Abstract
Shilajit is a mineral-rich complex organic compound used in the traditional system of Ayurvedic medicine for treating hypertension and improving the cardiac function with many herbomineral preparations. However, very little experimental evidence is available about its effect on the cardiac function. We used Daphnia as a model organism for observing the effect of shilajit on its heart due to its myogenic properties and its response to number of cardioactive drugs that are known to affect human heart function. Genome of Daphnia shows the strongest homology with the human genome. These characteristics of Daphnia make it an ideal organism for biomedical research. Our results suggest that this complex organic compound lowers the heart beats as its concentration increases from 1.0 to 100 ppm. The beats come to near normal condition at 1000 ppm. Above 1000 ppm, the beats are very fast and impossible to count. These results indicate a negative chronotropic effect on the Daphnia heart at low concentrations and a positive chronotropic effect to arrhythmia and finally failure at increasing higher concentrations of shilajit.
Keywords: Chronotropic effect, Daphnia, shilajit
INTRODUCTION
Shilajit (L. Asphaltum) also known as “mineral pitch” is a pale brown to blackish brown rock exudates found in many mountain ranges of the world, especially the Himalayas and Hindukush ranges of the Indian subcontinent.[1] Shilajit is a complex mixture of organic humic substances as well as plant and microbial metabolites which occur in the rock rhizospheres.[2] Substances identified in Shilajit include moisture, gums, albuminoids, resin, vegetable matter, benzoic acid, silica, minerals, vitamins, and many other substances.[3,4] It is used for the last thousands of years as a rejuvenator and as an adaptogen in traditional medicinal systems of many countries[5,6] and has been attributed with miraculous healing properties.[7] Recent reports have revealed that it has antioxidant,[8] anti-inflammatory,[9] and anxiolytic activity.[10] It has also demonstrated spermiogenic and ovogenic effects in mature rats.[11] Although many herbomineral preparations aimed to treat hypertension and cardiac hypertrophy use shilajit studies related to its effect on blood pressure and cardiac hypertrophy have not been reported so far which prompted the present investigations.
In this study, we used Daphnia [Figure 1] as a model due to several reasons such as their high responsiveness to pharmacological agents added to culture water,[12] their transparent body enabling easy observations of heart beats, heart being myogenic with a myogenic pacemaker inhibited by extracardiac cholinergic nerves,[13] similarity of the ultrastructural features to striated and cardiac muscles of other species.[14] In addition, the genome of Daphnia shows strong homology with the human genome[15,16] which provides a provisional clinical relevance. Daphnia magna responds to many cardioactive drugs known to affect human heart function.[17,18] Thus, the organism is suitable to be used as a model for studying disease process, toxicological studies, and for biomedical research; However, there are many species in the genus which are poorly described taxonomically at the species level.
Figure 1.
Daphnia (Encyclopedia Brittanica Image)
MATERIALS AND METHODS
Preparation of different concentrations of Shilajit
Shilajit Sat was purchased from a local distributor and stored at ambient room temperature. Different concentrations were prepared in distilled water. Due to lack of previously reported information on its lethal dose in Daphnia and in humans, a starting concentration of 1 ppm was chosen so that the highest concentration did not exceed 100–200 mg/L. The concentration of shilajit was logarithmically increased (1, 10, 100, and 1000 ppm). The stock and working solutions of shilajit were maintained under refrigeration at 4–8°C during the course of this study [Figure 2].
Figure 2.
