Abstract
Background:
Manilkara zapota (L.) Van Royen is an evergreen tree, native to the tropical Americas and introduced to Egypt as a fruiting tree in 2002. No previous study was reported on the plant cultivated in Egypt.
Materials and Methods:
In this study, the leaves of the plant cultivated in Egypt were subjected to phytochemical and biological investigations. The lipoidal matter was analyzed by GLC. Five compounds were isolated from the petroleum ether and ethyl acetate fractions of the alcoholic extract of the leaves by chromatographic fractionation on silica gel and sephadex, the structures of these compounds were identified using IR, UV, MS, 1H-NMR and 13C-NMR. The LD50 of the alcoholic and aqueous extracts of the leaves was determined and their antihyperglycemic, hypocholesterolemic and antioxidant activities were tested by enzymatic colorimetric methods using specific kits.
Results:
Unsaturated fatty acids represent 32.32 % of the total fatty acids, oleic acid (13.95%), linoleidic acid (10.18 %) and linoleic acid (5.96 %) were the major ones. The isolated compounds were identified as lupeol acetate, oleanolic acid, apigenin-7-O-α-L-rhamnoside, myricetin-3-O-α-L-rhamnoside and caffeic acid. This is the first report about isolation of these compounds from Manilkara zapota except myricetin-3-O-α-L-rhamnoside, which was previously isolated from the plant growing abroad. The LD50 recorded 80 g/Kg b. wt. for both the tested extracts, so they could be considered to be safe. They exhibited antihyperglycemic, hypocholesterolemic and antioxidant activities.
Conclusion:
The observed biological activities were attributed to the different chemical constituents present in the plant mainly its phenolic constituents.
Keywords: Antihyperglycemic, hypocholesterolemic, antioxidant, flavonoids, Manilkara zapota (L.) Van Royen, triterpenes
INTRODUCTION
Sapotaceae is a family of some 35–75 ill-defined genera and about 800 species, most are tropical trees.[1] Manilkara zapota (L.) Van Royen (synonyms: Manilkara zapotilla, Manilkara achras, Mimusopus manilkara, Achras zapota, and Achras sapota),[2] growing abroad, was subjected to chemical study resulted in isolation of flavonoids,[3,4] tannins (mainly from unripe fruits),[4–7] triterpenes,[8–10] and saponins (mainly from the seeds).[10,11] Also, the plant was reported to exhibit antioxidant,[12,13] antimicrobial,[13,14] and analgesic[15] activities. However, no previous study was carried out on the plant cultivated in Egypt, thus this study was performed aiming for isolation and identification of active constituents and investigation of biological activity, viz. antihyperglycemic, hypocholesterolemic, and antioxidant activities, of the leaves of M. zapota (L.) Van Royen cultivated in Egypt.
MATERIALS AND METHODS
General experimental
Electro thermal 9100 was used for the determination of melting point. IR spectra were run in KBr using a Perkin–Elmer infrared spectrophotometer FT-IR 1650. Beckman Du-7 and Shimadzu-265 spectrophotometers were used for the determination of ultraviolet absorption spectra. Mass spectrometer, Varian Mat 711 (USA), Finnigan SSQ 7000 was used for EI/MS. 1H-(300 MHz) and 13C-(75 MHz) NMR spectra were recorded on a Varian Mercury apparatus at 25°C using TMS as an internal standard and chemical shifts were given in δ values. TLC was performed on precoated silica gel plates using suitable solvent systems: S1 [Pet. ether: CHCl3 (1:2 v/v)], S2 [Pet. ether: EtOAC (7:3 v/v)], S3 [CHCl3: MeOH (9:1 v/v)], and S4 [EtOAC: MeOH: Formic acid (5:0.1:2 v/v/drops)]. The chromatograms were visualized under UV light (at λmax 254 and 366 nm) before and after exposure to ammonia vapor, as well as, spraying with p-anisaldehyde/sulfuric acid spray reagent. Reference samples for TLC comparison were obtained from E. Merck, Darmstadt, Germany.
GLC analysis of unsaponifiable fraction and fatty acid methyl esters was performed on a Trace GC Ultra with Thermo Scientific Trace TR-5MS capillary column packed with 5% phenyl polysilphenylene–siloxane and TR-FAME capillary columns packed with 70% cyanopropyl polysilphenylene–siloxane, respectively (30 m length × 0.25 mm ID × 0.25 μm film thickness). In the case of unsaponifiable fraction, the initial oven temperature was kept at 70 °C for 2 min, raised to 270 °C at 5 °C/min, then held for 5 min. The injector temperature was 270 °C; the detector (FID) temperature was 280 °C. Concerning fatty acid methyl esters, the initial oven temperature was 140 °C, raised to 200 °C at 5 °C/min, then held for 3 min. The injector temperature was 200 °C; the detector (FID) temperature was 220 °C. The air flow rate was 350 ml/min; hydrogen flow rate was 40 and 50 ml/min, respectively, and carrier gas (nitrogen) flow rate was 30 ml/min.
