 days),[28] and (iv) positive control group or statin Group (treated with atorvastatin–25 mg/kg body daily for 30 days).[29]
All compounds were administered orally.
Analysis: Estrous cyclicity of the animals in all the groups was monitored daily during treatment. After 30 days of treatment, 12 h fasting blood samples were collected for all groups of rats, and the rats were then killed. Plasma from the blood samples was used for the estimation of glucose and lipid profile parameters. Ovarian steroid enzymes activities were assayed from the mitochondrial fraction of ovarian tissue. HMG-CoA reductase activity from the liver tissue was determined.
Assessment of biochemical parameters
A oral glucose tolerance test (OGTT) was performed after 12 h fasting in all rats by the protocol of Buchanan et al.[30] Glucose (300 mg/kg body weight) was orally fed to the rats and blood samples were collected in NaF-coated tubes at the different time intervals (30′, 60′, 90′, and 120′). Steroidogenic enzyme assays were carried out according to a previously published procedure.[26] Analysis of the lipid profile was done by Enzopak kits which estimated total cholesterol content, triglycerides, HDL-C, and LDL-C from plasma. The kit reagents hydrolyze the fat in the sample, which is then acted upon by an oxidase and then a peroxidase which forms a colored complex that can be assayed spectrophotometrically. Liver lipid was extracted by the method proposed by Folch et al., and subsequent liver cholesterol was estimated by Zlatkis’ method.[31,32] HMG-CoA reductase is expressed as a ratio of absorbance of the substrate (HMG-CoA) to the absorbance of the product (Mevalonate). The higher the substrate:product ratio, the lower the activity and vice versa. HMG–CoA reductase analysis was carried out according to the protocol published by Rao and Ramakrishnan.[33] Lecithin cholesterol acyl transferase (LCAT) activity was expressed as a measure of the amount of free cholesterol liberated during the assay. The more the free cholesterol liberated, the lesser the LCAT activity. LCAT activity was estimated from the separated blood plasma using Hitz’ method.[34]
Statistics
Comparison of different groups was done using analysis of variance (ANOVA) and Student t-test. The analysis was carried out using Graph Pad version 3.0. P ≤ 0.05 was considered significant. All results are expressed as the mean ± SD for 6–8 animals in each group.
RESULTS
Effect of AVG formulation on estrous cyclicity and ovarian steroidogenesis enzymes
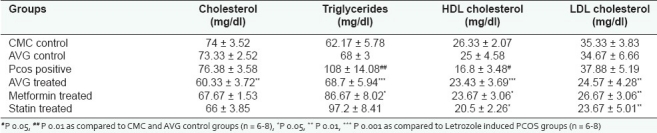
The disruption of estrous cyclicity caused by letrozole was observed in the PCOS positive group which showed an extended diestrous stage. The presence of cysts in PCOS rat ovaries led to an increase in ovarian weight as shown in Figure 1. Ovarian weight of PCOS positive rats showed a marginal nonsignificant decrease on treatment with AVG thus establishing that AVG reversed anovulation status and prevented further increases in cyst formation. Figure 2 demonstrates the efficacy of AVG on ovarian steroidal enzymes activities. Letrozole administration resulted in a significant increase in both 3β hydroxysteroid dehydrogenase (HSD) and 17β HSD activities. AVG treatment of PCOS positive rats caused in a significant decrease in 3β HSD activity wherein 17β HSD activity showed a nonsignificant decrease. The AVG extract decreases 3β HSD activity while keeping the 17β HSD activity near normal, thereby decreasing overall androgen production and increasing the flux of the steroidogenesis pathway toward estrogen synthesis. Metformin treatment had a similar effect wherein statin treatment did not bring about a change in any of these parameters.
Figure 1.