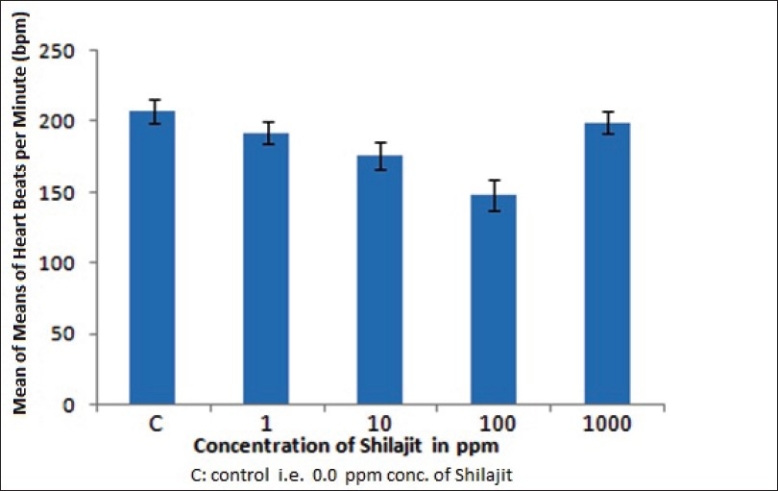
Observed mean of means of heart rate of daphnia following exposure to Shilajit at different concentrations
Collection of Daphnia
Daphnia were collected from the pond water from Ahmednagar College Campus without ascertaining the exact species. After the settling of debris, mud, and particulate matter, Daphnia were transferred to cavity block containing filtered pond water. Organism was maintained using pond culture method at ambient temperature in the laboratory till the completion of this study.
Counting of heart beats
After acclimatization for 10 minutes, an individual Daphnia was carefully transferred, to a cavity slide containing filtered pond water. Its heart beats were counted for 10 seconds, under the low power objective of Olympus compound optical microscope using a countdown timer with audible signal. In all, 25 such observations were taken and their mean was taken as a control reading. The organism was then carefully transferred to another cavity block containing 1 ppm Shilajit solution, acclimatized for 10 minutes, and 25 observations of heart beats for 10 second intervals were noted. The procedure was repeated for shilajit concentrations of 10, 100, and 1000 ppm using the same organism. Data were obtained for 20 individuals of Daphnia as described above.
Data analysis
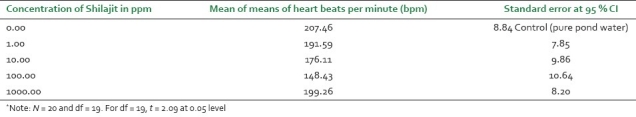
The mean value and standard error per organism per shilajit concentration were calculated. Similarly, the mean of means of heart beats per 10 seconds were calculated in each case and were converted to heart beats per minute with standard error. Both the data were tested using Students’ distribution (t-test) at 95% CI. The data of mean of means are shown in Table 1.
Table 1.
Mean of means of heart beats per minute of Daphnia at different concentrations of shilajit with standard error at 95 % confidence interval*
RESULTS AND DISCUSSION
The individual variations in the rhythm observed may be a result of body size, sex, and physiological factors. However, the individual rhythm appears to be fairly constant during treatment with shilajit concentrations of 1, 10, and 100 ppm. A rapid increase in heart beat frequency was observed when the organism was treated with shilajit concentration above 1000 ppm. The frequency increase was so rapid that heart beats could not be measured manually.
The data represented in Table 1 are the mean of means of heart beats of Daphnia per minute under control and test conditions with 95% CI. It was revealed that the frequency decreases by 7.65% at 1 ppm, 15% at 10 ppm, and 28.45% at 100 ppm treatments, respectively, indicating a negative chronotropic effect at low shilajit concentrations, whereas treatment with 1000 ppm showed a positive chronotropic effect. Although the exact mechanism of action is not yet analyzed, it is possible that the negative chronotropic effect may result from a direct effect on muscle or stimulation of the cholinergic nerves to the pacemaker.