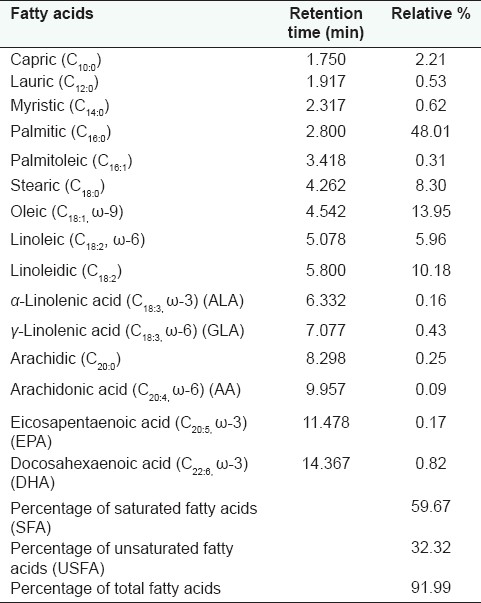
Identification of hydrocarbons, sterols and fatty acids component was based on comparison of the retention times of their peaks with those of the available authentic samples. The relative amount of each component was calculated via peak area measurement by means of a computing integrator (PU 4810, Philips, England). The obtained results are recorded in Tables 1 and 2.
Table 1.
GLC analysis of the unsaponifiable matter of M. zapota (L.) Van Royen leaves
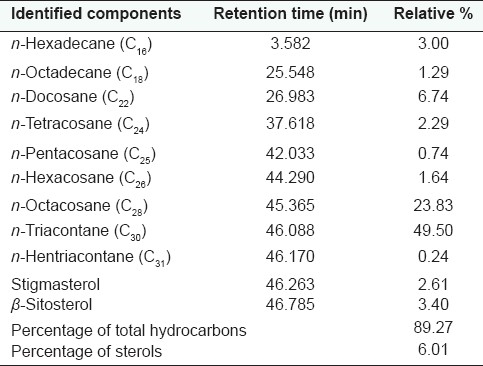
Table 2.
GLC analysis of the fatty acid methyl esters of M. zapota (L.) Van Royen leaves
Plant material
Manilkara zapota (L.) Van Royen leaves were supplied through the Horticulture Research Center, Ministry of Agriculture, Giza in June 2006. Identity was verified by Prof. Dr. Mohammed El-Sayed, Horticultural Research Institute, Agriculture Research Center, Ministry of Agriculture, Giza. Voucher specimens had been deposited in the Herbarium of the Department of Pharmacognosy, Faculty of Pharmacy, Cairo University, Egypt.
Preparation of the unsaponifiable matter and fatty acids methyl esters
The air-dried powdered leaves (1 kg) of M. zapota (L.) Van Royen were extracted with n-hexane till exhaustion (500 ml ×8), the solvent was evaporated under reduced pressure to produce 11 g of dry residue. The lipoidal matter (0.669 g) was prepared from the n-hexane extract (2 g) adopting the method of Association of Official Analytical Chemists (2000).[16] The lipid sample (0.5 g) was saponified according to the method described by Vogel (1975)[17] to yield 0.160 and 0.007 g of unsaponifiable and saponifiable matter, respectively. The fatty acids methyl esters were prepared from the saponifiable fraction according to the method of Vogel (1975).[17]
Extraction, fractionation, and isolation
The air dried powdered leaves (2 kg) of M. zapota (L.) Van Royen were macerated with 70% ethyl alcohol till exhaustion. The combined ethanol extracts were evaporated under reduced pressure at temperature not exceeding 60°C to yield 200 g dry extract. The residue obtained was suspended in distilled water (500 ml) and successively extracted with petroleum ether (8 × 500 ml), chloroform (3 × 500 ml), ethyl acetate (6 × 500 ml), and n-butanol (4 × 500 ml). The solvent in each case was evaporated under reduced pressure to yield 46, 3, 16, and 20 g, respectively.
Petroleum ether extract (20 g) was chromatographed on silica gel H type 60 using VLC (15 × 5 cm). Gradient elution was carried out with petroleum ether, petroleum ether–chloroform mixtures, chloroform, and chloroform–ethyl acetate mixtures. Fractions, 100 ml each, were collected. The obtained fractions were monitored with TLC, similar fractions were pooled and the solvent was evaporated under reduced pressure to yield two main fractions.
Fractions (6–8, 2.5 g) were rechromatographed on a silica gel 60 column (2.5 × 10 cm) using petroleum ether:methylene chloride mixtures of increasing polarity as eluent to yield compound 1 (40 mg).