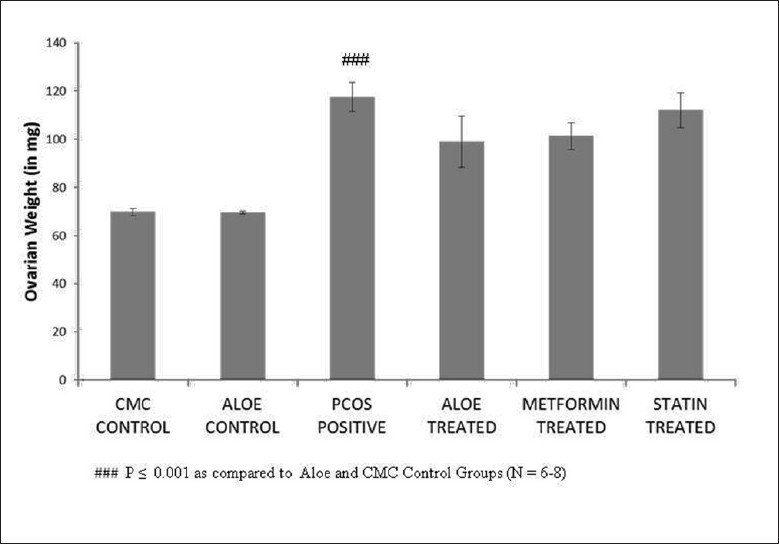
Effect of AVG extract on ovarian weight
Figure 2.
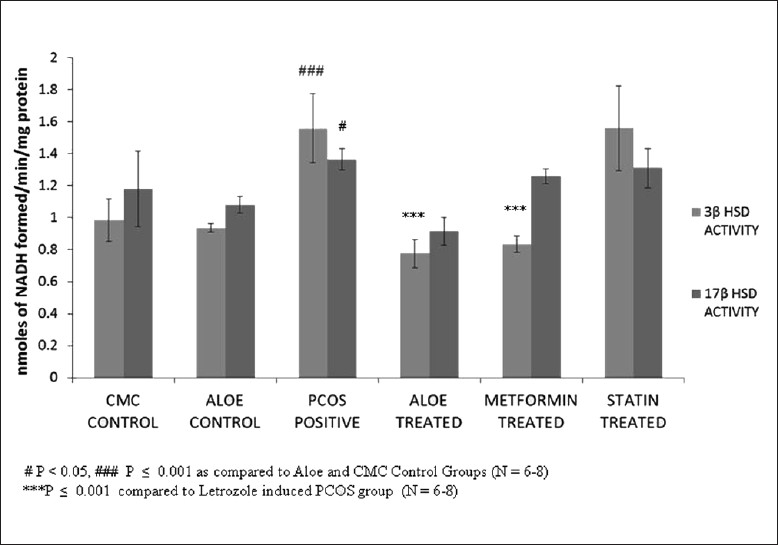
Effect of AVG extract on ovarian steroidogenesis enzyme activities
Effect of AVG formulation on glucose metabolism and body weight
An insulin-resistant phenotype was confirmed in PCOS positive rats by carrying out an oral glucose tolerance test (OGTT) in these rats. The OGTT profile in Figure 3 shows that a significant reduction glucose tolerance in PCOS rats was seen when compared to controls groups wherein AVG-treated PCOS rats showed a restored glucose tolerance comparable to that of the control groups. The body weights of these animals were also used as a marker of glucose homeostasis. Figure 4 shows the increase body weight in PCOS rat model when compared to CMC and Aloe control groups, whereas AVG-treated PCOS rats showed a significant decrease in body weight similar to the decrease shown by metformin and statin treatment groups.
Figure 3.
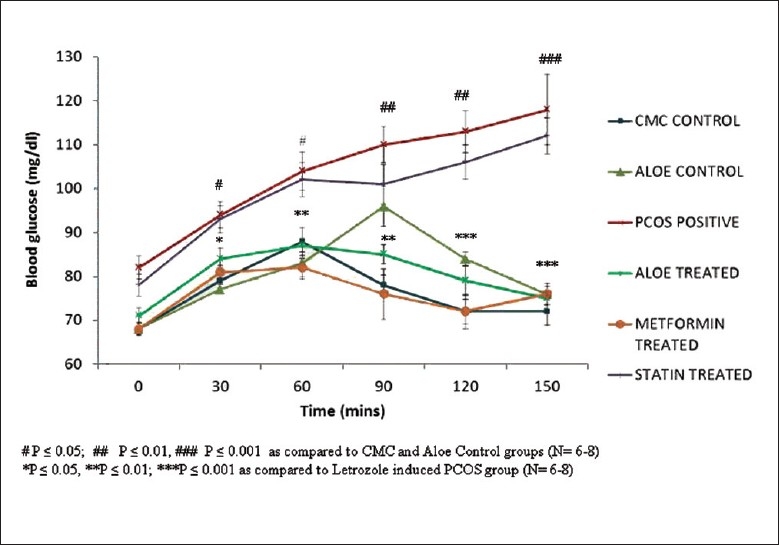
Effect of AVG extract on the oral glucose tolerance test (OGTT)
Figure 4.
