Abstract
1,3,4-Oxadizoles form a biologically important group of compounds having activities like analgesic, anti-inflammatory, bactericidal, antifungal, anticonvulsant, psychotropic, plant growth regulating and mono amino oxidase inhibition. This research has focused on the incorporation of the oxadiazole moiety into isoniazid because of their versatile biological action, to get 2-aryl-5-(4-pyridyl)-1,3,4-oxadiazole to explore the possibilities of some altered biological action. 1,3,4-Oxadiazole derivatives were synthesized by microwave-assisted synthesis and screened for their analgesic, anti-inflammatory activities. The synthesized compounds were characterized by Melting point, Thin layer chromatographyInfra red, Nuclear magnetic resonance spectroscopy, etc. Almost all the synthesized compounds possessed good activity as compared to the standard.
Keywords: 1,3,4-Oxadiazole; analgesic; anti-inflammatory; isoniazid
INTRODUCTION
Microwave-enhanced synthesis[1,2] represents a fundamental step forward in the capabilities of synthetic chemistry. It allows organic chemists to work faster, generating higher yields with increased product purity, and to scale experiments up reliably from milligrams to much larger quantities without the need to alter reaction parameters. It offers much more precise control over conditions of temperature and pressure than any previous technology. Ultimately, by eliminating much of the time and effort from the process of performing chemical reactions, it allows chemists to focus on what is most important—the development of new compounds, or refined methods for generating known products. In a solvent-less reaction all the microwave energy is directly absorbed by the reactant molecules.[3] Under these conditions, the non-thermal microwave effect will be operative at high efficiency.
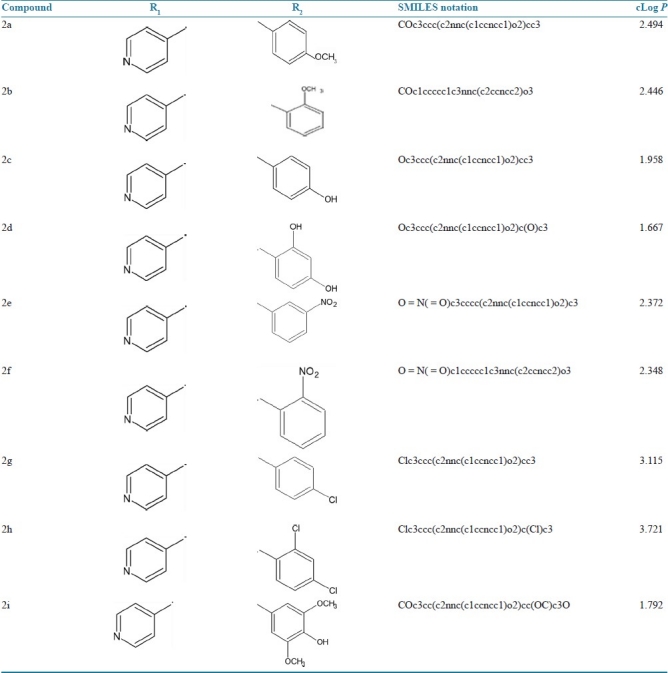
This work aims at the development of a newer isoniazid-based oxadiazole ring system. 1,3,4-Oxadiazole derivatives show a broad spectrum of biological activities, which include analgesic and anti-inflammatory, antimicrobial, anticonvulsant, antifungal, anticancer, antimycobacterial,[3,4] etc. The research envisages a meaningful exploration of this lead molecule for novel analgesic, anti-inflammatory activities with minimum toxicity and high potency.[5] The lead compound was structurally modified by incorporating various substitutions at the second and fifth position of the heterocyclic ring system [Table 1]. From a review of the literature it is clear that 2,5 disubstituted 1,3,4-oxadiazole derivatives of oxadiazole possess remarkable analgesic, anti-inflammatory activity.[5,6]
Table 1.
SMILES and cLog P values of proposed analogues (generated by molinspiration software)
MATERIALS AND METHODS
Microwave-assisted synthetic procedure
Step 1
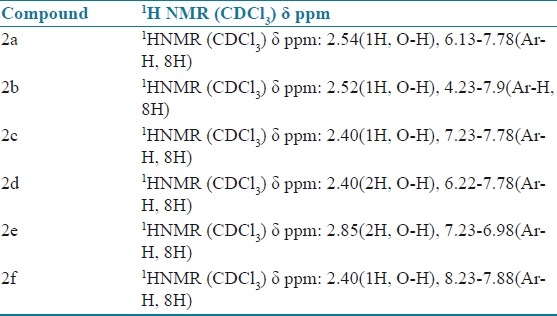
A mixture of (0.01 mole, 1.37 g) isoniazid, (0.01 mole) aromatic aldehyde and DMF (5 drops) was subjected to microwave irradiation at 300 w internally at 30-sec intervals for 3 min. The reaction mixture was cooled and treated with ice cold water. The resulting solid product was filtered, washed with water and recrystallized from ethanol [Table 2].[7–11]
Table 2.
