Abstract
Objectives:
To evaluate short- and long-term effects of Cinnamomum zeylanicum on food consumption, body weight, glycemic control, and lipids in healthy and diabetes-induced rats.
Materials and Methods:
The study was conducted in two phases (Phase I and Phase II), using Sprague-Dawley rats in four groups. Phase I evaluated acute effects on fasting blood glucose (FBG) (Groups 1 and 2) and on post-oral glucose (Groups 3 and 4) blood glucose. Groups 1 and 3 received distilled-water and Groups 2 and 4 received cinnamon-extracts. Phase II evaluated effects on food consumption, body weight, blood glucose, and lipids over 1 month. Group A (n = 8, distilled-water) and Group B (n = 8, cinnamon-extracts) were healthy rats, while Group C (n = 5, distilled-water) and Group D (n = 5, cinnamon-extracts) were diabetes-induced rats. Serum lipid profile and HbA1c were measured on D-0 and D-30. FBG, 2-h post-prandial blood glucose, body weight, and food consumption were measured on every fifth day.
Results:
Phase I: There was no significant difference in serial blood glucose values in cinnamon-treated group from time 0 (P > 0.05). Following oral glucose, the cinnamon group demonstrated a faster decline in blood glucose compared to controls (P < 0.05). Phase II: Between D0 and D30, the difference in food consumption was shown only in diabetes-induced rats (P < 0.001). Similarly, the significant difference following cinnamon-extracts in FBG and 2-h post-prandial blood glucose from D0 to D30 was shown only in diabetes-induced rats. In cinnamon-extracts administered groups, total and LDL cholesterol levels were lower on D30 in both healthy and diabetes-induced animals (P < 0.001).
Conclusions:
C. zeylanicum lowered blood glucose, reduced food intake, and improved lipid parameters in diabetes-induced rats.
Keywords: Blood glucose, Ceylon cinnamon, Cinnamomum zeylanicum, diabetes mellitus, lipids, Sprague-Dawley rats
INTRODUCTION
Diabetes mellitus is a leading cause of morbidity and mortality worldwide, with an estimated 80% of the world population with diabetes living in developing countries.[1] Most patients with the disease have type 2 diabetes to which the South Asians are known to have an increased predisposition.[2] The causes of type 2 diabetes are multi-factorial, and the diet plays an important role on its’ incidence, severity, and management.[3] Hence, studies have frequently focused on dietary components beneficial in the prevention and treatment of diabetes. Recent studies have demonstrated that herbal products have beneficial effects in patients with diabetes by improving glucose and lipid metabolism, antioxidant status, and capillary function.[4] Cinnamon is one such a dietary component that has shown to have biologically active substances with insulin-mimetic properties. In vitro[5,6] and in vivo[7,8] studies have shown that cinnamon enhances glucose uptake by activating the insulin receptor kinase activity, auto-phosphorylation of the insulin receptor, and glycogen synthase activity.
Cinnamon, the inner bark of a tropical evergreen tree has two main types, Ceylon cinnamon (Cinnamomum zeylanicum Blume) and Chinese Cassia (Cinnamomum aromaticum Ness) and which when dried, rolls into a tubular form known as a quill. Cinnamon is available in either its whole quill form (cinnamon sticks) or as ground powder. In native Ayurvedic medicine, cinnamon is considered a remedy for respiratory, digestive, and gynecological ailments. Recent studies emerging from western countries have shown many potentially beneficial health effects of cinnamon such as anti-inflammatory properties, anti-microbial activity, blood glucose control, reducing cardiovascular disease, boosting cognitive function, and reducing risk of colonic cancer.[9–11] Sri Lanka is a middle-income country in the South Asian region produces the largest quantity of C. zeylanicum and the best quality cinnamon. C. zeylanicum, also known as Ceylon cinnamon (the source of its Latin name, zeylanicum) or ‘true cinnamon’, is indigenous to Sri Lanka. One important difference between ‘true’ cinnamon and the ‘cassia’ cinnamon is their coumarin content.[12] Coumarins are naturally occurring plant compounds with strong anticoagulant properties. The coumarin content in Ceylon cinnamon appears to be very small to cause health risks, whereas the coumarin level in C. aromaticum appears to be much higher and may pose health risks if consumed in higher quantity on a regular basis.[12] In addition, coumarins also have potentially toxic effects on the liver.[13]
Previous studies have explored the anti-diabetic effects of Cinnamon cassia (C. aromaticum) extract in vivo and in vitro.[5–8] However, there is a relative lack of knowledge regarding the effects of the indigenous species of Sri Lankan Cinnamon (C. zeylanicum) on blood glucose and lipids. The aim of this study was to evaluate the short- and medium-term effects of C. zeylanicum extracts on food consumption, body weight, blood glucose and glycemic control, and lipids in both healthy and diabetes-induced rats as an animal model.