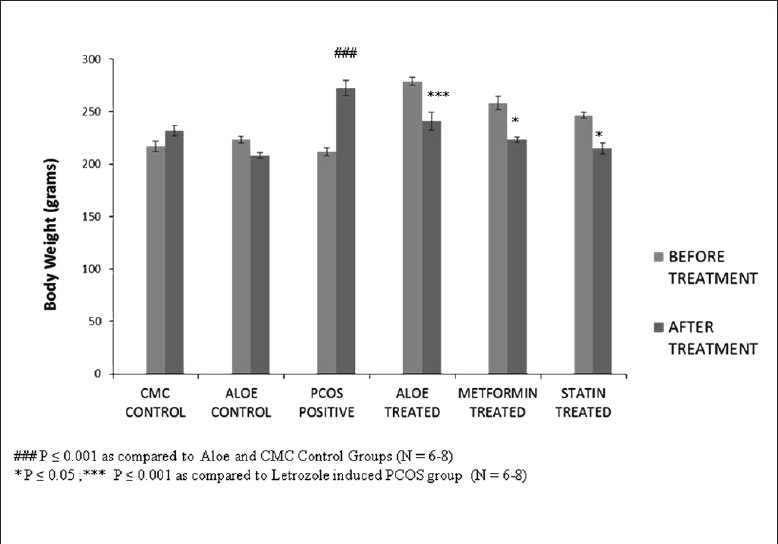
Effect of AVG extract on body weight
Effect of AVG formulation on the lipid profile of PCOS rats
As seen in Table 1, on administration of letrozole for 21 days, a marked increase was seen in the plasma triglyceride levels, decrease in HDL-C levels, whereas plasma cholesterol and LDL-C levels remained almost the same as compared to the various control groups. 90% of the experimental rats showed this phenotype and thus a dyslipidemic PCOS rat model was established. On treatment with Metformin and Statin for 30 days, a marked decrease in plasma cholesterol and LDL-C levels was seen, along with an increase in HDL-C levels. However, AVG treatment had a more significant effect in restoring of plasma cholesterol levels back to normal. In addition, AVG also led to a significant decrease in plasma triglyceride levels, thus bringing the complete lipid profile to normal.
Table 1.
Effect of AVG extract on lipid profile
Effect of AVG formulation on lipid metabolizing enzymes
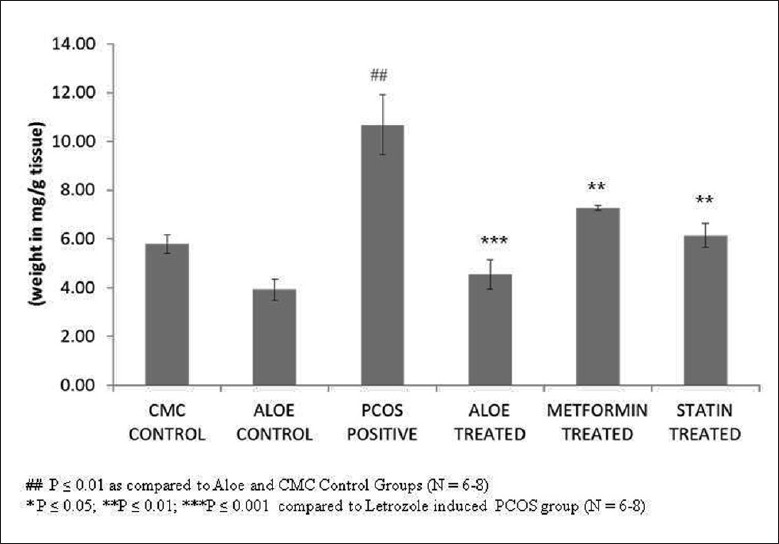
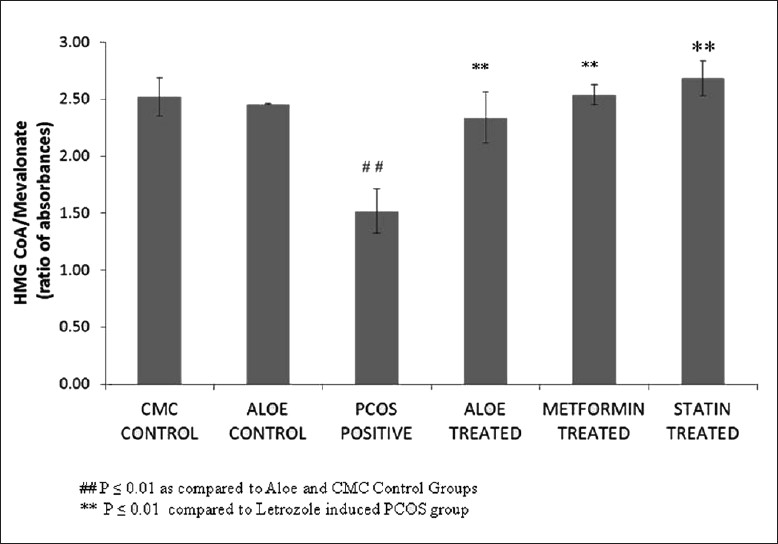