Fractions (11–15, 2 g) were purified on a silica gel 60 column (2.5 × 15 cm). Static elution was carried out using petroleum ether:ethyl acetate (1:1 v/v) to yield compound 2 (30 mg).
Ethyl acetate fraction (7 g) was chromatographed on 100 g silica gel H type 60 using VLC (15 × 5 cm). Gradient elution was carried out with chloroform, chloroform–ethyl acetate mixtures, ethyl acetate and ethyl acetate–methanol mixtures. Fractions, 100 ml each, were collected. The obtained fractions were monitored with TLC, similar fractions were pooled and the solvent was evaporated under reduced pressure to give two main fractions.
Fractions (23–27, 1.6 g) were rechromatographed on a column of sephadex LH-20 (2.5 × 20 cm). Gradient elution was carried out using water-methanol mixtures which afforded compound 3 (10 mg) and compound 4 (1 g).
Fractions (28–40, 1.66 g) were rechromatographed on a column of sephadex LH-20 (2.5 × 20 cm). Gradient elution was carried out using water–methanol mixtures to yield compound 5 (15 mg, yellow powder, Rf = 0.32 in S3).
Compound 1: White crystals, soluble in chloroform, m. p. 215-215°C, Rf = 0.80 (S1). IR: 2949 and 2845 cm-1 (-C-H stretching vibration), 1642 cm-1 (-C=O), 1447 and 1385 cm-1 (C-CH3 bending vibration) and 1020 cm-1 (C=C). EI/MS m/z: 468 (M+), 453 (M-CH3)+, 218, 189, 161, 135, 107 (100%), 81 and 55. 1H-NMR (CDCl3): δH 4.70 (1H, s, H-29b), 4.58 (1H, s, H-29a), 4.51 (m, H-3), 2.35 (2H, m, H-2a and H-2b), 2.05 (3H, s, CH3 of acetate group), 1.70 (3H, s, H-30), six methyl groups at 1.04 (3H, s), 0.99 (3H, s), 0.95 (3H, s), 0.88 (3H, s), 0.86 (3H, s), 0.79 (3H, s). 13C-NMR (CDCl3): δC 170.93 (C-31, O-C=O), 150.91 (C-20), 109.33 (C-29), 80.98 (C-3), 55.42 (C-5), 50.38 (C-9), 48.33 (C-18), 48.02 (C-19), 43.01 (C-17), 42.86 (C-14), 40.89 (C-8), 40.02 (C-22), 38.43 (C-1), 38.08 (C-4), 37.82 (C-10), 37.12 (C-13), 35.60 (C-16), 34.26 (C-7), 29.87 (C-21), 27.97 (C-23), 27.47 (C-15), 25.15 (C-12), 21.13 (C-2), 20.98 (C-11), 19.31 (C-30), 18.24 (C-6), 18.02 (C-28), 16.51 (C-24), 16.19 (C-25), 16.01 (C-26), 14.53 (C-27).
Compound 2: White crystal, soluble in chloroform, m. p. 308-310°C, Rf = 0.12 (S2). IR: 3428 cm-1 (-OH), 2931 and 2869 cm-1 (C-H stretching vibration), 1692 cm-1 (-C=O), 1460 and 1384 cm-1 (C-CH3 bending vibration) and 1034 cm-1 (C=C). EI/MS m/z: 456 (M+), 248 (100%), 219, 188 (248-COOH-CH3), 163, 118, 85, 83 and 57. 1H-NMR (CDCl3): δH 5.28 (1H, br. s, H-12), 3.22 (1H, m, H-3), 2.33 (2H, m, H-2a and H-2b), seven methyl groups at 1.27 (3H, s), 1.15 (3H, s), 1.10 (3H, s), 1.00 (3H, s), 0.94 (3H, s), 0.80 (3H, s), 0.79 (3H, s). 13C-NMR (CDCl3): δC 180.52 (C-28), 139.00 (C-13), 122.20 (C-12), 79.11 (C-3), 55.32 (C-5), 47.63 (C-9), 46.55 (C-17), 45.96 (C-19), 41.69 (C-14), 41.43 (C-18), 39.37 (C-8), 38.69 (C-4), 38.51 (C-1), 37.04 (C-10), 33.88 (C-21), 33.05 (C-29), 32.60 (C-7), 32.51 (C-22), 30.67 (C-20), 28.16 (C-23), 27.76 (C-15), 27.49 (C-2), 26.07 (C-27), 23.61 (C-30), 23.47 (C-16), 23.13 (C-11), 18.38 (C-6), 16.88 (C-26), 15.60 (C-24), 15.33 (C-25).
