Abstract
The present study was undertaken to study the sunscreen activity of herbal formulation. There is no evidence of the sun protection factor (SPF) studies on essential oil of Calendula flowers (Calendula officinalis L., Asteraceae). The study investigates the in vitro SPF by ultraviolet specrtophotometry method of Calendula flower oil in a cream formulation. Calendula oil was isolated by Clavenger's apparatus, compositions were identified by GC–MS and the cream of calendula flower oil was prepared by homogenization method followed by evaluation for physical parameters. The sun protection factor of cream was evaluated by in vitro method employing UV–visible spectrophotometer (Shimazdu-1600). The SPF of Calendula oil in cream formulation exhibited good activity (SPF = 14.84 ± 0.16). Finding of this study suggested that calendula oil cream can be used to protect the skin from UV radiations in form of sunscreen cream and to maintain the natural pigmentation of the skin.
Keywords: Calendula officinalis, calendula oil, essential oil, sun protection factor
INTRODUCTION
Applying a sunscreen to skin changes the way the body reacts to the sun's rays.[1] Sunscreens are like medicine we apply to our skin to keep it healthy.[2] The proof of sunscreen products efficacy is of great significance for the protection of public health as the UVB rays of solar radiation is the main contributor to skin sunburn, immunosuppression, and skin cancer.[3–5] Standard test methods for SPF evaluation may offer consumers worldwide consistent values efficacy of sunscreen products.[6] According to the USFDA and COLIPA guidelines, the SPF of a sunscreen product is calculated as the ratio of the minimal erythema dose (MED) of sunscreen-protected skin to the MED of unprotected skin and performed on in vivo on human volunteers.[7] In general, the test sunscreen sample is applied at a thickness of 2 mg/cm2 when evaluating the SPF.[8,9]
Calendula officinalis L. (Asteraceae) is an important plant of genus Calendula (marigolds), having several medicinal application in India and all over the world.[10] Calendula is fast growing annual herb, easy to germinate and simple to care. Calendula flower is often used in skin care products because of assistance in cell rejuvenation, wound healing, reducing inflammation, soothing, and softening the skin.[11] Oil of calendula flower has shown marked presence of flavonoids, coumarines, quinones, volatile oil, carotenoids, and amino acids.[12] The calendula oil is having great potential to quench the free radical reactions; hence, its application in area of antioxidant as cosmetics cannot be ignored.[13]
The present study includes the determination of SPF as cosmetic use of Calendula officinalis flower essential oil. In order to evaluate the composition of isolated oil, GC–MS analysis was performed. The cream base formulation containing isolated Calendula oil was evaluated for SPF in vitro by using ultraviolet spectrophotometric method.
Mansur et al. (1986) developed a very simple mathematical equation which is cost effective, rapid and easy in operation for in vitro determination of SPF utilizing UV Spectrophotometry with following equation.[14]

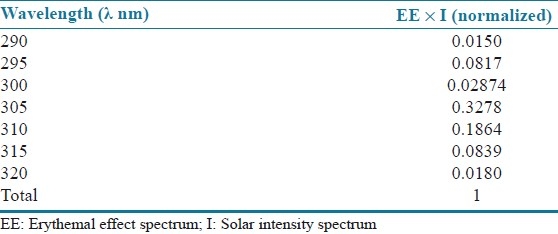
where EE(I) is the erythemal effect spectrum; I(I) is the solar intensity spectrum; Abs is the absorbance of sunscreen product; CF is the correction factor (=10). The value of EE × I are constant, determined by Sayre et al. (1986) and presented in [Table 1].[15]
Table 1.
Normalized product function used in the calculation of SPF
The present study was designed with an objective to in vitro determination of sun protection factor, using UV–visible spectrophotometer (Shimazdu-1600, Japan), of the investigational sunscreen cream sample.
MATERIALS AND METHODS
Plant material
The flowers from plant for proposed study were collected from botanical garden of IFTM; District Moradabad, Uttar Pradesh India in the month of January 2010. At the time of flower collection, plant was of three and half month old. The specimens were identified, authenticated and voucher specimen of herbarium (ref. no. IFTM/Pharmacog/Auth/10/2 dated 29.1.2010) is preserved in Pharmacognosy division of IFTM, Moradabad.