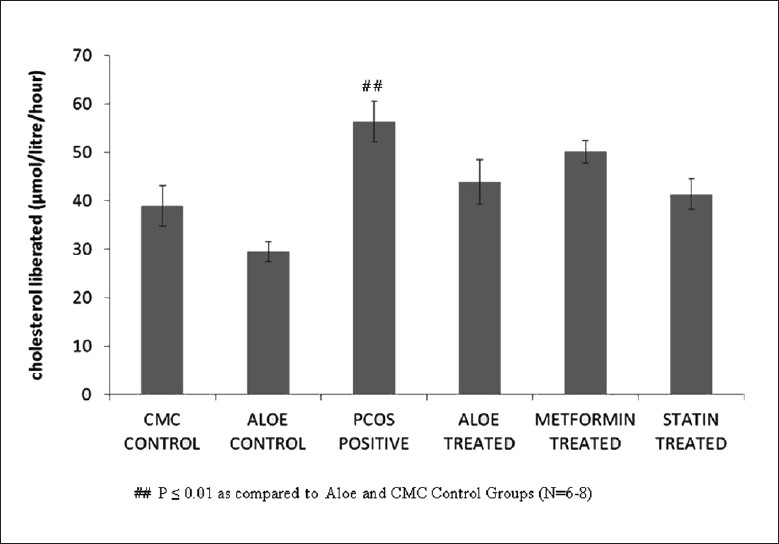
Figure 5 demonstrates the high levels of liver cholesterol seen in letrozole-induced PCOS rats as compared to the control group. Treatment with AVG, metformin and statin restored these levels back to normal. Decreased liver cholesterol levels were also reflected in the effect of AVG on activity of HMG-CoA reductase. Figure 6 shows that letrozole treatment increases HMG-CoA reductase activity, thus indicating an increased endogenous cholesterol production. Treatment with combined AVG, metformin and statin decreased its activity significantly. Ideal decrease in activity was seen on treatment with statin, which is a HMG-CoA reductase inhibitor. On administering letrozole, a significant decrease in LCAT activity was observed as compared to the control groups. This activity increased on treatment with combined AVG, metformin, and statin. However, the increase was not significant as indicated in Figure 7.
Figure 5.
Effect of AVG extract on liver cholesterol
Figure 6.
Effect of AVG extract on HMG CoA reductase activity
Figure 7.
