Abstract
Objective:
This study was undertaken to study in vitro H+ -K+ ATPase inhibitory potential of methanolic extract of Cissus quadrangularis Linn.
Materials and Mathods:
Total phenolic and flavonoid contents from extract was quantified and H+ -K+ ATPase inhibition assay was performed in presence of different concentrations of standard (omeprazole) and methanol extract.
Results:
Extract showed significant (*P < 0.05) proton pump inhibitory activity in the goat gastric mucosal homogenate which was comparable to standard.
Conclusions:
These findings showed that methanolic extract of C. quadrangularis Linn. is potent inhibitor of proton pump.
Keywords: Cissus quadrangularis Linn., H+ -K+ ATPase inhibition assay, in vitro
INTRODUCTION
Gastroduodenal ulcers are one of the most common problems faced by populace worldwide. Hyperchlorhydria is a condition characterized by uncontrolled hypersecretion of hydrochloric acid from parietal cells of gastric mucosa through proton pump.[1] A large number of therapeutic interventions are available for treatment of gastric ulcers, such as proton pump inhibitors, anticholinergics, histamine H2 receptor antagonist, antacids and anticholinergics. These drugs suffer from side effects including, allergic reaction, arrythmia, gynecomastia, etc.[2,3]
Nature serves to be a rich repository of medicinal plants[4–6] and macrofungi,[7] from time immortal man is using herbs for health benefits. A large number of chemical compounds from medicinal herbs express antiulcer activity.[8,9] Many plants in folk medicine are known for their antiulcer potential. Cissus quadrangularis Linn. (Vitaceae) commonly known as ‘bone setter’, is frequently used as a common food item in India.[10] The stout fleshy quandrangular stem of C. quadrangularis Linn. is an edible plant found throughout the hotter parts of India, Malaya, West Africa, and Sri Lanka.[11] The stem is used for the treatment of eye and ear diseases, irregular menstruation, asthma, piles, and tumors, fractures of bones, wounds, and scurvy.[12]
A large number of phytoconstituents such as α-amyrin and α-amyrone, β-sitosterol, ketosteroid, oxo-steroid, onocer-7-ene-3α, 21β-diol and onocer-7-ene3β, 21α-diol, stilbene derivatives, and quercitin are found in it,[13] herb is also rich in β-carotene.[14] The extract of C. quadrangularis Linn. was reported to show antiulcer and cytoprotective property in various experimental ulcer models,[15,16] but still its activity on enzyme H+ -K+ ATPase remains to be unknown. On the basis of these observations, the aim of the study was to determine the activity of plant extract on enzyme H+ -K+ -ATPase.
MATERIALS AND METHODS
Chemicals
Folin-Ciocalteu′s phenol reagent, aluminum trichloride, Tris-HCl were purchased from Sigma, Germany. MgCl2 , KCl, methanol, and ATP were purchased from Loba Chemie, India.
Plant material
The tubers of C. quadrangularis Linn were purchased from a local market of Jabalpur and identified and authenticated by Dr. A.B. Tiwari, Senior Scientist and Botanist, Department of Crop and Physiology, Jawaharlal Nehru Agricultural University, Jabalpur.
Extraction of plant material
The tubers of C. quadrangularis Linn. were dried for a week in shade and powdered. A 100 g of the powdered drug was extracted with 95% of methanol in asoxhlet apparatus. The extract was filtered using a muslin cloth and then with a filter paper (Whatmann No. 4) and concentrated at a rotary vacuum evaporator. The dried extract was stored at 4°C until use.
Phytochemical analysis
The phytochemical analysis of the plant was carried out by the standard methods.[17,18]
Phytoanalytical studies
Determination of total phenolic compounds
Total soluble phenolic compounds in the HEE were determined with the Folin-Ciocalteu reagent according to the method of Slinkard et al.,[19] using pyrocatechol as a standard phenolic compound. The total concentration of phenolic compounds in the extract determined as micrograms of pyrocatechol equivalent by using an equation that was obtained from a standard pyrocatechol graph:
Absorbance = 0.0054×total phenols [pyrocatechol equivalent (μg)] -0.0058.