Characteristic 1H NMR spectrum of the synthesized compounds
Step 2
To a solution of compound 1a (0.01 mole) in ethanol (15 ml), chloramine-T (0.01 mole) was added. The reaction mixture was exposed to microwave irradiation at 300W internally at 30-sec intervals for 4 min. The reaction mixture was cooled and digested with cold water. The solid thus obtained was filtered, washed with water and recrystallized from methanol to give the product [Figure 1].[9,11–13]
Figure 1.
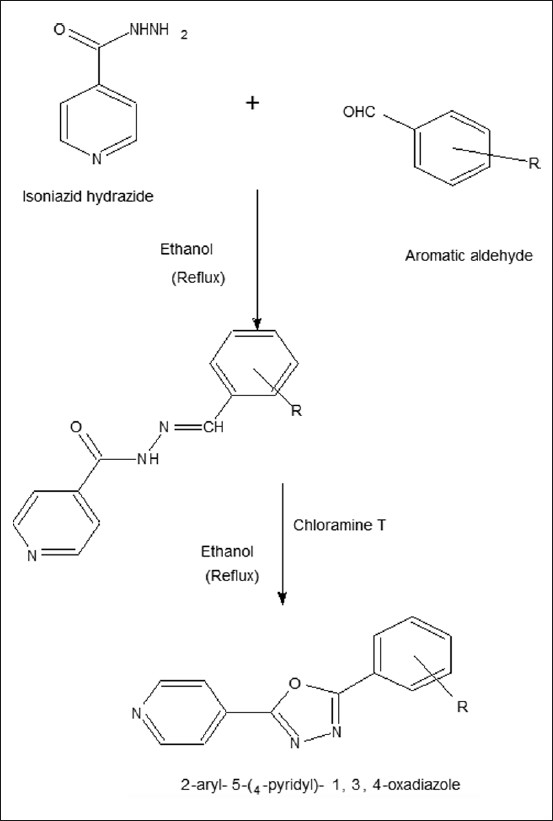
Synthetic scheme of 1,3,4 oxadiazole derivative
RESULTS AND DISCUSSION
The purity of the synthesized molecules was ascertained routinely by TLC, and melting points were noted with an open capillary tube method and are uncorrected.[12–16]
Infra-red spectral analysis
Infra-red (IR) spectra were recorded using KBr pellets in the range of 4000-500 cm–1 on Jasco FTIR model 4100 type A to elucidate the structure of the compounds [Table 3].
Table 3.
Characteristic IR peaks of the synthesized compounds

1H NMR spectral analysis
Proton NMR (300 MHz) spectra were recorded in CDCl3. Chemical shifts were recorded in parts per million downfield with reference to internal standard Tetra Methyl Silane (TMS) on BurkerAvance DPX 300. The total number of proton obtained from NMR spectra was in accordance with that of respective analogues.
PHARMACOLOGICAL SCREENING
Acute toxicity study
A prototype molecule was randomly selected for the study of the safety dose range of the analogues.[11,14,15] In this study, it was found that up to 1600 mg/kg dose, the compound is safe. i.e. there was no mortality or gross behavioral change in the animals used.[17–24]
SUMMARY AND CONCLUSION
This research work was focused on the rational approach in the design and development of 1,3,4 oxadiazole derivatives as novel analgesic, anti-inflammatory drugs.
The candidates which obeyed the Lipinski rule of five were taken for wet lab synthesis. Nine different analogues were synthesized by microwave methods and the purity of the compounds thus synthesized was ascertained by consistency in melting point and Rf value and characterized by UV, IR and 1H NMR spectral studies [Tables 4 and 5].
Table 4.
Physicochemical properties of the proposed analogues (generated by ACDLABS software)
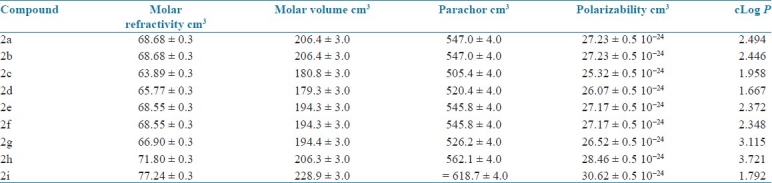
Table 5.