Isolation of calendula oil
Fresh calendula flowers were obtained from the botanical garden; the petals were separated and washed thoroughly under running water. The excess water was drained out completely and the petals were packed in distillation flask of Clavenger's apparatus with sufficient quantity of water and few pieces of porcelain chips to avoid bumping during distillation.
The extraction was continued for 8 h. The calendula oil was collected from graduated receiver and purified by anhydrous sodium sulphate for removing water traces. The yield of the oil obtained was found to be 1.25%.
Evaluation of physicochemical parameters
Upon successful isolation of oil, the physico-chemical parameters for oil was evaluated which included specific gravity (0.795 g/ml at 25°C), viscosity by Brookfield viscometer (15 Cp) and ester value (113.43), acid number (3.37), saponification value (116.8).[16] For acid value determination, 1 g of calendula oil was taken in a dried conical flask containing 25 ml of absolute alcohol and added (2–3) drops of phenolphthalein and resulting mixture was heated with shaking on water bath for 10 min, then cooled and finally titrated the solution against 0.1 N KOH until pink color appears (end point). The calculation was done as per Eq. (1).
For saponification value determination, it was performed by taking approximately 1 g of the oil into a 250 ml round bottom flask. In this, 25 ml of alcoholic potassium hydroxide solution (0.5 N) added and fitted with reflux condenser. Finally, flask contents were heated on a boiling water bath for 1 h with occasional shaking. While the solution was still hot, added three drops of phenolphthalein indicator and titrated with the excess potassium hydroxide with the 0.5 N hydrochloric acid (V ml of hydrochloric acid at end point represented by C). By same procedure, without sample, titration was performed (V ml of hydrochloric acid at end point represented by B). The saponification value was calculated by formula stated in Eq. (2). The ester value is defined as the mg of KOH required to react with glycerin (glycerol/or glycerin) after saponify 1 g of fat. It is calculated from the saponification value and the acid value as formula in Eq. (3).
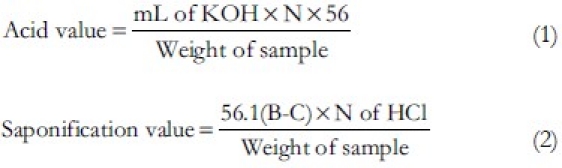
Where B is ml of HCl required by Blank, C is ml of HCl required by Sample.
Ester value = Saponification value - Acid value (3)
GC–MS analysis of calendula oil
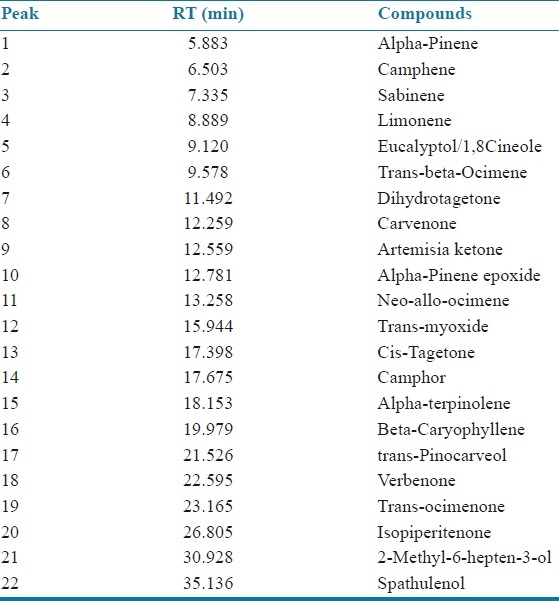
GC–MS analyses were performed on a capillary gas chromatograph directly coupled with mass spectrometer having thermal desorption system (model Shimadzu QP-2010 TD 20). AB-Innowax column (60 m length × 0.25 mm id × 0.25 μm film thickness) was used under the following conditions: Column oven temp: 70.0°C, injection temp: 260.00°C, pressure 156.7 kPa, total flow: 40.5 ml/min, column flow: 1.21 ml/min, the volume of injected sample 0.1 μl of oil, split ratio: 30.0, ion source temperature: 250.00°C, interface temp: 260°C with scan m/z starts from 40.00 and end at m/z: 950.00. MS spectra of separated compounds were compared with one from Wiley 7 Nist 05 mass spectral database. The compounds characterized from isolated calendula oil by GC–MS are presented in Figure 1 and Table 2.