Assay for the total flavonoid content
The total flavonoid content was determined using the method given elsewhere.[20,21] The concentrations of flavonoid compounds were calculated according to the following equation that was obtained from the standard quercetin graph:
Absorbance = 0.0338 quercetin (μg) - 0.0002; R2 = 0.9998
Assay of H+ -K+ ATPase activity
Proton potassium ATPase was prepared from mucosal scrapings of goat by the method reported by Cheon et al., with necessary modifications.[22] Stomach from freshly slaughtered goats was washed gently with tap water. The mucosal layer of fundus was scrapped and homogenized in ice-cold phosphate buffer, pH 7.4. The homogenate was centrifuged for 20 min at 18,000 rpm. The supernatant so obtained was recentrifuged for 60 min at 100,000 rpm. The pellet was resuspended in homogenisation buffer. Ficoll-sucrose discontinuous density gradient centrifugation was utilized to prepare H+K+ATPase. Protein was determined by the method of Lowry et al.[23]
Assay of H+ -K+ -ATPase
Different concentrations of the extract 10-50 μg/ml were incubated in the reaction mixture (40 mM Tris-HCl buffer, pH 7.4, containing 2 mM MgC12 and 10 μg membrane protein) to make a volume of 1 ml. Then, 2 mM ATP Tris salt was utilized to start the reaction, this preparation was incubated for 20 min for 37°C. The reaction was terminated by adding 1 ml of ice-cold trichloroacetic acid (10% v/v). The H+ -K+ ATPase activity was assayed[24] in the presence and the absence of different doses of the extract and omeprazole. The amount of inorganic phosphate released from ATP was determined spectrophotometrically at 400 nm.
Statistical analysis
The results are expressed as mean±standard error of mean. Experiments were always performed in triplicates. Statistical comparison was performed using analysis of variance (ANOVA) followed by Bonferroni's test (*P<0.05).
RESULTS
Phytochemical analysis
The results of the preliminary phytochemical analysis extract of C. quadrangularis Linn. showed abundant presence of alkaloids, terpenoids, saponins, tannins, and phenols.
Phytoanalytical studies
Screening of phenolic compounds with NaOH and FeCl3 revealed their presence and quantification was done. The total amount of the phenolic content present in the extract was found to be 715.2 ± 2.57 mg PE (pyrocatechol equivalent)/100g. By using the standard curve of quercetin (R2 = 0.9998), the total flavonoid content of the extract was found to be 169.2 ± 1.97 mg QE (Quercetin equivalent)/100g. The total alkaloidal content in the extract was found to be 17.2 mg/kg dry basis.
H+ -K+ ATPase activity
The extract showed significant (*P<0.05) proton pump inhibitory activity in the goat gastric mucosal homogenate. The inhibitory activity was concentration dependent, and the results were comparable to standard drug omeprazole. In vitro, the methanolic extract of C. quadrangularis Linn. potently reduced the hydrolysis of ATP by the goat gastric ATPase with IC50 of 38 μg/mL. Omeprazole (10-50μg/mL) used as positive control reduced H+ -K+ ATPase activity with an IC50 = 26 μg/mL [Figure 1].
Figure 1.
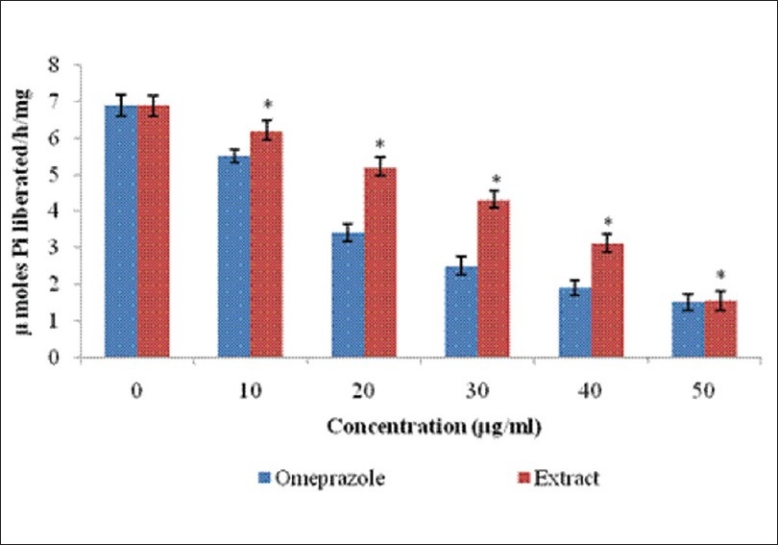
Effect of the ethanol extract of C. quadrangularis and omeprazole on H+-K+ ATPase activity: H+-K+ ATPase activity was measured with 10-50 µg/mL of the extract and omeprazole. Experiments were always performed in triplicates. The results are expressed as mean ± standard error of mean. Statistical comparison was performed using analysis of variance (ANOVA) followed by the Bonferroni′s test (*P< 0.05)
DISCUSSION