Physicochemical data of newly synthesized compounds
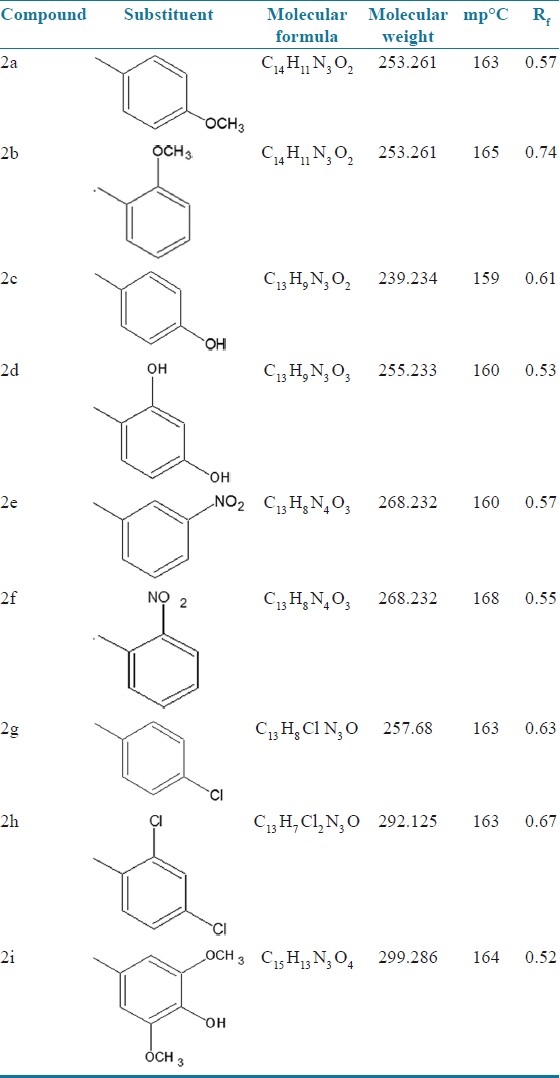
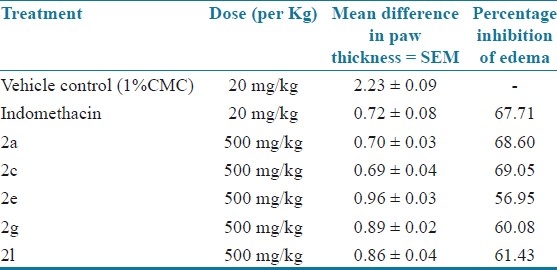
Among the newly synthesized 1,3,4 oxadiazole analogues five were screened for analgesic and anti-inflammatory activity and the compounds 2a, 2c and 2i showed good analgesic and anti-inflammatory activity. Acute toxicity studies showed that the analogues were safe with low toxicity. So these derivatives may be future leads for analgesic and anti-inflammatory drug discovery [Tables 6 and 7].
Table 6.
Analgesic activity (acetic acid-induced Writhing method)
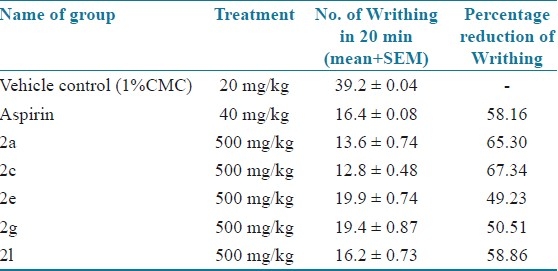
Table 7.