Effect of AVG on LCAT (lecithin cholesterol acyl transferase) activity
DISCUSSION
PCOS is manifested primarily by anovulation and hyperandrogenism. First, chronic anovulation and hyperplasia of the ovarian stroma are seen as a result of an increase in the LH/FSH ratio. An increase in LH concentration is seen due to the inappropriate estrogen feedback to the pituitary.[35] Second, almost 80% PCOS patients have high levels of free testosterone, DHEA (dehydroepiandrosterone), and androstenedione.[36,37] A deficiency in the activity of the aromatase enzyme is one of the many intra-ovarian disturbances thought to cause androgen excess in PCOS. Aromatase catalyzes the rate limiting step in the biosynthesis of estrogens from androgens. Letrozole, a nonsteroidal inhibitor of the aromatase enzyme, which results in androgen excess and promotes development of PCOS, was used to establish a PCOS rat model.[27]
The working of this model was confirmed by assaying for the activity of key androgen producing ovarian steroidogenesis enzymes. PCOS positive rats showed a higher 3β HSD and 17β HSD activities as compared to the control groups, indicating higher androgen production. The experiment also demonstrated the efficacy of AVG in bringing the activities of these enzymes back to control levels suggesting that the presence of polyphenols in the AVG could modulate the activities of these enzymes.[26]
Ovarian cyclicity was checked regularly, and it was concluded that PCOS positive animals showed an extended diestrous state as compared to control groups, thus suggesting that the model mimicked anovulation. Treatment with AVG restored estrous cyclicity back to normal levels. This hypoglycemic property may be attributed to nutritionally rich phytosterols and phytophenols in the plant. Ovarian weight in PCOS-positive animals was more than control rats and treatment with the gel prevented further increase in ovarian weight, thus suggesting that AVG somehow prevents ovarian hyperplasia and ovarian cyst formation.
A high level of androgens induces insulin resistance through the activation of the lipolytic cascade and by modifying muscle histology.[38,39] The OGTT was performed, and it was concluded that PCOS-positive rats showed a decreased tolerance to glucose. AVG and metformin treatment increased the glucose tolerance of these rats, suggesting that certain phytocomponents in AVG are able to affect the secretion and metabolism of insulin and its action. As glucose homeostasis is maintained in combined AVG treatment groups, the body weight is also maintained.
Hyperandrogenism also leads to dyslipidemia. Free testosterone levels have been implicated in lowering HDL cholesterol levels.[40] Androgens through the interaction with androgen receptor (AR) decrease the catabolic removal of LDL.[41] The overall lipid profile was carried out and 90% of all PCOS rats showed elevated plasma triglyceride levels and reduced HDL cholesterol levels. These rats were then selected for the rest of the study and divided into different treatment groups. AVG-treated groups showed a marked reduction in plasma triglyceride, cholesterol, and LDL cholesterol levels along with a significant increase in HDL cholesterol levels as compared to PCOS-positive rats.
The liver plays an important role in glucose and lipid homeostasis. It is also involved in cholesterol synthesis and secretion of plasma lipoproteins. Hepatic LCAT (lecithin cholesterol acyl transferase) acts on lipoprotein remnants discharged into the plasma from the liver. It scavenges the cholesterol from these remnants so as to form HDL cholesterol, which can be then taken to the liver for degradation. PCOS positive rats treated with AVG showed reduced level of LCAT activity and this result is confirmed by an increase in plasma HDL-C levels as shown by the lipid profile results. HMG-CoA reductase is the rate limiting enzyme involved in cholesterol biosynthesis in tissues such as liver, ovary, etc. It converts fatty acyl groups in the form of HMG-CoA to mevalonate. An increased HMG CoA activity seen in the AVG treated group as compared to controls could be due to decreased cholesterol absorption in the gastrointestinal tract. Thus, the decreasing cholesterol level in body under the influence of AVG formulation could have enhanced the enzymatic activity by a positive feedback mechanism.
It is well established that AVG is a strong hypoglycemic agent and reduces cholesterol levels.[23] Hypoglycemic effect of AVG helps to improve glucose uptake and metabolism in PCOS rats. Insulin resistance is also the main cause of hyperinsulinemia in PCOS and if left untreated can lead to Type II Diabetes Mellitus. AVG, with its various phytosterols, sensitizes the insulin receptor and improves the insulin secretion.[23] PCOS condition exhibits high FFA and elevated cholesterol levels due to an androgen excess as discussed before. AVG works to bring the lipid profile back to normal in PCOS rats. Dyslipidemia is also a major cause of obesity in PCOS and if left untreated can lead to CVD.