MATERIALS AND METHODS
The study was conducted in two phases from June to August 2010. Ethical approval was obtained from the Ethics Review Committee of the Faculty of Medicine, University of Colombo, Sri Lanka.
Preparation of cinnamon extract
The cinnamon extracts were prepared at the Industrial Technology Institute (ITI), Colombo, Sri Lanka. A cinnamon sample was obtained from the collection center of S.D.S. Spices (Pvt) Ltd, Kosgoda, Sri Lanka. The voucher specimen is deposited at the Herbal Technology Section of Industrial Technology Institute under voucher specimen number HTS-CIN-01 (Ham), and was identified by Dr Sirimal Premakumara. The cinnamon was extracted to distilled water from the stem barks of C. zeylanicum using a Soxhlet apparatus and the resulting hot water extract was freeze-dried to obtain crude water extract (yield 8.3% w/w dry weight basis). This solid freeze-dried extract in a dosage form in distilled water was administered to experimental rats. The dosage for rats was calculated according to the body surface area as shown below, using the human dosage (6 g) obtained from previous studies.[9] The daily cinnamon dose given for a 200 g rat was 120 mg (600 mg/kg) in 1 ml of distilled water administered via a metal oro-gastric tube. Dose (mg/kg) = Human dose (mg/kg) × [Human Km factor/Rat Km factor][14]
Phase I
The aim of Phase I was to determine the short-term effects of C. zeylanicum on blood glucose in the unfed and post-prandial state. Healthy Sprague-Dawley rats having a mean body weight of 190 ± 25 g and aged 3–4 months (n = 32, male:female = 1:1) were used as the animal model. They were divided into four equal groups of eight animals each. In Groups 1 and 2 blood glucose levels in the unfed state were measured, while in Groups 3 and 4 blood glucose levels following a standard oral glucose load (1.25 g/kg) was measured.
In all four groups, the animals were kept fasting for 12 h overnight. In Groups 1 and 2 the fasting blood glucose (FBG) value was determined at ‘0’ h and the cinnamon extract and distilled water, respectively, were administered immediately. Subsequently the blood glucose levels in the unfed state were measured at 0.5, 1, 2, 4, 8, 12, and 24 h. Similarly in Groups 3 and 4 the FBG values were determined at ‘0’ h and an oral glucose load was administered. The cinnamon and distilled water were administered after 0.5 h following the glucose load with measuring of blood glucose values at that time. Subsequently blood glucose levels were measured at 1, 2, 4, 8, 12 and 24 h. In all four groups, the animals were fed at 24 h and kept fasting for 12 h for repetition of this cycle the next day. This protocol was carried out for three consecutive cycles and mean blood glucose values were analysed. The percentage reductions of the blood glucose values of each hour from the respective FBG of Groups 1 and 2 were compared. The percentage reductions were calculated as follows:
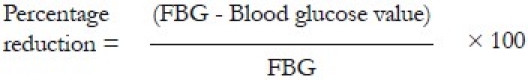
Phase II
The aim of Phase II was to determine the medium-term effects of C. zeylanicum on food consumption, body weight, blood glucose and glycemic control, and lipids. Group A (n = 8) and Group B (n = 8) were healthy Sprague-Dawley rats, while Group C (n = 5) and Group D (n = 5) were diabetes-induced Sprague-Dawley rats. In groups A and B distilled water and cinnamon were administered, respectively, on a daily basis for a period of 1 month. Similarly, in Groups C and D distilled water and cinnamon were administered, respectively, for 1 month. In all four groups, the animals were kept fasting overnight for a period of 12 h, and cinnamon or distilled water were administered, followed by a 30 min interval prior to commencing of the standard daily pellet feed. In all four groups’ blood for estimation of serum total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, and HbA1c were drawn on day 0 and day 30 from the tail vein of the rats. FBG and 2 h post-prandial blood glucose were measured from day 0 every fifth day till day 30.