Figure 1.
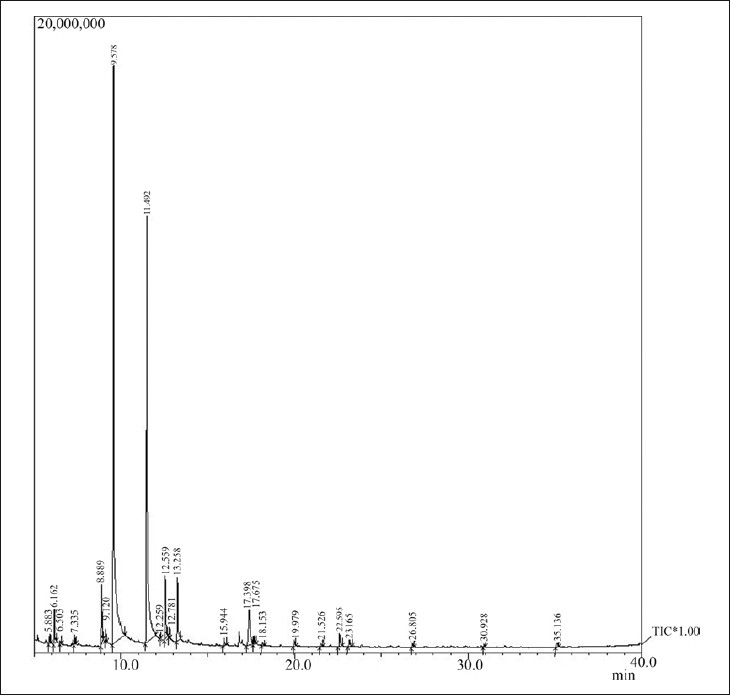
GC–MS chromatogram of essential oil of Calendula officinalis
Table 2.
Composition of calendula oil
Preparation of cream formulation
Reddening of the skin, as a direct result of UVB radiation, is an excellent warning sign to avoid further sun exposure.[17] As UVB is 1000 times more erythemogenic than UVA, SPF indicates chiefly the acute protection against UVB, but it predicts no indication of a product's protection against UVA.[18] Products with the same SPF may have quite different absorption spectral profiles and sunscreens with high SPF do not guarantee protection against UVA. In current market, there is a demand of such stable sunscreen cream but the same is a challenge because difficulty to stabilize the components, no irritancy, easy spreadibility, feel effect after application, etc. In the present study, for the formulation purpose, oil phase was comprised of stearyl alcohol, bees wax, sorbitan monooleate, whereas sorbitol solution, polysorbate 80, methyl paraben, propyl paraben, and deionized water constituted the aqueous phase. Calendula flower oil 5% was used as active sunscreening agent in cream formulation.
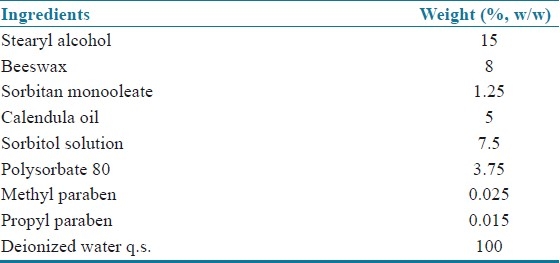
Cream formulation of isolated Calendula oil from flowers of Calendula officinalis were prepared using formula given in Table 3.
Table 3.
Formulation content
All the content of oil phase and water phase was heated up to 70°C separately. Afterwards the oil phase was added slowly to aqueous phase with continuous stirring to form a crude emulsion. It was cooled to about 55°C and homogenized. The resulting materials were cooled with agitation until congealed. The cream was stored at room temperature afterwards calendula oil was weighed (5%) and incorporated in the formed emulsion under constant homogenization. Whole formulation was stored in closed amber colored glass bottle.