Thus, in conclusion, our present study could highlight that the AVG used in our study has potential efficacy in prevention and management of PCOS. Beneficial effects of metformin were not as significant as that of the combined herbal extract with AVG. Also, administration of metformin brought about aggressiveness in the rats. Statin, on the other hand, showed a lipid lowering effect but did not act to manage the PCOS condition.
AVG has not only been confirmed to restore glucose metabolism in PCOS rats but has also provided strong evidence of its anti-hyperlipidemic effects by reversing the dyslipidemic status in these rats. Preliminary phytochemical experiments suggest that the phytosterols and polyphenols in AVG are the active components in controlling the hyperglycemic condition. However, detailed studies still need to be done in order to identify the active ingredient that could help in management of abnormal serum lipid levels in the PCOS condition. Effect of AVG on dyslipidemia in a non-PCOS model also needs to be checked in order to test its efficacy as an anti-hyperlipidemic therapeutic agent.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Padamnabhi S. Nagar (Botany Department, The M.S. University of Baroda, India) for his expertise and assistance in the preparation of the AVG. This work was funded by Department of Biotechnology (DBT), Ministry of Science and Technology, Government of India (DBT—BT/PR11279/GBD/27/154/2008).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Diamanti-Kandarakis E, Kouli CR, Bergiele AT, Filandra FA, Tsianateli TC, Spina GG, et al. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: Hormonal and metabolic profile. J Clin Endocrinol Metab. 1999;84:4006–11. doi: 10.1210/jcem.84.11.6148. [DOI] [PubMed] [Google Scholar]
- 2.Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: A prospective study. J Clin Endocrinol Metab. 1998;83:3078–82. doi: 10.1210/jcem.83.9.5090. [DOI] [PubMed] [Google Scholar]
- 3.Asuncion M, Calvo RM, San Millan JL, Sancho J, Avila S, Escobar-Morreale HF. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab. 2000;85:2434–8. doi: 10.1210/jcem.85.7.6682. [DOI] [PubMed] [Google Scholar]
- 4.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–9. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 5.Nidhi R, Padmalatha V, Nagarathna R, Amritanshu R. Prevalence of polycystic ovarian syndrome in indian adolescents. J Pediatr Adolesc Gynecol. 2011;24:223–7. doi: 10.1016/j.jpag.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Azziz R. Controversy in clinical endocrinology: Diagnosis of polycystic ovarian syndrome: The Rotterdam criteria are premature. J Clin Endocrinol Metab. 2006;91:781–5. doi: 10.1210/jc.2005-2153. [DOI] [PubMed] [Google Scholar]
- 7.Guzick DS. Polycystic ovary syndrome. Obstet Gynecol. 2004;103:181–93. doi: 10.1097/01.AOG.0000104485.44999.C6. [DOI] [PubMed] [Google Scholar]
- 8.Third Report of the National Cholesterol Education Program (NCEP). Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 9.Wild RA. Long-term health consequences of PCOS. Hum Reprod Update. 2002;8:231–41. doi: 10.1093/humupd/8.3.231. [DOI] [PubMed] [Google Scholar]
- 10.Wild RA, Rizzo M, Clifton S, Carmina E. Lipid levels in polycystic ovary syndrome: Systematic review and meta-analysis. Fertil Steril. 2011;95:1073–9. doi: 10.1016/j.fertnstert.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 11.Matsuzawa Y, Shimomura I, Nakamura T, Keno Y, Tokunaga K. Pathophysiology and pathogenesis of visceral fat obesity. Ann N Y Acad Sci. 1995;748:399–406. doi: 10.1111/j.1749-6632.1994.tb17336.x. [DOI] [PubMed] [Google Scholar]