Compound 3: Yellow powder, soluble in methanol, Rf = 0.34 (S3). UV λmax nm: MeOH: 265 and 333, MeOH / NaOMe: 272 and 396, MeOH / AlCl3: 274 and 351, MeOH /AlCl3 / HCl: 274 and 351, MeOH / NaOAc: 263 and 340, MeOH / NaOAc / H3BO3: 263 and 330. 1H-NMR (DMSO): δH 7.94 (2H, d, J=7.6 Hz, H-2’ and H-6’), 6.83 (2H, d, J=7.6 Hz, H-3’ and H-5’, 6.64 (1H, d, J=1.5 Hz, H-8), 6.37 (1H, d, J=1.5 Hz, H-6), 6.16 (1H, s, H-3), 5.09 (s, H-1”), 4.42 (br. s, H-2”), 3.32-3.84 (m, H-3”, H-4” and H-5”), 0.79 (3H, H-6”)
Compound 4: Yellow powder, soluble in methanol, Rf = 0.38 (S4). UV λmax nm: MeOH, 256 and 352, MeOH / NaOMe: 255 and 423, MeOH / AlCl3: 272 and 423, MeOH / AlCl3 / HCl: 272 and 363, MeOH / NaOAc: 269 and 409, MeOH / NaOAc / H3BO3: 259 and 374. 1H-NMR (CD3OD): δH 6.95 (2H, d, J =1.5 Hz, H-2’ and H-6’), 6.37 (1H, d, J=2.1 Hz, H-6), 6.21 (1H, d, J=2.1 Hz, H-8). 5.32 (br s, H-1”), 4.22 (br s, H-2”), 3.71-3.81 (m, H-3”, H-4” and H-5”), 0.97 (3H, d, J=6.3 Hz, H-6”). 13C-NMR (CD3OD): δC 179.63 (C-4), 165.84 (C-7), 163.17 (C-5), 159.41 (C-9), 158.48 (C-2), 146.82 (C-3’ and C-5’), 137.86 (C-4’), 136.28 (C-3), 121.92 (C-1’), 109.57 (C-2’ and C-6’), 105.86 (C-10), 103.60 (C-1”), 99.79 (C-6), 94.67 (C-8), 73.35 (C-4”), 72.13 (C-3”), 72.02 (C-2”), 71.88 (C-5”), 17.66 (C-6”)
Compound 5: Yellow powder, soluble in methanol, Rf = 0.32 (S3). 1H-NMR (CD3OD): δH 7.52 (1H, d, J=15.9 Hz, H-7), 7.03 (1H, d, J=2.1 Hz, H-2), 6.93 (1H, dd, J=2.1, 8.4 Hz, H-6), 6.77 (1H, d, J=8.4 Hz, H-5), 6.22 (1H, d, J=15.9 Hz, H-8).
Biological study
Plant extracts
The alcoholic and aqueous extracts were prepared by percolating 200 g of dried powdered leaves in each case with 70% ethyl alcohol and distilled water, respectively, and then the extracts were evaporated, separately, under reduced pressure.
Experimental animals and diets
Male albino mice weighing 25–30 g were used for determination of the median lethal dose (LD50). Wistar strain rats weighing 150–160 g, aged 3 months, were used for evaluation of biological activities. The animals were supplied from the Research Institute of Ophthalmology. They were individually housed in separate cages under standardized temperature (25–28 °C), humidity (50–60%) and light (12 h’ light/dark cycles) conditions. They were fed the standard laboratory diet consisting of vitamins mixture (1%), mineral mixture (4%), corn oil (10%), sucrose (20%), cellulose (0.2%), starch (54.3%), and casein (10.5%).
Drugs and chemicals
Alloxan solution: Alloxan powder (Sigma Co., Germany) was dissolved in normal saline (0.9 g NaCl in 100 ml distilled water) to obtain 10 mg alloxan in 0.1 ml solution.
Cholesterol and bile salts powders: Lab Plus, UK.
Metformin (Cidophage®): Chemical Industries Development Co. (CID Co.), Giza, Egypt, as antidiabetic.
Vitamin C (Vitacid C®): Chemical Industries Development Co. (CID Co.), Giza, Egypt, as antioxidant.
Atorvastatin (Lipitor®): Pfizer Company, Cairo, Egypt, as hypocholesterolemic.
Kits
Glucose Kits: Biocon, Germany.
Cholesterol Kit: Biocon, Germany.
Total Antioxidant Kit: Biodiagnostic, Cairo, Egypt.
Spectophotometer for biological evaluation
Spectophotometer (Perkin Elmer 3300, Germany) was used for evaluation of the total antioxidant, hypoglycemic, and hypocholesterolemic activities by measurement of the color intensity at 500–520 nm.