Anti-inflammatory activity (Carageenaninduced rat paw edema method)
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Khan MS, Chawla G, Mueed MA. Synthesis characterization and biological evaluation of substituted oxadiazole, and triazole derivatives. Indian J Chem. 2004;43B:1302–5. [Google Scholar]
- 2.Zheng L, Wang X, China PR, Wang X. Synthesis and antibacterial activities of 1,3,4-oxadiazole derivatives. Indian J Chem. 2003;42B:941. [Google Scholar]
- 3.Delhi: Controller of Publications; 1996. Govt. of India, Ministry of Health and Family Wefare, Indian Pharmacopoeia; p. 408. [Google Scholar]
- 4.Khan MS, Anther M. Microwave assisted synthesis of 2,5- disubstituted 1,3,4- oxadiazole derivatives. Indian J Chem. 2003;42B:900–4. [Google Scholar]
- 5.Hui XP, CnuCH , Zhang ZY. Synthesis and anti -inflammatory activities of 1,3,4-oxadiazole derivatives containing 5-methylisoxazole moiety. Indian J Chem. 2002;41B:2176–9. [Google Scholar]
- 6.David AW, Thomas LL. 5th ed. New York: Lippincott Williams and Wilkins; 2002. Foye's Principles of medicinal chemistry; pp. 12–7. [Google Scholar]
- 7.King FB. 2nd ed. UK: Royal Society of Chemistry; 2002. Medicinal chemistry Principles and practice. [Google Scholar]
- 8.Thomas G. New York: John Wiley and Sons; 2003. Fundamentals of medicinal chemistry; pp. 57–61. [Google Scholar]
- 9.Manfred EW. Synthesis and anti-inflammatory activity of 1,3,4-oxadiazole derivatives. 5th ed. New York: John Wiley and Sons; 2003. Burger's medicinal chemistry and drug discovery; pp. 10–6. [Google Scholar]
- 10.Patrick GL. New York: Oxford University Press; 2001. An introduction to medicinal chemistry; pp. 142–7. [Google Scholar]
- 11.Rand HP, Dale MM, Ritter MM, Moore PK. 5th ed. NewYork: Churchill Livingstone; 2003. Pharmacology; pp. 51–63. [Google Scholar]
- 12.David AW, Thomas LL. 5th ed. New York: Lippincott Williams and Wilkins; 2002. Foye's Principles of medicinal chemistry; pp. 751–94. [Google Scholar]
- 13.Jaime ND, William AR. 10th ed. New York: Lippincott-Raven; 1998. Wilson and Gisvold's text book of organic medicinal and pharmaceutical chemistry; pp. 687–795. [Google Scholar]
- 14.11th ed. New york: Mc Graw Hill company Publication; 1998. Goodman and Gilman's, The Pharmacological basis of therapeutics; pp. 617–47. [Google Scholar]
- 15.Jaime ND, William AR. 11th ed. New York: Lippincott-Raven; 2004. Wilson and Gisvold's text book of organic medicinal and pharmaceutical chemistry; pp. 822–9. [Google Scholar]
- 16.Chemical and Engineering News, American Chemical Soc. 2002;80:16. [Google Scholar]
- 17.Patrick GL. 2nd ed. New York: Oxford University Press; 2001. An introduction to medicinal chemistry; pp. 258–88. [Google Scholar]
- 18.Kappe CO, Dallinger D. The impact of microwave synthesis on drug discovery. Nat Rev Drug Discov. 2006;5:51–63. doi: 10.1038/nrd1926. [DOI] [PubMed] [Google Scholar]
- 19.Abd el-Samii ZK. Synthesis and anti-inflammatory activity of some novel 1,3,4-oxadiazole derivatives. J Chem Technol Biotechnol. 1992;53:143–6. doi: 10.1002/jctb.280530206. [DOI] [PubMed] [Google Scholar]
- 20.Mullican MD, Wilson MW, Connor DT, Kostlan CR, Schrier DJ, Dyer RD. Design of 5-(3,5-di-tert-butyl-4-hydroxyphenyl)-1,3,4-thiadiazoles, -1,3,4-oxadiazoles, and -1,2,4-triazoles as orally-active, nonulcerogenicantiinflammatory agents. J Med Chem. 1993;36:1090–9. doi: 10.1021/jm00060a017. [DOI] [PubMed] [Google Scholar]
- 21.Boschelli DH, Connor DT, Bornemeier DA, Dyer RD, Kennedy JA, Kuipers PJ, et al. 1,3,4-Oxadiazole, 1,3,4-thiadiazole, and 1,2,4-triazole analogs of the fenamates: In vitro inhibition of cyclooxygenase and 5-lipoxygenase activities. J Med Chem. 1993;36:1802–10. doi: 10.1021/jm00065a002. [DOI] [PubMed] [Google Scholar]
- 22.Omar FA. Synthesis of some novel 1,3,4-oxadiazole derivatives for anti diabetic activity. EurJ Med Chem. 1996;31:819–25. doi: 10.1016/0223-5234(96)83976-6. [DOI] [PubMed] [Google Scholar]
- 23.Palaska E, Sahin G, Kelicen P, Durlu NT, Altinok G. Synthesis and anti-inflammatory activity of 1-acylthiosemicarbazides, 1,3,4-oxadiazoles, 1,3,4-thiadiazoles and 1,2,4-triazole-3-thiones. Farmaco. 2002;57:101–7. doi: 10.1016/s0014-827x(01)01176-4. [DOI] [PubMed] [Google Scholar]
- 24.Jakubkiene V, Burbuliene MM, Mekuskiene G, Udrenaite E, Gaidelis P, Vainilavicius P. Synthesis and anti-inflammatory activity of 5-(6-methyl-2-substituted 4-pyrimidinyloxymethyl)-1,3,4-oxadiazole-2-thiones and their 3-morpholinomethyl derivatives. Farmaco. 2003;58:323–8. doi: 10.1016/s0014-827x(02)00022-8. [DOI] [PubMed] [Google Scholar]