A large number of phytochemicals such as tannins, flavonoids, tannins, and triterpenes from plants have previously demonstrated potential antiulcerogenic activity.[25,26] Catechin and epicatechin are reported to be potent non-competitive inhibitors of H+ -K+ -ATPase.[27] Plant polyphenols and flavonoids are used in treatment of gastric ulcers.[27] Flavonoids are capable of defending gastric damage. Flavonoids are excellent antioxidant; some of them are capable of enhancing mucosal content of prostaglandins. Apart from this, they preserve capillary integrity and restore normal function of mucus membrane.[28]
Quantitative estimation on the plant extract showed the presence of phytoconstituents such as phenolics and flavonoids. In this study, the possible mechanism of protection to gastric ulcer was evaluated. H+ -K+ ATPase is a key enzyme in inducing acidity; in this study, the ability of methanolic extract to inhibit H+ -K+ ATPase in vitro isolated from goat stomach was studied. In vitro studies are considered necessary in order to evaluate the potential of phytochemicals to enter in the cell and additionally to exemplify their interaction with the gastric ATPase. Enzyme H+ -K+ ATPase is an important enzyme system located on apical secretory membrane of partial cell. In this study, dose-dependent inhibition of enzyme by omeprazole and extract was observed, suggesting that the C. quadrangularis Linn. extract was significantly (*P<0.05) able to inhibit enzyme H+ -K+ ATPase, responsible for the secretion of acid and effect was comparable to omeprazole.
Therefore, it could be concluded that the inactivation of H+ -K+ ATPase is the major gastroprotective mechanisms of action of C. quadrangularis Linn., which indicates its protective role against inhibiting gastric proton pump and opens a door for isolation and characterization of active compounds responsible for it.
ACKNOWLEDGMENTS
The authors are thankful to Dr. A.B. Tiwari for authenticating the sample of C. quadrangularis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Sachs G, Maton PN, Wallmark B. Pharmacology of Peptic ulcer disease. In: Collin MJ, Benjamin SB, editors. New York: Springer Verlag; 1991. Pharmacology of Peptic ulcer disease. [Google Scholar]
- 2.Chan FK, Leung WK. Peptic ulcer disease. Lancet. 2002;360:933–41. doi: 10.1016/s0140-6736(02)11030-0. [DOI] [PubMed] [Google Scholar]
- 3.Schöll I, Untersmayr E, Bakos N, Roth-Walter F, Gleiss A, Boltz-Nitulescu G, et al. Antiulcer drugs promote oral sensitization and hypersensitivity to hazelnut allergens in BALB/c mice and humans. Am J Clin Nutr. 2005;81:154–60. doi: 10.1093/ajcn/81.1.154. [DOI] [PubMed] [Google Scholar]
- 4.Pardhi P, Jain AP, Ganeshpurkar A, Rai G. Anti-microbial, anti-oxidant and anthelmintic activity of crude extract of Solanum xanthocarpum. Pharmacogn J. 2010;2:400–4. [Google Scholar]
- 5.Bhadoriya SS, Ganeshpurkar A, Narwaria J, Rai G, Jain AP. Tamarindus indica: Extent of explored potential. Pharmacogn Rev. 2011;5:73–81. doi: 10.4103/0973-7847.79102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sen S, Chakraborty R, De B, Mazumder J. Plants and phytochemicals for peptic ulcer: An overview. Pharmacogn Rev. 2009;3:270–9. [Google Scholar]
- 7.Ganeshpurkar A, Rai G, Jain AP. Medicinal mushrooms: Towards a new horizon. Pharmacogn Rev. 2010;4:127–35. doi: 10.4103/0973-7847.70904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gadekar R, Singour PK, Chaurasiya PK, Pawar RS, Patil UK. A potential of some medicinal plants as an antiulcer agents. Pharmacogn Rev. 2010;4:136–46. doi: 10.4103/0973-7847.70906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borrelli F, Izzo AA. The plant kingdom as a source of antiulcer remedies. Phytother Res. 2000;14:581–91. doi: 10.1002/1099-1573(200012)14:8<581::aid-ptr776>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 10.Sivarajan VV, Balachandran I. New Delhi: Oxford and India Book Publishing Co. Pvt; 1994. Ayurvedic drugs and their plant sources. [Google Scholar]