Evaluation of physical parameters of cream
The prepared formulation was evaluated for its sensory evaluation, pH, spreadibility, specific gravity, conductivity, zeta potential, poly dispersity index (PDI), globule size, and skin irritancy, as per official methods, and results are mentioned in [Table 4].
Table 4.
Physical parameters of calendula oil cream

Determination of in vitro sun protection factor
A quantity of 1.0 g of prepared cream was weighed, transferred to 100 ml volumetric flask, and finally diluted to volume with ethanol. Further, it was kept for ultrasonication for 5 min and filtered through cotton filter, discarding the ten first ml. A 5.0 ml aliquot was transferred to 25 ml volumetric flask and the volume was adjusted with ethanol.[19]
The absorbance spectra of sample in solution form were obtained in range of 290–320 nm, every 5 nm interval, and three determinations were made at each point, followed by application of Mansur equation.
RESULTS
The isolated calendula oil was studied for its composition by employing GC–MS study. The compositions of essential oils are shown in Table 1. Twenty two compounds were characterized out of which trans-beta-Ocimene (46.18%), dihydrotagetone (31.66%), Cis-Tagetone (4.63%), Artemisia ketone (3.42%) Neo-allo-ocimene (3.74%), Limonene (2.69%), Verbenone (0.93%) are in major amount. The compounds characterized from isolated calendula oil are presented in Table 3. After formulating the cream of essential oil of Calendula officinalis, physical parameters were evaluated for all physical parameters of cream as well as for in vitro sun protection factor. The results of physical parameters of cream and sun protection factor test summarized in Tables 4 and 5 showed that cream parameters complies as per official acceptance criteria's, and SPF for calendula oil cream formulation 14.84 ± 0.16 shows a good sun protection activity to protect the skin from sunlight and erythema [Table 5].
Table 5.
Result of SPF determination of calendula oil cream

DISCUSSION
The SPF is a quantitative approach to measure the effectiveness of a sunscreen formulation.[20] So far as the performance of topical sunscreens is concerned, persons are more conscious about the effectiveness of sunscreens. Hence, for a cream to be an effective one, it must be capable in preventing sunburn and should be having a wide range of absorbance between 290 and 400 nm. Since a long time, in vivo tests are performed with human volunteers for evaluation of SPF of sunscreen formulations.[21] In vivo test is time consuming and includes various degree of variability. The in vitro SPF is useful for screening test during product development as a supplement of the in vivo measure. No evidence was found from the literature survey on the sun protection factor studies on Calendula officinalis flower essential oil but it was reported that tribal persons uses the calendula extract and oil to cure various dermatological ailments as skin injuries and in some cases of burns. By the present work, it was novel finding that Calendula officinalis flower oil is having sun protection activity.
CONCLUSION
The study showed that calendula oil cream is having good sun protection activity and hence it can be used in sun protecting formulations. The proposed UV spectrophotometric method is simple, rapid and cost effective and can be used in the in vitro determination of SPF values in many cosmetic formulations during production process.
ACKNOWLEDGEMENTS
Authors would like to express sincere gratitude towards Prof. R. M. Dubey MD, IFTM-Moradabad for providing necessary facilities and Mr. Ajai Kumar Incharge GC–MS, AIRF, JNU, New Delhi to complete this work with great ease.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bonda C. Sunscreen Photostability. Happi J. 2009;10:1–4. [Google Scholar]
- 2.Draelos ZD. Helpful ideas for enhancing patient sunscreen compliance. Cosmet Dermatol. 2005;18:638–40. [Google Scholar]