Toxicity study
The LD50 for the tested aqueous and alcoholic extracts of the leaves of M. zapota (L.) Van Royen was determined following Karber's[18] and Klaassen.[19] The animals were divided into groups each of six animals. Preliminary experiments were done to determine the minimal dose that kills all animals (LD100) and the maximal dose that fails to kill any animal. Several doses at equal logarithmic intervals were chosen in between these two doses, each dose was injected i.p. in a group of animals. Twenty-four hours after injection, the number of dead animals in each group was determined and the LD50 was calculated. Accordingly, preliminary pilot experiments were carried out to determine the minimal effective dose of these extracts.
Antihyperglycemic, hypocholesterolemic, and total antioxidant (TAO) activities[20,21]
Animal grouping
The animals were divided into seven groups, ten animals each. The first group was the negative control group, received 1 ml saline orally. The second group was the hyperglycemic and hypercholesterolemic untreated model. The third group was the model treated with standard drugs, metformin, atorvastatin, and vitamine C (20, 5, and 100 mg/kg b. wt, respectively). The fourth and fifth groups were control groups received the tested extracts. The sixth and seventh groups were the model treated with the extracts under investigation. The dose for each treated control and model groups was a single daily oral dose of 53.6 mg/kg b. wt.
Model induction
Hypercholesterolemia and hyperglycemia were induced by feeding the male Wistar rats with standard laboratory diet mixed with 1% cholesterol and 0.25% bile salts powders from the diet weight[22] and intraperitoneal injection with a single dose of alloxan (100 mg/kg b. wt.),[23] respectively.
Blood samples were taken at the sixth week of experiment and then centrifuged at 4000 rpm for 10 min. The supernatant (serum) was separated and divided into three portions to measure the serum glucose,[24] cholesterol levels[25] and total antioxidant capacity,[26] calorimetrically using specific enzymatic kits.
Statistical analysis
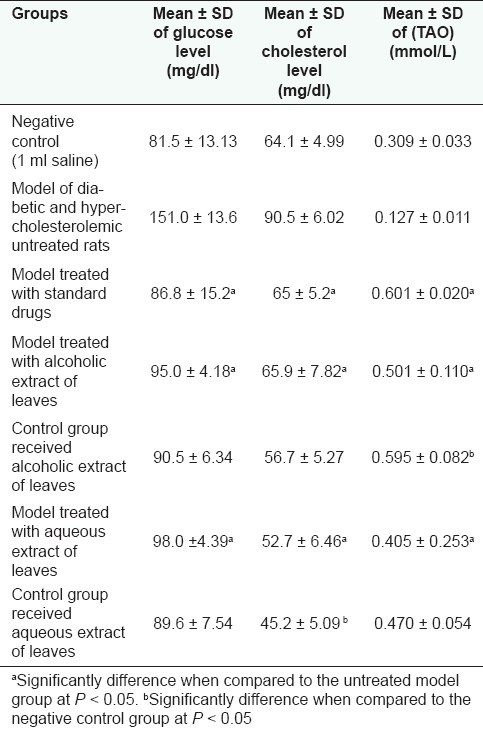
Results were expressed as mean ± SD. Comparison between groups was statistically analyzed by one way ANOVA followed by post hoc tests and Student's t tests.[27] The obtained results are compiled in Table 3. The results were considered significant at P < 0.05.
Table 3.
Antihyperglycemic, hypocholesterolemic, and antioxidant, activities of the aqueous and alcoholic extracts of the leaves of M. zapota (L.) Van Royen
RESULTS AND DISCUSSION
GLC analysis of unsaponifiable matter
Nine hydrocarbons were identified which constituted 89.303% of the unsaponifiable matter. n-Triacontane (49.50%) and n-octacosane (23.83%) were the major identified hydrocarbons. Two sterols, β-sitosterol (3.40%) and stigmasterol (2.61%), were identified.
GLC analysis of fatty acids methyl esters
Fifteen fatty acids were identified which constituted 92.05%. The saturated fatty acids represented 59.95%, among which palmitic acid (48.01%) was the major one. The percentage of the unsaturated fatty acids was 32.09%. The polyunsaturated fatty acids constituted 17.82%. Oleic acid (13.95%), linoleidic acid (10.18%) and linoleic acid (5.96%) were the major components.
Identification of the isolated compounds
Compounds 1 and 2 were identified by comparing their spectral data (IR, MS, 1H-NMR, and 13C -NMR) with the published data, as lupeol-3-acetate[28] and oleanolic acid.[29] The identification was further confirmed by direct comparison with authentic samples and m.p.
UV and 1H-NMR spectral data of compound 3 revealed the presence of seven substituted apigenin nucleus.[30] Broad singlet at δH 5.09 corresponding to the anomeric proton (H-1”) of a sugar molecule. Signal at δH 0.79 (3H) corresponding to the methyl group of L-rhamnose.[30] Thus, compound 3 was identified as apigenin-7-O-α-L-rhamnoside. The identification was also confirmed by acid hydrolysis[31] and comparison with authentic samples.