The probable reason for the positive chronotropic effect may be due to mimicking of adrenaline- and noradrenaline-like effect or a change in Ca2+ levels which needs to be assessed during further studies. At increasing higher concentrations, shilajit may be leading to hypercalcemia in daphnia. This condition is associated with abnormal heart rhythm (arrhythmia) leading to cardiac arrest. We feel that the accelerating action of this drug on daphnian heart is of less physiological significance because it occurs only with high concentrations (>1000 ppm). This study being a preliminary investigation does not satisfactorily explain the related phenomena, but the results are still encouraging and of provisional clinical relevance prompting further in-depth studies for which authors intend to include echocardiographic analysis to strengthen their observations along with biochemical studies.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Ghosal S, Reddy JP, Lal VP. “Shilajit I: Chemical constituents”. J Pharm Sci. 1976;65:772–3. doi: 10.1002/jps.2600650545. [DOI] [PubMed] [Google Scholar]
- 2.Ghosal S, Lal J, Singh SK, Goel RK, Bhattacharya SK. “The need for formulation of shilajit by its isolated active constituents”. Phytother Res. 1991;5:211–6. [Google Scholar]
- 3.Ghosal S, Baumi KB, Chattopadhyay S. “Shilajit induced morphometric and functional changes in mouse peritoneal macrophages”. Phytother Res. 2006;9:194–8. [Google Scholar]
- 4.Ghosal S, Singh SK, Kumar Y, Srivastav R, Goel RK, Dey R, et al. “Antiulcerogenic activity of fulvic acids and 4- methoxy-6-carbomethoxybiphenyl isolated from shilajit”. Phytother Res. 2006;2:187–91. [Google Scholar]
- 5.Froton MH, Acharya SB. “Pharmacological studies of shilajit”. Indian J Pharmacol. 1984;16:45. [Google Scholar]
- 6.Acharya SB, Frotan MH, Goel RK, Tripathi SK, Das PK. “Pharmacological actions of shilajit”. Indian J Exp Biol. 1988;26:775–7. [PubMed] [Google Scholar]
- 7.Agarwal SP, Khanna R, Karmarkar R, Anwer MK, Khar RK. “Shilajit: A review”. Phytother Res. 2007;21:401–5. doi: 10.1002/ptr.2100. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharya SK, Sen AP. “Effects of shilajit on biogenic free radicals”. Phytother Res. 1995;9:56–9. [Google Scholar]
- 9.Goel RS, Acharya SB. Antiulcerogenic and anti-inflammatory studies with shilajit. J Ethnopharmacol. 1990;29:95–103. doi: 10.1016/0378-8741(90)90102-y. [DOI] [PubMed] [Google Scholar]
- 10.Jaiswal AK, Bhattacharya SK. “Effects of shilajit on memory, anxiety and brain mono amines in rats”. Indian J Pharmacol. 1992;24:12–7. [Google Scholar]
- 11.Park JS, Kim GY, Han K. “The spermiogenic and ovogenic effects of chronically administered shilajit to rats”. J Ethnopharmacol. 2006;107:349–53. doi: 10.1016/j.jep.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 12.Campbell AK, Wann KT, Matthews SB. Lactose causes heart arrhythmia in the water flea: Daphnia pulex. Comp Biochem Physiol B Biochem Mol Biol. 2004;139:225–34. doi: 10.1016/j.cbpc.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Bekker JM, Krijgsman BJ. Physiological investigations in to the heart function of daphnia. J Physiol. 1951;115:249–57. doi: 10.1113/jphysiol.1951.sp004669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stein RJ, Richter WR, Zussman RA, Brynjolfsson G. Ultrastructural characterization of daphnia heart muscle. J Cell Biol. 1966;29:168–70. doi: 10.1083/jcb.29.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daphnia. [Last accessed on 2010 Oct 19]. Available from: http: //www.nih.gov/science/models .
- 16.Daphnia genome annotation and analysis notes by Don Gilbert. [Last accessed on 2010 Oct 05]. Available from: http://www.gilbert@indiana.edu .
- 17.Villegas-Navarro A, Rosas-L E, Reyes JL. The heart of daphnia magna: Effect of four cardioactive drugs. Comp Biochem Physiol C Toxicol Pharmacol. 2003;136:127–34. doi: 10.1016/s1532-0456(03)00172-8. [DOI] [PubMed] [Google Scholar]
- 18.Postmes TJ, Prick R, Brorens I. The deceleration of the heart frequency in the water flea Daphnia magna by adrenoreceptor agonists and antagonists. Hydrobiologia. 1987;171:141–8. [Google Scholar]