Induction of diabetes
In Groups C and D (n = 16), diabetes was induced by a single intravenous injection of streptozotocin (Sigma Chemicals Co, St. Louis, MO, USA) to the tail vein at a dose of 75 mg/kg body weight. Confirmatory FBG tests were performed after 14 days of injection and animals with a FBG of >200 mg/dl (six females and four males) after induction were randomly allocated to Groups C (n = 5) and D (n = 5) with an equal gender distribution.
Measurements: Biochemical and physiological
The blood glucose values were determined using an Optium Xceed glucometer and strips (Abbott Diabetes Care Ltd, Oxon, UK). A cross-validation to establish the accuracy of the glucometer was done by comparing with 10 laboratory plasma glucose tests. Blood for estimation of lipid parameters were collected to plain tubes while for estimation of HbA1c blood was collected to K3 EDTA tubes. HbA1c was determined by the HPLC Bio-Rad variant method (Bio-Rad, USA), cholesterol by the CHOD-PAP method (Bio System S.A., Barcelona, Spain), HDL cholesterol by an enzymatic colorimetric direct HDL assay (Analyticon Biotechnologies AG, Lichtenfels, Germany), LDL cholesterol by a homogeneous enzymatic direct LDL assay (Analyticon Biotechnologies AG, Lichtenfels, Germany) and triglycerides by the GPO-PAP method (Bio System S.A., Barcelona, Spain).
During the study period, the animals were fed with a standard pellet diet and water daily with added vitamins as required. The animals were housed in separate cages in a barrier procedure room at ambient temperature with a relative humidity of 40–60% and a 12 h light/dark cycle. The daily food and water consumption was measured, while body weight was measured on every fifth day.
Determination of the effects on organs
On completion of Phase II, a histo-pathological study was conducted on 3 animals from each group. The selected animals were dissected under ether anesthesia by a trained researcher and the liver (10% formalin preservative), both kidneys (modified Bouin's solution) and pancreas (10% formalin preservative) were harvested to determine the effects of cinnamon on these organs. The liver was examined for spotty/zonal liver cell necrosis, hepatocyte swelling, fatty changes, and inflammation. In the kidenys, tubular epithelial cell damadge, presence of casts/crystals, glomular changes, and inflammation were examined. The pancreas was examined for cell necrosis, inflammation, islet cell hyperplasia, and/or atrophy.
Statistical analysis
Data from the experiments are presented as mean ± standard deviation. Statistical analysis was performed using the Statistical Package for Social Science (SPSS) software for windows version 14 (SPSS Inc., Chicago, Illinois, USA). In all statistical analysis a P values of <0.05 were considered significant.
RESULTS
Phase I
The mean body weight of animals in Groups 1–4 were 185 ± 12 g, 192 ± 8 g, 180 ± 14 g and 188 ± 10 g, respectively. There was no statistically significant difference in the mean body weights of the respective groups. There were no statistically significant differences observed between the mean blood glucose values at each hour of Groups 1 and 2 [Table 1]. When comparing the percentage reductions of the blood glucose values of each hour from the respective FBG of Groups 1 and 2 only the cinnamon administered group demonstrated a greater mean percentage reduction at 0.5, 1 and 2 h. However, this difference was not statistically significant [Figure 1].
Table 1.
Effect of Cinnamomum zeylanicum on fasting blood glucose and post-oral glucose load
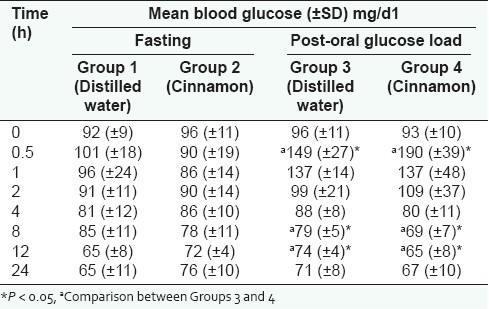
Figure 1.