UV and 1H-NMR spectral data of compound 4 indicated the presence of myricetin nucleus.[30] Signals at δH 5.32 (br. s) and δC 103.60 assigned for H-1” and C-1”, respectively, of a sugar molecule. Signals at δH 0.97 (3H, d, J = 6.3 Hz) and δC 17.66 corresponding to the methyl group of L-rhamnose.[30,32] Thus compound 4 was identified as 5,7,3’,4’,5’-pentahydroxyflavon-3-O-α-L-rhamnoside (myricetin-3-O-α-L-rhamnoside, myricetrin).
Compound 5 was identified as caffeic acid by comparing its spectral data (UV and 1H-NMR) with the published data.[33] The identification was further confirmed by co-chromatography with the authentic sample.
This study is the first report about the isolation of lupeol-3-acetate, oleanolic acid, apigenin-7-O-α-L-rhamnoside, and caffeic acid from M. zapota (L.) Van Royan, while, myricetin-3-O-α-L-rhamnoside (myricetrin) was previously isolated from both the leaves[3] and fruits[4] of M. zapota (L.) Van Royen growing abroad.
Toxicity study
The LD50 for the tested aqueous and alcoholic extracts of the leaves of M. zapota (L.) Van Royen was found to be 8 g/kg b. wt. Thus the tested extracts could be considered to be safe according to Buck et al.[34] The minimal effective dose was found 8 mg/kg b. wt. subcutaneously and 16 mg/kg b. wt. orally for both the alcoholic and aqueous extracts.
Biological activities
In this study the antihyperglycemic and hypocholesterolemic activities of M. zapota (L.) Van Royen leaves were estimated for the first time. The tested alcoholic and aqueous extracts exhibited significant decrease in the blood glucose level of the model group comparing to metformin; however, these extracts showed no significant change in the blood glucose level of the normal rats (control group). Thus, the mechanism of their antihyperglycemic activity could be similar to that of biguanides, e.g. metformin, it is an antihyperglycemic compound which do not affect the blood glucose level in the normal state.[35]
The model group treated with the alcoholic and aqueous extracts of the leaves showed a marked decrease in the cholesterol level close to that of atorvastatin. Concerning the control group, the aqueous extract showed a significant decrease in the cholesterol level, while the alcoholic extract showed no significant change.
The antioxidant activity of M. zapota (L.) Van Royen leaves from India was previously studied.[12,13] The methanolic extract of the leaves showed week antioxidant activity,[12] while the acetone extract exhibited high activity which was attributed to its phenolic content.[13] In this study, the alcoholic extract of the leaves showed marked significant improvement of TAO parameter in both the model group and the control group comparing to that of vitamin C. The aqueous extract of the leaves exhibited a significant improvement of TAO levels in the model group, a non significant improvement in the control group.
The observed biological activities of the alcoholic and aqueous extracts of M. zapota (L.) Van Royen leaves could be attributed mainly to their phenolic content (apigenin-7-O-α-L-rhamnoside, myricetin-3-O-α-L-rhamnoside, and caffeic acid). This is in agreement with the published data which revealed that phenolic compounds showed promising antioxidant,[13,36,37] antidiabetic,[38,39] and hypocholesterolemic[40,41] activities.
Other plant constituents may also participate in the observed biological activities, sterol, and triterpenes were reported to possess antihyperglycemic[42] and hypocholesterolemic effects.[43,44] Therefore, sterols (β-sitosterol and stigmasterol) identified in the unsaponifiable matter and triterpenes (lupeol-3-acetate and oleanolic acid) isolated from the petroleum ether fraction may participate in the observed hypocholesterolemic effect of the alcohol extract.
Also, unsaturated fatty acids were reported to possess hypocholesterolemic activity.[45] Thus, oleic acid, linoleidic acid, and linoleic acid, which represent the major identified fatty acids in the lipoidal matter of the leaves, may also attribute to the hypocholesterolemic effect of the alcoholic extract.