- 3.Dogra S, Prasad D, Handa S. Narrowband ultraviolet B in air borne contact dermatitis: A ray of hope. Br J Dermatol. 2004;150:373–4. doi: 10.1111/j.1365-2133.2004.05724.x. [DOI] [PubMed] [Google Scholar]
- 4.Weissman AM. Regulating protein degradation by ubiquination. Immunol Today. 1997;18:189–98. doi: 10.1016/s0167-5699(97)84666-x. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin CL, Ananthaswamy HN. p53 and the pathogenesis of skin cancer. Toxicol Appl Pharmacol. 2007;224:241–8. doi: 10.1016/j.taap.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bendova H, Akrman J, Krejc A, Kuba L, Jırova D, Kejlova K, et al. In-vitro approaches to evaluation of sun protection factor. Toxicol In Vitro. 2007;21:1268–75. doi: 10.1016/j.tiv.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 7.Diffey BL, Robson J. A new substrate to measure sunscreen protection factors throughout the ultraviolet spectrum. J Soc Cosmet Chem. 1989;40:127–33. [Google Scholar]
- 8.Haywood R, Wardman P, Sanders R, Linge C. Sunscreens inadequately protect against ultraviolet-A-induced free radicals in skin: Implications for skin aging and melanoma? J Invest Dermatol. 2003;121:862–8. doi: 10.1046/j.1523-1747.2003.12498.x. [DOI] [PubMed] [Google Scholar]
- 9.Stephens TJ, Herndon JH, Colon LE, Gottschalk RW. The impact of natural sunlight exposure on the UVB-sun protection factor (UVB-SPF) and UVA protection factor (UVA-PF) of a UVA/UVB SPF 50 sunscreen. J Drugs Dermatol. 2001;10:150–5. [PubMed] [Google Scholar]
- 10.Braga PC, Dal SM, Culici M, Spallino A, Falchi M, Bertelli A, et al. Antioxidant activity of Calendula officinalis extract: Inhibitory effects on chemiluminescence of human neutrophil bursts and electron paramagnetic resonance spectroscopy. Pharmacology. 2009;83:348–55. doi: 10.1159/000217583. [DOI] [PubMed] [Google Scholar]
- 11.Mishra AK, Mishra A, Chattopadhayay P. Calendula officinalis: An important herb with valuable therapeutic dimensions-An overview. J Glob Pharm Technol. 2010;2:14–23. [Google Scholar]
- 12.Kasprzyk Z, Pyrek J. Triterpenic alcohols of Calendula officinalis L.flowers. Phytochemistry. 1968;7:1631–9. [Google Scholar]
- 13.Guinot P, Gargadennec A, Valette G, Fruchier A, Andary C. Primary flavonoids in marigold dye: Extraction, structure and involvement in the dyeing process. Phytochem Anal. 2008;19:46–51. doi: 10.1002/pca.1014. [DOI] [PubMed] [Google Scholar]
- 14.Mansur JS, Breder MN, Mansur MC, Azulay RD. Determination of Sun protection factor by spectrophotometry. An Bras Dermatol. 1986;61:121–4. [Google Scholar]
- 15.Sayre RM, Agin PP, Levee GJ, Marlowe E. Comparison of in vivo and in vitro testing of sunscreening formulas. Photochem Photobiol. 1979;29:559–66. doi: 10.1111/j.1751-1097.1979.tb07090.x. [DOI] [PubMed] [Google Scholar]
- 16.Government of India: Controller of Publications. Vol. 2. Government of India, Ministry of Health and Family Welfare; 1996. Indian Pharmacopoeia; pp. A70–4. [Google Scholar]
- 17.Lavker RM, Gerberick GF, Veres D. Cumulative effects from repeated exposures to suberythemal doses of UVB and UVA in human skin. J Am Acad Dermatol. 1995;32:53–62. doi: 10.1016/0190-9622(95)90184-1. [DOI] [PubMed] [Google Scholar]
- 18.Rai R, Srinivas CR. Photoprotection. Indian J Dermatol Venereol Leprol. 2007;73:73–9. doi: 10.4103/0378-6323.31889. [DOI] [PubMed] [Google Scholar]
- 19.Dutra EA, Oliveira DA, Kedor-Hackmann ER, Miritello MI. Determination of sun protection factor (SPF) of sunscreens by ultraviolet spectrophotometry. Braz J Pharm Sci. 2004;40:381–5. [Google Scholar]
- 20.Pissavini M, Ferrero L. In vitro determination of sun protection factor. Glob Cosmet Manuf. 2004;4:1–5. [Google Scholar]
- 21.COLIPA, CTFA-SA, JCIA. International Sun Protection Factor (SPF) test method (COLIPA–The European Cosmetic Toiletry and Perfumery Association; CTFA-SA– Cosmetic, Toiletry and Fragrance Association of South Africa; JCIA – Japan Cosmetic Industry Association) 2003 [Google Scholar]


