Abstract
Introduction:
The tuberculosis control program is based on a felt need–oriented basis. The diagnosis is mainly microbiological. However, sputum smear-negative Acid Fast Bacilli (AFB) cases with suspected radiological findings can be problematic in diagnosis.
Objectives:
To confirm the diagnosis of tuberculosis early, in smear-negative AFB cases by using a Fiberoptic Bronchoscope.
Materials and Methods:
We embarked on Fiberoptic Bronchoscopy (FOB) and Spot Scopy smear Microscopy (SSM) for 533 suspected Pulmonary Tuberculosis (PT) cases (sputum smear negative and radiologically suggestive) from February 2007 to May 2010. FOB was performed using a special device, a Trans Oro Pharyngeal Spacer (TOPS), as a conduit.
Results:
The yield for positivity for AFB was 341 (64%) out of 533 cases.
Conclusion and Recommendation:
The specimens collected by using the fiberoptic bronchoscope confirmed the disease in the smear-negative cases. Hence, FOB was recommended in smear-negative cases, to avoid delay in the treatment of tuberculosis.
Keywords: Fiberoptic bronchoscopy, pulmonary tuberculosis, spot scopy smear microscopy, trans oro pharyngeal spacer
INTRODUCTION
Microbiological diagnosis is the main stay for the effective treatment of Pulmonary Tuberculosis (PT). For obtaining the correct sputum sample, patient education is imperative. However, even if the correct sample is expectorated, the bacillary population has to be at least 10000 per milliliter, to get the smear positive for Acid Fast Bacilli (AFB).[1] Moreover, it depends on the previous treatment, default behavior, and effective cough. Again 31% of the new cases may be smear-negative for AFB.[2] As such, it opens a Pandora's box in the diagnosis, as there are no authentic radiological diagnosis criteria for PT. The Computed Tomography (CT) of the chest only increases the guessing probability for the clinician, but does not lead to a photo-finish diagnosis.
However, a fiberscope can reach the pathological site of the bronchopulmonary segments and the lavage, brush smear, and post scopy sputum can reflect the correct collection of the specimen. Hence Fiberoptic Bronchoscopy (FOB) was done in cases having undiagnosed radiological panorama. All were sputum smear-negative for AFB twice and exfoliative cytology was suggestive of inflammation. The highlight of this study was the usage of the Trans Oro Pharyngeal Spacer (TOPS), which was a multipurpose one. This avoided contamination with the nasal and oral flora, and if necessary, respiratory toilet or emergency intubation could be done through this. This further induced cough, tickling the pharynx resulting in better expectoration.
The objective of the study was to confirm the presence of AFB in the pulmonary lesions in smear-negative pulmonary tuberculosis cases for earlier initiation of the treatment.
MATERIALS AND METHODS
This study has been conducted by the Department of Microbiology and Department of Respiratory Medicine, Ganni Subba Lakshmi (GSL) Medical College, Rajahmundry, from February 2007 to May 2010. Our teaching hospital attached to the medical college has thousand beds and is a referral center for TB treatment in this area.
In this study we considered patients with suspected PT, that is, cough of more than two weeks. All the cases — new, old, and default, were considered. Both male and female patients were included. Patients who were not cooperative and children below 14 years were excluded from the study (getting sputum sample was difficult). First, smears were made with the sputum samples to detect AFB as per the Revised National Tuberculosis Control Program (RNTCP) guidelines.[3] All smears positive for AFB cases were referred to the clinician for anti-tuberculosis treatment (ATT) and smears negative for AFB cases were preceded by FOB, and were then included in the study. Written informed consent was taken from all the individuals participating in the study, as also an endorsement from the Ethical Committee of the institute. An algorithm explains the exact procedures of this study [Figure 1].
Figure 1.