Percentage reduction of hourly blood glucose values from fasting blood glucose in Groups 1 (distilled water) and 2 (cinnamon extract)
When a standard loading dose of glucose was administered to Groups 3 and 4, in both groups the peak plasma glucose following the standard oral glucose load was recorded at 0.5 h. When the test substance (cinnamon or distilled water) was administered to the respective groups at 0.5 h, Group 4 (cinnamon) demonstrated a faster decline in blood glucose in comparison to Group 3 (distilled water) [Figure 2]. The mean percentage reduction in blood glucose from 0.5 to 1 h in Groups 3 and 4 were 7.7% and 27.7%, respectively, with the cinnamon administered group demonstrating a significantly higher percentage reduction (P < 0.001). In addition, the cinnamon group also demonstrated a lower blood glucose value at 8 and 12 h post-oral glucose load (P < 0.05) [Table 1].
Figure 2.
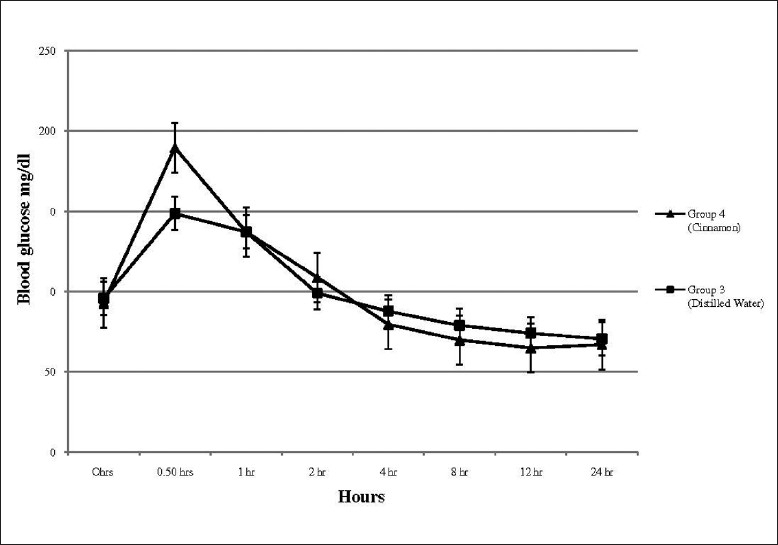
Blood glucose values following post-oral glucose load in Groups 3 (distilled water) and 4 (cinnamon extract)
Phase II
In healthy animals the mean body weight (±SD) of the cinnamon group and control group on day 0 was 193.9 ± 9.2 g and 191.6 ± 8.6 g, respectively. At the end of the study period (D-30), the mean weight of the control group increased significantly (P < 0.001) to 210.0 ± 16.8 g, while in the cinnamon group the weight gain was not significant (202.5 ± 15.7 g, P > 0.05). The mean food consumption of healthy rats on day 0 in the cinnamon and control groups was 10.9 ± 1.2 g and 11.4 ± 1.9 g, respectively, whereas on day 30 it was 10.3 ± 1.5 g and 11.4 ± 1.3 g, respectively, indicating no significant difference in the mean food consumption between day 0 and day 30 [Figure 3a].
Figure 3.
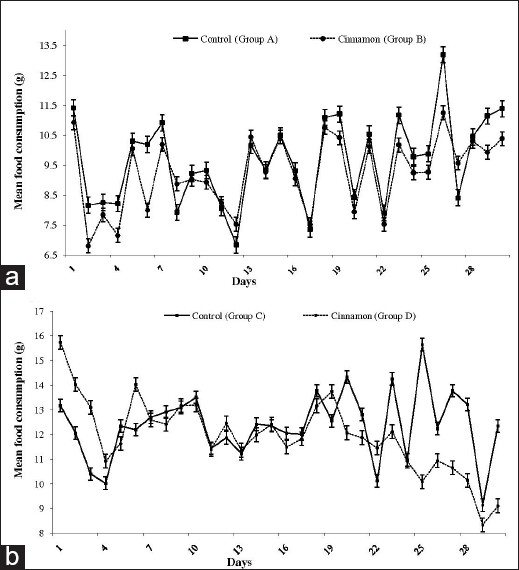
Mean food consumption of animals during the study period, (a) in healthy animals and (b) diabetes-induced animals
The diabetes-induced animals had mean body weights of 176.5 ± 13.9 g in the cinnamon group and 169.9 ± 6.5 g in the control group at the beginning of the study (day 0). On day 30, the mean body weight of the cinnamon group was 168.8 ± 33.4 g and in the control group it was 163.1 ± 17.6 g. The weight reduction observed in both groups during the study period was not statistically significant. The mean food consumption per animal had significantly reduced from day 0 to day 30 in the cinnamon group, but not in the control group of diabetes-induced animals (P < 0.001) (cinnamon group D0: 15.7 ± 1.2 g, D30: 9.1 ± 0.5 g; control group D0: 13.2 ± 1.6 g, D30:12.3 ± 1.3 g) [Figure 3b].