- 12.Sathyapalan T, Kilpatrick ES, Coady AM, Atkin SL. Atorvastatin pretreatment augments the effect of metformin in patients with polycystic ovary syndrome (PCOS) Clin Endocrinol. 2010;4:566–8. doi: 10.1111/j.1365-2265.2009.03678.x. [DOI] [PubMed] [Google Scholar]
- 13.Kazerooni T, Shojaei-Baghini A, Dehbashi S, Asadi N, Ghaffarpasand F, Kazerooni Y. Effects of metformin plus simvastatin on polycystic ovary syndrome: A prospective, randomized, double-blind, placebo-controlled study. Fertil Steril. 2010;6:2208–13. doi: 10.1016/j.fertnstert.2009.11.045. [DOI] [PubMed] [Google Scholar]
- 14.Sangraula H, Paudel KR, Sharma M. Metformin and troglitazone in the treatment of female infertility associated with polycystic ovarian syndrome. JNMA J Nepal Med Assoc. 2009;176:335–9. [PubMed] [Google Scholar]
- 15.Rizzo M, Berneis K, Carmina E, Rini GB. How should we manage atherogenic dyslipidemia in women with polycystic ovary syndrome? Am J Obstet Gynecol. 2008;198:28. doi: 10.1016/j.ajog.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann IS, Roa M, Torrico F, Cubeddu LX. Ondansetron and metformin-induced gastrointestinal side effects. Am J Ther. 2003;10:447–51. doi: 10.1097/00045391-200311000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Corrao G, Zambon A, Bertù L, Botteri E, Leoni O, Contiero P. Lipid lowering drugs prescription and the risk of peripheral neuropathy: An exploratory case-control study using automated databases. J Epidemiol Community Health. 2004;58:1047–51. doi: 10.1136/jech.2003.013409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golomb BA, Kwon EK, Koperski S, Evans MA. Amyotrophic lateral sclerosis-like conditions in possible association with cholesterol-lowering drugs: An analysis of patient reports to the University of California, San Diego (UCSD) statin effects study. Drug Saf. 2009;32:649–61. doi: 10.2165/00002018-200932080-00004. [DOI] [PubMed] [Google Scholar]
- 19.Vasu VT, Modi H, Thaikoottathil JV, Gupta S. Hypolipidaemic and antioxidant effect of Enicostemma littorale Blume aqueous extract in cholesterol fed rats. J Ethnopharmacol. 2005;101:277–82. doi: 10.1016/j.jep.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Ram A, Lauria P, Gupta R, Kumar P, Sharma VN. Hypocholesterolaemic effects of Terminalia arjuna tree bark. J Ethnopharmacol. 1997;55:165–9. doi: 10.1016/s0378-8741(96)01493-6. [DOI] [PubMed] [Google Scholar]
- 21.Sortibrán AC, Téllez MG, Ocotero VM, Carballo-Ontiveros MA, García AM, Valdés RJ, et al. Chronic toxicity, genotoxic assay, and phytochemical analysis of four traditional medicinal plants. J Med Food. 2011;14:1018–22. doi: 10.1089/jmf.2010.0178. [DOI] [PubMed] [Google Scholar]
- 22.Pérez YY, Jiménez-Ferrer E, Zamilpa A, Hernández-Valencia M, Alarcón-Aguilar FJ, Tortoriello J, et al. Effect of a polyphenol-rich extract from Aloe vera gel on experimentally induced insulin resistance in mice. Am J Chin Med. 2007;35:1037–46. doi: 10.1142/S0192415X07005491. [DOI] [PubMed] [Google Scholar]
- 23.Rajasekaran S, Ravi K, Sivagnanam K, Subramanian S. Beneficial effects of aloe vera leaf gel extract on lipid profile status in rats with streptozotocin diabetes. Clin Exp Pharmacol Physiol. 2006;33:232–7. doi: 10.1111/j.1440-1681.2006.04351.x. [DOI] [PubMed] [Google Scholar]
- 24.Habeeb F, Stables G, Bradbury F, Nong S, Cameron P, Plevin R, et al. The inner gel component of Aloe vera suppresses bacterial-induced pro-inflammatory cytokines from human immune cells. Methods. 2007;42:388–93. doi: 10.1016/j.ymeth.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Anilakumar KR, Sudarshanakrishna KR, Chandramohan G, Ilaiyaraja N, Khanum F, Bawa AS. Effect of Aloe vera gel extract on antioxidant enzymes and azoxymethane-induced oxidative stress in rats. Indian J Exp Biol. 2010;48:837–42. [PubMed] [Google Scholar]