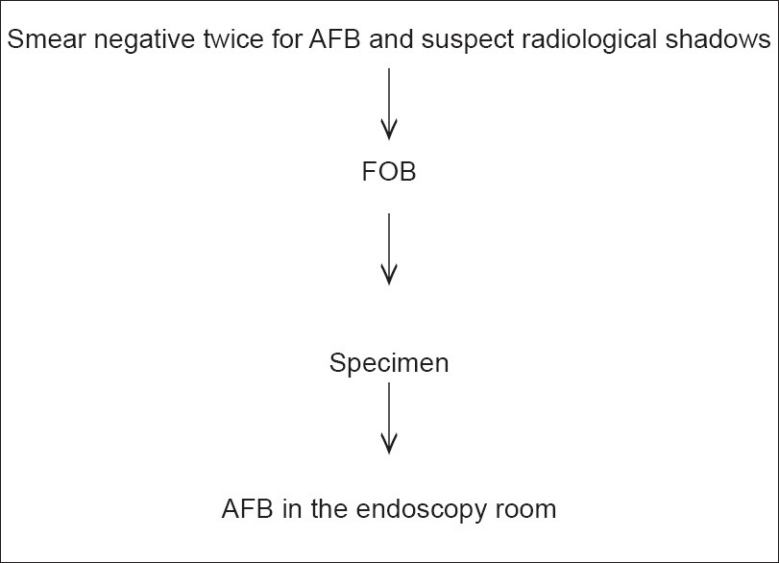
Algorithm of the study
The FOB was done by FUJINON-BRO YL2, Japan, transorally, TOPS was used to avoid contamination. The subject was kept fasting for three hours before the procedure. Syrup Codine phosphate 30 ml was given to suppress the cough. Injection Atropine 0.01mg/kg IM was given half an hour before the procedure. Midazolam was given IV, 0.05 mg/kg, as and when required. Bronchodilator, Salbutamol 1 ml + 1 ml normal saline was given via a nebulizer to all these subjects as they had bronchospasm earlier. The subjects were directed to rinse the oral cavity with 2 ml of Lidocaine jelly before the procedure and Lidocaine 2% was liberally used through the channel of FOB, not exceeding 200 mg in total.
Bronchial lavage from the affected segment, brush smear, and post scopy sputum were also collected for smear examination to detect AFB by Ziehl Neelsen (ZN) staining.
The main features of the study design were:
The microbiologist picked up the correct portion of the bronchial lavage in the endoscopy room and the samples were examined immediately after ZN staining.
The total Xylocaine used, on an average, was 3 ml from the vocal cords down to the bronchial tree.
FOB was done through TOPS, patented by the senior author and manufactured by the Pran Clinic and Co., Rajahmundry, 2004 model. This was a mechanical devise to deliver the aerosol medicine for the symptomatic treatment of bronchial asthma. This was made of ethylene vinyl acetate copolymer. It was 10 cm in length, with a 10 mm internal diameter [Figure 2]. There was a universal adapter in front, which had a straight part and an arched section that bent twice with angles of 110° and 150°. As per the Mallampati classification, the oropharynx could be classified into classes I, II, III, and IV, depending upon the patency of the oropharyngeal lumen.[4] Classes III and IV were narrower. In such cases the TOPS was much more useful [Figure 3], as it lifted the uvula [Figure 4], and FOB or blind intubation could be done easily through TOPS. It also snugly fitted into the pharynx, the distal end being just above the epiglottis. The advantage of using this was avoidance of contamination with the oropharyngeal / nasal flora and easy passage of the scope. If there was bleeding, effective toileting could be done and intubation also could be achieved.
Figure 2.
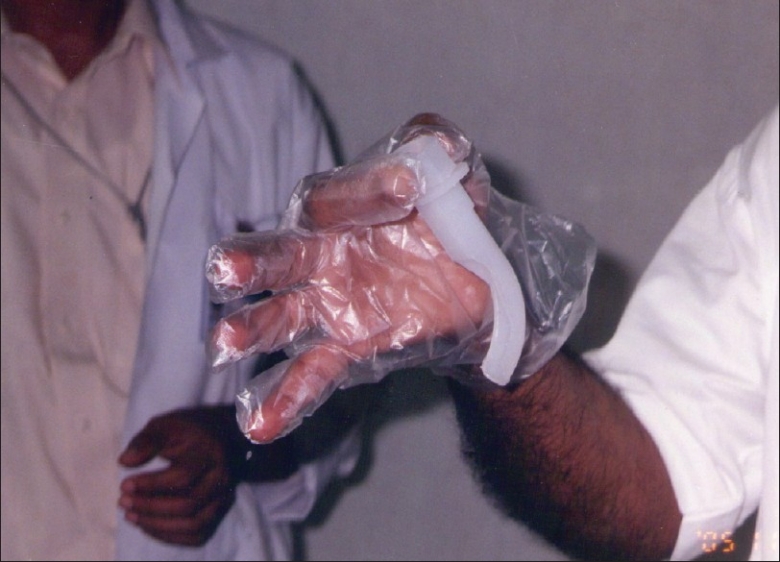
TOPS
Figure 3.