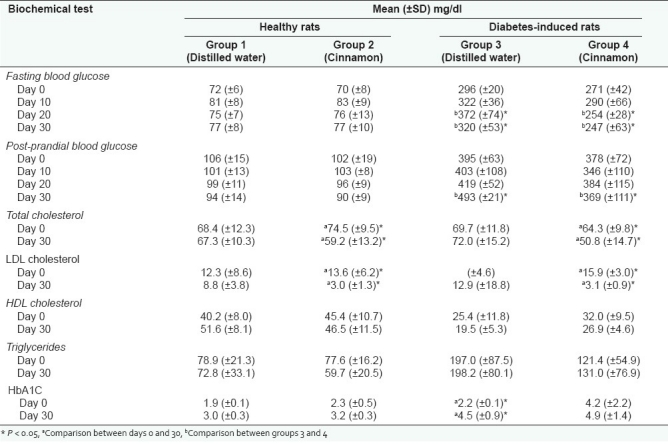
In healthy animals, there was no significant difference in the FBG or 2-h post-prandial blood glucose between the cinnamon and control groups from day 0 to day 30. However, in the diabetes-induced animals the FBG was significantly lower in the cinnamon group than the control group on day 20 and day 30, while the 2-h post-prandial glucose was significantly lower on day 30 in the cinnamon group [Table 2]. In both healthy and diabetes-induced animals, the cinnamon group showed a significantly lower total and LDL cholesterol level on day 30 compared to day 0. However, this difference was not observed in the control groups [Table 2]. The HDL cholesterol and triglycerides remained unchanged in all four groups of animals in Phase II. The mean HbA1C remained unchanged in healthy animals in day 0 and day 30, while in diabetes-induced animals the mean HbA1C in the control group increased significantly from 2.2% (±0.1) in day 0 to 4.5% (±0.9) on day 30 (P < 0.001). In the cinnamon group of diabetes-induced animals, the mean HbA1C on day 0 and day 30 was not significantly different [Table 2]. The histological analysis of the liver, kidney, and pancreas were within normal histological limits with no evidence indicative of toxicity. The liver histology demonstrated no evidence of spotty/zonal liver cell necrosis, hepatocyte swelling, fatty changes, and inflammation. In the kidneys, tubular epithelial cell damage, presence of casts/crystals, glomerular changes, and inflammation were not seen. The pancreas demonstrated no evidence of cell necrosis, inflammation, islet cell hyperplasia, and/or atrophy.
Table 2.
Effect of Cinnamomum zeylanicum on blood glucose, lipids, and HbA1C in healthy and diabetes-induced rats
DISCUSSION
To our knowledge, this is the first report on the effects of authentic Ceylon cinnamon collected from native cultivators on blood glucose, although there are studies on C. zeylanicum.[10] Our results demonstrate that C. zeylanicum improves the body's ability to handle a glucose load as demonstrated by the rapid metabolism shown in the cinnamon group of healthy animals following a standard oral glucose load. This probably occurs as a result of the ability of cinnamon to potentiate insulin-regulated glucose utilization via enhancing insulin signaling.[5,8,15] In the study by Qin et al.,[8] enhanced cellular glucose uptake due to the stimulation of the insulin receptor activity by the increasing concentrations of the phosphorylated intracellular protein IRS-1 and the increasing of PI 3-kinase has been shown with cinnamon. Studies have shown that this potentiating effect of cinnamon on insulin causes a dose-dependent reduction in the serum insulin concentration.[16] This rapid reduction in blood glucose concentration and the reduced insulin requirement could help prevent β-cell destruction occurring as a result of β-cell exhaustion from chronic hyperglycemia in diabetes.[17]
The cinnamon group of diabetes-induced animals demonstrated a significant reduction in mean food consumption implying that cinnamon also tends to promote satiety. In addition with long-term usage in diabetes-induced animals the cinnamon group demonstrated a lower FBG (on day 20 and 30) and 2-h post-prandial blood glucose (on day 30). These results are compatible with results from previous studies.[9,18] However, the same result was not observed in healthy animals where normal glucose homeostatic mechanisms are intact indicating that the effects of cinnamon are under the regulation of normal physiological feedback mechanisms. The fact that cinnamon lowers blood glucose usually within physiological levels without hypoglycemia and increases satiety leads to the hypothesis that it may act by potentiating the effects of incretin hormones.[19]
The cinnamon extracts also promoted a better glycemic control in diabetes-induced animals as demonstrated by the stable HbA1c in the cinnamon group as opposed to the diabetic control group. According to the United Kingdom Prospective Diabetes Study (UKPDS), a drop of HbA1C from 7.9% to 7% lowers the risk of macro- and micro-vascular disease significantly;[20] thus, a better control of HbA1c levels in patients with cinnamon supplementation might be expected to yield similar beneficial effects on the long-term complications of diabetes. In addition, both in healthy and diabetes-induced animals, cinnamon caused a very significant reduction in the total and LDL cholesterol levels. This cholesterol lowering effect of cinnamon together with its antioxidant properties[21] has the potential to reduce the long-term cardiovascular complications associated with diabetes,[22] while also being effective as a supplement in patients with hyperlipdiemia and cardiovascular diseases without diabetes.