- 11.Udupa KN, Chaturvedi GN, Tripathi SN. Vol. 12. Varanasi: Banaras Hindu University; 1970. Advances in research in Indian medicine. [Google Scholar]
- 12.Kritikar KR, Basu BD. 3rd ed. Allahabad, India: 2000. Indian Medicinal Plants. [Google Scholar]
- 13.Attawish A, Chavalttumrong D, Chivapat S, Chuthaputti S, Rattarajarasroj S, Punyamong S. Subchronic toxicity of Cissus quadrangularis Linn. Songklanakarin J Sci Tech. 2003;24:39–51. [Google Scholar]
- 14.Murthy KN, Vanitha A, Swamy MM, Ravishankar GA. Antioxidant and antimicrobial activity of Cissus quadrangularis Linn. J Med Food. 2003;6:99–105. doi: 10.1089/109662003322233495. [DOI] [PubMed] [Google Scholar]
- 15.Jainu M, Devi CS. Potent antiulcerogenic activity of methanol extract of Cissus quadrangularis Linn by antioxidative mechanism. J Clin Biochem Nutr. 2003;34:43–7. [Google Scholar]
- 16.Jainu M, Devi CS. Effect of Cissus quadrangularis Linn on gastric mucosal defensive factors in experimentally induced gastric ulcer-a comparative study with sucralfate. J Med Food. 2004;7:372–6. doi: 10.1089/jmf.2004.7.372. [DOI] [PubMed] [Google Scholar]
- 17.Harborne JB. New York: Chapman and Hall; 1983. Phytochemical Method: A guide to modern techniques of plants analysis. [Google Scholar]
- 18.Trease EG, Evans WC. London: Bailliere Tindal; 1989. Textbook of Pharmacognosy. [Google Scholar]
- 19.Slinkard K, Singleton VL. Total phenol analyses: Automation and comparison with manual methods. Am J Enol Vitic. 1977;28:49–55. [Google Scholar]
- 20.Dewanto V, Wu XZ, Adom KK, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem. 2002;50:3010–4. doi: 10.1021/jf0115589. [DOI] [PubMed] [Google Scholar]
- 21.Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–9. [Google Scholar]
- 22.Cheon HG, Lim H, Lee DH. Biochemical properties of a newly synthesized H+/K+ ATPase inhibitor, 1-(2-methyl-4-methoxyphenyl)-4- [(3-hydroxypropyl) amino]-6-methyl-2,3-dihydropyrrolo[3,2-c] quinoline. Eur J Pharmacol. 2001;41:181–6. doi: 10.1016/s0014-2999(00)00919-5. [DOI] [PubMed] [Google Scholar]
- 23.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 24.Ricardo RC, Cristiane HB, Dagoberto S, Elizabeth EM, Frederick CK, Rosa IS, et al. Inhibition of gastric H + -K + ATPase activity by flavonoids, coumarins and xanthones from mexican medicinal plants. J Ethnopharmacol. 2006;105:167–72. doi: 10.1016/j.jep.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 25.Souza-Formigoni ML, Oliveira MG, Monteiro MG, da Silveira-Filho NG, Braz S, Carlini EA. Antiulcerogenic effects of two Maytenus species in laboratory animals. J Ethnopharmacol. 1991;34:21–7. doi: 10.1016/0378-8741(91)90185-g. [DOI] [PubMed] [Google Scholar]
- 26.Jorge RM, Leite JP, Oliveira AB, Tagliati CA. Evaluation of antinociceptive, anti-inflammatory and antiulcerogenic activities of Maytenus ilicifolia. J Ethnopharmacol. 2004;94:93–100. doi: 10.1016/j.jep.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 27.Borrelli F, Izzo AA. The plant kingdom as a source of anti-ulcer remedies. Phytother Res. 2000;14:581–91. doi: 10.1002/1099-1573(200012)14:8<581::aid-ptr776>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 28.Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry. 2000;55:481–504. doi: 10.1016/s0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]