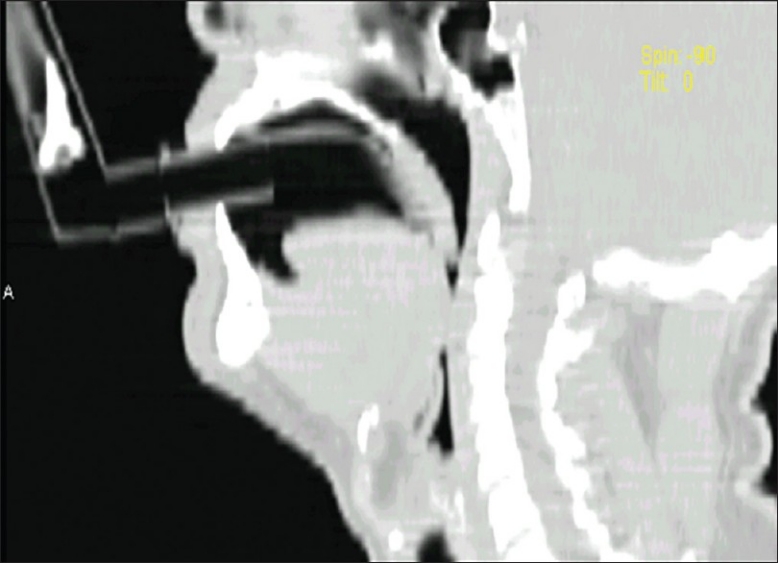
TOPS in class III oropharynx
Figure 4.
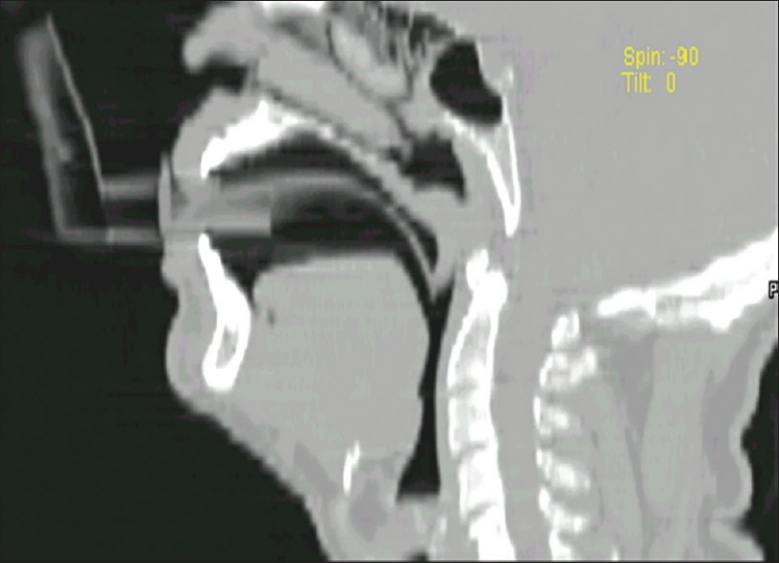
TOPS lifting Uvula
The highlight of the study is the use of the Trans Oro Pharyngeal Spacer (TOPS), which is an innovative product, by the senior author. It helps in easy passage of the tube, and bronchoscope bite is avoided. Adding to this, there have been no complaints of discomfort from the patients while passing the scope. As TOPS is the first of its kind no other references are available.
OBSERVATIONS
Out of 533 individuals in our study, there were 322 males and 211 females (mean age was 39.4 years). The radiological profile [Table 1] revealed that 291 (54.5%) patients had unilateral minimal radiological extent and the remaining 242 (45.5%) had bilateral moderately advanced disease.[5] In the bilateral disease, the lavage and brush biopsy were apparently obtained from the most affected area.
Table 1.
Radiological profile

The samples of lavage, brush smear, plus post scopy sputum were positive for AFB, in 241 cases. Only post scopy collection of sputum within 24 hours was positive for AFB in 100 cases [Table 2]. Thus in 341(64%) subjects, the bacteriological diagnosis was clinched. In the AFB-positive patients the quantitative analysis showed scanty (1 – 9 AFB in 100 fields) results in 260 (76%) and 1+ (10 – 99 AFB in 100 fields) bacillary load in the remaining 81 (24%) patients.[3] This spectrum of cases, after establishing the cause and effect, were put on effective ATT.
Table 2.
Acid Fast Bacilli yield by fiberoptic bronchoscopy

DISCUSSION
An editorial quoted that 40% of the cases with initial sputum AFB-negative status continued to be sputum negative, while 60% of them had progressive disease later.[6] Previous reports in non-immunocompromised patients with sputum smear-negative TB indicated that bronchoscope specimens and post bronchoscope sputum are helpful in establishing the diagnosis.[7,8] As per Adelaithi M et al.,[9] diagnostic FOB is often performed before the final results of the sputum analysis are available, to exclude other common infections, such as Pneumocystis carinii pneumonia, to confirm PT. Hence, the present study adumbrated a similar picture helping the clinician to treat the cases immediately not withstanding the radiological shadows.