The usage of cinnamon as a regular supplement with meals was not advocated or the daily dosage was restricted in many countries due to the toxic effects of C. aromaticum on the liver and coagulation.[23] In contrast, C. zeylanicum has shown to contain a lesser content of coumarin[11,24] and thus it may be possible that Ceylon cinnamon could be used in higher doses without toxic effects for longer durations. In addition our pathological analysis also revealed no evidence indicative of toxicity of C. zeylanicum. Our study has several limitations, the study was conducted with a single dose of cinnamon the dose-dependent effect of cinnamon extracts on the serum insulin level was not evaluated. The serum insulin levels that were measured in Phase II were <2 mIU/l in all animals (the sensitivity limit of the assay). The study involved both healthy and diabetes-induced male and female rats, the difference in the body composition and lipid profile between genders were not considered during analysis, however since all comparative groups had equivalent numbers of males and females this effect is likely to be minimal.
CONCLUSION
In conclusion, C. zeylanicum lowered blood glucose, reduced food intake, and reduced atherogenic LDL cholesterol. Its effect on humans and the possibility of incretin mimetic effects need further study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Mather HM, Keen H. The Southall Diabetes Survey: Prevalence of known diabetes in Asians and Europeans. Br Med J (Clin Res Ed) 1985;291:1081–4. doi: 10.1136/bmj.291.6502.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association. Nutrition Recommendations and Interventions for Diabetes: A position statement of the American Diabetes Association. Diabetes Care. 2007;30:S48–65. doi: 10.2337/dc07-S048. [DOI] [PubMed] [Google Scholar]
- 4.Bailey CJ, Day C. Traditional plant medicines as treatments for diabetes. Diabetes Care. 1989;12:553–64. doi: 10.2337/diacare.12.8.553. [DOI] [PubMed] [Google Scholar]
- 5.Imparl-Radosevich J, Deas S, Polansky MM, Baedke DA, Ingebritsen TS, Anderson RA, et al. Regulation of PTP-1 and insulin receptor kinase by fractions from cinnamon: Implications for cinnamon regulation of insulin signaling. Horm Res. 1998;50:177–82. doi: 10.1159/000023270. [DOI] [PubMed] [Google Scholar]
- 6.Jarvill–Taylor KJ, Anderson RA, Graves DJ. A hydroxychalcone derivative from cinnamon functions as a mimetic for insulin in 3T3–L1 adipocytes. J Am Coll Nutr. 2001;20:327–36. doi: 10.1080/07315724.2001.10719053. [DOI] [PubMed] [Google Scholar]
- 7.Cao H, Polansky MM, Anderson RA. Cinnamon extract and polyphenols affect the expression of tristetraprolin, insulin receptor, and glucose transporter 4 in mouse 3T3–L1 adipolytes. Arch Biochem Biophys. 2007;459:214–22. doi: 10.1016/j.abb.2006.12.034. [DOI] [PubMed] [Google Scholar]
- 8.Qin B, Nagasaki M, Ren M, Bajotto G, Oshida Y, Sato Y. Cinnamon extract (traditional herb) potentiates in vivo insulin regulated glucose utilization via enhanced insulin signaling in rats. Diabetes Res Clin Pract. 2003;62:139–48. doi: 10.1016/s0168-8227(03)00173-6. [DOI] [PubMed] [Google Scholar]