The variation change in the antihyperglycemic and hypocholesterolemic effects of the alcoholic and aqueous extracts in the control groups could be explained based on the difference in type, concentration, and relative potency of their bioactive constituents.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Evans WC. 14th ed. London, Philadelphia, Toronto, Sydney and Tokyo: WB Saunders Company Ltd; 1996. Trease and Evans, Pharmacognosy; p. 50. [Google Scholar]
- 2.Quattrocchi FL. Boca Raton, London, New York, Washington, DC: CRC Press; 2000. CRC World Dictionary of Plant Names-Common Names, Scientific Names, Eponyms, Synonyms and Etymology; pp. 1609–10. [Google Scholar]
- 3.Subramanian SS, Nair AG. Myricetin and myricetin-3-O-L-rhamnoside from leaves of Madhuca indica and Achras sapota. Phytochemistry. 1972;11:3090–1. [Google Scholar]
- 4.Ma J, Luo X, Protiva P, Yang H, Ma C, Basile MJ, et al. Bioactive Novel Polyphenols from the fruit of Manilkara zapota (Sapodilla) J Nat Prod. 2003;66:983–6. doi: 10.1021/np020576x. [DOI] [PubMed] [Google Scholar]
- 5.Mathew AG, Lakshminarayana S. Polyphenols of immature sapota fruit. Phytochemistry. 1969;8:507–9. [Google Scholar]
- 6.Pontes PV, Moreira RF, Trugo LC, Maria CA. The content of chlorogenic acids in tropical fruits. J Sci Food Agric. 2002;82:1171–81. [Google Scholar]
- 7.Shui G, Wong SP, Leong LP. Characterization of antioxidants and change of antioxidant levels during storage of Manilkara zapota L. J Agric Food Chem. 2004;52:7834–41. doi: 10.1021/jf0488357. [DOI] [PubMed] [Google Scholar]
- 8.Misra G, Nigam SK, Mitra CR. Constituents of Mimusops manilkara leaves and saponins of mimusops seed kernels. Phytochemistry. 1969;8:2255–6. [Google Scholar]
- 9.Misra G, Mitra CR. Mimusops manilkara, constituents of fruit and seed. Phytochemistry. 1969;8:249–52. [Google Scholar]
- 10.Hart NK, Lamberton JA, Triffett AC. Triterpenoids of Achras sapota (Sapotaceae) Aust J Chem. 1973;26:1827–9. [Google Scholar]
- 11.Ahmed R, Rashid F, Ahmed VU, Mohammad FV, Noorwala M, Bibi N, et al. Saponins from the seeds of Achras sapota. J Asian Nat Prod Res. 2008;10:7–16. doi: 10.1080/10286020701276026. [DOI] [PubMed] [Google Scholar]
- 12.Kaneria M, Baravalia Y, Vaghasiya Y, Chanda S. Determination of antibacterial and antioxidant potential of some medicinal plants from Saurashtra region, India. Indian J Pharm Sci. 2009;71:406–12. doi: 10.4103/0250-474X.57289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chanda SV, Nagani KV. Antioxidant capacity of Manilkara zapota leaves extracts evaluated by four in vitro methods. Nat Sci. 2010;8:260–6. [Google Scholar]
- 14.Abu Osman M, Abdul Aziz M, Rowshanul M, Rezaul M. Antimicrobial investigation on Manilkara zapota (L.) P. Royen. Int J Drug Dev Res. 2011;3:185–90. [Google Scholar]
- 15.Pankaj KJ, Prashant S, Neeraj U, Yogesh S. Evaluation of analgesic activity of Manilkara zapota (leavea) Eur J Exp Biol. 2011;1:14–7. [Google Scholar]
- 16.14th ed. Washington, D.C: 2000. A.O.A.C. Official Methods of Analysis of the Association of Official Analytical Chemist. [Google Scholar]
- 17.Vogel AJ. 3rd ed. London: English Language Book Society and Longman Group Ltd; 1975. A Text Book of Practical Organic Chemistry; pp. 969–71. [Google Scholar]
- 18.Karber J. Beitrag zur kollektiven behandlung pharmakologischer reihen versuche. Arch Exp Pathol Pharmacol. 1931;162:480–3. [Google Scholar]
- 19.Klaassen CD. Principles of Toxicology and Treatments of Poisoning in Pharmacological Basis of Therapeutics. In: Goodman, Gilman, editors. New York: McGraw-Hill; 2005. p. 1740. Ch. 64. [Google Scholar]
- 20.Odetola AA, Akinloye O, Egunjobi C, Adekunle WA, Ayoola AO. Possible antidiabetic and antihyperlipidaemic effect of fermented Parkia biglobosa (JACQ) extract in alloxan-induced diabetic rats. Clin Exp Pharmacol Physiol. 2006;33:808–12. doi: 10.1111/j.1440-1681.2006.04444.x. [DOI] [PubMed] [Google Scholar]
- 21.Owens D. Spontaneous, Surgically and Chemically Induced Models of Disease in the Laboratory Rats. In: Suckow MA, Weisbroth SH, Franklin CL, editors. Elsevier Academic Press; 2006. pp. 711–32. [Google Scholar]