Fiberoptic Bronchoscopy through TOPS ensured ease of procedure and the secretions adhering inside the conduit were useful for microscopic examination. An immediate diagnosis of PT in smear-negative cases could be established with the bronchoscope, which was 45.3% in our study. Similar results were reported by Steve H[10] et al. in a study. However, there was some difference in the AFB yield of post scopy sputum. In our study 19% of the post scopy smears were positive for AFB, whereas, it was 32% in the study by Steve H[10] et al.
The usage of brush for smear is mandatory when doing bronchoscopy.[11] We have improved over this recommendation by examining the bronchial lavage, brush smear, and post scopy sputum with a stand-by microbiologist. The specimens were examined and collated by both the pulmonologist and microbiologist, avoiding any false negative reports.
One feature of this study was that the residual 191 (36%) cases (negative for AFB by all means) were not administered treatment for PT and saved the grace. They were managed symptomatically. It was quite possible that they had treatment elsewhere inadvertently or non-tubercular granulomatous lung disease had to be entertained.
CONCLUSION
The specimens collected by bronchoalveolar lavage, brush smear, and post scopy sputum, should be evaluated together for AFB positivity in a smear negative for AFB cases. They have to be examined immediately avoiding transit delay, contamination, and false reading. Collection of post scopy sputum in the next 24 hours and examining post scopy sputum for AFB is also an important aspect of this study.
As per the Revised National Tuberculosis Control Program (RNTCP) algorithm, in a symptomatic patient, if sputum smear is negative for Acid Fast Bacilli (AFB), we have to wait for 10 – 14 days, with broad spectrum antibiotics. After this time period also, if the sputum sample is negative for AFB and if the chest X-ray is suggestive of TB, then the patient will be given anti-TB treatment by categorizing him/her as sputum negative pulmonary Tb. In this manner, the two-week time period is wasted and without AFB patient may have to start ATT. Hence, the bronchoscope can help us in the rapid diagnosis of the TB.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil
REFERENCES
- 1.Katoch VM. Newer diagnostic techniques for tuberculosis. Indian J Med Res. 2004;120:418–28. [PubMed] [Google Scholar]
- 2. Annual status report, Revised National TB Control Programme, TB India 2011.
- 3.Revised National Tuberculosis Control Programme, Central TB division, directorate general of health services. Nirman Bhavan, New Delhi, India: 2005. Technical guidelines for Tuberculosis control. [Google Scholar]
- 4.Mallampati classification. Dept. of Anasthesiology, University of Florida. [Last accessed on 2010 Sep 14]. Available from: http://www.anest.edu/at/cases/mallappati.html .
- 5.Rawat J, Sindhwani G, Juyal R. Clinico- radiological profile of new smear positive pulmonary tuberculosis cases among young adult and elderly people in a tertiary care hospital at Deheradun (Uttarakhand) Indian J Tuberc. 2008;55:84–90. [PubMed] [Google Scholar]
- 6.Anonymous sputum negative patients (editorial) Indian J Tuberc. 1979;26:173–4. [Google Scholar]
- 7.Jell JR, Cortese DA, Dines DE. The value of bronchoscopy in the diagnosis of mycobacterial disease. Chest. 1981;80:575–8. doi: 10.1378/chest.80.5.575. [DOI] [PubMed] [Google Scholar]
- 8.Danek SJ, Bower JS. Diagnosis of pulmonary tuberculosis by flexible fiberoptic bronchoscopy. Am Rev Respir Dis. 1979;119:677–9. doi: 10.1164/arrd.1979.119.4.677. [DOI] [PubMed] [Google Scholar]
- 9.Miro AM, Gibilara E, Powell S, Kamholz SL. The role of fiberoptic bronchoscopy for diagnosis of pulmonary tuberculosis in patients at risk for AIDS. Chest. 1992;101:1211–4. doi: 10.1378/chest.101.5.1211. [DOI] [PubMed] [Google Scholar]
- 10.Salzman SH, Schindel ML, Aranda CP, Smith RL, Lewis ML. The role of bronchoscopy in the diagnosis of pulmonary tuberculosis in patients at risk for HIV infection. Chest. 1992;102:143–6. doi: 10.1378/chest.102.1.143. [DOI] [PubMed] [Google Scholar]
- 11.Swarnakar JS. Proceedings of the VI national update on Fibreoptic Bronchoscopy. Hyderabad: India: 1991. Dec 8th, Fibreoptic Bronchoscopy in the diagnosis of smear-negative pulmonary tuberculosis; p. 6. [Google Scholar]