- 9.Khan A, Safdar M, Ali Khan MM, Khattak KN, Anderson RA. Cinnamon improves glucose and lipids in people with type 2 diabetes. Diabetes Care. 2003;26:3215–8. doi: 10.2337/diacare.26.12.3215. [DOI] [PubMed] [Google Scholar]
- 10.Shen Y, Fukushima M, Ito Y, Muraki E, Hosono T, Seki T, et al. Verification of the antidiabetic effects of cinnamon (Cinnamomum zeylanicum) using insulin-uncontrolled type 1 diabetic rats and cultured adipocytes. Biosci Biotechnol Biochem. 2010;74:2418–25. doi: 10.1271/bbb.100453. [DOI] [PubMed] [Google Scholar]
- 11.Ouattara B, Simard RE, Holley RA, Piette GJ, Bégin A. Antibacterial activity of selected fatty acids and essential oils against six meat spoilage organisms. Int J Food Microbiol. 1997;37:155–62. doi: 10.1016/s0168-1605(97)00070-6. [DOI] [PubMed] [Google Scholar]
- 12.Archer AW. Determination of cinnamaldehyde, coumarin and cinnamyl alcohol in cinnamon and cassia by high-performance liquid chromatography. J Chromatogr. 1988;447:272–6. [Google Scholar]
- 13.Ghosh P, Markin RS, Sorrell MF. Coumarin-induced hepatic necrosis. Am J Gastroenterol. 1997;92:348–9. [PubMed] [Google Scholar]
- 14.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–61. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 15.Khan A, Bryden NA, Polansky MM, Anderson RA. Insulin potentiating factor and chromium content of selected foods and spices. Biol Trace Elem Res. 1990;24:183–8. doi: 10.1007/BF02917206. [DOI] [PubMed] [Google Scholar]
- 16.Plexopathy DL. Cinnamon dose-dependently reduces insulin concentration. Am J Clin Nutr. 2009;89:815–21. [Google Scholar]
- 17.Donath MY, Ehses JA, Maedler K, Schumann DM, Ellingsgaard H, Eppler E, et al. Mechanisms of β-Cell Death in Type 2 Diabetes. Diabetes. 2005;54:S108–13. doi: 10.2337/diabetes.54.suppl_2.s108. [DOI] [PubMed] [Google Scholar]
- 18.Vanderweele DA. Insulin and satiety from feeding in pancreatic-normal and diabetic rats. Physiol Behav. 1993;54:477–85. doi: 10.1016/0031-9384(93)90239-c. [DOI] [PubMed] [Google Scholar]
- 19.Perfetti R, Brown TA, Velikina R, Busselen S. Control of glucose homeostasis by incretin hormones. Diabetes Technol Ther. 1999;1:297–305. doi: 10.1089/152091599317215. [DOI] [PubMed] [Google Scholar]
- 20.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 21.Mancini-Filho J, Van-Koiij A, Mancini DA, Cozzolino FF, Torres RP. Antioxidant activity of cinnamon (Cinnamomum zeylanicum, Breyne) extracts. Boll Chim Farm. 1998;137:443–7. [PubMed] [Google Scholar]
- 22.Roussel A, Hininger I, Benaraba R, Ziegenfuss TN, Anderson RA. Antioxidant effects of a cinnamon extract in people with impaired fasting glucose that are overweight or obese. J Am Coll Nutr. 2009;28:16–21. doi: 10.1080/07315724.2009.10719756. [DOI] [PubMed] [Google Scholar]
- 23.Lungarini S, Aureli F, Coni E. Coumarin and cinnamaldehyde in cinnamon marketed in Italy: A natural chemical hazard? Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2008;25:1297–305. doi: 10.1080/02652030802105274. [DOI] [PubMed] [Google Scholar]
- 24.Rychlik M. Quantification of free coumarin and its liberation from glucosylated precursors by stable isotope dilution assays based on liquid chromatography-tandem mass spectrometric detection. J Agric Food Chem. 2008;56:796–801. doi: 10.1021/jf0728348. [DOI] [PubMed] [Google Scholar]