- 26.Maharjan R, Nagar PS, Nampoothiri L. Effect of Aloe vera gel extract on Letrozole induced Polycystic Ovarian Syndrome (PCOS) Rat Model. J Ayurveda Integr Med. 2010;4:273–9. doi: 10.4103/0975-9476.74090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kafali H, Iriadam M, Ozardali I, Demir N. Letrozole induced polycystic ovaries in the Rat: A new model for Cystic Ovarian Disease. Arch Med Res. 2004;35:103–8. doi: 10.1016/j.arcmed.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Ali M, Eftekhar M, Taheripanah R. Effect of administration of metformin on lipid profile changes and insulin resistance in patients with PCOS. Middle East Fertil Soc J. 2007;12:174–8. [Google Scholar]
- 29.Dostal LA, Whitfield LR, Anderson JA. Fertility and general reproduction studies in rats with the HMG-CoA reductase inhibitor, atorvastatin. Fundam Appl Toxicol. 1996;32:285–92. doi: 10.1006/faat.1996.0132. [DOI] [PubMed] [Google Scholar]
- 30.Buchanan TA, Sipos GF, Gadalah S, Yip KP, Marsh DJ, Hsueh W, et al. Glucose tolerance and insulin action in rats with renovascular hypertension. Hypertension. 1991;18:341–7. doi: 10.1161/01.hyp.18.3.341. [DOI] [PubMed] [Google Scholar]
- 31.Folch J, Lees M, Stanley GH Sloane. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 32.Zlatkis A, Zak B, Boyle AJ. A new method for the direct determination of serum cholesterol. J Lab Clin Med. 1953;41:486–92. [PubMed] [Google Scholar]
- 33.Rao AV, Ramakrishnan S. Indirect assessment of HMG-CoA Reductase (NADPH) activity in liver. Clin Chem. 1975;21:1523–5. [PubMed] [Google Scholar]
- 34.Hitz J, Steinmetz J, Siest G. Plasma lecithin cholesterol acyl transferase – Reference values and effects of xenobiotics. Clin Chim Acta. 1983;133:85–93. doi: 10.1016/0009-8981(83)90023-2. [DOI] [PubMed] [Google Scholar]
- 35.Yen SS. The polycystic ovary syndrome. Clin Endocrinol (Oxf) 1980;12:177–208. doi: 10.1111/j.1365-2265.1980.tb02132.x. [DOI] [PubMed] [Google Scholar]
- 36.Azziz R, Sanchez LA, Knochenhauer ES, Moran C, Lazenby J, Stephens KC, et al. Androgen excess in women: Experience with over 1000 consecutive patients. J Clin Endocrinol Metab. 2004;89:453–62. doi: 10.1210/jc.2003-031122. [DOI] [PubMed] [Google Scholar]
- 37.Liepa GU, Sengupta A, Karsies D. Polycystic ovary syndrome (PCOS) and other androgen excess related conditions: Can change in dietary intake make a difference? Nutr Clin Pract. 2008;23:63. doi: 10.1177/011542650802300163. [DOI] [PubMed] [Google Scholar]
- 38.Bjorntorp P. The regulation of adipose tissue distribution in humans. Int J Obes Relat Metab Disord. 1996;20:291–302. [PubMed] [Google Scholar]
- 39.Conway GS, Agrawal R, Betteridge DJ, Jacobs HS. Risk factors for coronary artery disease in lean and obese women with the polycystic ovary syndrome. Clin Endocrinol (Oxf) 1992;37:119–25. doi: 10.1111/j.1365-2265.1992.tb02295.x. [DOI] [PubMed] [Google Scholar]
- 40.Von Eckardstein A. Androgens, cardiovascular risk factors, and atherosclerosis. In: Nieschlag E, Behre HM, editors. Testosterone: Action, Deficiency, Substitution. 2nd ed. Berlin Heidelberg New: Springer; 1998. pp. 229–58. [Google Scholar]
- 41.Croston GE, Milan LB, Marschke KB, Reichman M, Briggs MR. Androgen receptor-mediated antagonism of estrogen-dependent low density lipoprotein receptor transcription in cultured hepatocytes. Endocrinology. 1997;138:3779–86. doi: 10.1210/endo.138.9.5404. [DOI] [PubMed] [Google Scholar]