- 22.Berrougui H, Ettaib MD, Herrera G, Alvarez de Sotomayor M, Bennani-Kabchi N, Hmamouchi M. Hypolipidemic and hypocholesterolemic effect of argan oil (Argania spinosa) in Meriones shawi rats. J Ethnopharmacol. 2003;89:15–8. doi: 10.1016/s0378-8741(03)00176-4. [DOI] [PubMed] [Google Scholar]
- 23.Eliasson SG, Samet JM. Alloxane induced neuropathies: Lipid changes in nerve and root fragments. Life Sci. 1969;8:493–8. doi: 10.1016/0024-3205(69)90442-1. [DOI] [PubMed] [Google Scholar]
- 24.Glick MR, Ryder KW, Jackson SA. Graphical comparisons of interferences in clinical chemistry instrumentation. Clin Chem. 1986;24:863–9. [PubMed] [Google Scholar]
- 25.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–5. [PubMed] [Google Scholar]
- 26.Koracevic D, Koracevic G, Jordjevic VD. Method for the measurement of antioxidant activity in human fluids. J Clin Pathol. 2001;54:356–61. doi: 10.1136/jcp.54.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snedecor GW, Cochran WG. 10th ed. Iowa State, USA: University Press; 1982. Statistical Methods; p. 91. [Google Scholar]
- 28.Good JL, Akisha T. 1st ed. Blackie Academic and Professional Press, Champan and Hall; 1997. Analysis of Sterols. [Google Scholar]
- 29.Mahato SB, Kundu AP. 13CNMR spectra of pentacyclic triterpenoids. A compilation and some salient features. Phytochemistry. 1994;37:1517–75. [Google Scholar]
- 30.Mabry TJ, Markham KR, Thomas MB. New York: Springer Verlag; 1970. The Systematic Identification of Flavonoids; p. 280. [Google Scholar]
- 31.Harborne JB, Mabry TJ, Mabry H. New York, San Francisco: Acad. Press, Inc; 1975. The Flavonoids. London: Chapman and Hall. [Google Scholar]
- 32.Agrawal PK. New York: Elsevier; 1989. Carbon-13 NMR of Flavonoids. [Google Scholar]
- 33.Maritza H, Julio A, Pedro A, Magalis B, Jose B, Mario S, et al. New caffeic acid esters from Plazia daphnoides. Naturforsch. 2003;58c:39–41. doi: 10.1515/znc-2003-1-206. [DOI] [PubMed] [Google Scholar]
- 34.Buck WB, Osweiled GD, Van Gelder AG. 2nd ed. Iowa: Kendall/Hunt Publishing Company; 1976. Clinical and Diagnostic Vetrinary Toxicology. [Google Scholar]
- 35.Olorunnisola DS, Amao IS, Ehigie DO, Ajayi ZA. Antihyperglycemic and hypolipidemic effect of ethanolic extract of Chrysophyllum albidum seed cotyledon in alloxan induced diabetic rats. Res J Appl Sci. 2008;3:123–7. [Google Scholar]
- 36.Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20:933–56. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 37.Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74:2157–84. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hnatyszyn O, Mino J, Ferraro G, Acevedo C. The hypoglycemic effect of Phyllanthus sellowianus fractions in streptozotocin-induced diabetic mice. Phytomedicine. 2002;9:558–9. doi: 10.1078/09447110260573209. [DOI] [PubMed] [Google Scholar]
- 39.Addae-Mensah I, Munenge RW. Quercetin-3-neohesperidoside (rutin) and other flavonoids as the active hypoglycemic agents of Bridelia ferruginea. Fitoterapia. 1989;60:359–62. [Google Scholar]
- 40.Uhrman B, Volkova N, Kaplan M, Presser D, Attias J, Hayek T, et al. Antiatherosclerotic effects of licorice extract supplementation to hypercholesterolemic patients. Nutrition. 2002;18:268–73. doi: 10.1016/s0899-9007(01)00753-5. [DOI] [PubMed] [Google Scholar]
- 41.Koshy AS, Anila L, Vijayalakshmi NR. Flavonoids from garcinia cambogia lower lipid levels in hypercholesterolemic rats. Food Chem. 2001;72:289–94. [Google Scholar]
- 42.Abdel-Sattar E, Abdel-Monem AR, Sleem AA. Biological and chemical study of Cleome paradoxa B.Br. Pharmacognosy Res. 2009;1:175–8. [Google Scholar]
- 43.Wang HX, Ng TB. Natural products with hypoglycemic, hypotensive, hypocholesterolemic, antiatherosclerotic and antithrombotic activities. Life Sci. 1999;65:2663–77. doi: 10.1016/s0024-3205(99)00253-2. [DOI] [PubMed] [Google Scholar]
- 44.Howard BV, Kritchevsky D. Phytochemicals and cardiovascular disease. Circulation. 1997;95:2591–3. doi: 10.1161/01.cir.95.11.2591. [DOI] [PubMed] [Google Scholar]
- 45.Peifer JJ. Hypocholesterolemic effects induced in the rats by specific types of fatty acid unsaturation. Nutrition. 1966;88:351–8. doi: 10.1093/jn/88.3.351. [DOI] [PubMed] [Google Scholar]


